Na2SeO3 Exposure Inhibits Locomotion and Reproduction via Oxidative Stress Mechanism in Bioindicators C. elegans and Acrobeloides sp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Test Nematodes
2.3. Nematodes Maintenance and Treatments
2.4. Analysis of Se and Oxidative Stress in Nematodes
2.4.1. Total Se Quantification in Nematodes
2.4.2. Se Speciation in Nematodes
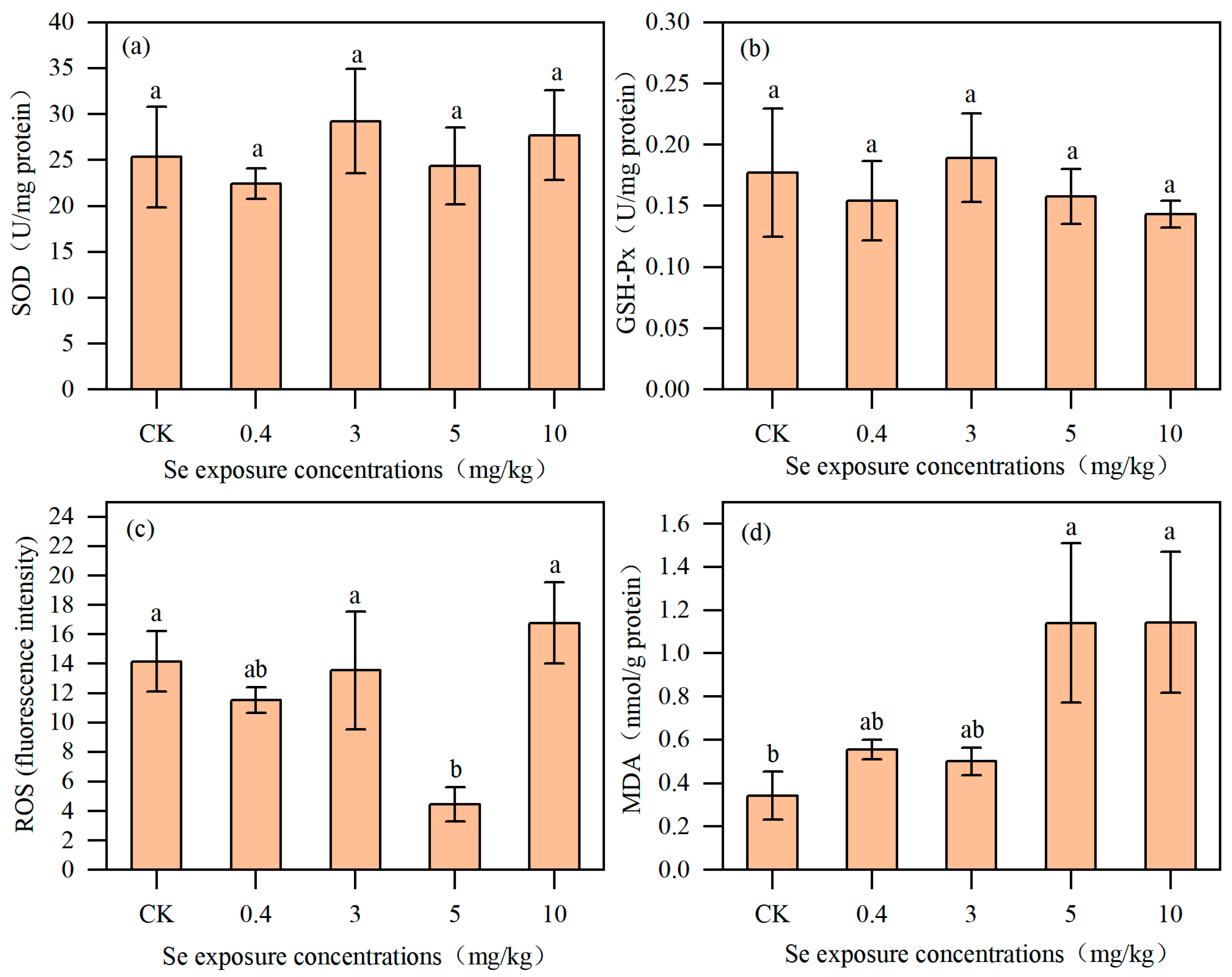
2.4.3. Determination of Oxidative Stress in Nematodes
2.5. Analysis of Locomotion and Reproduction
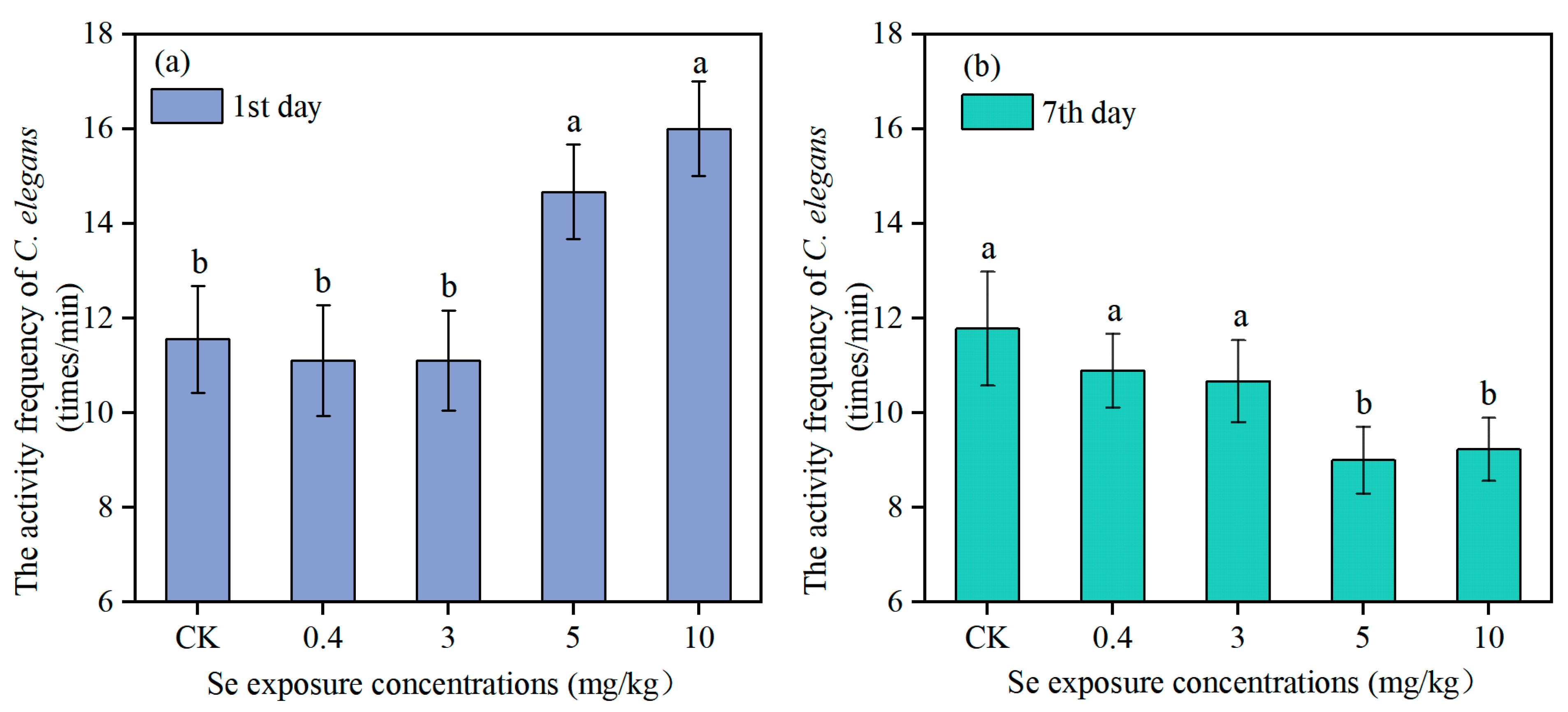
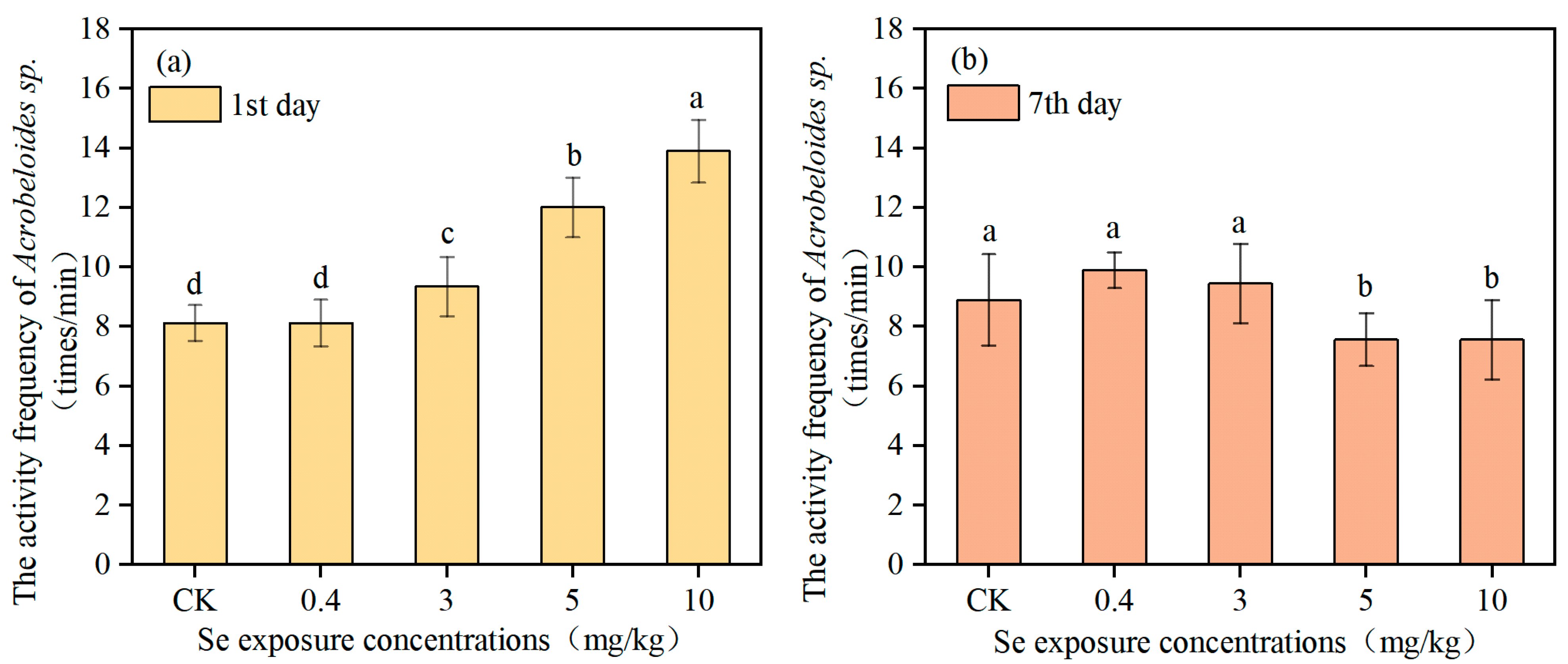
2.5.1. Body Activity Frequency
2.5.2. Reproductive Profile
2.6. Statistical Analysis
2.7. Method Validation
3. Results
3.1. Total Se Content and Distribution of Se Speciation in Nematodes
3.2. Effects of Se on Physiological Indexes of Nematodes
3.3. Effects of Se on Antioxidant Indexes of Nematodes
4. Discussion
4.1. Se Application Threshold Based on Toxicological Response of Nematodes
4.2. Acrobeloides sp. Is a Suitable Bioindicator to Se
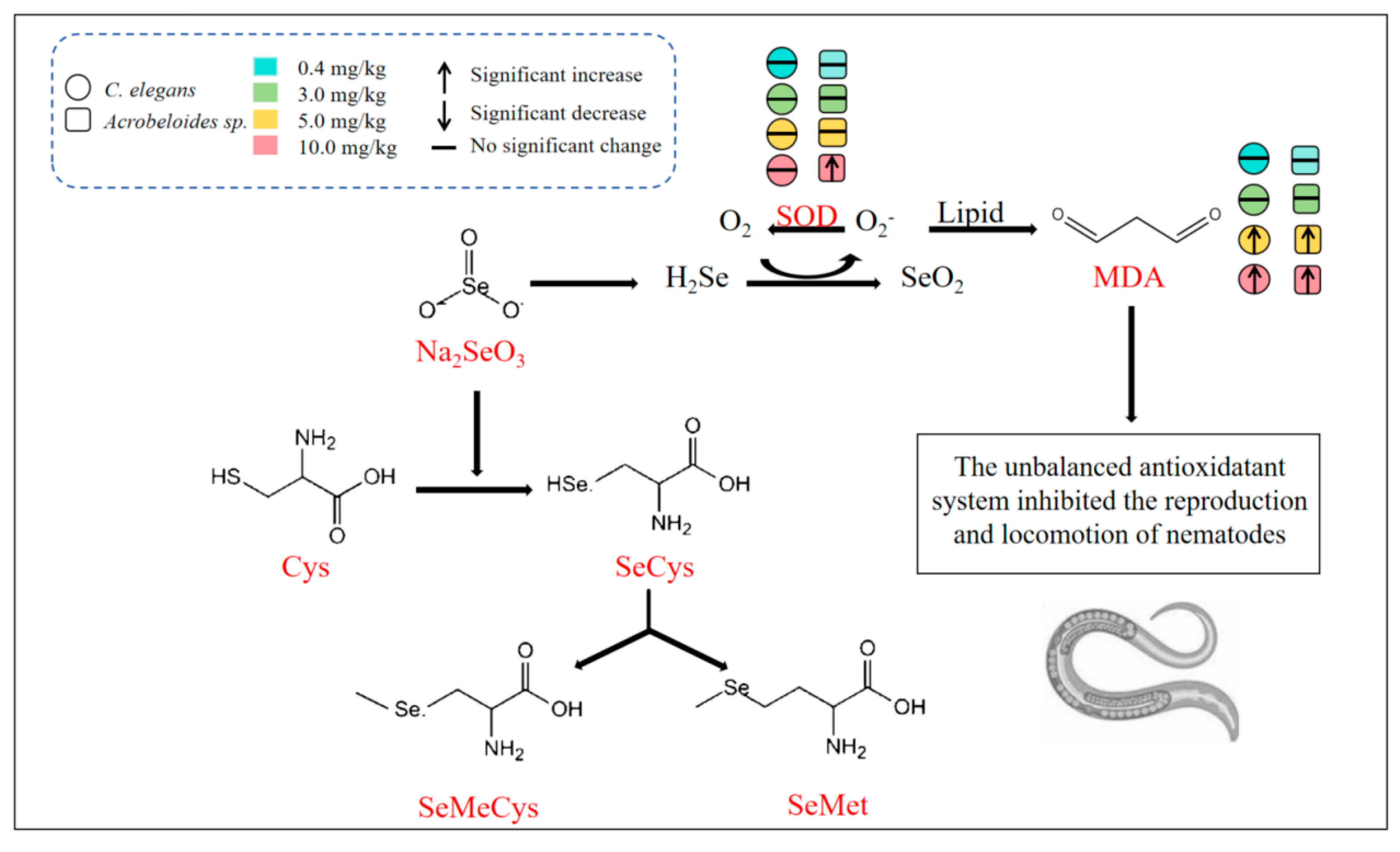
4.3. Inhibitory Effect of Se-Induced Oxidative Stress Mechanism on Nematodes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, S.; Sun, S.; Cai, J.; Jiang, J. Advances in the synthesis and bioactivity of polysaccharide Selenium nanoparticles: A review. Mini-Rev. Med. Chem. 2024, 24, 1535–1554. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and impact of Selenium on human health, a review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in research on the toxicological effects of Selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Du, Q.; Yao, H.; Chen, X.; Zhang, Z.; Xu, S. Roles of oxidative stress and endoplasmic reticulum stress in Se deficiency-induced apoptosis in chicken liver. Biometals 2015, 28, 255–265. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bakonyi, G.; Nagy, P.; Kádár, I. Long-term effects of heavy metals and microelements on nematode assemblage. Toxicol. Lett. 2003, 140, 391–401. [Google Scholar] [CrossRef]
- Kong, L.; Gao, X.; Zhu, J.; Zhang, T.; Xue, Y.; Tang, M. Reproductive toxicity induced by nickel nanoparticles in Caenorhabditis elegans. Environ. Toxicol. 2017, 32, 1530–1538. [Google Scholar] [CrossRef]
- Nigon, V.M.; Félix, M.A. History of research on C. elegans and other free-living nematodes as model organisms. In WormBook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2018; pp. 1–84. [Google Scholar]
- Yu, Y.; Hua, X.; Chen, H.; Wang, Y.; Li, Z.; Han, Y.; Xiang, M. Toxicity of lindane induced by oxidative stress and intestinal damage in Caenorhabditis elegans. Environ. Pollut. 2020, 264, 114731. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Wang, D. Regulation of the response of Caenorhabditis elegans to simulated microgravity by p38 mitogen-activated protein kinase signaling. Sci. Rep. 2018, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Rohn, I.; Marschall, T.A.; Kroepfl, N.; Jensen, K.B.; Aschner, M.; Tuck, S.; Kuehnelt, D.; Schwerdtle, T.; Bornhorst, J. Selenium species-dependent toxicity, bioavailability and metabolic transformations in Caenorhabditis elegans. Metallomics 2018, 10, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Hsu, F.L.; Liu, J.T.; Liao, V.H.C. The ameliorative and toxic effects of selenite on Caenorhabditis elegans. Food Chem. Toxicol. 2011, 49, 812–819. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Meyer, D.; Williams, P.L. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B 2014, 17, 284–306. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.; Tang, M.; Wang, D. The in vivo underlying mechanism for recovery response formation in nano-titanium dioxide exposed Caenorhabditis elegans after transfer to the normal condition. Nanomed. Nanotechnol. 2014, 10, 89–98. [Google Scholar] [CrossRef]
- Félix, M.A.; Braendle, C. The natural history of Caenorhabditis elegans. Curr. Biol. 2010, 20, R965–R969. [Google Scholar] [CrossRef]
- Huo, Z.; Li, Z.; Guan, P.; Shi, F.; Jiang, H.; He, C.; Wang, Z. Effect of heavy metal contamination on soil nematode communities in urban brownfields. Glob. Ecol. Conserv. 2024, 49, e02787. [Google Scholar] [CrossRef]
- Hao, W.; Li, Q.; Zhang, J.; Jiang, Y.; Liang, W. Utility of nematode Acrobeloides nanus for assessing subacute toxicity of heavy metals. Environ. Monit. Assess. 2010, 164, 273–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Zeng, S.; Zhang, Z.; Li, W.; Li, H.; Hu, F.; Xu, L. Toxic effects of phenanthrene on Caenorhabditis elegans, Mesorhabditis sp. and Acrobeloides sp. Soils 2017, 49, 935–940. [Google Scholar]
- Bendoy, C.P.; Tumang, V.M.C.; Moneva, C.S.O.; Albutra, Q.B.; Ganzon, M.A.M. Effects of cadmium on the interactions between bacterivorous nematode species, Acrobeloides. J. Multidiscip. Stud. 2014, 3, 48–59. [Google Scholar] [CrossRef]
- Song, J.P.; Yuan, L.X.; Liu, X.D.; Liu, Y.; Wang, Z.M.; Chen, Q.Q.; Zhang, Z.Z.; Long, Z.D.; Lin, J.Y.; Yin, X.B. Nematode structure and its indication in natural Selenium-rich paddy soil of Guiping City, Guangxi Zhuang Autonomous Region. Chin. Sci. Bull. 2022, 67, 537–547. [Google Scholar] [CrossRef]
- Xu, T.; Tao, M.; Li, R.; Tang, S.; Huang, Y.; Wu, T.; Kamiloglu, S.; Pan, S.; Xu, X. The ameliorative effects of ginger against heat-induced oxidative stress and mitochondrial dysfunction in Caenorhabditis elegans. Food Sci. Hum. Wellness 2025. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [PubMed]
- Farooq, M.R.; Zhang, Z.; Yuan, L.; Liu, X.; Li, M.; Song, J.; Yin, X. Characterization of selenium speciation in Se-enriched crops: Crop selection approach. J. Agric. Food Chem. 2024, 72, 3388–3396. [Google Scholar] [CrossRef]
- Tsalik, E.L.; Hobert, O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J. Neurobiol. 2003, 56, 178–197. [Google Scholar] [CrossRef]
- Morgan, K.L.; Estevez, A.O.; Mueller, C.L.; Cacho-Valadez, B.; Miranda-Vizuete, A.; Szewczyk, N.J.; Estevez, M. The glutaredoxin GLRX-21 functions to prevent Selenium-induced oxidative stress in Caenorhabditis elegans. Toxicol. Sci. 2010, 118, 530–543. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of Selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef]
- Lemly, A.D. Aquatic Selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef]
- Li, W.H.; Ju, Y.R.; Liao, C.M.; Liao, V.H.C. Assessment of Selenium toxicity on the life cycle of Caenorhabditis elegans. Ecotoxicology 2014, 23, 1245–1253. [Google Scholar] [CrossRef]
- Chew, Y.L.; Walker, D.S.; Towlson, E.K.; Vértes, P.E.; Yan, G.; Barabási, A.-L.; Schafer, W.R. Recordings of Caenorhabditis elegans locomotor behaviour following targeted ablation of single motorneurons. Sci. Data 2017, 4, 170156. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Mazur, E. Femtosecond laser ablation of neurons in C. elegans for behavioral studies. Appl. Phys. A 2009, 96, 335–341. [Google Scholar] [CrossRef]
- Song, J.P.; Liu, X.D.; Wang, Z.M.; Zhang, Z.Z.; Chen, Q.Q.; Lin, Z.Q.; Yuan, L.X.; Yin, X.B. Selenium effect threshold for soil nematodes under rice biofortification. Front. Plant Sci. 2022, 13, 889459. [Google Scholar] [CrossRef] [PubMed]
- Tejeda-Benitez, L.; Olivero-Verbel, J. Caenorhabditis elegans, a biological model for research in toxicology. Rev. Environ. Contam. Toxicol. 2016, 237, 1–35. [Google Scholar]
- Xiao, K.; Song, M.; Liu, J.; Chen, H.; Li, D.; Wang, K. Differences in the bioaccumulation of Selenium by two earthworm species (Pheretima guillemi and Eisenia fetida). Chemosphere 2018, 202, 560–566. [Google Scholar] [CrossRef]
- Monteiro, L.C.; Van Butsel, J.; De Meester, N.; Traunspurger, W.; Derycke, S.; Moens, T. Differential heavy-metal sensitivity in two cryptic species of the marine nematode Litoditis marina as revealed by developmental and behavioural assays. J. Exp. Mar. Biol. Ecol. 2018, 502, 203–210. [Google Scholar] [CrossRef]
- Boyd, W.A.; Williams, P.L. Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ. Toxicol. Chem. 2003, 22, 2768–2774. [Google Scholar] [CrossRef]
- Kammenga, J.; Van Gestel, C.; Bakker, J. Patterns of sensitivity to cadmium and pentachlorophenol among nematode species from different taxonomic and ecological groups. Arch. Environ. Contam. Toxicol. 1994, 27, 88–94. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–163. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Boehler, C.J.; Raines, A.M.; Sunde, R.A. Toxic-Selenium and low-Selenium transcriptomes in Caenorhabditis elegans: Toxic Selenium up-regulates oxidoreductase and down-regulates cuticle-associated genes. PLoS ONE 2014, 9, e101408. [Google Scholar] [CrossRef]
- Xu, Q.; Shao, X.; Shi, Y.; Qian, L.; Zhou, X.; Qin, W.; Zhang, M. Is Selenium beneficial or detrimental to earthworm? Growth and metabolism responses of Eisenia Fetida to Na2SeO3 exposure. Sci. Total Environ. 2022, 807, 150770. [Google Scholar] [CrossRef]
- Suzuki, K.; Itoh, M. Metabolism of selenite labelled with enriched stable isotope in the bloodstream. J. Chromatogr. B Biomed. Sci. Appl. 1997, 692, 15–22. [Google Scholar] [CrossRef]
- Sugino, N. The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta 2007, 28, S133–S136. [Google Scholar] [CrossRef]
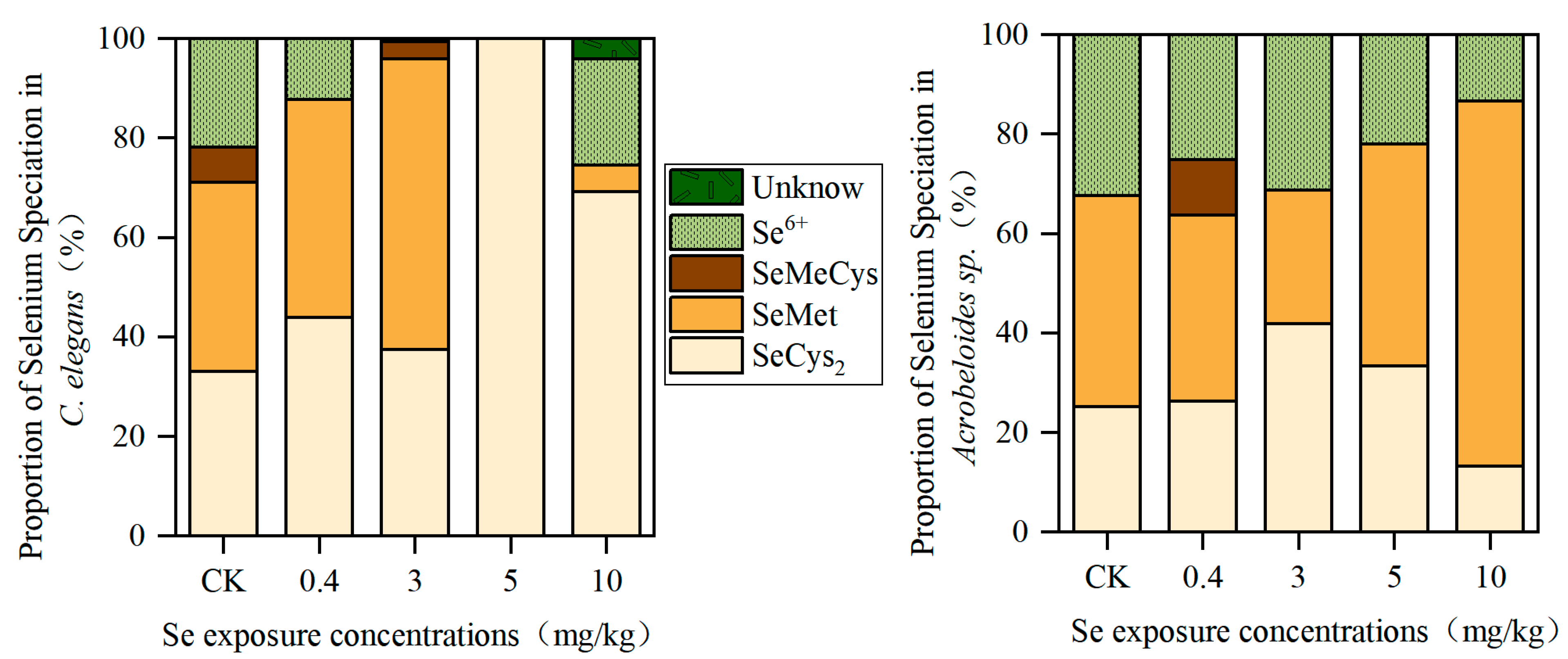


| Se Exposure Concentration (mg/kg) | Se Content in C. elegans (μg/mg Protein) | Se content in Acrobeloides sp. (μg/mg Protein) |
|---|---|---|
| Control (0) | 0.06 ± 0.01 c | 0.34 ± 0.15 c |
| 0.4 | 0.78 ± 0.18 b | 0.87 ± 0.14 c |
| 3 | 4.78 ± 1.28 a | 6.08 ± 2.13 bc |
| 5 | 5.04 ± 0.85 a | 9.77 ± 3.55 ab |
| 10 | 5.70 ± 2.63 a | 15.26 ± 2.91 a |
| Activity Frequency on Seventh Day | Laid Eggs | SOD | GSH-Px | ROS | MDA | ||
|---|---|---|---|---|---|---|---|
| C. elegans | Total Se content | −0.80 * | −0.87 * | 0.10 | −0.11 | −0.25 | 0.61 * |
| SeCys2 | −0.92 * | −0.86 * | 0.04 | −0.15 | −0.42 | 0.72 * | |
| SeMet | 0.13 | −0.09 | 0.12 | 0.12 | 0.06 | −0.22 | |
| SeMeCys | 0.15 | −0.06 | 0.13 | 0.15 | 0.03 | −0.26 | |
| Se6+ | −0.47 | −0.58 * | 0.07 | −0.18 | 0.31 | 0.54 * | |
| Acrobeloides sp. | Total Se content | −0.78 * | −0.90 * | 0.91 * | 0.23 | 0.59 | 0.84 * |
| SeCys2 | −0.63 * | −0.64 * | 0.69 * | 0.54 | 0.51 | 0.67 * | |
| SeMet | −0.72 * | −0.88 * | 0.86 * | 0.05 | 0.52 | 0.77 * | |
| SeMeCys | 0.53 * | 0.51 | −0.35 | −0.23 | −0.19 | −0.22 | |
| Se6+ | −0.68 * | −0.77 * | 0.81 * | 0.42 | 0.60 | 0.76 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, F.; Song, J.; Niu, S.; Yin, X. Na2SeO3 Exposure Inhibits Locomotion and Reproduction via Oxidative Stress Mechanism in Bioindicators C. elegans and Acrobeloides sp. Toxics 2025, 13, 870. https://doi.org/10.3390/toxics13100870
Jin F, Song J, Niu S, Yin X. Na2SeO3 Exposure Inhibits Locomotion and Reproduction via Oxidative Stress Mechanism in Bioindicators C. elegans and Acrobeloides sp. Toxics. 2025; 13(10):870. https://doi.org/10.3390/toxics13100870
Chicago/Turabian StyleJin, Fan, Jiaping Song, Shanshan Niu, and Xuebin Yin. 2025. "Na2SeO3 Exposure Inhibits Locomotion and Reproduction via Oxidative Stress Mechanism in Bioindicators C. elegans and Acrobeloides sp." Toxics 13, no. 10: 870. https://doi.org/10.3390/toxics13100870
APA StyleJin, F., Song, J., Niu, S., & Yin, X. (2025). Na2SeO3 Exposure Inhibits Locomotion and Reproduction via Oxidative Stress Mechanism in Bioindicators C. elegans and Acrobeloides sp. Toxics, 13(10), 870. https://doi.org/10.3390/toxics13100870






