Assessing the Kynurenine–Tryptophan Ratio (KTR) and CYP1 Activity in Longnose (Catostomus catostomus) and White Suckers (Catostomus commersonii) Exposed to Petroleum-Derived Contaminants from the Alberta Oil Sands Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection and Tissue Processing
2.2. Kynurenine–Tryptophan Ratio (KTR) Assessment
2.2.1. Chemicals and Standards
2.2.2. Sample Preparation and Extraction
2.2.3. High Performance Liquid Chromatography Tandem Mass Spectrometry
2.3. Data and Statistical Analysis
3. Results and Discussion
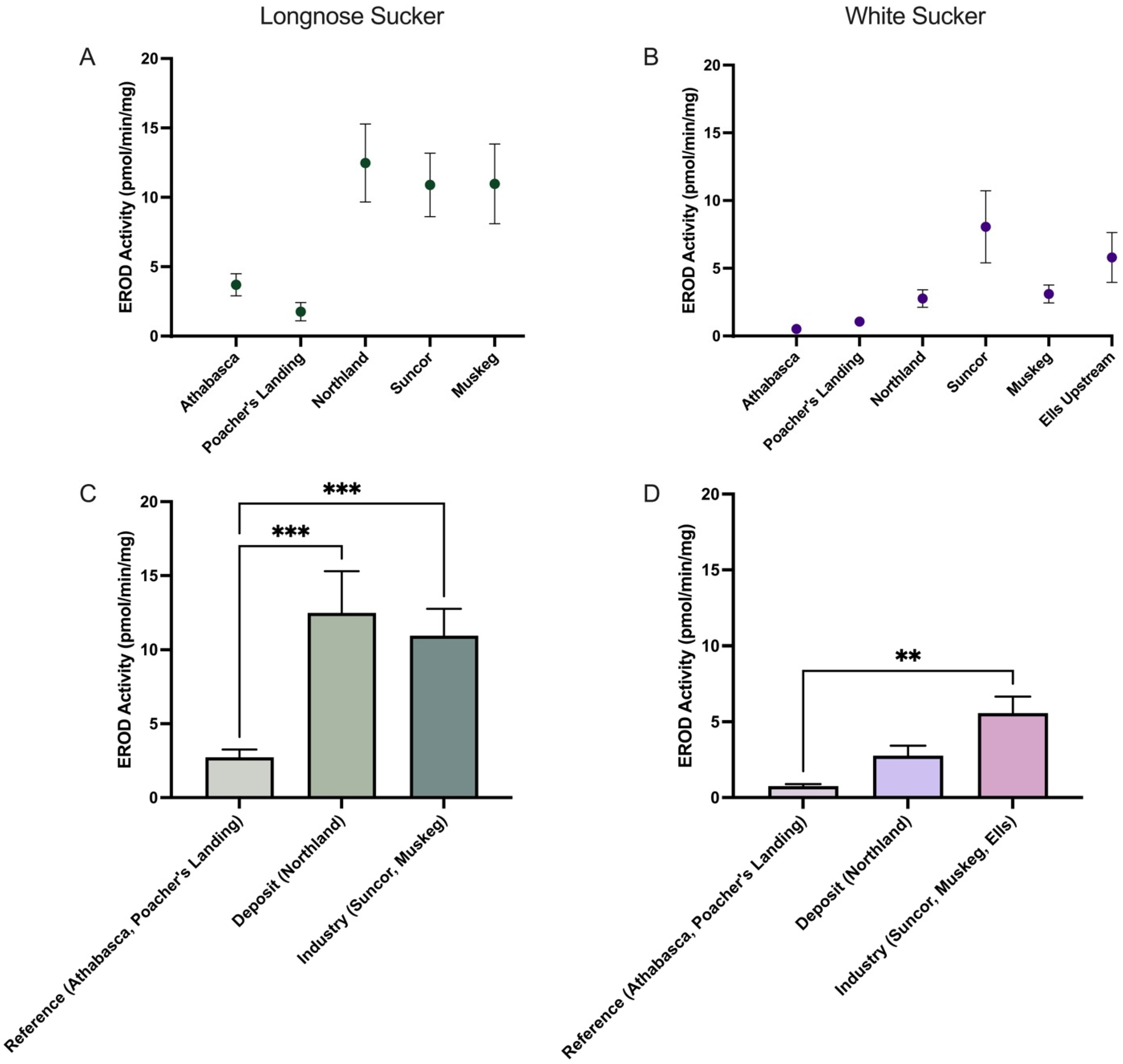
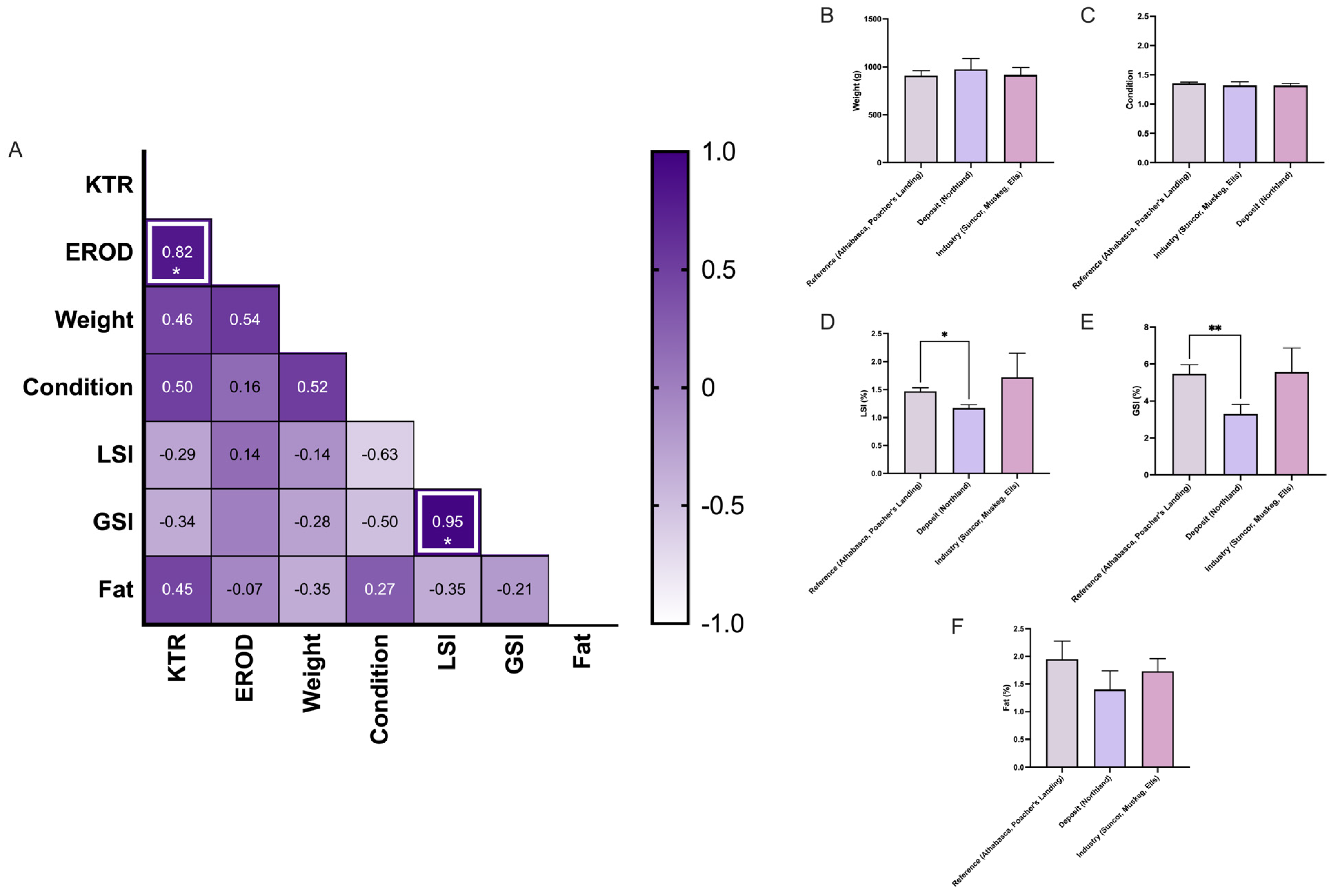
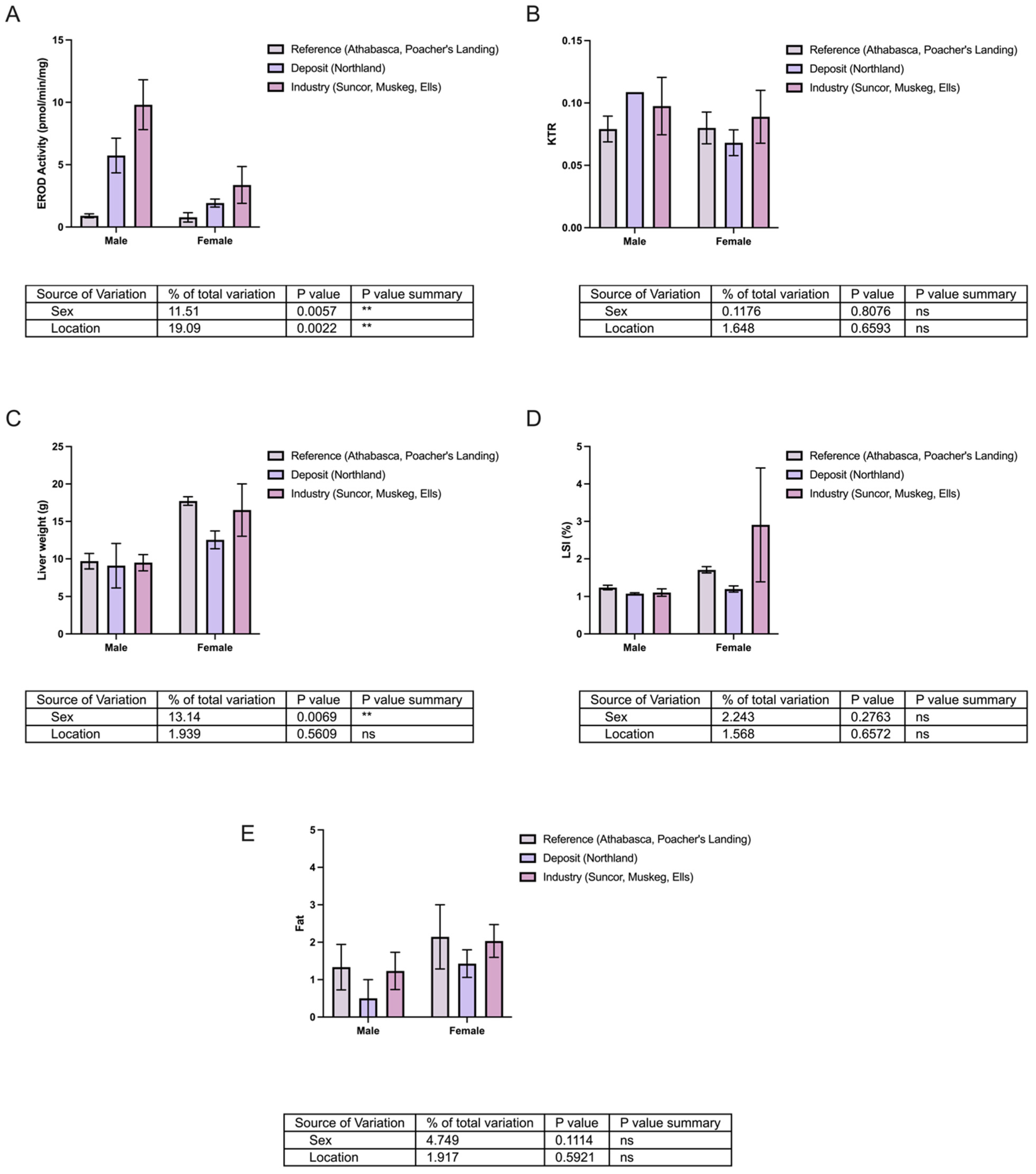
3.1. Comparative Analysis of Kynurenine–Tryptophan Ratio and EROD Activity in Longnose and White Sucker
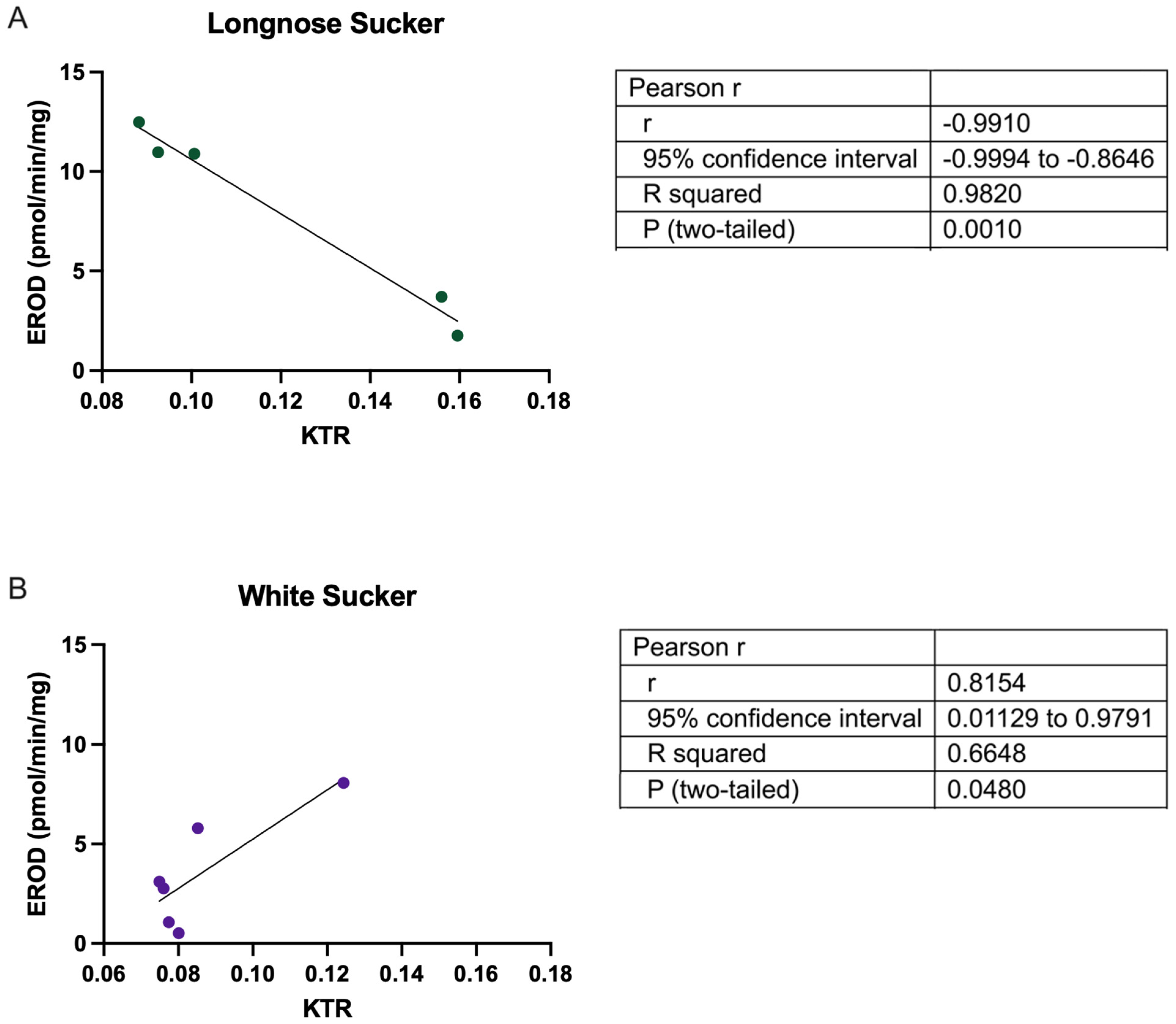
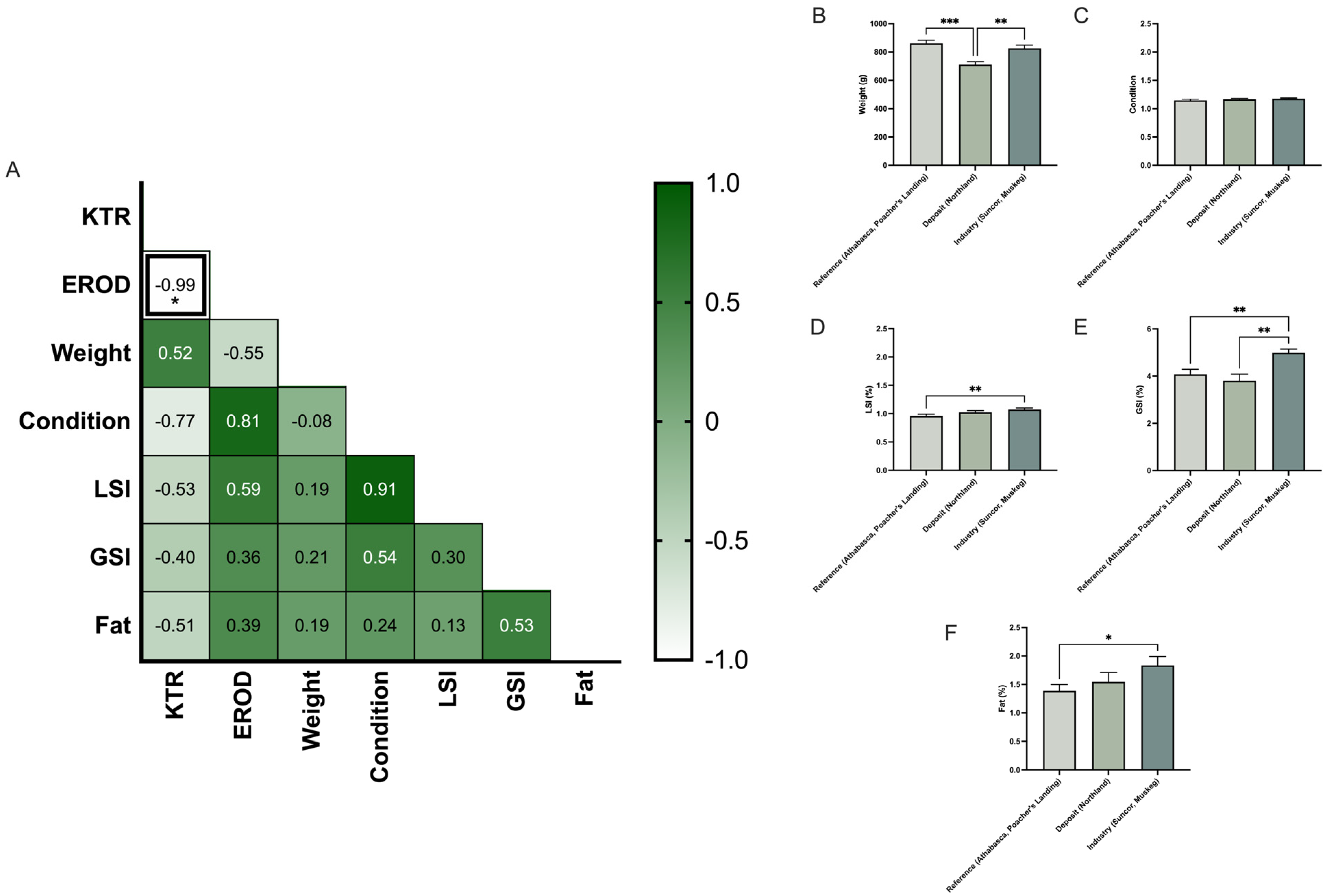
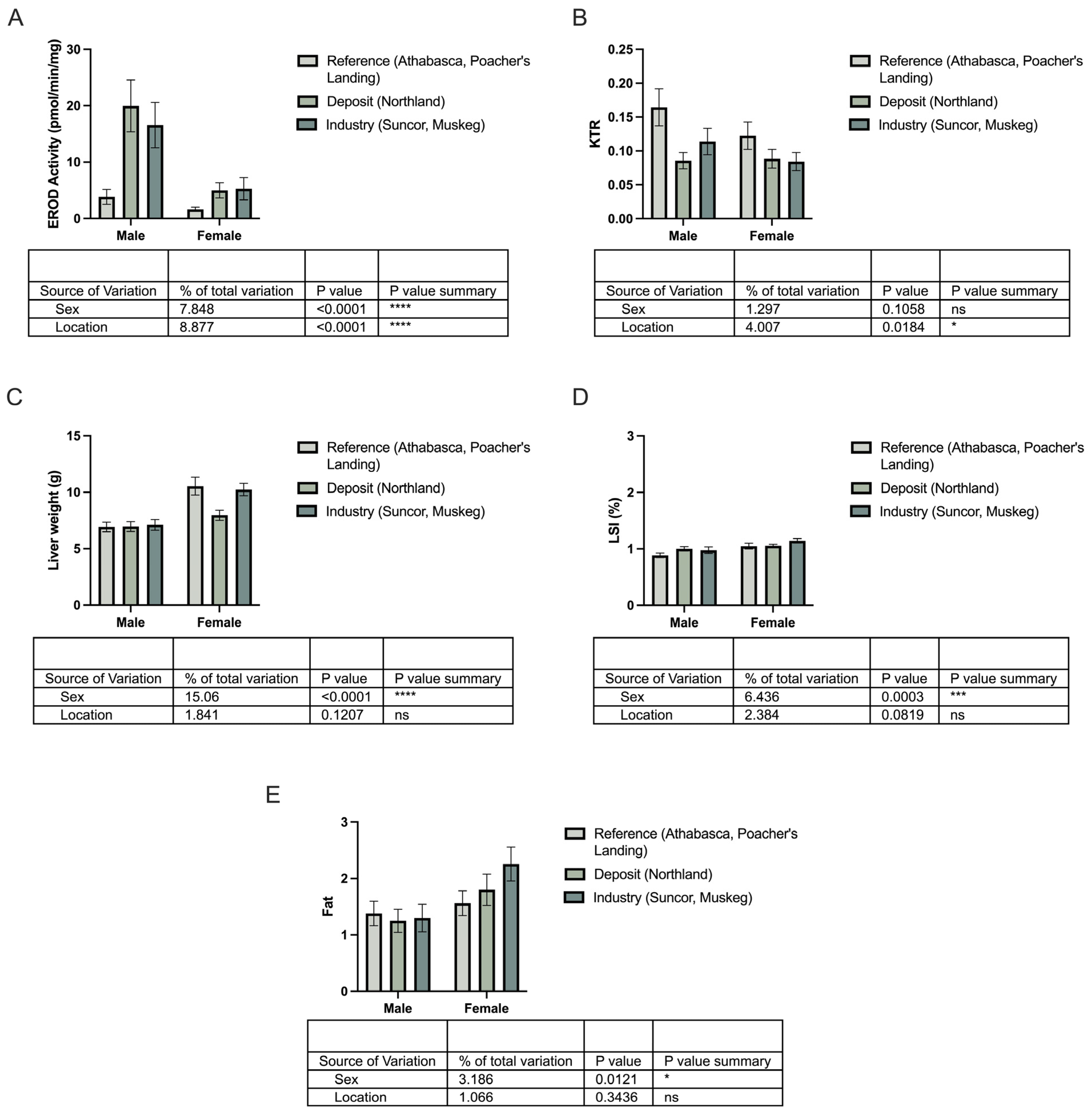
3.2. Does KTR Correlate with Biometric Data?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KTR | Kynurenine:Tryptophan Ratio |
| AOSR | Alberta Oil Sands Region |
| EROD | Ethoxyresorufin-O-deethylase |
| HSI | Hepatic somatic index |
| GSI | Gonadosomatic index |
| PAC | Polycyclic aromatic compound |
| ECCC | Environment and Climate Change Canada |
| TRP | Tryptophan |
| KYN | Kynurenine |
| SC | Sympatho-chromaffin |
| HPI | Hypothalamic-pituitary-interrenal |
| AhR | Aryl hydrocarbon receptor |
References
- Government of Canada. Fisheries Act; Vol. c. F-14. Available online: https://laws-lois.justice.gc.ca/eng/acts/f-14/ (accessed on 28 November 2024).
- Government of Canada. Alberta Oil Sands Environmental Monitoring. Available online: https://www.canada.ca/en/environment-climate-change/services/oil-sands-monitoring.html (accessed on 28 November 2024).
- Fennell, J.; Arciszewski, T.J. Current Knowledge of Seepage from Oil Sands Tailings Ponds and Its Environmental Influence in Northeastern Alberta. Sci. Total Environ. 2019, 686, 968–985. [Google Scholar] [CrossRef]
- Arens, C.J.; Hogan, N.S.; Kavanagh, R.J.; Mercer, A.G.; Kraak, G.J.V.D.; van den Heuvel, M.R. Sublethal Effects of Aged Oil Sands-Affected Water on White Sucker (Catostomus Commersonii): Effects of Aged Oil Sands Affected Waters on White Sucker. Environ. Toxicol. Chem. 2015, 34, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. White Sucker. Available online: https://www.dfo-mpo.gc.ca/species-especes/profiles-profils/white-sucker-meunier-noir-eng.html (accessed on 28 November 2024).
- Mcmaster, M.E.; Tetreatult, G.R.; Clark, T.; Bennett, J.; Cunningham, J.; Evans, M. Aquatic Ecosystem Health Assessment of the Athabasca River Mainstem Oil Sands Area Using White Sucker Health. WIT Trans. Ecol. Environ. 2018, 215, 411–420. [Google Scholar]
- Corsi, I.; Mariottini, M.; Sensini, C.; Lancini, L.; Focardi, S. Fish as Bioindicators of Brackish Ecosystem Health: Integrating Biomarker Responses and Target Pollutant Concentrations. Oceanol. Acta 2003, 26, 129–138. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A Protocol for Identifying Suitable Biomarkers to Assess Fish Health: A Systematic Review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological Roles of Tryptophan in Teleosts: Current Knowledge and Perspectives for Future Studies. Rev. Aquac. 2019, 11, 3–24. [Google Scholar] [CrossRef]
- Jamshed, L.; Debnath, A.; Jamshed, S.; Wish, J.V.; Raine, J.C.; Tomy, G.T.; Thomas, P.J.; Holloway, A.C. An Emerging Cross-Species Marker for Organismal Health: Tryptophan-Kynurenine Pathway. Int. J. Mol. Sci. 2022, 23, 6300. [Google Scholar] [CrossRef]
- Badawy, A.A.-B.; Guillemin, G.J. Species Differences in Tryptophan Metabolism and Disposition. Int. J. Tryptophan Res. 2022, 15, 11786469221122511. [Google Scholar] [CrossRef]
- Wish, J.; Bulloch, P.; Oswald, L.; Halldorson, T.; Raine, J.C.; Jamshed, L.; Marvin, C.; Thomas, P.J.; Holloway, A.C.; Tomy, G.T. Kynurenine to Tryptophan Ratio as a Biomarker of Acute Stress in Fish. Chemosphere 2022, 288, 132522. [Google Scholar] [CrossRef]
- Zielińska, E.; Kocki, T.; Saran, T.; Borbely, S.; Kuc, D.; Vilagi, I.; Urbańska, E.M.; Turski, W.A. Effect of Pesticides on Kynurenic Acid Production in Rat Brain Slices. Ann. Agric. Environ. Med. 2005, 12, 177–179. [Google Scholar]
- Seifert, J.; Casida, J.E. Relation of Yolk Sac Membrane Kynurenine Formamidase Inhibition to Certain Teratogenic Effects of Organophosphorus Insecticides and of Carbaryl and Eserine in Chicken Embryos. Biochem. Pharmacol. 1978, 27, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, J.; Chen, R.; Zhao, Z.; Ying, Z.; Wang, L.; Chen, J.; Hao, K.; Kinney, P.L.; Chen, H.; et al. Particulate Matter Exposure and Stress Hormone Levels: A Randomized, Double-Blind, Crossover Trial of Air Purification. Circulation 2017, 136, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Li, X.; Petriello, M.C.; Wang, C.; Morris, A.J.; Hennig, B. Application of Metabolomics to Characterize Environmental Pollutant Toxicity and Disease Risks. Rev. Environ. Health 2019, 34, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.A.; Liu, T.; Snider, J.M.; Tfaily, M.M.; Itson, C.; Beitel, S.; Parsawar, K.; Keck, K.; Galligan, J.; Walker, D.I.; et al. Evaluating Changes in Firefighter Urinary Metabolomes after Structural Fires: An Untargeted, High Resolution Approach. Sci. Rep. 2023, 13, 20872. [Google Scholar] [CrossRef]
- Monteiro, V.; Dias Da Silva, D.; Martins, M.; Guedes De Pinho, P.; Pinto, J. Metabolomics Perspectives of the Ecotoxicological Risks of Polycyclic Aromatic Hydrocarbons: A Scoping Review. Environ. Res. 2024, 249, 118394. [Google Scholar] [CrossRef]
- Hu, H.; Lu, X.; Wu, M.; Bai, Z.; Liu, X. Effects of Environmental Pollutants on Tryptophan Metabolism. Toxics 2025, 13, 311. [Google Scholar] [CrossRef]
- Sarma, S.N.; Thomas, P.J.; Naz, S.; Pauli, B.; Crump, D.; Zahaby, Y.; O’Brien, J.M.; Mallory, M.L.; Franckowiak, R.P.; Gendron, M.; et al. Metabolomic Profiles in Relation to Benchmark Polycyclic Aromatic Compounds (PACs) and Trace Elements in Two Seabird Species from Arctic Canada. Environ. Res. 2022, 204, 112022. [Google Scholar] [CrossRef]
- Arens, C.J.; Arens, J.C.; Hogan, N.S.; Kavanagh, R.J.; Berrue, F.; Van Der Kraak, G.J.; Van Den Heuvel, M.R. Population Impacts in White Sucker (Catostomus commersonii) Exposed to Oil Sands–Derived Contaminants in the Athabasca River. Environ. Toxicol. Chem. 2017, 36, 2058–2067. [Google Scholar] [CrossRef]
- Arciszewski, T.J.; McMaster, M.E. Potential Influence of Sewage Phosphorus and Wet and Dry Deposition Detected in Fish Collected in the Athabasca River North of Fort McMurray. Environments 2021, 8, 14. [Google Scholar] [CrossRef]
- Van Den Heuvel, M.R.; Dixon, D.G.; Munkittrick, K.R.; Stegeman, J.J. Second-round Interlaboratory Comparison of Hepatic Ethoxyresorufin- O -deethylase Activity in White Sucker (Catostomus commersoni) Exposed to Bleached-kraft Pulp Mill Effluent. Environ. Toxicol. Chem. 1995, 14, 1513–1520. [Google Scholar] [CrossRef]
- Munkittrick, K.R.; Dixon, D.G. Growth, Fecundity, and Energy Stores of White Sucker (Catostomus commersoni) from Lakes Containing Elevated Levels of Copper and Zinc. Can. J. Fish. Aquat. Sci. 1988, 45, 1355–1365. [Google Scholar] [CrossRef]
- Hossen, M.M.; Fleiss, B.; Zakaria, R. The Current State in Liquid Chromatography-Mass Spectrometry Methods for Quantifying Kynurenine Pathway Metabolites in Biological Samples: A Systematic Review. Crit. Rev. Clin. Lab. Sci. 2025, 62, 437–453. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and Degradation Products of Tryptophan in Solution and Proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: Bucharest, Romania, 2014; Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 28 November 2024).
- Wester, P.W.; Van Der Ven, L.T.M.; Vethaak, A.D.; Grinwis, G.C.M.; Vos, J.G. Aquatic Toxicology: Opportunities for Enhancement through Histopathology. Environ. Toxicol. Pharmacol. 2002, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Meador, J.P.; Yeh, A.; Gallagher, E.P. Adverse Metabolic Effects in Fish Exposed to Contaminants of Emerging Concern in the Field and Laboratory. Environ. Pollut. Barking Essex 1987 2018, 236, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Bunt, C.M.; Hamilton, S.J.; Jennings, C.A.; Pearson, M.P.; Cooperman, M.S.; Markle, D.F. Threats, Conservation Strategies, and Prognosis for Suckers (Catostomidae) in North America: Insights from Regional Case Studies of a Diverse Family of Non-Game Fishes. Biol. Conserv. 2005, 121, 317–331. [Google Scholar] [CrossRef]
- Quinn, A.L.; Rasmussen, J.B.; Hontela, A. Physiological Stress Response of Mountain Whitefish (Prosopium williamsoni) and White Sucker (Catostomus commersoni) Sampled along a Gradient of Temperature and Agrichemicals in the Oldman River, Alberta. Environ. Biol. Fishes 2010, 88, 119–131. [Google Scholar] [CrossRef]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Haxton, T.; Friday, M.; Cano, T.; Hendry, C. Large-bodied Fish Assemblage Characteristics in Large Rivers across Ontario. River Res. Appl. 2017, 33, 1044–1051. [Google Scholar] [CrossRef]
- Childress, E.S.; Papke, R.; McIntyre, P.B. Spawning Success and Early Life History of Longnose Suckers in Great Lakes Tributaries. Ecol. Freshw. Fish 2016, 25, 393–404. [Google Scholar] [CrossRef]
- Wilhite, J.W.; Hubert, W.A. Longitudinal Variation and Habitat Associations of Small Fishes in the Bighorn River, Wyoming: Influences of River Regulation and Introduced Fishes. J. Freshw. Ecol. 2011, 26, 163–170. [Google Scholar] [CrossRef][Green Version]
- Arciszewski, T.J.; Munkittrick, K.R.; Kilgour, B.W.; Keith, H.M.; Linehan, J.E.; McMaster, M.E. Increased Size and Relative Abundance of Migratory Fishes Observed near the Athabasca Oil Sands. FACETS 2017, 2, 833–858. [Google Scholar] [CrossRef]
- Gutierrez-Villagomez, J.M.; Lara-Jacobo, L.R.; Gauthier, C.; Patey, G.; Xin, Q.; Triffault-Bouchet, G.; Dettman, H.D.; Langlois, V.S. Diluted Bitumen Weathered under Warm or Cold Temperatures Is Equally Toxic to Freshwater Fish. Front. Environ. Sci. 2024, 12, 1328313. [Google Scholar] [CrossRef]
- Li, C.; Fu, L.; Stafford, J.; Belosevic, M.; Gamal El-Din, M. The Toxicity of Oil Sands Process-Affected Water (OSPW): A Critical Review. Sci. Total Environ. 2017, 601–602, 1785–1802. [Google Scholar] [CrossRef]
- Barrow, M.P.; Peru, K.M.; Fahlman, B.; Hewitt, L.M.; Frank, R.A.; Headley, J.V. Beyond Naphthenic Acids: Environmental Screening of Water from Natural Sources and the Athabasca Oil Sands Industry Using Atmospheric Pressure Photoionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 1508–1521. [Google Scholar] [CrossRef]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-Deethylase (EROD) Activity in Fish as a Biomarker of Chemical Exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef]
- Colavecchia, M.V.; Hodson, P.V.; Parrott, J.L. CYP1A Induction and Blue Sac Disease in Early Life Stages of White Suckers (Catostomus commersoni) Exposed to Oil Sands. J. Toxicol. Environ. Health A 2006, 69, 967–994. [Google Scholar] [CrossRef]
- Walczak, K.; Langner, E.; Makuch-Kocka, A.; Szelest, M.; Szalast, K.; Marciniak, S.; Plech, T. Effect of Tryptophan-Derived AhR Ligands, Kynurenine, Kynurenic Acid and FICZ, on Proliferation, Cell Cycle Regulation and Cell Death of Melanoma Cells-In Vitro Studies. Int. J. Mol. Sci. 2020, 21, 7946. [Google Scholar] [CrossRef]
- Nuti, R.; Gargaro, M.; Matino, D.; Dolciami, D.; Grohmann, U.; Puccetti, P.; Fallarino, F.; Macchiarulo, A. Ligand Binding and Functional Selectivity of Tryptophan Metabolites at the Mouse Aryl Hydrocarbon Receptor (mAhR). J. Chem. Inf. Model. 2014, 54, 3373–3383. [Google Scholar] [CrossRef]
- Prasad, P.; Ogawa, S.; Parhar, I.S. Role of Serotonin in Fish Reproduction. Front. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamshed, L.; Debnath, A.; Marie-Lucas, A.; Tomy, T.; Tomy, G.T.; Arciszewski, T.J.; McMaster, M.E.; Holloway, A.C. Assessing the Kynurenine–Tryptophan Ratio (KTR) and CYP1 Activity in Longnose (Catostomus catostomus) and White Suckers (Catostomus commersonii) Exposed to Petroleum-Derived Contaminants from the Alberta Oil Sands Region. Toxics 2025, 13, 862. https://doi.org/10.3390/toxics13100862
Jamshed L, Debnath A, Marie-Lucas A, Tomy T, Tomy GT, Arciszewski TJ, McMaster ME, Holloway AC. Assessing the Kynurenine–Tryptophan Ratio (KTR) and CYP1 Activity in Longnose (Catostomus catostomus) and White Suckers (Catostomus commersonii) Exposed to Petroleum-Derived Contaminants from the Alberta Oil Sands Region. Toxics. 2025; 13(10):862. https://doi.org/10.3390/toxics13100862
Chicago/Turabian StyleJamshed, Laiba, Amrita Debnath, Amica Marie-Lucas, Thane Tomy, Gregg T. Tomy, Tim J. Arciszewski, Mark E. McMaster, and Alison C. Holloway. 2025. "Assessing the Kynurenine–Tryptophan Ratio (KTR) and CYP1 Activity in Longnose (Catostomus catostomus) and White Suckers (Catostomus commersonii) Exposed to Petroleum-Derived Contaminants from the Alberta Oil Sands Region" Toxics 13, no. 10: 862. https://doi.org/10.3390/toxics13100862
APA StyleJamshed, L., Debnath, A., Marie-Lucas, A., Tomy, T., Tomy, G. T., Arciszewski, T. J., McMaster, M. E., & Holloway, A. C. (2025). Assessing the Kynurenine–Tryptophan Ratio (KTR) and CYP1 Activity in Longnose (Catostomus catostomus) and White Suckers (Catostomus commersonii) Exposed to Petroleum-Derived Contaminants from the Alberta Oil Sands Region. Toxics, 13(10), 862. https://doi.org/10.3390/toxics13100862







