The Unhappy Effects of the Antidepressant Fluoxetine on the Freshwater Microalga Raphidocelis subcapitata
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Exposure of R. subcapitata to FLX
2.3. Nuclei Counting
2.4. Assessment of Cell Membrane Integrity
2.5. Determination of Metabolic Activity
2.6. Evaluation of Pigments Concentration and Photosynthetic Performance
2.7. Mitochondrial Membrane Potential Assessment
2.8. Determination of Oxidative Stress Biomarkers
2.9. Reproducibility and Statistical Analysis of the Results
3. Results
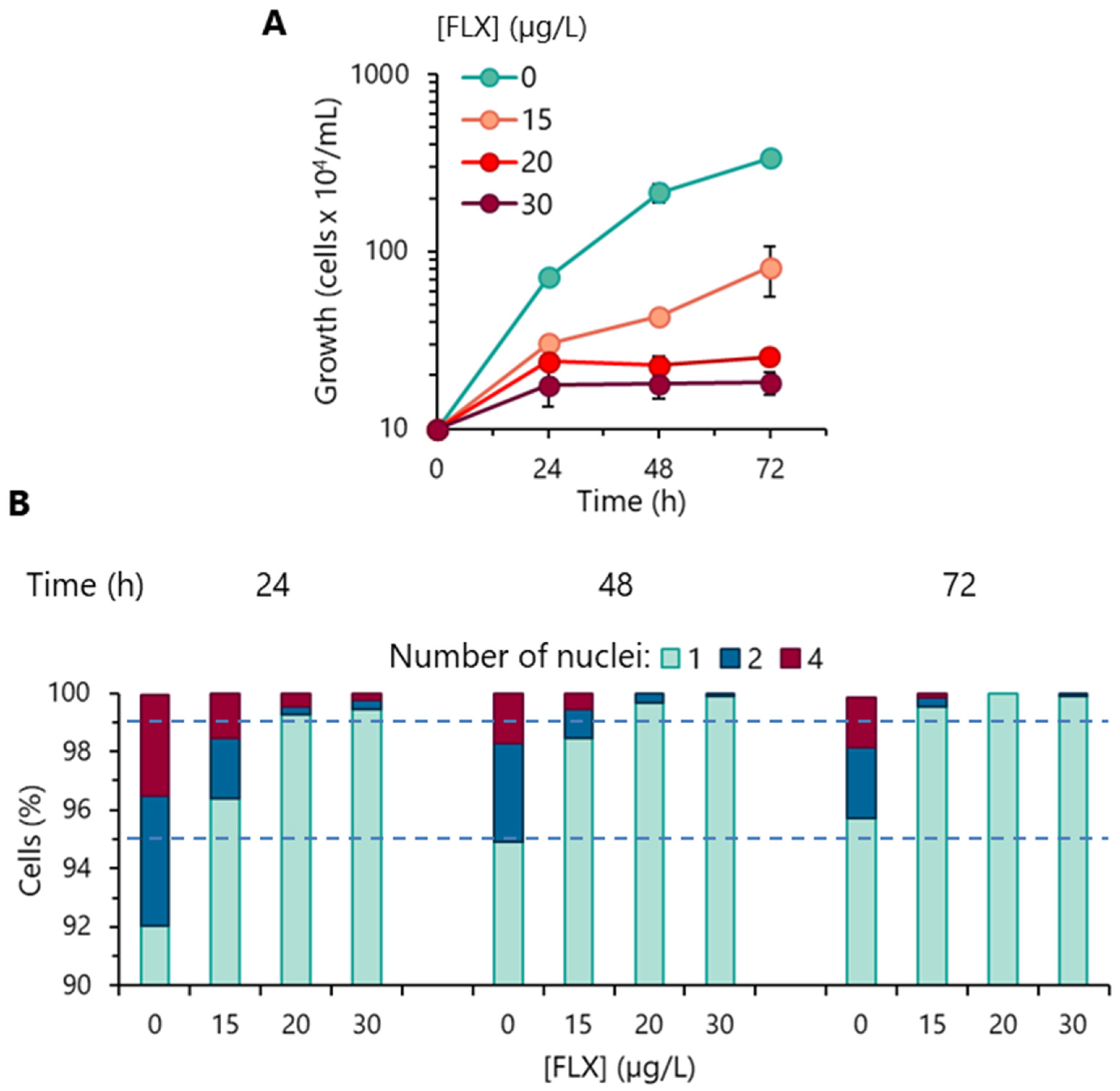
3.1. Algal Growth Inhibition
3.2. Cell-Cycle Progression Analysis
3.3. Impact on Cell Membrane Integrity
3.4. Overall Metabolic Activity Assessment
3.5. Photosynthetic Pigments Content
3.6. Photosynthetic Performance
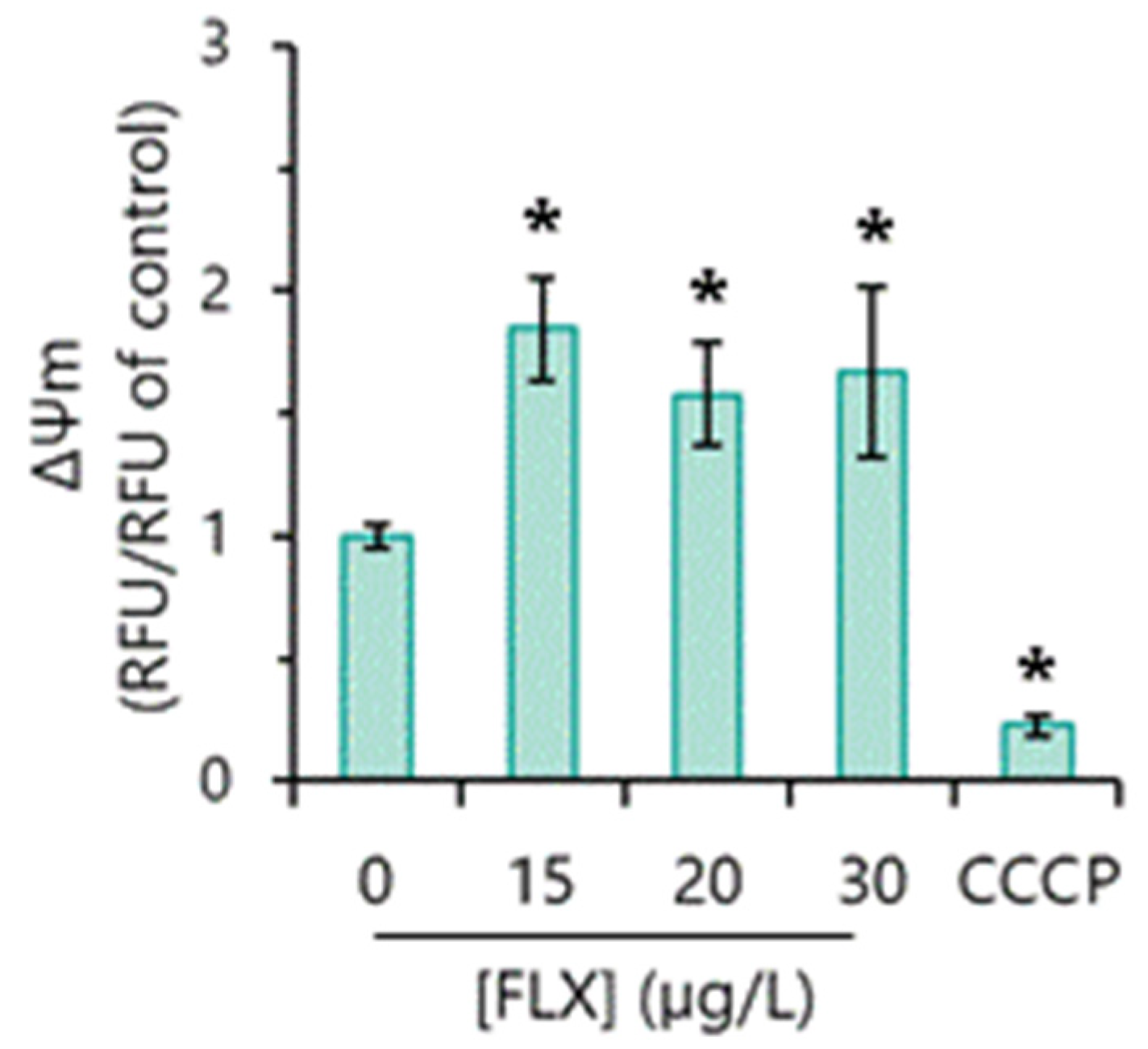
3.7. Mitochondrial Function
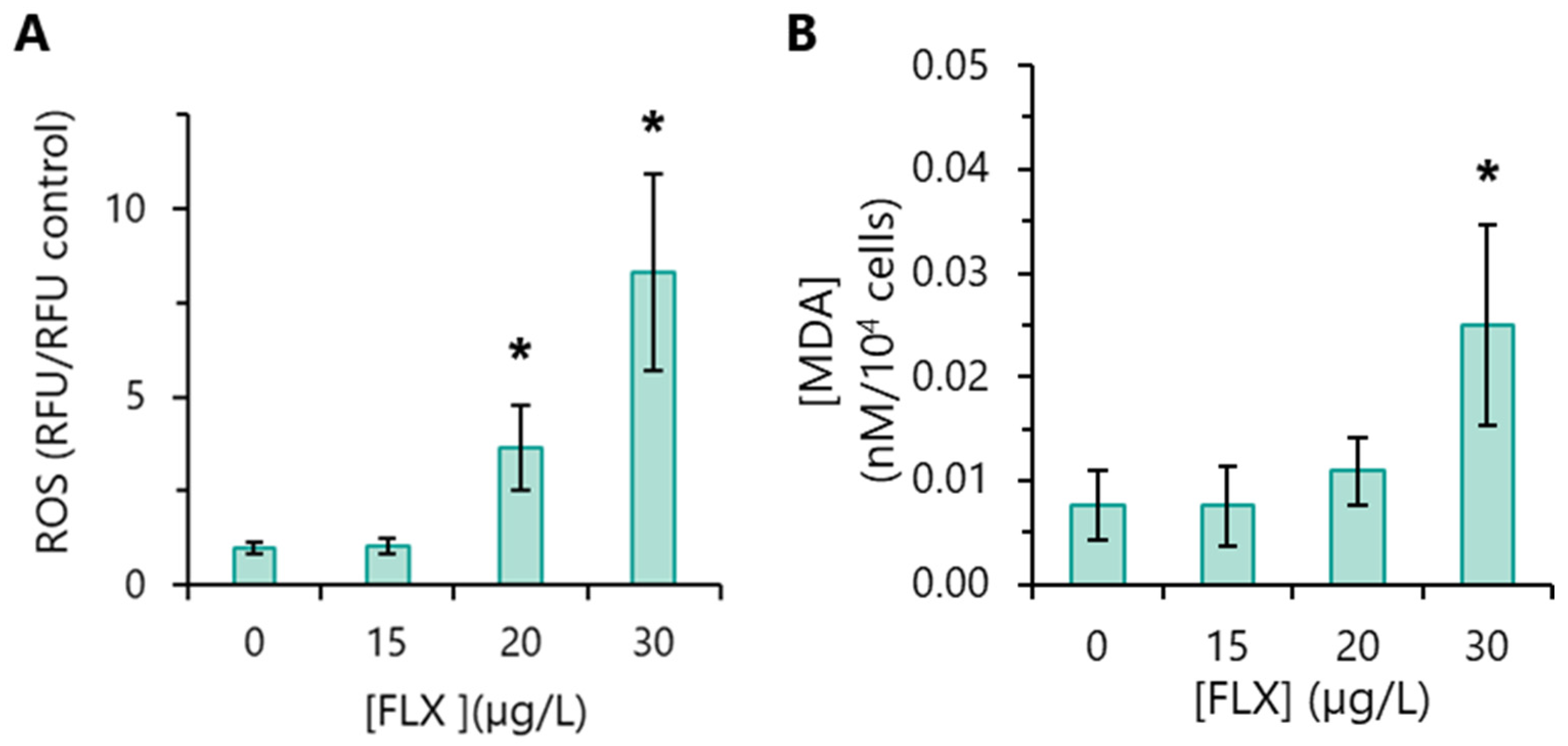
3.8. Oxidative Stress Markers Detection
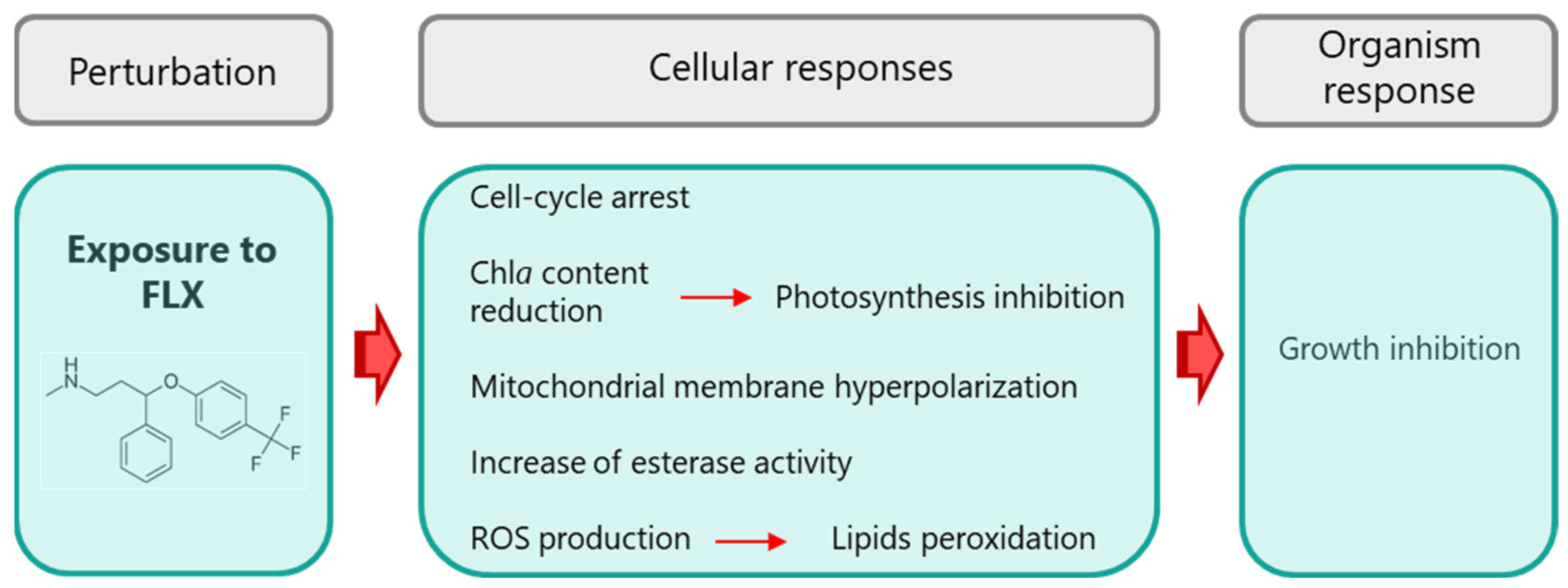
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamal, K.M.; Chiumente, M.; Nakagawa, S.; Giannetti, V.; Marlin, T. Disposal practices for unused and expired medications: Pilot data from three cities in three countries. GMS Health Innov. Technol. 2022, 16, Doc01. [Google Scholar] [CrossRef]
- Sangkuhl, K.; Klein, T.E.; Altman, R.B. Selective serotonin reuptake inhibitors pathway. Pharmacogenet Genom. 2009, 19, 907–909. [Google Scholar] [CrossRef]
- DrugBank Fluoxetine. Available online: https://go.drugbank.com/drugs/DB00472 (accessed on 13 January 2025).
- Clincal Fluoxetine. Drug Usage statistics, United States, 2013–2022. Available online: https://Clincalc.Com/DrugStats/Drugs/Fluoxetine (accessed on 13 January 2025).
- Kwon, J.-W.; Armbrust, K.L. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ. Toxicol. Chem. 2006, 25, 2561–2568. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Wu, R.; Ruan, Y.; Lin, H.; Yuen, C.N.T.; Feng, H.; Lam, P.K.S. Occurrence and fate of psychiatric pharmaceuticals in wastewater treatment plants in Hong Kong: Enantiomeric profiling and preliminary risk assessment. ACS EST Water 2021, 1, 542–552. [Google Scholar] [CrossRef]
- Correia, D.; Domingues, I.; Faria, M.; Oliveira, M. Effects of fluoxetine on fish: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2023, 857, 159486. [Google Scholar] [CrossRef]
- Yin, L.; Ma, R.; Wang, B.; Yuan, H.; Yu, G. The degradation and persistence of five pharmaceuticals in an artificial climate incubator during a one year period. RSC Adv. 2017, 7, 8280–8287. [Google Scholar] [CrossRef]
- Bean, T.G.; Boxall, A.B.A.; Lane, J.; Herborn, K.A.; Pietravalle, S.; Arnold, K.E. Behavioural and physiological responses of birds to environmentally relevant concentrations of an antidepressant. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130575. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grossman, L.; Nguyen, M.; Maximino, C.; Rosemberg, D.B.; Echevarria, D.J.; Kalueff, A.V. Aquatic toxicology of fluoxetine: Understanding the knowns and the unknowns. Aquat. Toxicol. 2014, 156, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Foran, C.M.; Richards, S.M.; Weston, J.; Turner, P.K.; Stanley, J.K.; Solomon, K.R.; Slattery, M.; La Point, T.W. Aquatic ecotoxicology of fluoxetine. Toxicol. Lett. 2003, 142, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Turner, P.K.; Stanley, J.K.; Weston, J.J.; Glidewell, E.A.; Foran, C.M.; Slattery, M.; La Point, T.W.; Huggett, D.B. Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere 2003, 52, 135–142. [Google Scholar] [CrossRef]
- Péry, A.R.R.; Gust, M.; Vollat, B.; Mons, R.; Ramil, M.; Fink, G.; Ternes, T.; Garric, J. Fluoxetine effects assessment on the life cycle of aquatic invertebrates. Chemosphere 2008, 73, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Minguez, L.; Pedelucq, J.; Farcy, E.; Ballandonne, C.; Budzinski, H.; Halm-Lemeille, M.-P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in Northwestern France. Environ. Sci. Pollut. Res. 2016, 23, 4992–5001. [Google Scholar] [CrossRef]
- De Lange, H.J.; Noordoven, W.; Murk, A.J.; Lürling, M.; Peeters, E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006, 78, 209–216. [Google Scholar] [CrossRef]
- Weinberger, J.; Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- Johnson, D.J.; Sanderson, H.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae. Ecotoxicol. Environ. Saf. 2007, 67, 128–139. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Fleming, J. Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 2008, 54, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Neuwoehner, J.; Escher, B.I. The pH-dependent toxicity of basic pharmaceuticals in the green algae Scenedesmus vacuolatus can be explained with a toxicokinetic ion-trapping model. Aquat. Toxicol. 2011, 101, 266–275. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, X.; Gan, Y.; Cheng, H.; Fan, S.; Li, X.; Tang, J. Ecotoxicological effects of the antidepressant fluoxetine and its removal by the typical freshwater microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2022, 244, 114045. [Google Scholar] [CrossRef] [PubMed]
- Feijão, E.; Cruz de Carvalho, R.; Duarte, I.A.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Fluoxetine arrests growth of the model diatom Phaeodactylum tricornutum by increasing oxidative stress and altering energetic and lipid metabolism. Front. Microbiol. 2020, 11, 1803. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Features of the microalga Raphidocelis subcapitata: Physiology and applications. Appl. Microbiol. Biotechnol. 2024, 108, 219. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamaguchi, H.; Nakajima, N.; Kawachi, M. Raphidocelis subcapitata (=Pseudokirchneriella subcapitata) provides an insight into genome evolution and environmental adaptations in the Sphaeropleales. Sci. Rep. 2018, 8, 8058. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; Organization for Economic Cooperation and Development: Paris, France, 2011. [Google Scholar]
- Machado, M.D.; Soares, E.V. Development of a short-term assay based on the evaluation of the plasma membrane integrity of the alga Pseudokirchneriella subcapitata. Appl. Microbiol. Biotechnol. 2012, 95, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Life and death of Pseudokirchneriella subcapitata: Physiological changes during chronological aging. Appl. Microbiol. Biotechnol. 2022, 106, 8245–8258. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Optimization of a microplate-based assay to assess esterase activity in the alga Pseudokirchneriella subcapitata. Water. Air. Soil Pollut. 2013, 224, 1358. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association (APHA): Washington, DC, USA, 1989; 1516p. [Google Scholar]
- Strickland, J.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Sipka, G.; Magyar, M.; Mezzetti, A.; Akhtar, P.; Zhu, Q.; Xiao, Y.; Han, G.; Santabarbara, S.; Shen, J.-R.; Lambrev, P.H.; et al. Light-adapted charge-separated state of Photosystem II: Structural and functional dynamics of the closed reaction center. Plant Cell 2021, 33, 1286–1302. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.D.; Soares, E.V. Impact of erythromycin on a non-target organism: Cellular effects on the freshwater microalga Pseudokirchneriella subcapitata. Aquat. Toxicol. 2019, 208, 179–186. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Short- and long-term exposure to heavy metals induced oxidative stress response in Pseudokirchneriella subcapitata. Clean–Soil Air Water 2016, 44, 1578–1583. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Biomembranes—Part C: Biological Oxidations; Fleischer, S., Packer, L., Eds.; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Regel, R.H.; Ferris, J.M.; Ganf, G.G.; Brookes, J.D. Algal esterase activity as a biomeasure of environmental degradation in a freshwater creek. Aquat. Toxicol. 2002, 59, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Van der Grinten, E.; Pikkemaat, M.G.; van den Brandhof, E.-J.; Stroomberg, G.J.; Kraak, M.H.S. Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere 2010, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Modification of cell volume and proliferative capacity of Pseudokirchneriella subcapitata cells exposed to metal stress. Aquat. Toxicol. 2014, 147, 1–6. [Google Scholar] [CrossRef]
- Schiphorst, C.; Bassi, R. Chlorophyll-xanthophyll antenna complexes: In between light harvesting and energy dissipation. In Photosynthesis in Algae: Biochemical and Physiological Mechanisms, Advances in Photosynthesis and Respiration; Larkum, A.W.D., Grossman, A.R., Raven, J.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 27–55. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature: Biosynthesis, Regulation and Function; Stange, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 111–139. ISBN 978-3-319-39126-7. [Google Scholar]
- Li, X.; Zhao, Y.; Yin, J.; Lin, W. Organic fluorescent probes for detecting mitochondrial membrane potential. Coord. Chem. Rev. 2020, 420, 213419. [Google Scholar] [CrossRef]
- Curti, C.; Mingattao, F.E.; Polizello, A.C.M.; Galastri, L.O.; Uyemura, S.A.; Santos, A.C. Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol. Cell. Biochem. 1999, 199, 103–109. [Google Scholar] [CrossRef]
- De Oliveira, M.R. Fluoxetine and the mitochondria: A review of the toxicological aspects. Toxicol. Lett. 2016, 258, 185–191. [Google Scholar] [CrossRef]
- Charles, E.; Hammadi, M.; Kischel, P.; Delcroix, V.; Demaurex, N.; Castelbout, C.; Vacher, A.-M.; Devin, A.; Ducret, T.; Nunes, P.; et al. The antidepressant fluoxetine induces necrosis by energy depletion and mitochondrial calcium overload. Oncotarget 2017, 8, 3181–3196. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.D.; Soares, E.V. The Unhappy Effects of the Antidepressant Fluoxetine on the Freshwater Microalga Raphidocelis subcapitata. Toxics 2025, 13, 876. https://doi.org/10.3390/toxics13100876
Machado MD, Soares EV. The Unhappy Effects of the Antidepressant Fluoxetine on the Freshwater Microalga Raphidocelis subcapitata. Toxics. 2025; 13(10):876. https://doi.org/10.3390/toxics13100876
Chicago/Turabian StyleMachado, Manuela D., and Eduardo V. Soares. 2025. "The Unhappy Effects of the Antidepressant Fluoxetine on the Freshwater Microalga Raphidocelis subcapitata" Toxics 13, no. 10: 876. https://doi.org/10.3390/toxics13100876
APA StyleMachado, M. D., & Soares, E. V. (2025). The Unhappy Effects of the Antidepressant Fluoxetine on the Freshwater Microalga Raphidocelis subcapitata. Toxics, 13(10), 876. https://doi.org/10.3390/toxics13100876







