Bioaccessibility-Based Fuzzy Health Risk Assessment and Integrated Management of Toxic Metals Through Multimedia Environmental Exposure near Urban Industrial Complexes
Abstract
1. Introduction
2. Materials and Methods
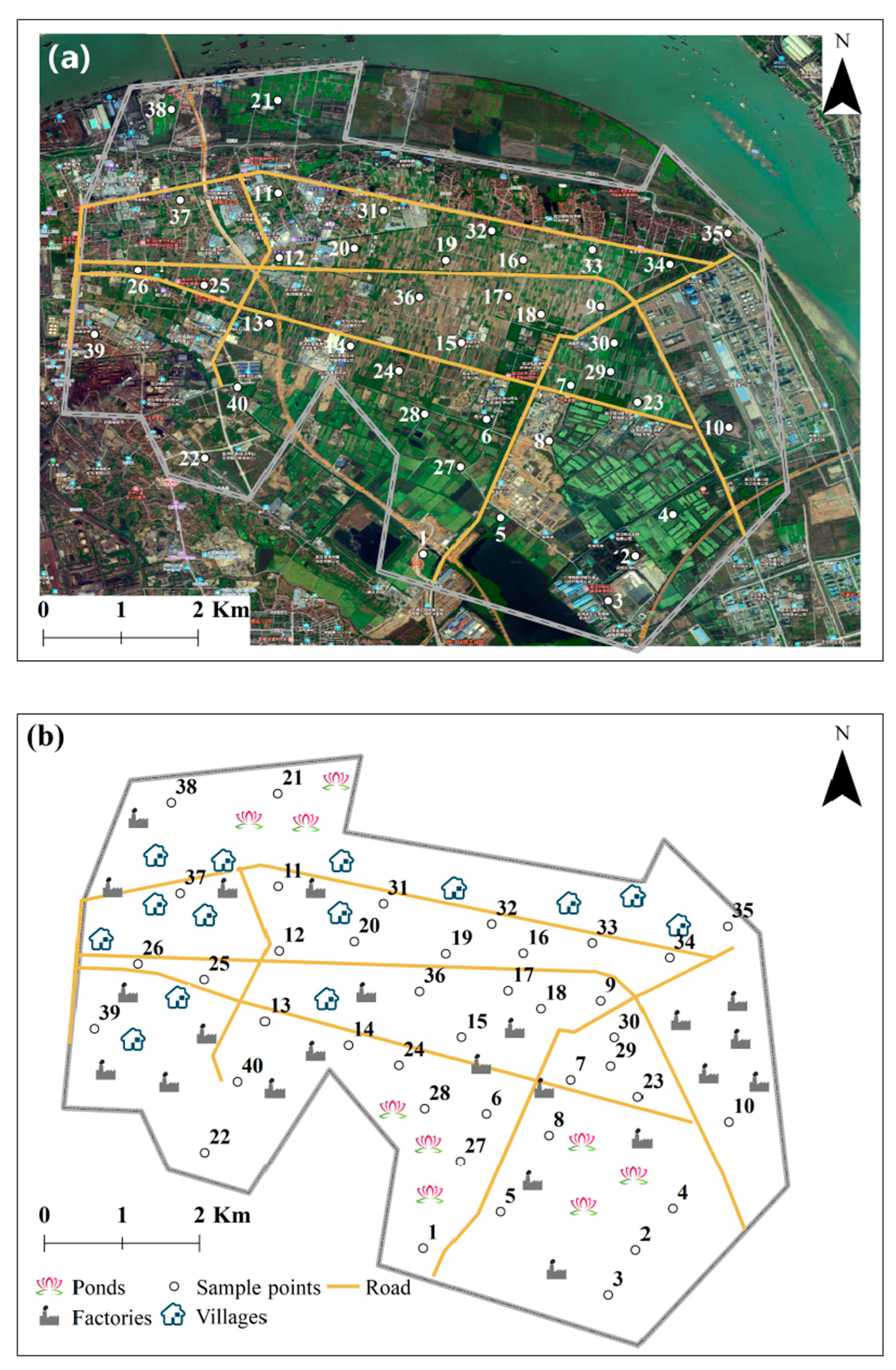
2.1. Study Area and Multimedia Environment Sampling
2.2. Pre-Treatment and Sample Analysis
2.2.1. Atmospheric Particles and Dust
2.2.2. Soil and Vegetables
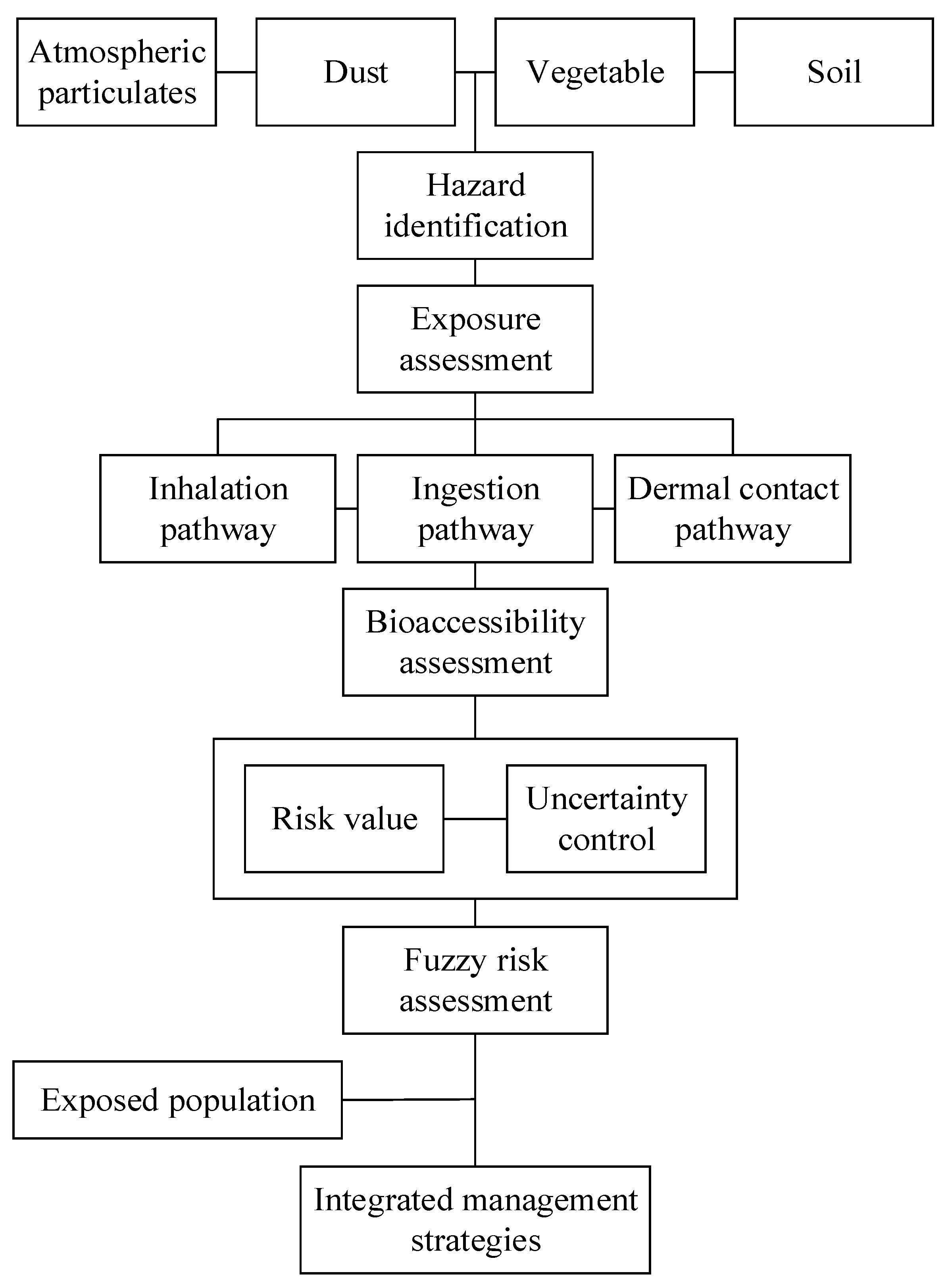
2.3. Bioaccessibility-Based Fuzzy Health Risk Assessment Method
2.3.1. Health Risk Characterization
2.3.2. Uncertainty Control Based on TFN
3. Results and Discussion
3.1. TMs in QCD Multimedia Environment
3.1.1. Atmospheric Particulates
3.1.2. Dust
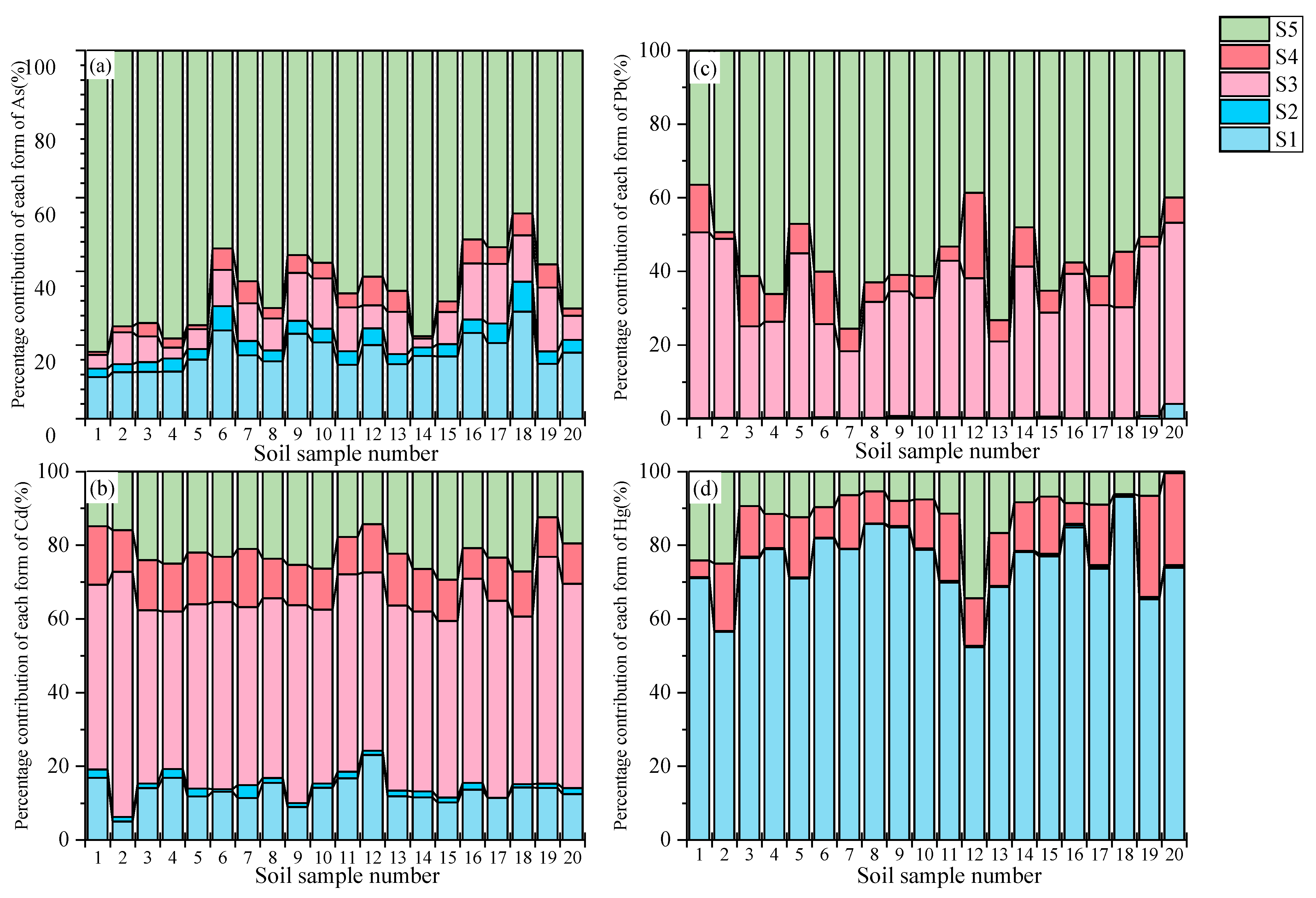
3.1.3. Soil
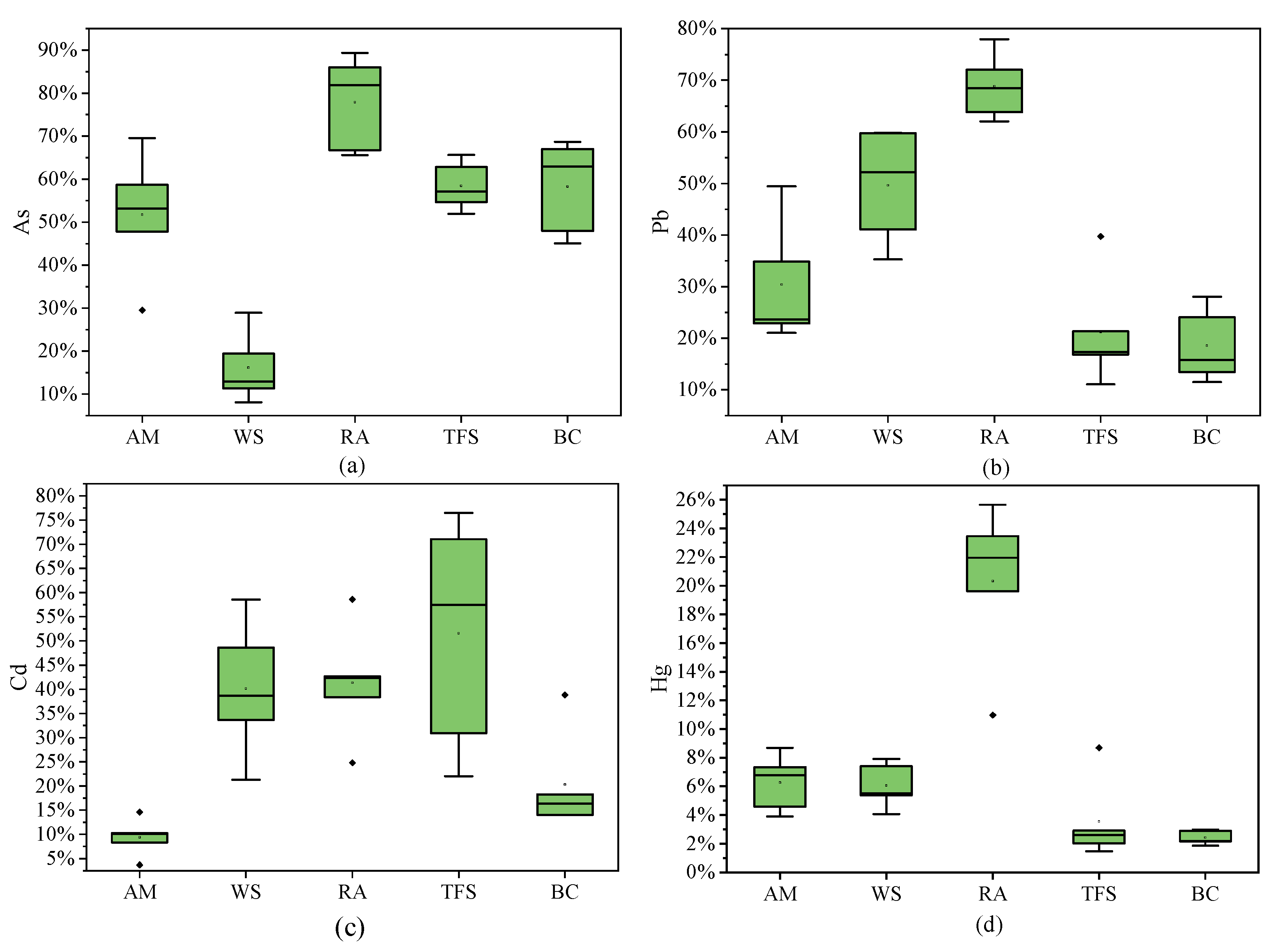
3.1.4. Locally Grown Vegetables
3.2. Bioaccessibility of TMs in a Multimedia Environment
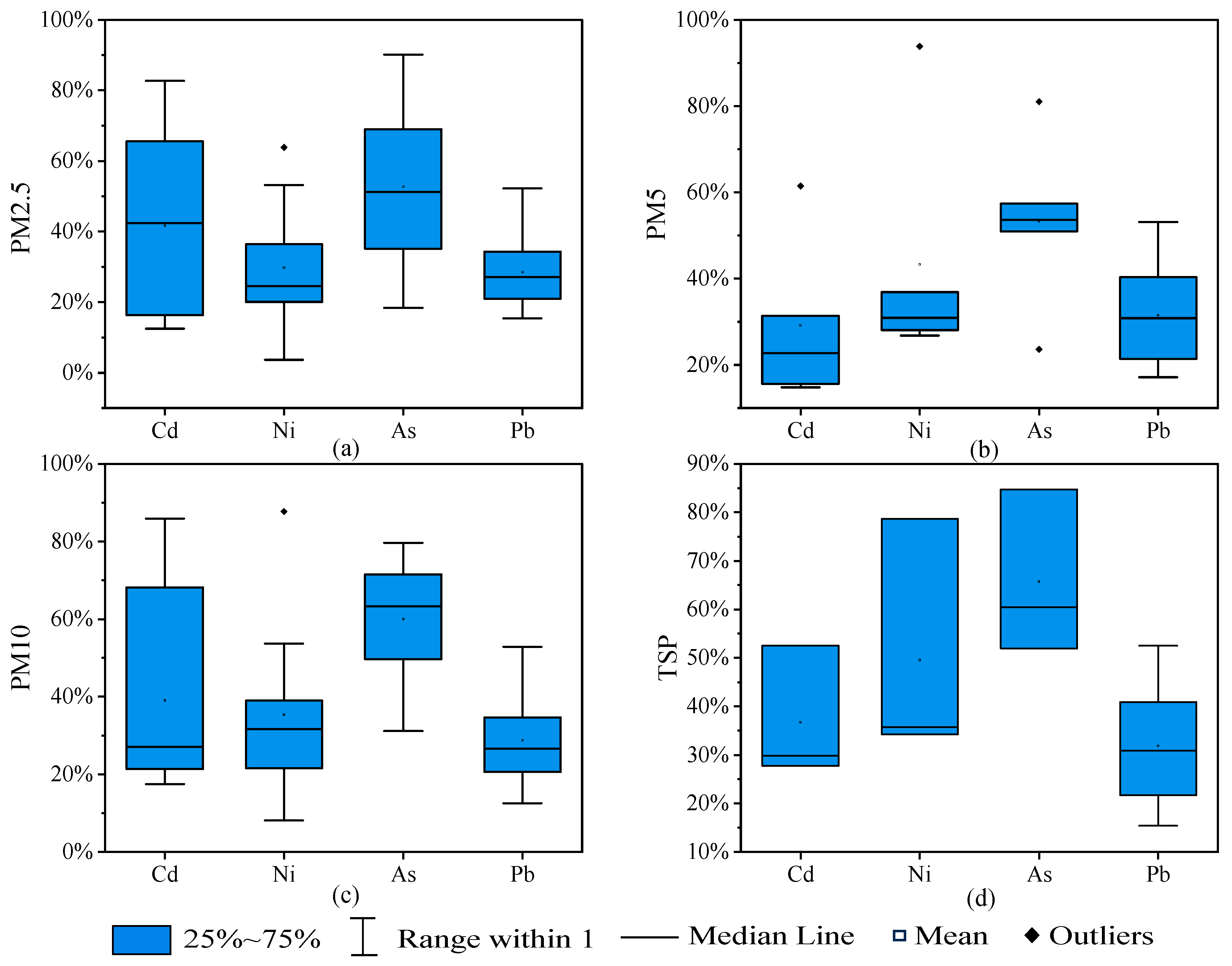
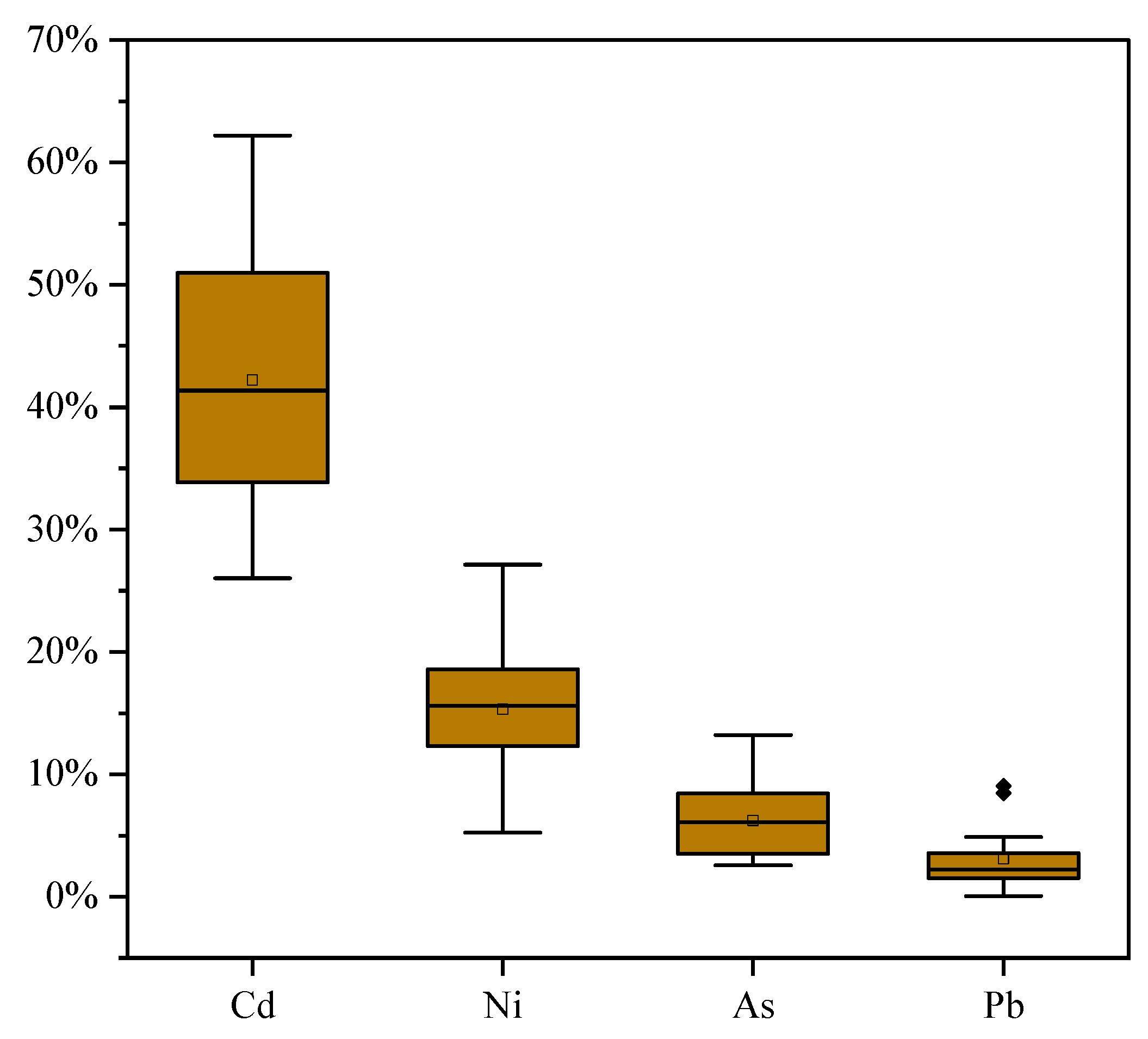
3.2.1. Atmospheric Particulates
3.2.2. Dust
3.2.3. Soil
3.2.4. Vegetables
3.3. Bioaccessibility-Based Fuzzy Health Risk Assessment of Multimedia Environmental TM Exposure
3.3.1. Ingestion Exposure
3.3.2. Inhalation Exposure
3.3.3. Dermal Contact Exposure
3.3.4. Comprehensive Review of Multi-Pathway TM Health Risk Assessment
3.3.5. Comprehensive Risk Management Policy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kastner, C.A.; Lau, R.; Kraft, M. Quantitative tools for cultivating symbiosis in industrial parks. Appl. Energy 2015, 155, 599–612. [Google Scholar] [CrossRef]
- Islam, A.M.T.; Islam, H.M.T.; Mia, M.U.; Khan, R.; Habib, M.A.; Bodrud-Doza, M.; Siddique, M.A.B.; Chu, R. Co-distribution, possible origins, status and potential health risk of trace elements in surface water sources from six major river basins, Bangladesh. Chemosphere 2020, 249, 126180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, F.; Du, H.Z.; Liu, X.L.; Liu, S.Q.; Zhang, J.D. Fuzzy health risk assessment and integrated management of toxic elements exposure through soil-vegetables-farmer pathway near urban industrial complexes. Sci. Total Environ. 2021, 764, 142817. [Google Scholar] [CrossRef]
- Qishlaqi, A.; Moore, F.; Forghani, G. Characterization of metal pollution in soils under two landuse patterns in the Angouran region, NW Iran; a study based on multivariate data analysis. J. Hazard Mater. 2009, 172, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.D.; Liu, W.C.; Liu, J.; Huang, J.H.; Zeng, G.M. An exploration of an integrated stochastic-fuzzy pollution assessment for heavy metals in urban topsoil based on metal enrichment and bioaccessibility. Sci. Total Environ. 2018, 644, 649–660. [Google Scholar] [CrossRef]
- Yan, J.; Qu, Z.; Li, F.; Li, H. Heavy metals in the water environment of Yangtze River Economic Belt: Status, fuzzy environmental risk assessment and management. Urban Clim. 2021, 40, 100981. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Ali Khan, A.A.; abdul Manan, H.; Shahid, R.; Altaf, M. Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Masri, S.; LeBrón, A.M.W.; Logue, M.D.; Valencia, E.; Ruiz, A.; Reyes, A.; Wu, J. Risk assessment of soil heavy metal contamination at the census tract level in the city of Santa Ana, CA: Implications for health and environmental justice. Environ. Sci. Process. Impacts 2021, 23, 812–830. [Google Scholar] [CrossRef]
- Yuan, X.H.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- Kumari, S.; Jain, M.K.; Elumalai, S.P. Assessment of Pollution and Health Risks of Heavy Metals in Particulate Matter and Road Dust Along the Road Network of Dhanbad, India. J. Health Pollut. 2021, 11, 210305. [Google Scholar] [CrossRef]
- Cai, Y.; Li, F.; Zhang, J.; Zhu, X.; Li, Y.; Fu, J.; Chen, X.; Liu, C. Toxic metals in size-fractionated road dust from typical industrial district: Seasonal distribution, bioaccessibility and stochastic-fuzzy health risk management. Environ. Technol. Innov. 2021, 23, 101643. [Google Scholar] [CrossRef]
- Wang, P.; Yu, F.; Lv, H.; Wu, L.; Zhou, H. Potential risk of heavy metals release in sediments and soils of the Yellow River Basin (Henan section): A perspective on bioavailability and bioaccessibility. Ecotoxicol. Environ. Saf. 2025, 291, 117799. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Q.C.; Kang, S.G.; Li, M.Y.; Zhang, M.Y.; Xu, W.M.; Xiang, P.; Ma, L.Q. Heavy metal(loid)s in agricultural soil from main grain production regions of China: Bioaccessibility and health risks to humans. Sci. Total Environ. 2023, 858, 159819. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Wang, J.; Xu, L.; Zheng, X.; Zhang, Y.; Hu, C. Dominant environmental factors influencing soil metal concentrations of Poyang Lake wetland, China: Soil property, topography, plant species and wetland type. CATENA 2021, 207, 105601. [Google Scholar] [CrossRef]
- Zhou, W.T.; Li, Z.; Liu, Y.J.; Shen, C.Y.; Tang, H.Z.; Huang, Y.F. Soil type data provide new methods and insights for heavy metal pollution assessment and driving factors analysis. J. Hazard. Mater. 2024, 480, 135868. [Google Scholar] [CrossRef]
- Le, T.V.; Nguyen, B.T. Heavy metal pollution in surface water bodies in provincial Khanh Hoa, Vietnam: Pollution and human health risk assessment, source quantification, and implications for sustainable management and development. Environ. Pollut. 2024, 343, 123216. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Bai, L.; Li, C.; He, Z.; Liu, X. Assessment of heavy metal contamination levels and health risks in environmental media in the northeast region. Sustain. Cities Soc. 2022, 80, 103796. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Zhu, C.; Tang, X. Analysis of Heavy Metal Pollution in Cultivated Land of Different Quality Grades in Yangtze River Delta of China. Int. J. Environ. Res. Public Health 2021, 18, 9876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Y.; Liu, Y.; Wang, Q.; Liang, Y. Farmers’ adaptation to heavy metal pollution in farmland in mining areas: The effects of farmers’ perceptions, knowledge and characteristics. J. Clean. Prod. 2022, 365, 132678. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Zhang, M.; Lin, Y.; Yang, J.; Lv, J.; Han, N.; Xie, J.; Jia, H.; Su, B.L.; Roeffaers, M.; et al. Photo-assisted technologies for environmental remediation. Nat. Rev. Clean Technol. 2025, 1, 201–215. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Levizou, E.; Shahid, M.; Niazi, N.K.; Vithanage, M.; Ok, Y.S.; Bolan, N.; Rinklebe, J. A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: Are they protective concerning health risk assessment?—A review. Environ. Int. 2019, 127, 819–847. [Google Scholar] [CrossRef]
- Tessier, A.P.; Campbell, P.G.C.; Bisson, M.X. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Davidson, C.M.; Duncan, A.L.; Littlejohn, D.; Ure, A.M.; Garden, L.M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 1998, 363, 45–55. [Google Scholar] [CrossRef]
- Hassoun, M.; Royall, P.G.; Parry, M.; Harvey, R.D.; Forbes, B. Design and development of a biorelevant simulated human lung fluid. J. Drug Deliv. Sci. Technol. 2018, 47, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Cui, Y.; Liu, J.; Xi, C.; Xue, Y.; Wang, W. Emission characteristics of PM2.5 and risk assessment of heavy metal exposure in cremation Workshop. Environ. Chem. 2022, 41, 3279–3287. [Google Scholar] [CrossRef]
- Pavlíková, D.; Zemanová, V.; Pavlík, M. Health Risk and Quality Assessment of Vegetables Cultivated on Soils from a Heavily Polluted Old Mining Area. Toxics 2023, 11, 583. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, Y.; Mu, L.; Liu, W.; Wang, Q.; Su, T.; Yang, Q.; Milinga, A.; Zhang, Y. Health Risk Assessment of Heavy Metals in Agricultural Soils Based on Multi-Receptor Modeling Combined with Monte Carlo Simulation. Toxics 2024, 12, 643. [Google Scholar] [CrossRef]
- Wekumbura, C.; Hettiarachchi, G.M.; Sobin, C. Estimating the proportion of bioaccessible lead (BaPb) in household dust wipe samples: A comparison of IVBA and PBET methods. J. Environ. Sci. Health Part A Toxic/Hazard Subst. Environ. Eng. 2023, 58, 127–138. [Google Scholar] [CrossRef]
- Giuliano, C.; Frizzarin, S.; Beuttel, C.; Powell, K.; Alonzi, A.; Stimamiglio, V.; Ortiz-Romero, P. Percutaneous Absorption of Chlormethine Gel in Human Skin: In Vitro Permeation Testing. J. Investig. Dermatol. 2021, 141, S171. [Google Scholar] [CrossRef]
- Sharma, J.B.; Bhatt, S.; Saini, V.; Kumar, M. Development and validation of uv-visible spectrophotometric method for the estimation of curcumin and tetrahydrocurcumin in simulated intestinal fluid. Res. J. Pharm. Technol. 2021, 14, 2971–2975. [Google Scholar] [CrossRef]
- Qu, M.K.; Li, W.D.; Zhang, C.R.; Wang, S.Q.; Yang, Y.; He, L.Y. Source Apportionment of Heavy Metals in Soils Using Multivariate Statistics and Geostatistics. Pedosphere 2013, 23, 437–444. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, Y.; Zhang, C.T.; Zhang, R.X.; Liao, X.S.; Liu, Y.Y. Heavy metal contamination in surface soils of the industrial district of Wuhan, China. Hum. Ecol. Risk Assess. 2016, 22, 126–140. [Google Scholar] [CrossRef]
- Wan, T.; Huo, Q.; Qi, Z.; Cao, Y.; Hu, H. Contents of heavy metals in soils and vegetables around Wuhan iron and steel corporation. J. Huazhong Agric. Univ. 2014, 33, 77–83. (In Chinese) [Google Scholar] [CrossRef]
- Coufalík, P.; Mikuška, P.; Matoušek, T.; Večeřa, Z. Determination of the bioaccessible fraction of metals in urban aerosol using simulated lung fluids. Atmos. Environ. 2016, 140, 469–475. [Google Scholar] [CrossRef]
- Mukhtar, A.; Limbeck, A. Recent developments in assessment of bio-accessible trace metal fractions in airborne particulate matter: A review. Anal. Chim. Acta 2013, 774, 11–25. [Google Scholar] [CrossRef] [PubMed]
- GBW 07405; Certified Reference Material for the Chemical Composition of Soil and Stream Sediments. Institute of Geophysical and Geochemical Exploration: Langfang, China, 2019. (In Chinese)
- HJ 1111-2020; Technical Guidelines for Eco-Environmental Health Risk Assessment—General Principles. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2020. (In Chinese)
- Tong, R.P.; Yang, X.Y.; Su, H.R.; Pan, Y.; Zhang, Q.Z.; Wang, J.; Long, M.C. Levels, sources and probabilistic health risks of polycyclic aromatic hydrocarbons in the agricultural soils from sites neighboring suburban industries in Shanghai. Sci. Total Environ. 2018, 616, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Orosun, M.M. Assessment of arsenic and its associated health risks due to mining activities in parts of North-central Nigeria: Probabilistic approach using Monte Carlo. J. Hazard Mater. 2021, 412, 125262. [Google Scholar] [CrossRef]
- Orosun, M.M.; Adewuyi, A.D.; Salawu, N.B.; Isinkaye, M.O.; Orosun, O.R.; Oniku, A.S. Monte Carlo approach to risks assessment of heavy metals at automobile spare part and recycling market in Ilorin, Nigeria. Sci. Rep. 2020, 10, 22084. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.D.; Liu, C.Y.; Xiao, M.S.; Wu, Z.X. Distribution, bioavailability and probabilistic integrated ecological risk assessment of heavy metals in sediments from Honghu Lake, China. Process Saf. Environ. Prot. 2018, 116, 169–179. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Furuichi, T.; Ishii, K.; Tanikawa, N. A fuzzy analytic network process for multi-criteria evaluation of contaminated site remedial countermeasures. J. Environ. Manag. 2008, 88, 479–495. [Google Scholar] [CrossRef]
- van Laarhoven, P.J.M.; Pedrycz, W. A fuzzy extension of Saaty’s priority theory. Fuzzy Sets Syst. 1983, 11, 229–241. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Zeng, G.; Tang, X.; Yuan, X.-Z.; Liang, J.; Zhu, H. An integrated assessment model for heavy metal pollution in soil based on triangular fuzzy numbers and chemical speciation of heavy metal. Acta Sci. Circumstantiae 2012, 32, 432–439. (In Chinese) [Google Scholar] [CrossRef]
- Li, R.Z. Assessment for environmental health of urban water supply source based on uncertain information. J. Hydraul. Eng. 2007, 38, 895–900. (In Chinese) [Google Scholar] [CrossRef]
- Li, F.; Lu, Y.C.; Zhang, J.D.; Wang, Y.L.; Chen, X.Y.; Yan, J.J.; Liu, C.Y. Investigation and regional fuzzy health risk management of lead and cadmium in best-selling cigarettes across China. J. Clean. Prod. 2020, 261, 121005. [Google Scholar] [CrossRef]
- HJ 25.3-2019; Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2019. (In Chinese)
- GB 3095-2012; Ambient Air Quality Standards. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2016. (In Chinese)
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. (In Chinese)
- Wilding, L.P. Spatial variability: Its documentation, accommodation and implication to soil survey. In Proceedings of the Workshop of the ISSS and the SSA, Las Vegas, NV, USA, 30 November–1 December 1984; pp. 166–197. [Google Scholar]
- GB 2762-2022; National Food Safety Standard—Limits for Contaminants in Foodstuffs. National Health Commission of the People’s Republic of China: Beijing, China, 2022. (In Chinese)
| Sites | Latitude | Longitude |
|---|---|---|
| 1 | 30°39′24.51″ | 114°25′40.06″ |
| 2 | 30°36′43.17″ | 114°25′55.58″ |
| 3 | 30°34′47.98″ | 114°27′03.46″ |
| 4 | 30°36′32.00″ | 114°28′43.16″ |
| As | Cd | Pb | Ni | |
|---|---|---|---|---|
| Mean | 3.88 | 0.53 | 78.41 | 33.66 |
| Min | 2.47 | 0.31 | 18.63 | 13.50 |
| Max | 7.62 | 1.30 | 250.36 | 151.81 |
| Standard deviation | 1.65 | 0.31 | 79.45 | 37.67 |
| Coefficient of variation | 42% | 58% | 101% | 107% |
| Hubei Province’s background values a | 12.3 | 0.17 | 26.7 | 29 |
| Risk screening values b | 30, 25 | 0.3, 0.6 | 120, 170 | 100, 190 |
| As | Cd | Pb | Hg | |
|---|---|---|---|---|
| Mean | 3.84 | 0.24 | 18.82 | 0.29 |
| Min | 2.15 | 0.10 | 7.60 | 0.13 |
| Max | 5.11 | 0.65 | 67.30 | 0.57 |
| Standard deviation | 0.86 | 0.13 | 13.58 | 0.11 |
| Coefficient of variation | 23% | 55% | 72% | 38% |
| Hubei Province background values a | 12.3 | 0.17 | 26.7 | 0.08 |
| Risk screening values b | 30, 25 | 0.3, 0.6 | 120, 170 | 2.4, 3.4 |
| Vegetable Varieties | As | Cd | Pb | Hg | ||||
|---|---|---|---|---|---|---|---|---|
| Gastric Stage | Enteric Phase | Gastric Stage | Enteric Phase | Gastric Stage | Enteric Phase | Gastric Stage | Enteric Phase | |
| Amaranth | 51.74% | 17.67% | 9.38% | 2.36% | 30.39% | 1.73% | 6.26% | 2.99% |
| Water spinach | 16.13% | 7.90% | 40.17% | 7.90% | 49.61% | 6.18% | 6.05% | 2.82% |
| Radish | 77.90% | 21.03% | 41.36% | 17.02% | 68.86% | 31.83% | 20.32% | 21.95% |
| Tender flower stalk | 58.45% | 8.60% | 51.59% | 6.04% | 21.24% | 2.70% | 3.54% | 2.41% |
| Bok choy | 58.30% | 13.09% | 20.32% | 3.56% | 18.55% | 1.61% | 2.42% | 1.57% |
| Exposure Medium | As | Cd | Pb | Hg | Ni | |
|---|---|---|---|---|---|---|
| Soil | [2.20 × 10−3, 1.10 × 10−2] | [6.60 × 10−4, 3.30 × 10−3] | [1.89 × 10−4, 9.43 × 10−4] | [3.21 × 10−4, 1.81 × 10−3] | NA | |
| Dust | [8.54 × 10−2, 2.83 × 10−1] | [2.85 × 10−2, 3.13] | [8.09 × 10−3, 4.78 × 10−2] | NA | [7.51 × 10−3, 1.3] | |
| Vegetables | Amaranth | [8.67 × 10−2, 1.06 × 10−1] | [1.72 × 10−2, 2.10 × 10−2] | [5.66 × 10−3, 6.91 × 10−3] | [8.08 × 10−4, 9.88 × 10−4] | NA |
| Water spinach | [3.40 × 10−1, 4.15 × 10−1] | [6.85 × 10−2, 8.37 × 10−2] | [2.08 × 10−2, 2.54 × 10−2] | [3.56 × 10−3, 4.35 × 10−3] | NA | |
| Radish | [1.01 × 10−1, 1.23 × 10−1] | [1.64 × 10−2, 2.01 × 10−2] | [5.32 × 10−3, 6.50 × 10−3] | [3.60 × 10−3, 4.40 × 10−3] | NA | |
| Tender flower stalk | [6.90 × 10−2, 8.44 × 10−2] | [2.22 × 10−2, 2.71 × 10−2] | [3.63 × 10−3, 4.44 × 10−3] | [7.90 × 10−4, 9.65 × 10−4] | NA | |
| Bok choy | [1.85 × 10−1, 2.26 × 10−1] | [3.27 × 10−2, 3.99 × 10−2] | [1.06 × 10−2, 1.30 × 10−2] | [1.59 × 10−3, 1.94 × 10−3] | NA | |
| Total | [8.69 × 10−1, 1.25] | [1.86 × 10−1, 3.33] | [4.61 × 10−2, 5.63 × 10−2] | [1.07 × 10−2, 1.45 × 10−2] | [7.51 × 10−3, 1.3] | |
| Exposure Medium | As | Cd | Pb | |
|---|---|---|---|---|
| Soil | [2.39 × 10−6, 1.72 × 10−5] | [1.06 × 10−8, 6.06 × 10−8] | [1.32 × 10−8, 8.13 × 10−8] | |
| Dust | [3.67 × 10−6, 2.53 × 10−5] | [3.57 × 10−8, 2.01 × 10−8] | [9.46 × 10−8, 5.59 × 10−7] | |
| Vegetables | Amaranth | [2.12 × 10−4, 2.59 × 10−4] | [3.26 × 10−6, 3.98 × 10−6] | [8.41×10−8, 1.03×10−7] |
| Water spinach | [8.31 × 10−4, 1.02 × 10−3] | [1.30 × 10−5, 1.59 × 10−5] | [3.09×10−7, 3.78×10−7] | |
| Radish | [2.46 × 10−4, 3.01 × 10−4] | [3.12 × 10−6, 3.82 × 10−6] | [7.91×10−8, 9.67×10−8] | |
| Tender flower stalk | [1.69 × 10−4, 2.06 × 10−4] | [4.21 × 10−6, 5.14 × 10−6] | [5.41×10−8, 6.61×10−8] | |
| Bok choy | [4.52 × 10−4, 5.53 × 10−4] | [6.21 × 10−6, 7.59 × 10−6] | [1.58×10−7, 1.94×10−7] | |
| Total | [1.92 × 10−3, 2.38 × 10−3] | [2.98 × 10−5, 3.67 × 10−5] | [7.92 × 10−7, 1.48 × 10−6] | |
| Exposure Medium | As | Cd | Pb | Hg | Ni |
|---|---|---|---|---|---|
| Soil | [1.18 × 10−3, 3.77 × 10−3] | [3.37 × 10−4, 8.55 × 10−4] | [1.41 × 10−5, 3.84 × 10−5] | [5.00 × 10−5, 1.26 × 10−4] | NA |
| Dust | [1.81 × 10−3, 5.55 × 10−3] | [1.13 × 10−3, 2.83 × 10−3] | [1.01 × 10−4, 2.64 × 10−4] | NA | [5.69 × 10−5, 8.17 × 10−5] |
| PM2.5 | [6.47 × 10−2, 7.94 × 10+1] | [2.96 × 10−3, 4.24] | [1.38 × 10−3, 1.51 × 10−3] | NA | [1.83 × 10−3, 2.58] |
| PM10 | [3.74 × 10−2, 3.78 × 10+1] | [9.47 × 10−3, 5.60] | [1.93 × 10−3, 2.24 × 10−3] | NA | [6.78 × 10−3, 2.76] |
| Total | [1.05 × 10−1, 1.17 × 10+2] | [1.39 × 10−2, 9.84] | [3.43 × 10−3, 4.05× 10−3] | [5.00 × 10−5, 1.26 × 10−4] | [8.67 × 10−3, 5.34] |
| Exposure Medium | As | Cd | Ni |
|---|---|---|---|
| Soil | [5.51 × 10−9, 1.76 × 10−8] | [4.77 × 10−9, 1.21 × 10−8] | NA |
| Dust | [8.45 × 10−9, 2.59 × 10−8] | [1.60 × 10−8, 4.00 × 10−8] | [4.57 × 10−10, 6.56 × 10−10] |
| PM2.5 | [4.35 × 10−8, 8.89 × 10−8] | [4.06 × 10−8, 1.34 × 10−6] | [5.17 × 10−7, 5.74 × 10−7] |
| PM10 | [6.81 × 10−8, 9.71 × 10−6] | [6.77 × 10−8, 6.54 × 10−6] | [1.17 × 10−6, 1.33 × 10−6] |
| Total | [1.40 × 10−8, 4.35 × 10−8] | [2.08 × 10−8, 5.21 × 10−8] | [1.69 × 10−6, 1.90 × 10−6] |
| Exposure Medium | As | Cd | Pb | Hg | Ni |
|---|---|---|---|---|---|
| Dust | [6.40 × 10−5, 7.27 × 10−5] | [2.58 × 10−4, 9.12 × 10−4] | [5.48 × 10−4, 2.23 × 10−3] | NA | [2.03 × 10−4, 2.92 × 10−4] |
| Soil | [4.96 × 10−3, 2.69 × 10−2] | [1.50 × 10−2, 2.69 × 10−2] | [2.91 × 10−3, 5.35 × 10−4] | [1.63 × 10−2, 1.89 × 10−2] | NA |
| Total | [5.02 × 10−3, 2.70 × 10−2] | [1.53 × 10−2, 2.78 × 10−2] | [3.45 × 10−4, 2.77 × 10−3] | [1.63 × 10−2, 1.89 × 10−2] | [2.03 × 10−4, 2.92 × 10−4] |
| Exposure Route | As | Cd | Pb | Ni | Hg |
|---|---|---|---|---|---|
| Ingestion | 16.87% | 29.33% | 99.95% | 38.79% | 99.95% |
| Inhalation | 81.89% | 70.30% | 0.00% | 61.21% | 0.05% |
| Dermal contact | 1.24% | 0.37% | 0.05% | NA | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Zhu, D.; An, M.; Wang, H.; Guo, J.; Wang, Y.; Wei, Y.; Li, F. Bioaccessibility-Based Fuzzy Health Risk Assessment and Integrated Management of Toxic Metals Through Multimedia Environmental Exposure near Urban Industrial Complexes. Toxics 2025, 13, 861. https://doi.org/10.3390/toxics13100861
Xu S, Zhu D, An M, Wang H, Guo J, Wang Y, Wei Y, Li F. Bioaccessibility-Based Fuzzy Health Risk Assessment and Integrated Management of Toxic Metals Through Multimedia Environmental Exposure near Urban Industrial Complexes. Toxics. 2025; 13(10):861. https://doi.org/10.3390/toxics13100861
Chicago/Turabian StyleXu, Siqi, Donghua Zhu, Miao An, Haoyu Wang, Jinyuan Guo, Yazhu Wang, Yongchang Wei, and Fei Li. 2025. "Bioaccessibility-Based Fuzzy Health Risk Assessment and Integrated Management of Toxic Metals Through Multimedia Environmental Exposure near Urban Industrial Complexes" Toxics 13, no. 10: 861. https://doi.org/10.3390/toxics13100861
APA StyleXu, S., Zhu, D., An, M., Wang, H., Guo, J., Wang, Y., Wei, Y., & Li, F. (2025). Bioaccessibility-Based Fuzzy Health Risk Assessment and Integrated Management of Toxic Metals Through Multimedia Environmental Exposure near Urban Industrial Complexes. Toxics, 13(10), 861. https://doi.org/10.3390/toxics13100861








