Advanced Catalytic Peroxymonosulfate Activation via Zeolite-Supported Cu3Mn-Layered Double Hydroxide for Enhanced Oxidative Degradation of Bisphenol A (BPA)
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cu3Mn-LDH and Zeolite/Cu3Mn-LDH(Z-LDH) Preparation
2.2.1. Cu3Mn-LDH Preparation
2.2.2. Z-LDH Preparation
2.3. Analysis and Characterization Methods
2.4. Evaluation of Catalytic Performance: BPA Degradation and Reaction Kinetics
2.5. Reactive Species Identification
3. Results and Discussion
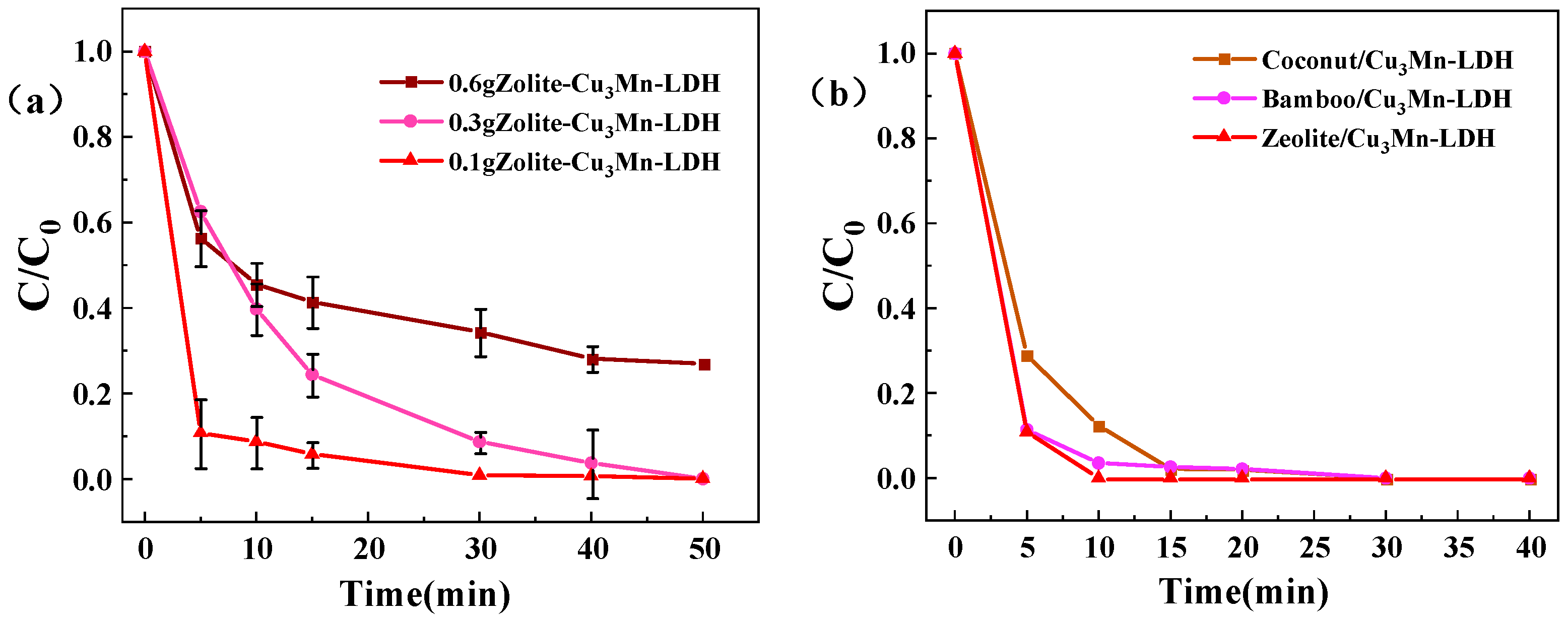
3.1. Material Stoichiometry Optimization
3.2. Z-LDH Catalysts Characterization
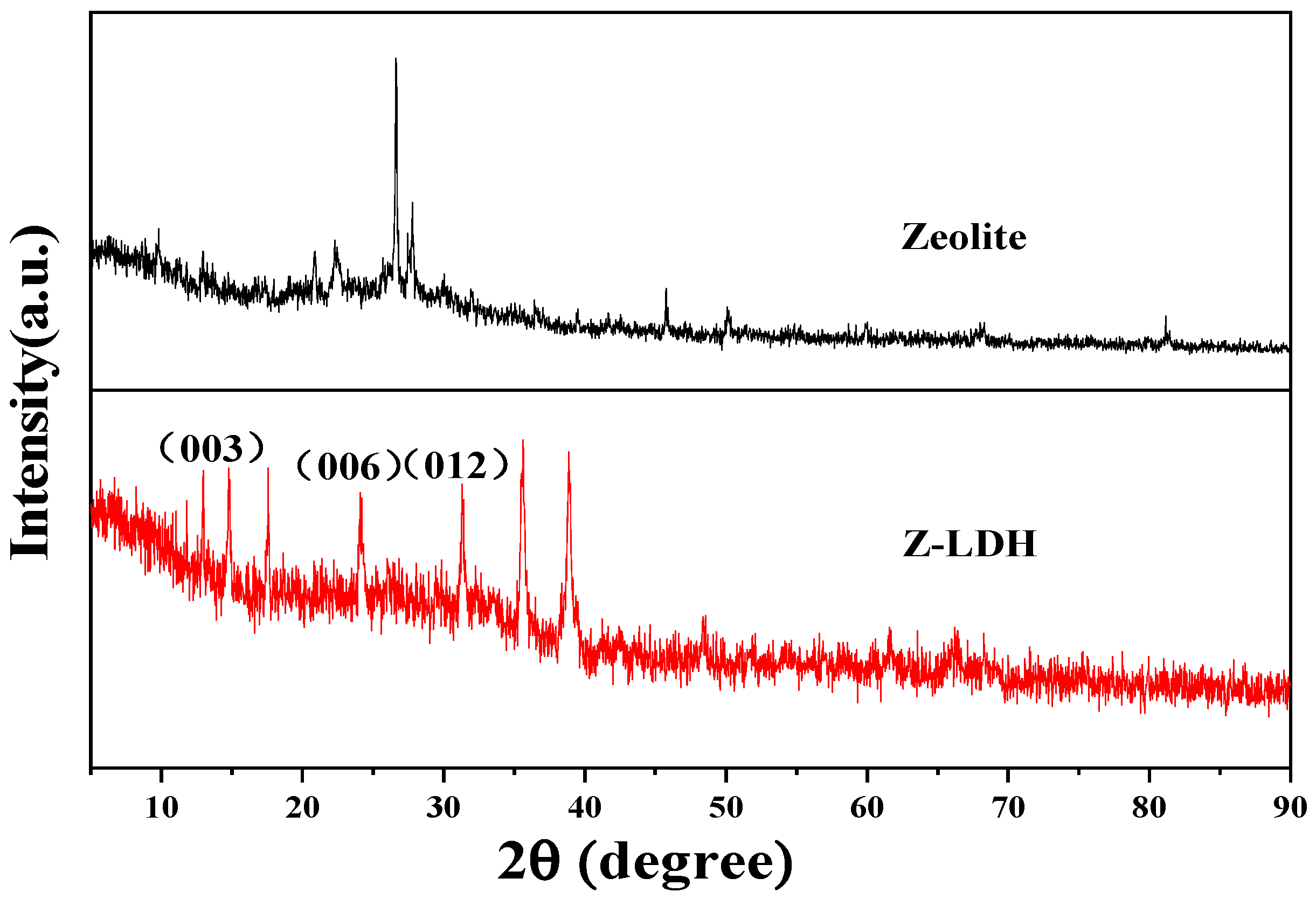
3.2.1. XRD Analysis
3.2.2. Zeolite FTIR Spectra
3.2.3. SEM and TEM Analysis
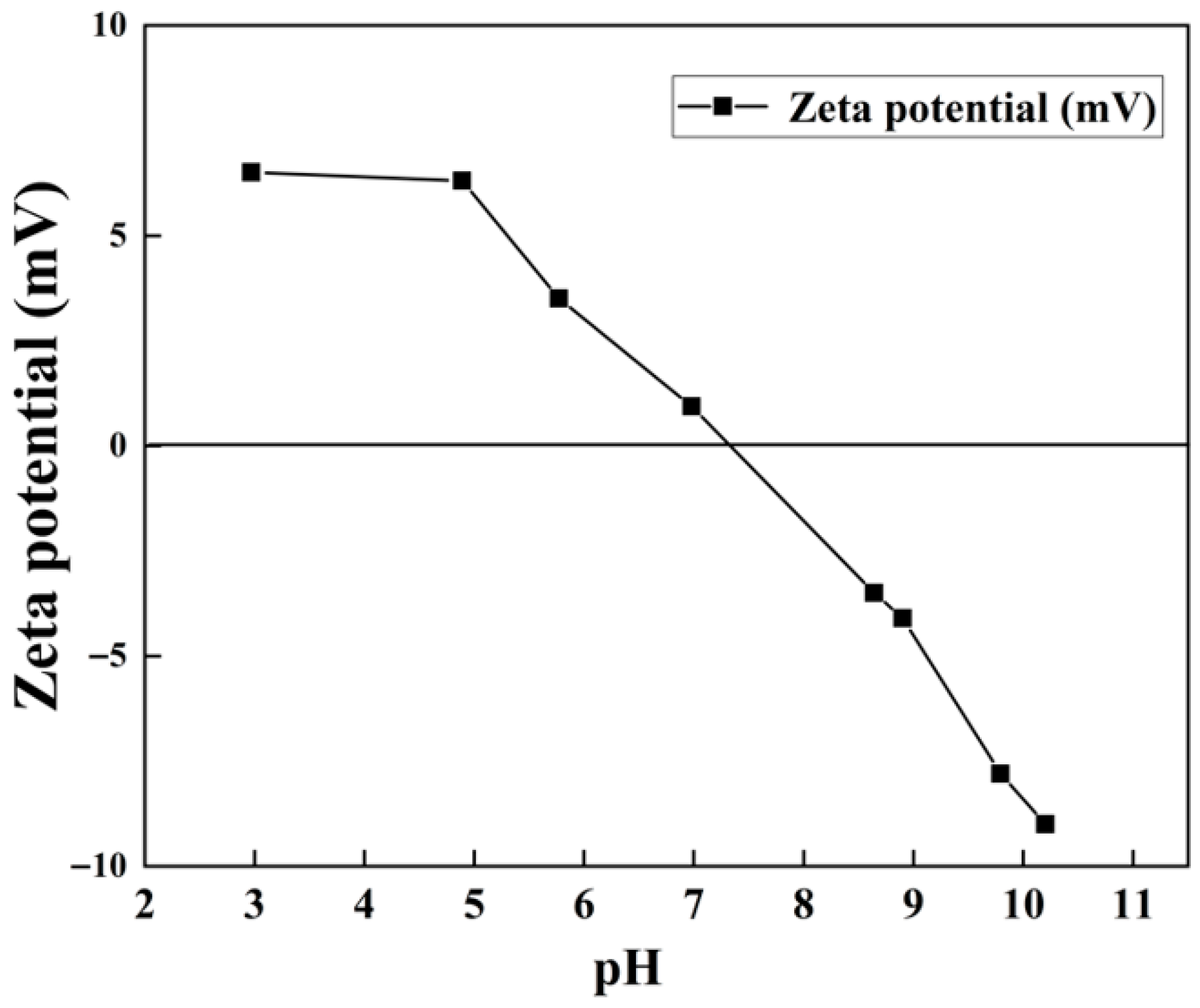
3.2.4. Specific Surface Area Analysis and Zeta Potential
3.3. BPA Catalytic Oxidation in Z-LDH+PMS
3.3.1. Effect of Materials Dosage
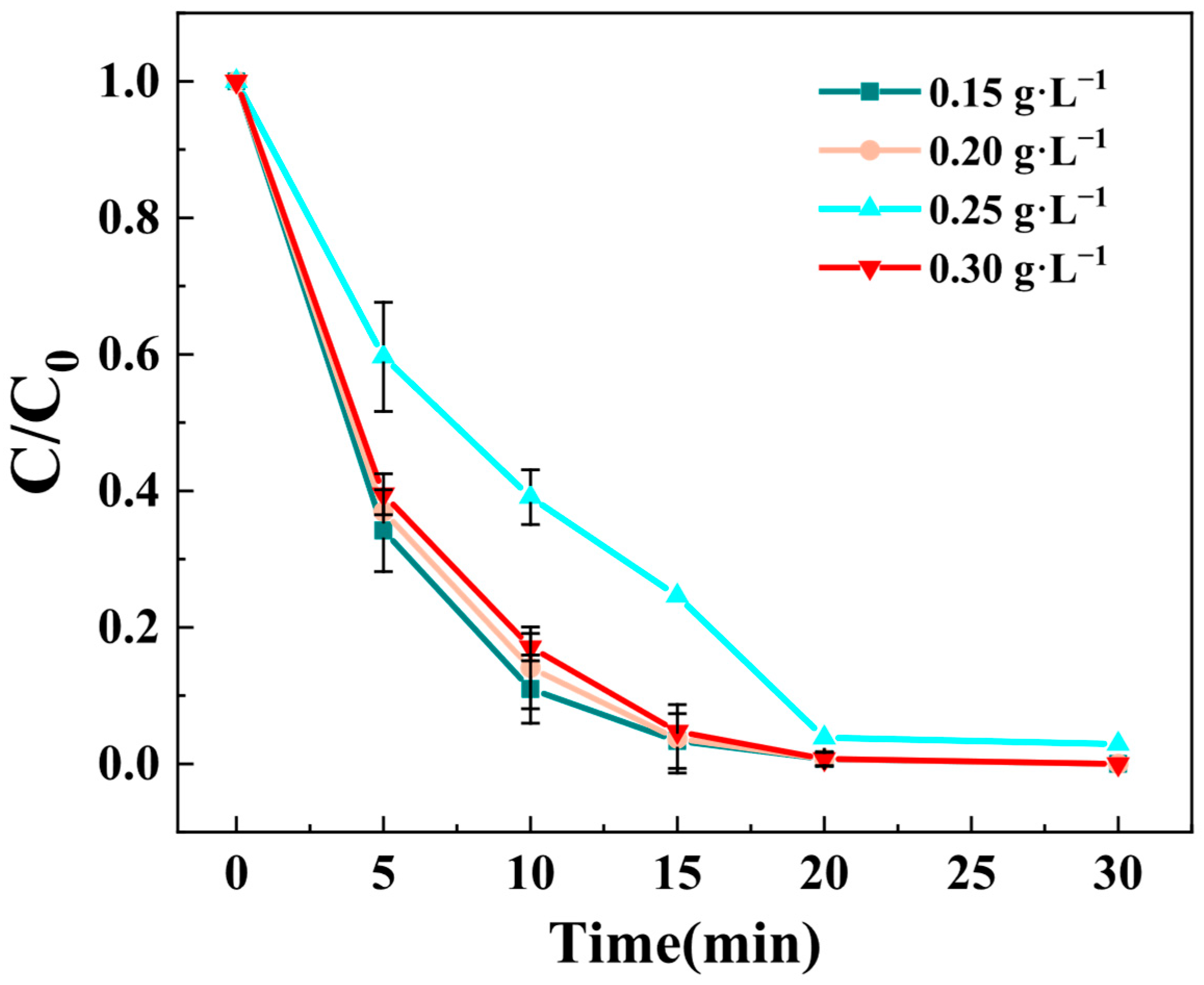
3.3.2. Effect of PMS Concentration
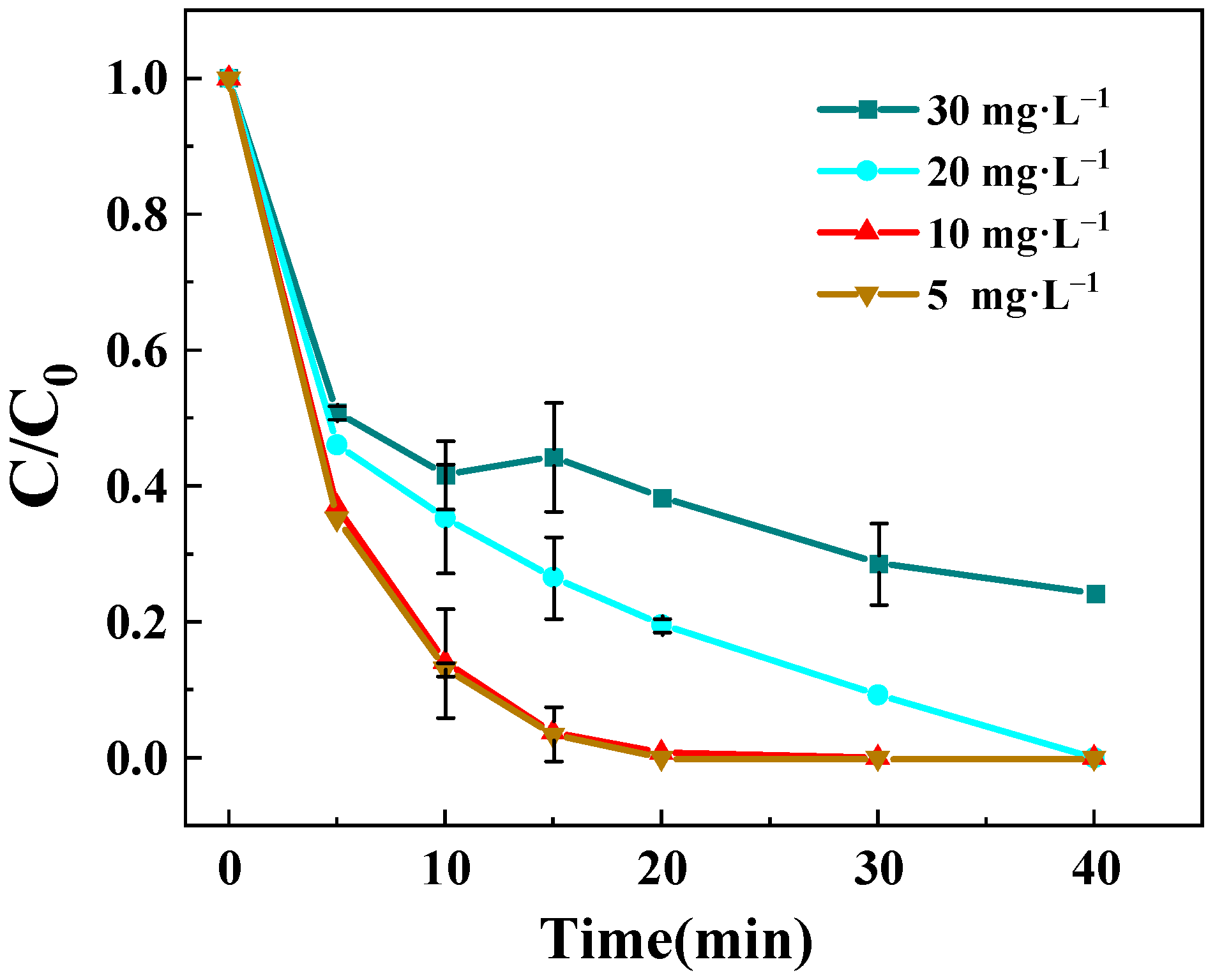
3.3.3. Effect of BPA Concentration
3.3.4. Effect of Coexisting Anions
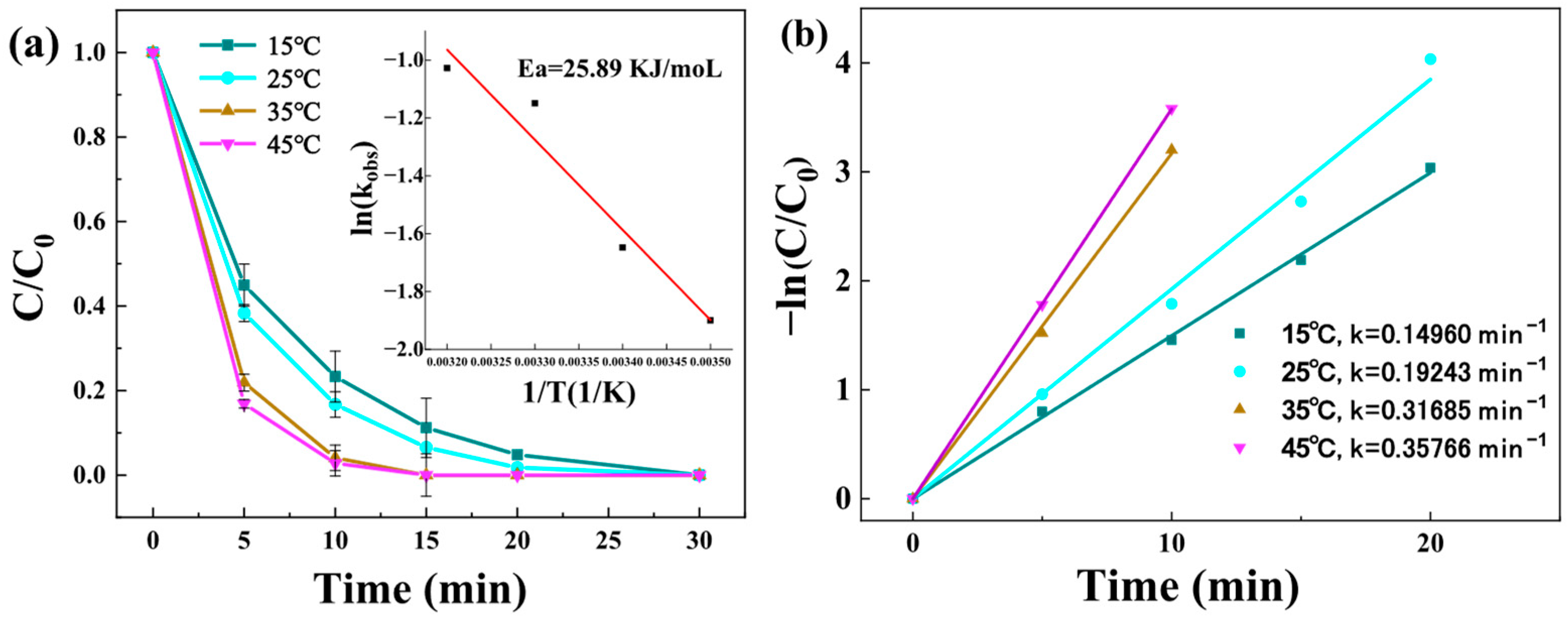
3.3.5. Effect of Solution Temperature
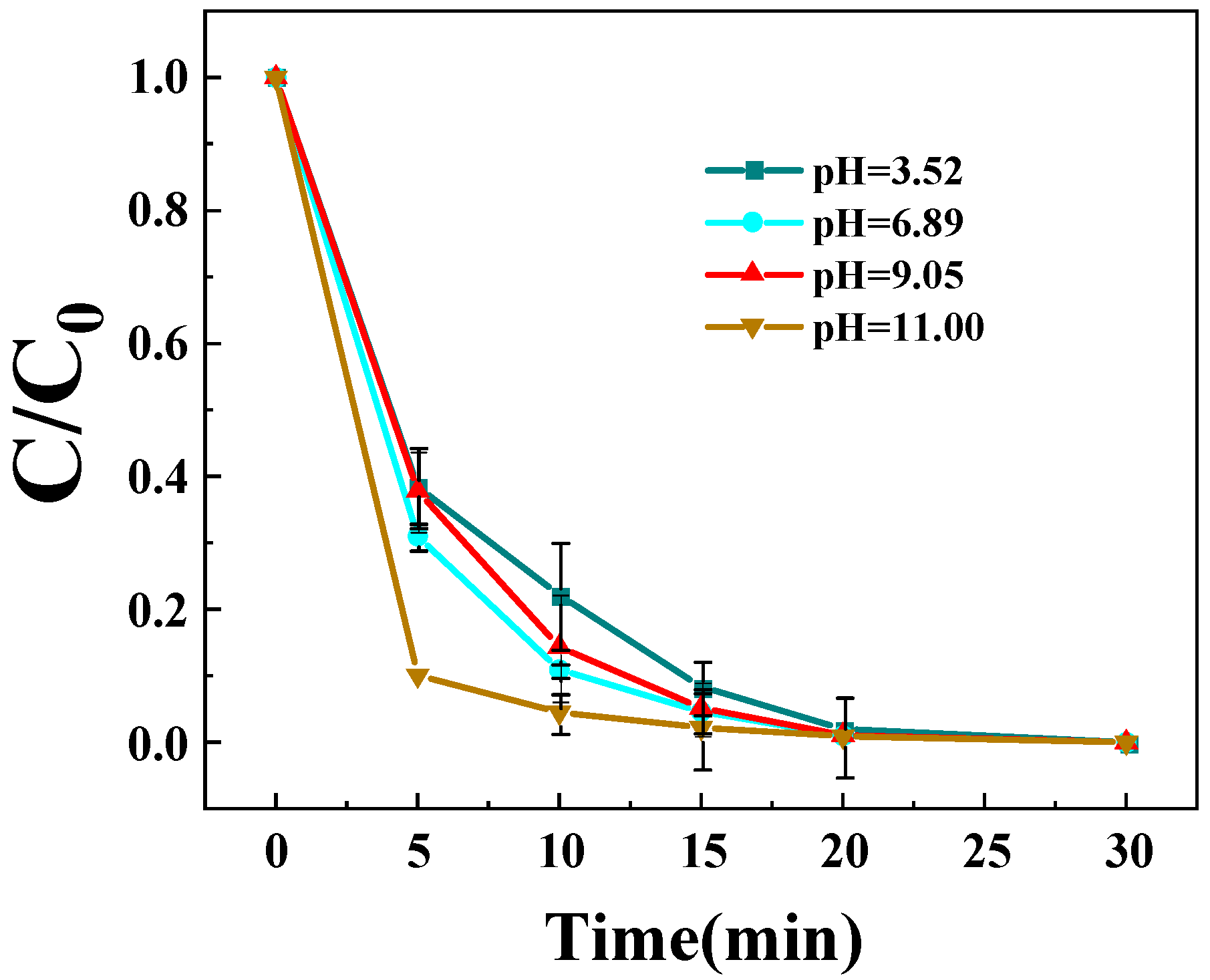
3.3.6. Impact of Initial pH
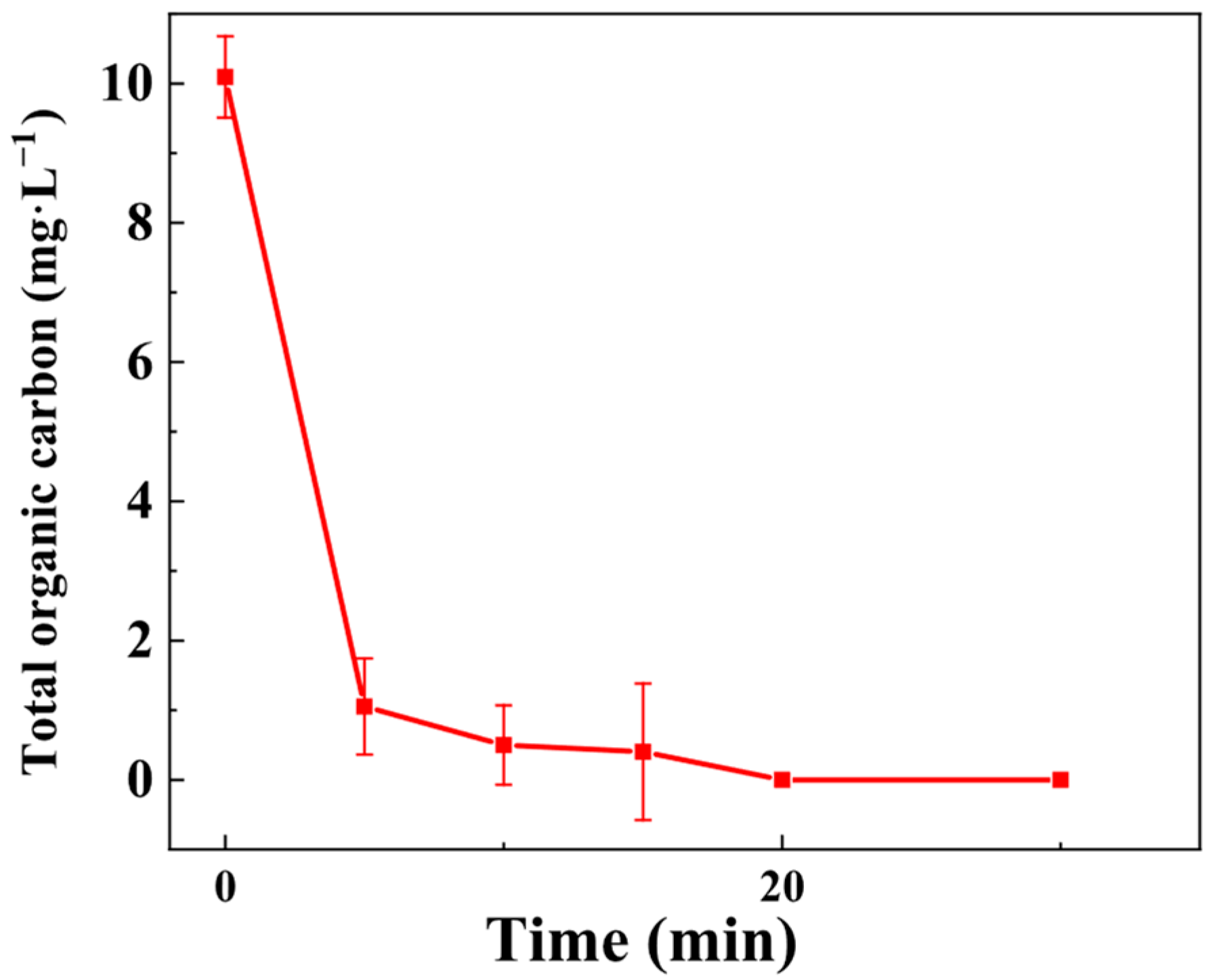
3.3.7. Mineralization Performance: TOC Analysis
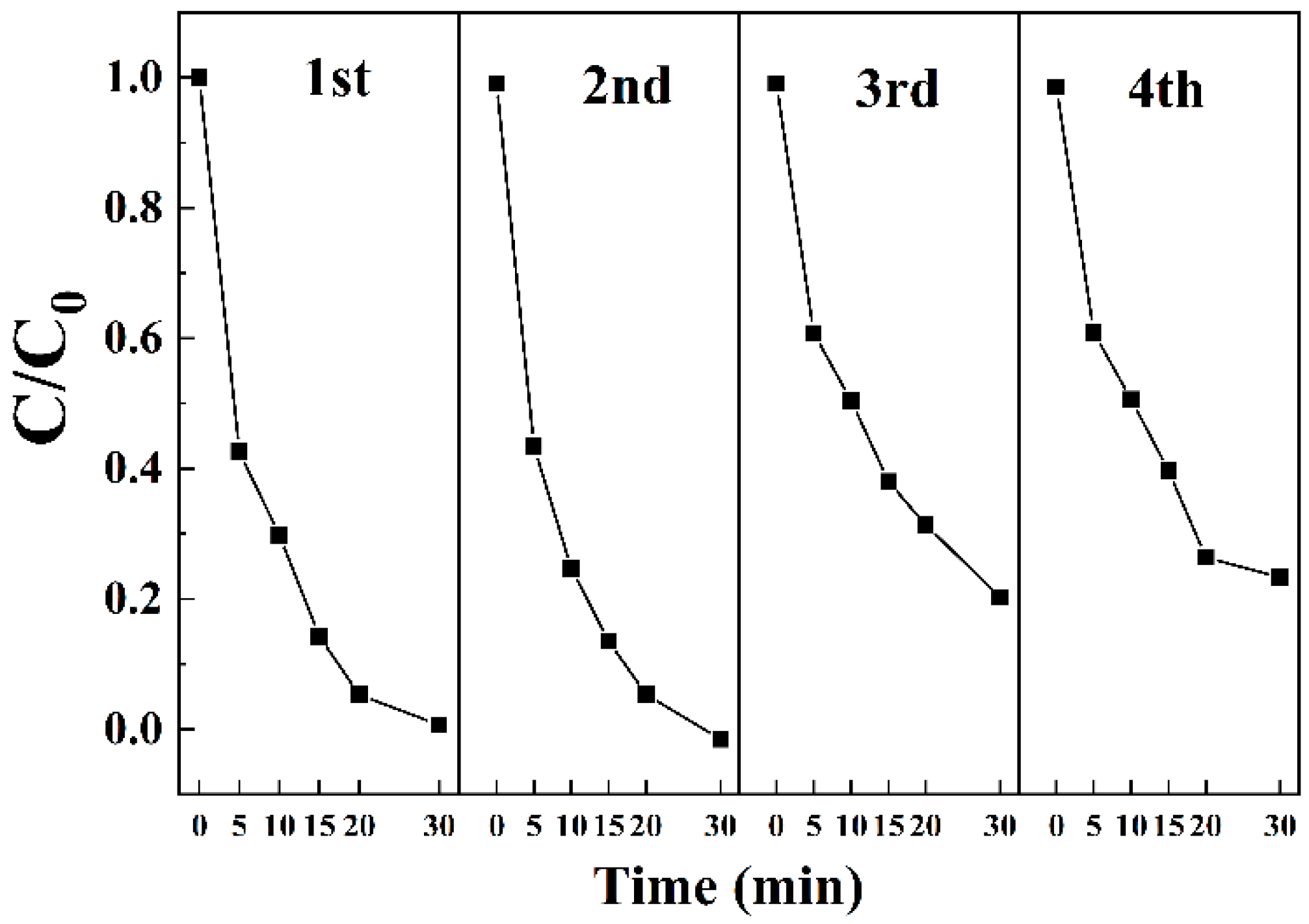
3.3.8. Recycling Performance of the Material
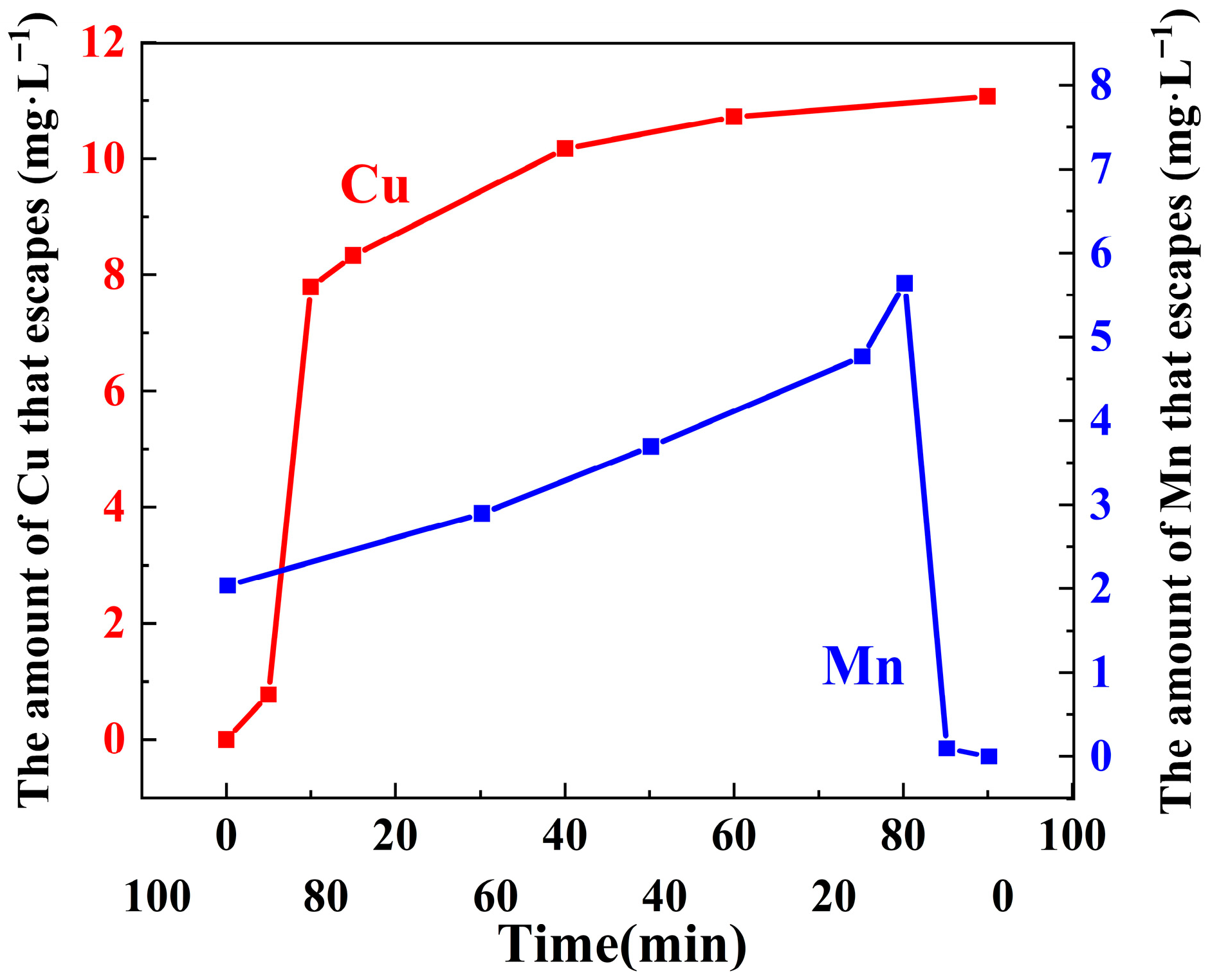
3.3.9. Metal Escape Experiment
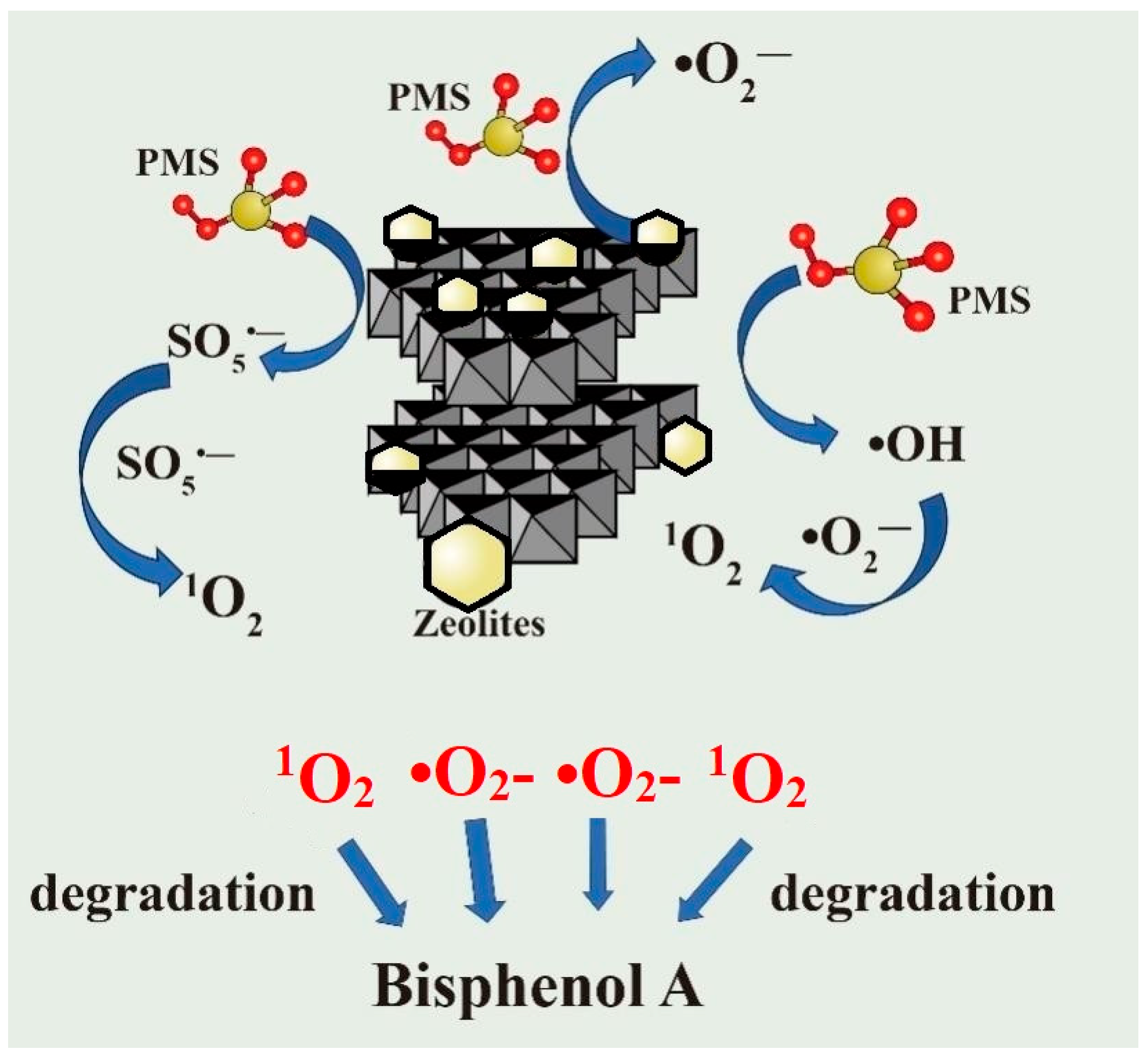
3.4. Possible Catalytic Mechanism for BPA Removal
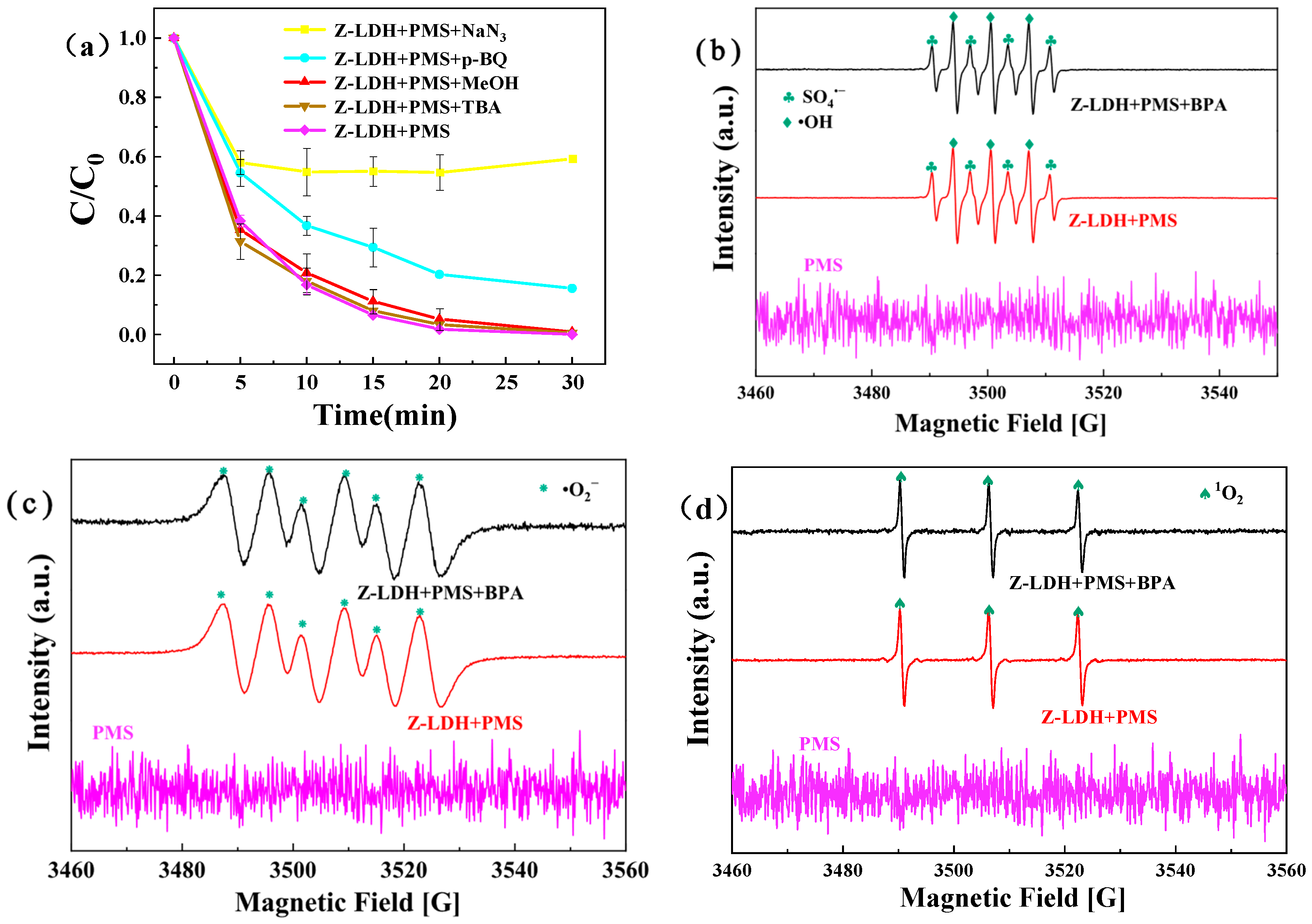
3.4.1. Identification of ROS
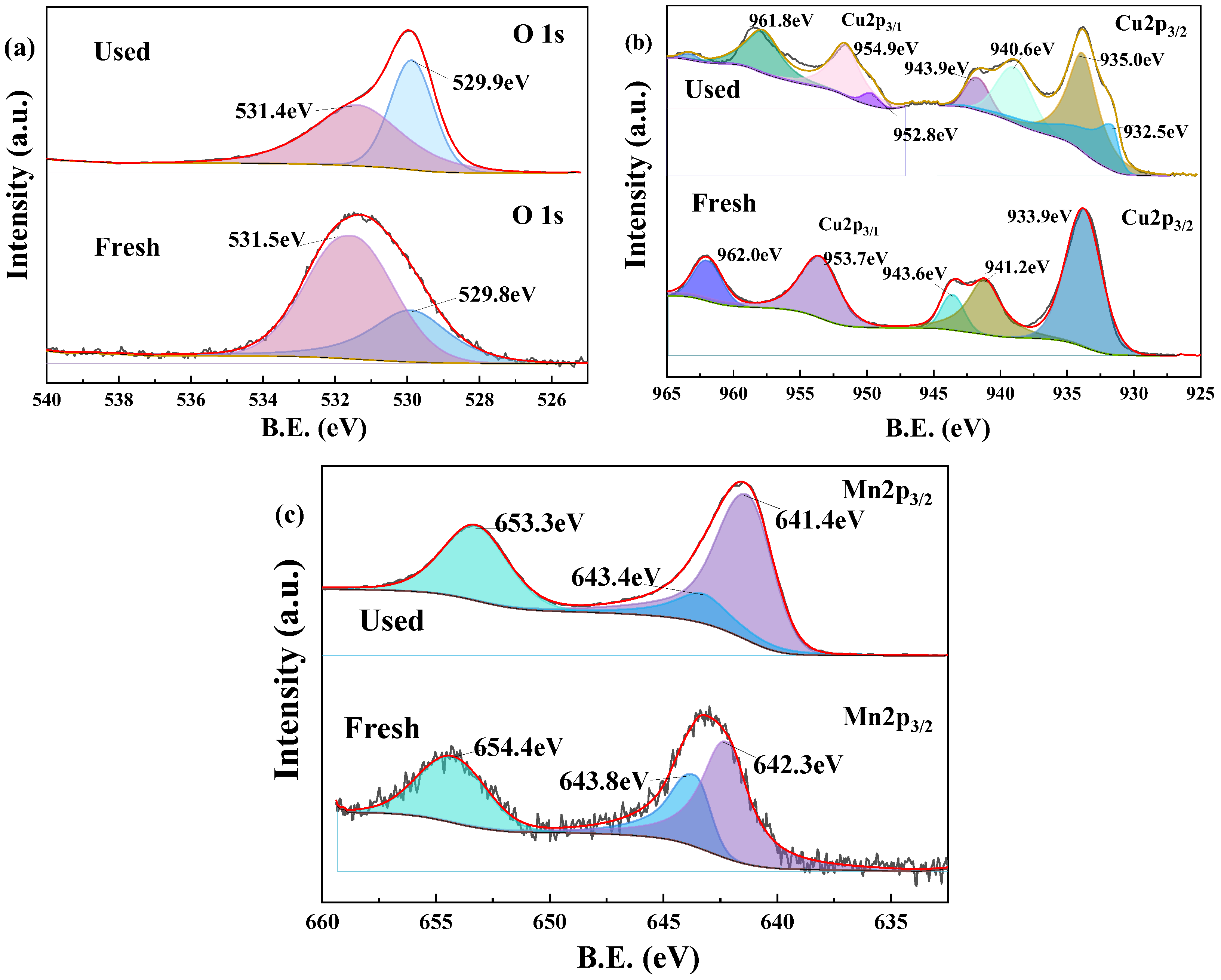
3.4.2. XPS Analysis
3.4.3. Analysis of the Degradation Pathway of BPA by Z-LDH+PMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol A and human chronic diseases: Current evidences, possible mechanisms, and future perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bicalho-Silva, S.; Grigio, V.; Ruiz, T.F.R.; de Freitas Calmon, M.; Rahal, P.; Taboga, S.R.; dos Santos, F.C.A.; Vilamaior, P.S.L. Perinatal exposure to BPA leads to pronounced prostatic morphophysiological disorders in a rodent model of induced hyperplasia. Mol. Cell. Endocrinol. 2025, 610, 112664. [Google Scholar] [CrossRef] [PubMed]
- Torres-García, J.L.; Ahuactzin-Pérez, M.; Fernández, F.J.; Cortés-Espinosa, D.V. Bisphenol A in the environment and recent advances in biodegradation by fungi. Chemosphere 2022, 303, 134940. [Google Scholar] [CrossRef]
- Si, W.; Cai, Y.; Liu, J.; Shen, J.; Chen, Q.; Chen, C.; Ning, L. Investigating the role of colloids on the distribution of bisphenol analogues in surface water from an ecological demonstration area, China. Sci. Total Environ. 2019, 673, 699–707. [Google Scholar] [CrossRef]
- Shi, J.; Liu, X.; Chen, Q.; Zhang, H. Spatial and seasonal distributions of estrogens and bisphenol A in the Yangtze River Estuary and the adjacent East China Sea. Chemosphere 2014, 111, 336–343. [Google Scholar] [CrossRef]
- Ding, M.; Zhou, H.; Ge, M.; Jiang, Z.; Xu, H.; Jia, X.; Gao, E.; Mallya, D.; Gao, L. MXene-based catalysts for water purification in the persulfate-based Fenton-like system. J. Water Process Eng. 2025, 77, 108291. [Google Scholar] [CrossRef]
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. A review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, C.; Zhang, C.; Zheng, H.; Ding, W.; Li, H. Mn/Fe bimetallic MOF-driven sulfite activation for synergistic non-radical oxidation and chemisorption of arsenite: Involvement of high-valent Fe(IV)/Mn(V) species. J. Hazard. Mater. 2025, 496, 139234. [Google Scholar] [CrossRef]
- Yang, S.; Ye, S.; Hu, J.; Zhao, Q.; Chen, J.; Li, C.; Chen, L.; Xiong, J.; Zhu, H.; Wang, S. From solid wastes to functional catalysts for persulfate Activation: Biochar with structured Fe mediated non-radical pathway for chlorinated phenols removal. J. Clean. Prod. 2025, 523, 146443. [Google Scholar] [CrossRef]
- Ge, L.; Shao, B.; Liang, Q.; Huang, D.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; et al. Layered double hydroxide based materials applied in persulfate based advanced oxidation processes: Property, mechanism, application and perspectives. J. Hazard. Mater. 2022, 424, 127612. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Li, Y.; Lu, X.; Chen, Z.; Liu, W.; Yin, G. Controlled leaching with prolonged activity for Co–LDH supported catalyst during treatment of organic dyes using bicarbonate activation of hydrogen peroxide. J. Hazard. Mater. 2015, 289, 165–173. [Google Scholar] [CrossRef]
- Ren, X.; Tian, M.; Fu, N.; Chen, H.; Chen, Z.; Wei, Y.; Wang, Q. Mechanistic study on the enhanced activation of peroxymonosulfate by the bimetallic Mo/Co Co-doped g-C3N4 composite catalyst 8-CNMC for phenol degradation: Applicable for neutral and alkaline condition. J. Environ. Chem. Eng. 2025, 13, 118359. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, H.; Wu, D.; Shang, X.; Lv, M.; Yu, H. Single Co atoms incorporated on Fe-Ni-LDH with enriched oxygen vacancies for efficient peroxymonosulfate activation: Investigation of electron transfer mechanism. J. Environ. Chem. Eng. 2024, 12, 113723. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Li, Q.; Liu, H.; Yao, W.; Wang, Q.; Chai, L. Design of a high-performance ternary LDHs containing Ni, Co and Mn for arsenate removal. J. Hazard. Mater. 2022, 427, 127865. [Google Scholar] [CrossRef]
- Zhao, L.-X.; Li, M.-H.; Jiang, H.-L.; Xie, M.; Zhao, R.-S.; Lin, J.-M. Activation of peroxymonosulfate by a stable Co-Mg-Al LDO heterogeneous catalyst for the efficient degradation of ofloxacin. Sep. Purif. Technol. 2022, 294, 121231. [Google Scholar] [CrossRef]
- Guo, R.; Nengzi, L.-c.; Chen, Y.; Li, Y.; Zhang, X.; Cheng, X. Efficient degradation of sulfamethoxazole by CuCo LDH and LDH@fibers composite membrane activating peroxymonosulfate. Chem. Eng. J. 2020, 398, 125676. [Google Scholar] [CrossRef]
- Sadeghi, M.; Moradi, M. Mg–Al LDH@MoS2 nanocomposite as promising recyclable nanosorbent for high-efficient removal of endocrine disrupting chemicals from aqueous environment. Colloids Surf. A Physicochem. Eng. Asp. 2025, 710, 136247. [Google Scholar] [CrossRef]
- Yan, J.; Chen, Y.; Qian, L.; Gao, W.; Ouyang, D.; Chen, M. Heterogeneously catalyzed persulfate with a CuMgFe layered double hydroxide for the degradation of ethylbenzene. J. Hazard. Mater. 2017, 338, 372–380. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, D.; Zhao, Y.; Jiang, J.; Wang, B.; Wei, S.; Li, C.; Zhang, F.; Lei, X.; Wang, Y. Synergistic activation of peroxymonosulfate by the laminate metals of MgMnFe-layered double hydroxides for imidacloprid degradation. Sep. Purif. Technol. 2025, 354, 129194. [Google Scholar] [CrossRef]
- Xie, M.; Liang, M.; Liu, C.; Xu, Z.; Yu, Y.; Xu, J.; You, S.; Wang, D.; Rad, S. Peroxymonosulfate activation by CuMn-LDH for the degradation of bisphenol A: Effect, mechanism, and pathway. Ecotoxicol. Environ. Saf. 2024, 270, 115929. [Google Scholar] [CrossRef]
- Li, T.; Zou, J.; Hong, L.; Zhang, J.; Sun, W.; Qiu, Z.; Younas, M. Facile synthesis of carbon doped CoAl-LDH for peroxymonosulfate activation and antibiotic degradation: Pilot scale and hospital wastewater application. J. Environ. Chem. Eng. 2025, 13, 118933. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Yin, R.-B.; Wei, Y.-F.; Fan, Z.-Y.; Gao, H.-L. Innovative hydrothermal synthesis of NiAl LDHs and their enhanced electrochemical performance via graphene oxide composites. Inorg. Chem. Commun. 2025, 182, 115602. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Dai, Z.; Jiang, W.; Ma, S.; Yao, L.; Chen, Y.; Zhou, Q.; Zheng, J. Zeolite-induced defect engineering for synthesis of CuBTC-derived novel carbon-zeolite bifunctional support catalyst for multicomponent VOCs catalytic oxidation removal. Chem. Eng. J. 2024, 500, 156830. [Google Scholar] [CrossRef]
- Chen, D.; Bai, Q.; Ma, T.; Jing, X.; Tian, Y.; Zhao, R.; Zhu, G. Stable metal–organic framework fixing within zeolite beads for effectively static and continuous flow degradation of tetracycline by peroxymonosulfate activation. Chem. Eng. J. 2022, 435, 134916. [Google Scholar] [CrossRef]
- Ramos, M.J.; Casas, A.; Rodríguez, L.; Romero, R.; Pérez, Á. Transesterification of sunflower oil over zeolites using different metal loading: A case of leaching and agglomeration studies. Appl. Catal. A Gen. 2008, 346, 79–85. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Yu, J. Advances in Catalytic Applications of Zeolite-Supported Metal Catalysts. Adv. Mater. 2021, 33, 2104442. [Google Scholar] [CrossRef]
- Peng, C.; Qian, M.; Li, R.; Hardacre, C.; Chansai, S.; Liu, Z. Recent advances in the catalytic oxidation of VOCs over core–shell and yolk-shell structured catalysts. Fuel 2025, 399, 135585. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Van, H.-T.; Dang, V.M.; Ha Tran, T.N.; Nguyen, T.T.; Hoang, T.K. Insight into chromium adsorption from contaminated soil using Mg/Al LDH-zeolite. Heliyon 2024, 10, e31084. [Google Scholar] [CrossRef] [PubMed]
- Metkar, P.S.; Balakotaiah, V.; Harold, M.P. Experimental study of mass transfer limitations in Fe- and Cu-zeolite-based NH3-SCR monolithic catalysts. Chem. Eng. Sci. 2011, 66, 5192–5203. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Wang, X. Removal mechanism of lead by the composite of clinoptilolite, biochar, and Ca(OH)2 and its application in acid wastewater. Environ. Technol. Innov. 2025, 37, 104024. [Google Scholar] [CrossRef]
- Gunawan, T.; Widiastuti, N.; Widyanto, A.R.; Fansuri, H.; Akhlus, S.; Salleh, W.N.W.; Ismail, A.F.; Sazali, N.; Lin, R.; Motuzas, J.; et al. Fine-tuning zeolite pore structures with carbon coating for enhanced gas separation in polyimide-based mixed matrix membrane. Chem. Eng. Res. Des. 2024, 204, 556–571. [Google Scholar] [CrossRef]
- Lundegaard, L.F.; Berdiell, I.C.; König, N.; Junge, N.H.; Beato, P.; Chernyshov, D.; Svelle, S.; Wragg, D.S. Tracking deactivation of zeolite beta with and without a detailed structure model: XRD analysis and in situ studies. Microporous Mesoporous Mater. 2024, 366, 112911. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, Z.; Ashraf, G.; Wang, J.; Luo, H.; Chen, X.; Xiao, F.; Liu, H. Hierarchical CNTs@CuMn Layered Double Hydroxide Nanohybrid with Enhanced Electrochemical Performance in H2S Detection from Live Cells. Anal. Chem. 2019, 91, 3912–3920. [Google Scholar] [CrossRef]
- Huang, X.; Lang, L.; Li, J.-s.; Poon, C.S. Synthesis of Na-A zeolite loaded bentonite and its application for removal of Cu(II) from aqueous solutions. J. Water Process Eng. 2023, 56, 104359. [Google Scholar] [CrossRef]
- Asano, N.; Asahina, S.; Lu, J.; Xu, J.; Shen, Y.; Qin, Z.; Mintova, S. Advanced scanning electron microscopy techniques for structural characterization of zeolites. Inorg. Chem. Front. 2022, 9, 4225–4231. [Google Scholar] [CrossRef]
- Belviso, C.; Piancastelli, A.; Sturini, M.; Belviso, S. Synthesis of composite zeolite-layered double hydroxides using ultrasonic neutralized red mud. Microporous Mesoporous Mater. 2020, 299, 110108. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, M.; Gu, P.; Huang, W.; Yu, C.-P.; Zheng, Z.; Wang, Y.; Yao, N.; Li, Y. Layered double hydroxide@zeolite composite modified sponge: A versatile biocarrier for enhancing nitrogen removal in biofilters. Chem. Eng. J. 2024, 481, 148686. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-geopolymer composite materials: Production and characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- Kumar, P.; Agrawal, K.V.; Tsapatsis, M.; Mkhoyan, K.A. Quantification of thickness and wrinkling of exfoliated two-dimensional zeolite nanosheets. Nat. Commun. 2015, 6, 7128. [Google Scholar] [CrossRef]
- Diaz, I.; Mayoral, A. TEM studies of zeolites and ordered mesoporous materials. Micron 2011, 42, 512–527. [Google Scholar] [CrossRef]
- Mkaddem, H.; Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Benamor, H.; Sanromán, M.A. Efficient degradation of organic pollutants using MnCuFe-LDH as a photo-fenton catalyst. Chem. Eng. Sci. 2025, 311, 121611. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Wang, J. Photo-Fenton degradation of rhodamine B using Fe2O3–Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid. Interface Sci. 2014, 433, 1–8. [Google Scholar] [CrossRef]
- Feng, J.; Mu, J.; Peng, Q.; Xiao, Y.; Liu, B. In situ interfacial engineering modulated adsorption and electron transfer boosting peroxymonosulfate activation for micro-contaminates degradation over CoOx nanosheet. Chem. Eng. J. 2023, 476, 146724. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Li, Q.; Ren, A.; Li, H.; Gong, Y.; Wu, J.; Ye, Y.; Li, W.; Pan, F. Enhanced bisphenol A degradation by CuMnO2 via peroxymonosulfate activation: Dominant 1O2-mediated pathway and electron transfer promotion. Sep. Purif. Technol. 2025, 377, 134211. [Google Scholar] [CrossRef]
- Bian, H.; OuYang, Z.; Zhang, X.; Wang, J. Efficient activation of peroxymonosulfate by CoCuAl-LDH nanosheets for the degradation of RhB. J. Solid. State Chem. 2025, 349, 125414. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Han, Q.; Wang, M.; Sun, F.; Yu, B.; Dong, Z.; Li, P.; Luo, J.; Li, M.; Jin, X.; Dai, Z. Effectiveness and degradation pathways of bisphenol A (BPA) initiated by hydroxyl radicals and sulfate radicals in water: Initial reaction sites based on DFT prediction. Environ. Res. 2023, 216, 114601. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Zhou, D.; Chen, X.; Zhang, M.; Lin, K. Degradation of bisphenol a using peroxymonosulfate activated by single-atomic cobalt catalysts: Different reactive species at acidic and alkaline pH. Chem. Eng. J. 2022, 439, 135002. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, G.; Liu, M.; Wang, Q.; Wang, P. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix. Chem. Eng. J. 2018, 338, 300–310. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Ahn, Y.-Y.; Kim, M.; Choi, J.; Min, D.; Kim, J.; Moon, G.-H.; Lee, J. Role of inorganic anions in improving the oxidizing capacity of heat-activated peroxymonosulfate: Identification of primary degradative pathways. Chem. Eng. J. 2023, 478, 147472. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, C.; Zhang, L.; Guo, H.; Wen, X.; Liang, C.; Zeng, G. Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere 2018, 204, 11–21. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B-Environ. 2012, 123–124, 117–126. [Google Scholar] [CrossRef]
- Wu, M.; Tang, S.; Wang, Z.; Zhang, Q.; Yuan, D. Molybdenum carbide activated calcium sulfite for antibiotic decontamination at near-neutral pH: Dissolved oxygen promoted bisulfite adsorption for singlet oxygen generation. Chin. Chem. Lett. 2025, 36, 110613. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Liu, S.; Li, R. Efficient decolorization of Methylene Blue catalyzed by MgFe-layered double hydroxides in the presence of hydrogen peroxide. Water Sci. Technol. 2020, 81, 781–789. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, T.M.P.; Van, H.T.; Nguyen, V.Q.; Nguyen, L.H.; Nguyen, T.D.; Nguyen, T.H.V.; Chu, T.H.H.; Nguyen, T.H.; Ha, L.T.; et al. Adsorption removal of ammonium from aqueous solution using Mg/Al layered double hydroxides-zeolite composite. Environ. Technol. Innov. 2022, 25, 102244. [Google Scholar] [CrossRef]
- Mi, X.; Ma, R.; Pu, X.; Fu, X.; Geng, M.; Qian, J. FeNi-layered double hydroxide (LDH)@biochar composite for, activation of peroxymonosulfate (PMS) towards enhanced degradation of doxycycline (DOX): Characterizations of the catalysts, catalytic performances, degradation pathways and mechanisms. J. Clean. Prod. 2022, 378, 134514. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Yu, X.; Sun, Z.; Wang, H.; Guo, J. Ultraefficient water purification with low peroxymonosulfate input by a polymerization-driven single-atom iron dispersed layered double hydroxide membrane. J. Membr. Sci. 2025, 732, 124250. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Gao, Z.; He, J.; Liu, Z.; Duan, L.; Sun, T.; Wu, J.; Liang, D.; Wei, C.; et al. High-efficiency Cu2O/CuFe-LDH nanocomposite for Fenton degradation of organic pollutants: Boosted dual redox cycles. Appl. Catal. A Gen. 2025, 707, 120516. [Google Scholar] [CrossRef]
- Momin, Z.H.; Lingamdinne, L.P.; Kulkarni, R.; Pal, C.A.K.; Choi, Y.-L.; Koduru, J.R.; Chang, Y.-Y. Improving U(VI) retention efficiency and cycling stability of GCN-supported calcined-LDH composite: Mechanism insight and real water system applications. Chemosphere 2024, 346, 140551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Z.; Liu, B.; Han, D.; Zhang, X.; Liu, J.; Huang, Y.; Xing, C. Study on the performance regulation mechanism of in-situ Bi doping on the degradation of tetracycline by Prussian blue analogue activated PMS catalytic system. J. Water Process Eng. 2025, 76, 108085. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Zhang, X.; Wang, J.; Hong, M.; Liang, W.; Zhang, X.; Liu, G. Efficient removal of bisphenol A by BiFeO3/CuMnFe-LDHs Z-scheme heterojunctions in a peroxymonosulfate/visible light system: A mechanism study. Environ. Res. 2025, 285, 122295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Wei, J.; Liu, Z.; Wang, H.; Zhu, Y.; Shao, Q.; Xie, P. Efficient bimetallic activation of sulfite by CoFe-LDH for bisphenol A removal: Critical role of Co/Fe ratio on ROS generation. Chem. Eng. J. 2024, 492, 152171. [Google Scholar] [CrossRef]
- Geng, H.; Wang, F.; Yan, C.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J. Hazard. Mater. 2020, 383, 121136. [Google Scholar] [CrossRef]
- Wang, X.-y.; Xiao, X.-y.; Guo, Z.-h.; Peng, C.; Richmond, A.; Xue, S.-g.; Bridget, A. Influence of pH on the leaching behaviour of heavy metal(loid)s in copper smelting flue dust and mineralogical control mechanism. J. Cent. South Univ. 2024, 31, 1121–1135. [Google Scholar] [CrossRef]
- GB 25467-2010; Emission Standard of Pollutants for Copper, Nickel, Cobalt Industry. Environmental Science Press: Beijing, China, 2010.
- Yang, C.; Li, X.; Li, M.; Liang, G.; Jin, Z. Anchoring oxidation co-catalyst over CuMn2O4/graphdiyne S-scheme heterojunction to promote eosin-sensitized photocatalytic hydrogen evolution. Chin. J. Catal. 2024, 56, 88–103. [Google Scholar] [CrossRef]
- Li, T.; Sun, W.; Zhang, J.; Liu, S.; Chen, H.; Qiu, Z.; Lam, J.C.-H. Co3O4 confined in activated carbon-supported Mg/Al-LDH for µM peroxymonosulfate activation with minute-level antibiotics degradation. Chem. Eng. J. 2025, 516, 163872. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef]
- He, S.; Yin, R.; Chen, Y.; Lai, T.; Guo, W.; Zeng, L.; Zhu, M. Consolidated 3D Co3Mn-layered double hydroxide aerogel for photo-assisted peroxymonosulfate activation in metronidazole degradation. Chem. Eng. J. 2021, 423, 130172. [Google Scholar] [CrossRef]
- Cao, J.; Sun, S.; Li, X.; Yang, Z.; Xiong, W.; Wu, Y.; Jia, M.; Zhou, Y.; Zhou, C.; Zhang, Y. Efficient charge transfer in aluminum-cobalt layered double hydroxide derived from Co-ZIF for enhanced catalytic degradation of tetracycline through peroxymonosulfate activation. Chem. Eng. J. 2020, 382, 122802. [Google Scholar] [CrossRef]
- Ji, Y.; Song, Z.; Xu, Y.; Zhang, Y. Cu-Fe LDHs/Bi2WO6 composite for superior photo-Fenton Rhodamine B removal through combination of photogenerated electrons and multivalent bimetal redox for accelerating Fe3+/Fe2+ cycles. J. Alloys Compd. 2022, 925, 166655. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.-Y.; Peng, J.-F.; Wang, L.-H.; Peng, D.-Y.; Liu, F.-Y.; Xu, S.; Yang, Z.-L. Fabrication of Cu2O/ZnTi-LDH p-n heterostructure by grafting Cu2O NPs onto the LDH host layers from Cu-doped ZnTi-LDH and insight into the photocatalytic mechanism. Compos. Part B Eng. 2023, 250, 110447. [Google Scholar] [CrossRef]
- Xia, W.; Luan, X.; Zhang, W.; Wu, D. Sulfuration of hierarchical Mn-doped NiCo LDH heterostructures as efficient electrocatalyst for overall water splitting. Int. J. Hydrog. Energy 2023, 48, 27631–27641. [Google Scholar] [CrossRef]
- Jiang, Z.-R.; Wang, P.; Zhou, Y.-X.; Wang, C.; Jiang, J.; Lan, Y.; Chen, C. Fabrication of a 3D-blocky catalyst (CoMnOx@sponge) via mooring Co-Mn bimetallic oxide on sponge to activate peroxymonosulfate for convenient and efficient degradation of sulfonamide antibiotics. Chem. Eng. J. 2022, 446, 137306. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, G.; Fan, Y.; Xia, Y.; Li, J.; Zhang, J.; Shih, K.; Tang, Y. Continuous degradation of plasticizer bisphenol A by catalytic Cu(FexAl2−x)O4@sediment ceramic membranes combined with peroxymonosulfate activation. Chem. Eng. J. 2023, 469, 143867. [Google Scholar] [CrossRef]
- Chen, X.; Duan, C.; Zhou, Y.; Yang, L.; Zhou, Y. Peroxymonosulfate activation by β-cyclodextrin modified amorphous manganese oxides for a high-efficiency bisphenol A degradation. J. Clean. Prod. 2023, 395, 136323. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhou, W. Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: Key role of superoxide radicals. J. Colloid Interface Sci. 2022, 610, 504–517. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, K.; Zhang, Z.; Gu, P.; Liu, S.; Li, M.; Wang, X.; Yin, Y.; Zhang, Z.; Wang, T.; et al. Heterogeneous activation of peroxymonosulfate by magnetic hybrid CuFe2O4@N-rGO for excellent sulfamethoxazole degradation: Interaction of CuFe2O4 with N-rGO and synergistic catalytic mechanism. Chemosphere 2023, 313, 137392. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Barzegar, G.; Ghanbari, F. Photo assisted electro-peroxone to degrade 2,4-D herbicide: The effects of supporting electrolytes and determining mechanism. Process Saf. Environ. Prot. 2017, 111, 520–528. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Li, J.; You, W.; Zhong, K.; Feng, L.; Han, S.; Zhang, X.; Pan, T.; Liu, W.; et al. PMS coupled Mn(II) mediated electrochemistry processes (E-Mn(II)-PMS) on the efficient RB19 wastewater treatment: Focus on the regulation and reinforcement of Mn(III)/Mn(II). Environ. Res. 2024, 240 Pt 1, 117220. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Cheng, Z.; Zhang, X.; Yan, C.; Wei, J.; Qiu, F.; Liu, Y. Fe–Mn bimetallic catalyst to activate peroxymonosulfate (PMS) for efficient degradation of tetracycline: Mechanism insights and application for pharmaceutical wastewater. J. Clean. Prod. 2024, 445, 141365. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, H.; Wang, P.; Zhang, M.; Yang, P. Multiwall carbon nanotube decorated hemin/Mn-MOF towards BPA degradation through PMS activation. J. Environ. Chem. Eng. 2022, 10, 108426. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yang, B.; Su, Y.; Xu, J.; Deng, J.; Zhou, T. Promoted BPA degradation in food waste leachate via alkali-fluffed CoFe2O4@CoSiOx activated PMS under the assistance of inherent acetate. Sep. Purif. Technol. 2023, 306, 122566. [Google Scholar] [CrossRef]





| Material | Degradation Efficiency | TOC Removal Rate | Dosage | Loop Count | Source |
|---|---|---|---|---|---|
| Z-LDH | 100% within 20 min | 97% within 20 min | [Z-LDH] = 0.15 g·L−1, [PMS] = 0.25 g·L−1, [BPA] = 10 mg·L−1 | 80% after 3 cycles | This work |
| Cu3Mn-LDH | 100% within 40 min | 95% within 40 min | [Cu3Mn-LDH] = 0.10 g·L−1, [PMS] = 0.25 g·L−1, [BPA] = 10 mg·L−1 | 97% after 4 cycles | [21] |
| 0.5BFO-LDHs | 99.3% within 15 min (with light) | 97.6% within 15 min | [BFO-LDHs] = 0.4g·L−1, [PMS] = 0.4 mmol·L−1, [BPA]0 = 20 mg·L−1 | 96.9% after 5 cycles | [62] |
| CoFe-LDH/S(IV) | 84.8% within 20 min | Not reported | [CoFe-LDH] = 0.1 g·L−1, [S(IV)] = 1.5 mmol·L−1, [BPA]0 = 2 μmol·L−1 | 80% after 5 cycles | [63] |
| FeNi-LDH@biochar | Not specified (60% TOC removal in 120 min) | 60% within 120 min | [FeNi-LDH@biochar] = 0.50 g·L−1, [PMS] = 0.75 g·L−1, [DOX]0 = 35 mg·L−1 | 80% after 3 cycles | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, C.; Liang, M.; Feng, M.; Xu, Z.; Wang, D.; Rad, S. Advanced Catalytic Peroxymonosulfate Activation via Zeolite-Supported Cu3Mn-Layered Double Hydroxide for Enhanced Oxidative Degradation of Bisphenol A (BPA). Toxics 2025, 13, 889. https://doi.org/10.3390/toxics13100889
Li Q, Liu C, Liang M, Feng M, Xu Z, Wang D, Rad S. Advanced Catalytic Peroxymonosulfate Activation via Zeolite-Supported Cu3Mn-Layered Double Hydroxide for Enhanced Oxidative Degradation of Bisphenol A (BPA). Toxics. 2025; 13(10):889. https://doi.org/10.3390/toxics13100889
Chicago/Turabian StyleLi, Qiuyi, Chongmin Liu, Meina Liang, Mi Feng, Zejing Xu, Dunqiu Wang, and Saeed Rad. 2025. "Advanced Catalytic Peroxymonosulfate Activation via Zeolite-Supported Cu3Mn-Layered Double Hydroxide for Enhanced Oxidative Degradation of Bisphenol A (BPA)" Toxics 13, no. 10: 889. https://doi.org/10.3390/toxics13100889
APA StyleLi, Q., Liu, C., Liang, M., Feng, M., Xu, Z., Wang, D., & Rad, S. (2025). Advanced Catalytic Peroxymonosulfate Activation via Zeolite-Supported Cu3Mn-Layered Double Hydroxide for Enhanced Oxidative Degradation of Bisphenol A (BPA). Toxics, 13(10), 889. https://doi.org/10.3390/toxics13100889







