Association of Bisphenol Exposure and Serum Hypothalamic–Pituitary–Thyroid Axis Hormone Levels in Adults and Pregnant Women: A Systematic Review and Meta-Analysis
Highlights
- Bisphenol exposure is significantly associated with altered thyroid hormone levels in adults and pregnant women.
- Subgroup analyses reveal differences by gender and region.
- Bisphenol exposure may contribute to thyroid dysfunction, highlighting the need for stronger regulations and targeted public health measures, especially for vulnerable groups such as pregnant women.
Abstract
1. Introduction
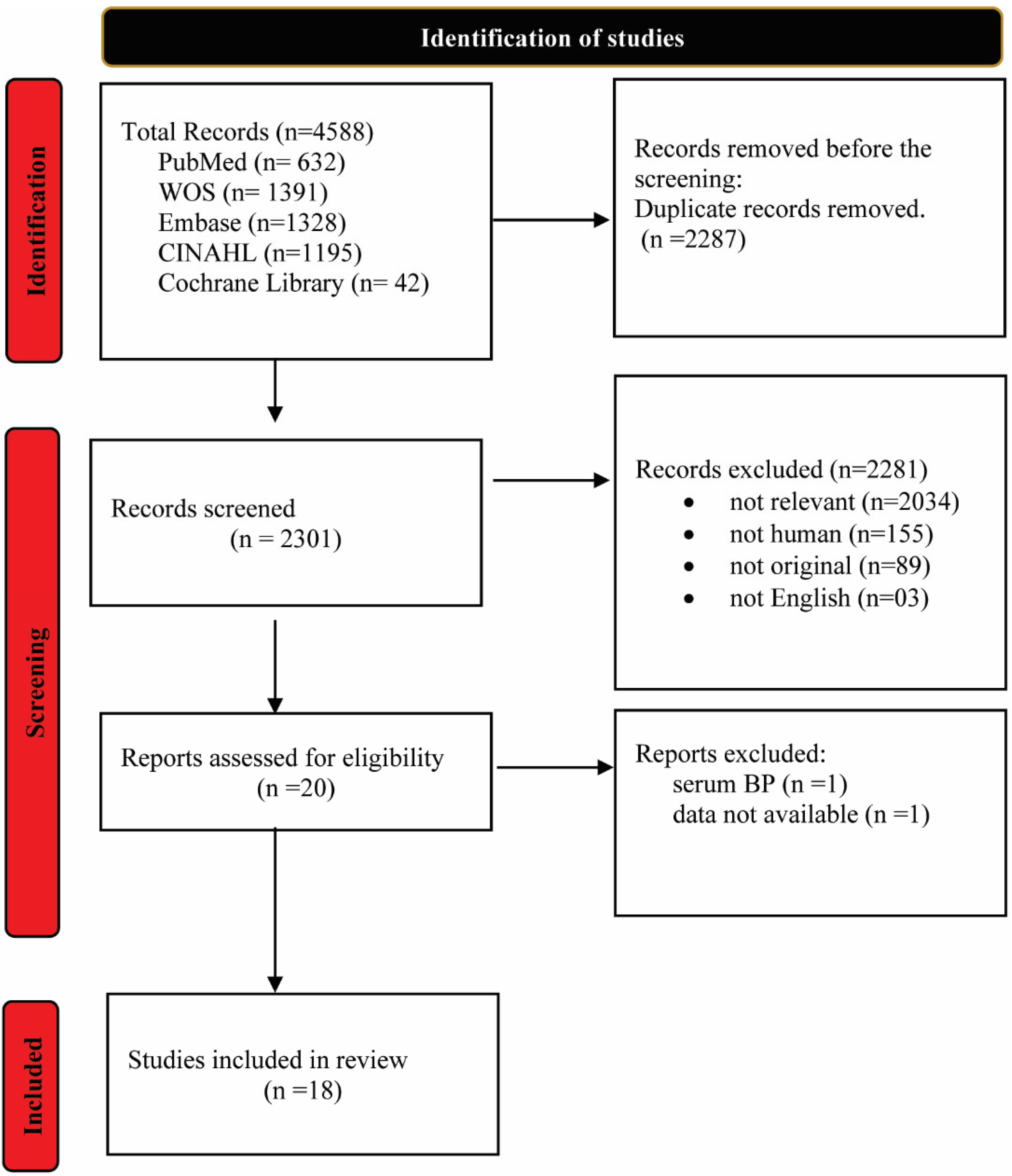
2. Materials and Methods
2.1. Literature Search, Inclusion Criteria, and Data Extraction
2.2. Quality Assurance
2.3. Statistical Analysis
3. Results
3.1. Characteristics Overview
3.2. Quality of Included Studies
Systematic Review
4. Meta-Analysis Results
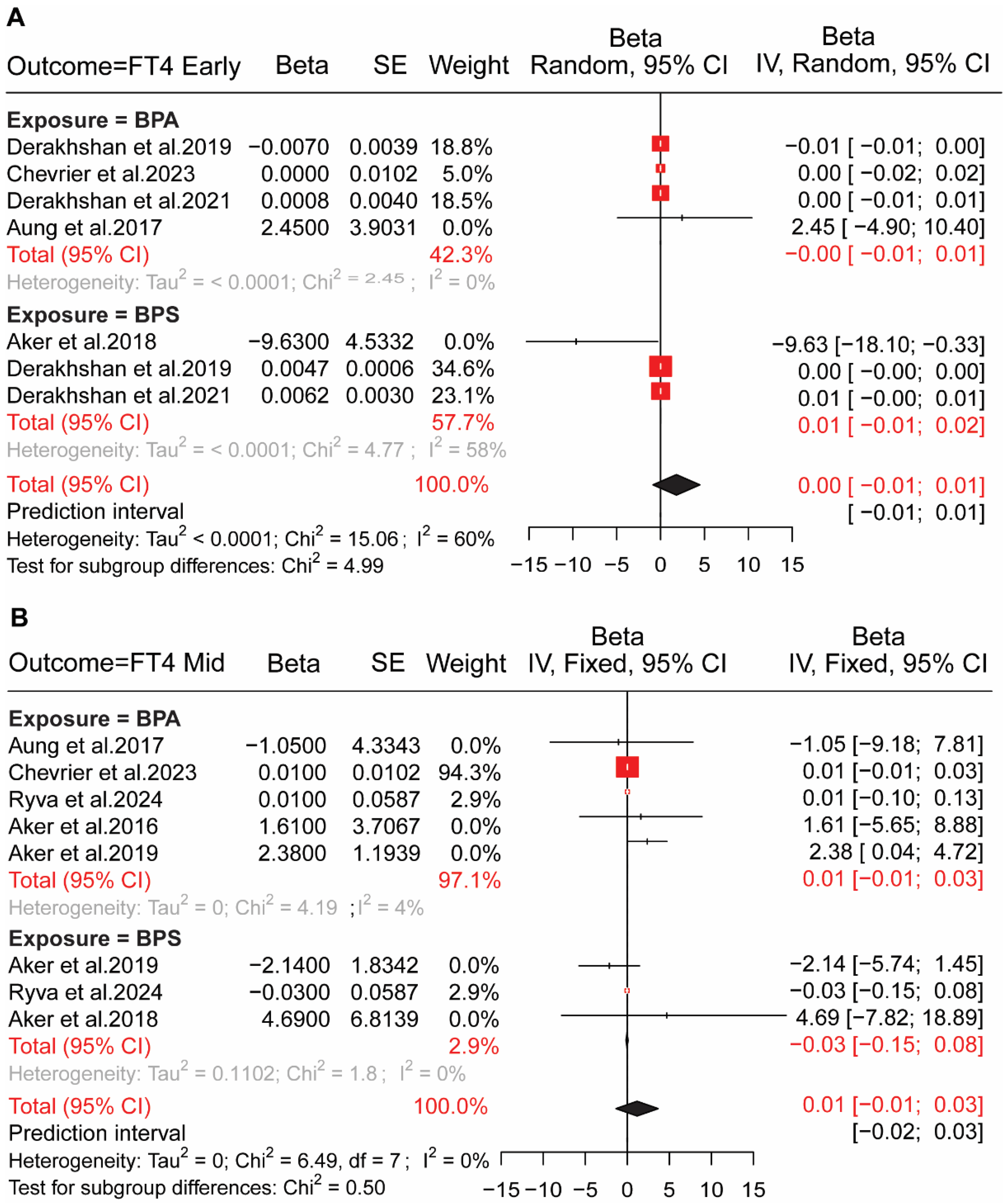
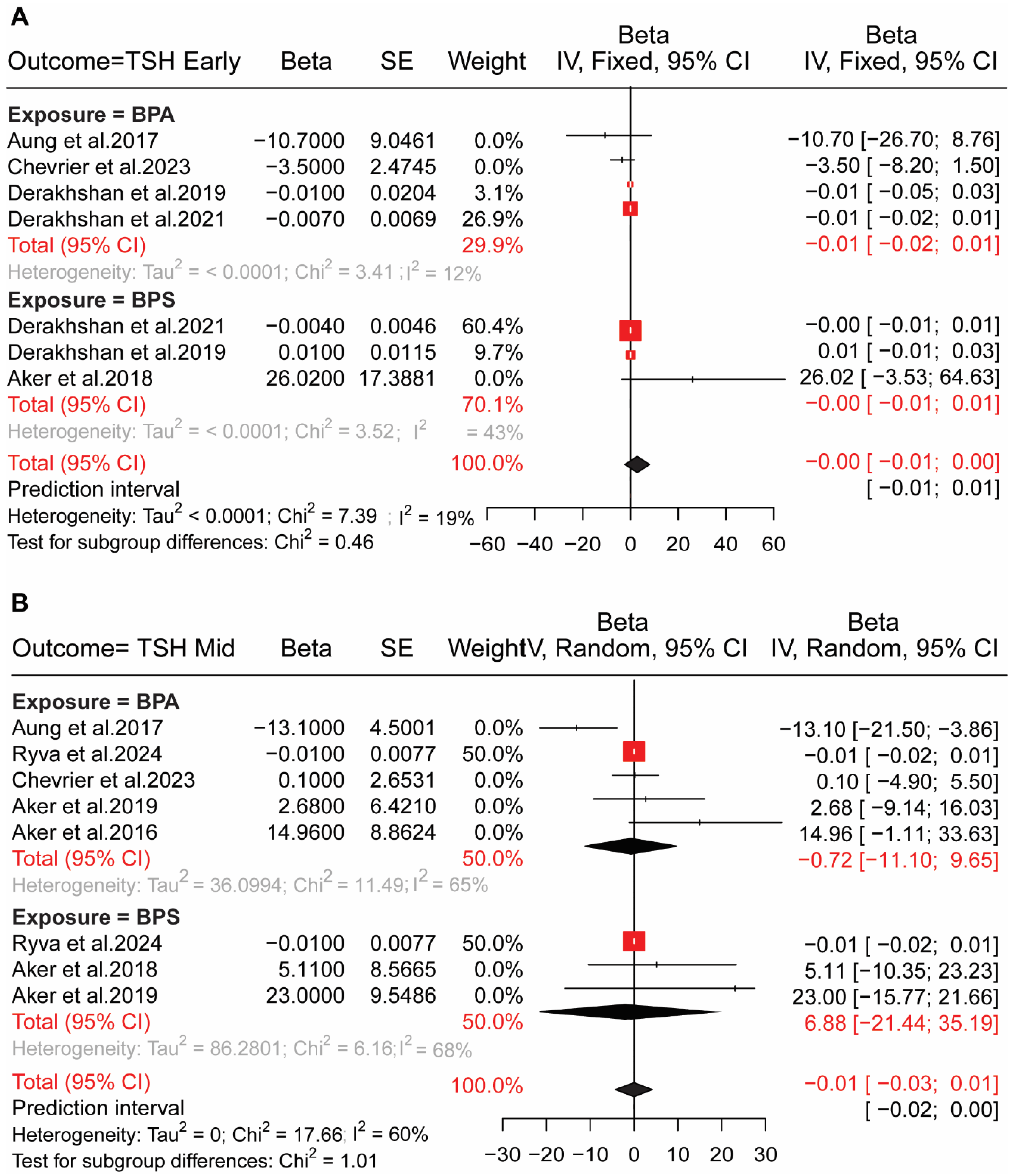
4.1. BP Exposure in Pregnant Women and HPT Axis Hormones
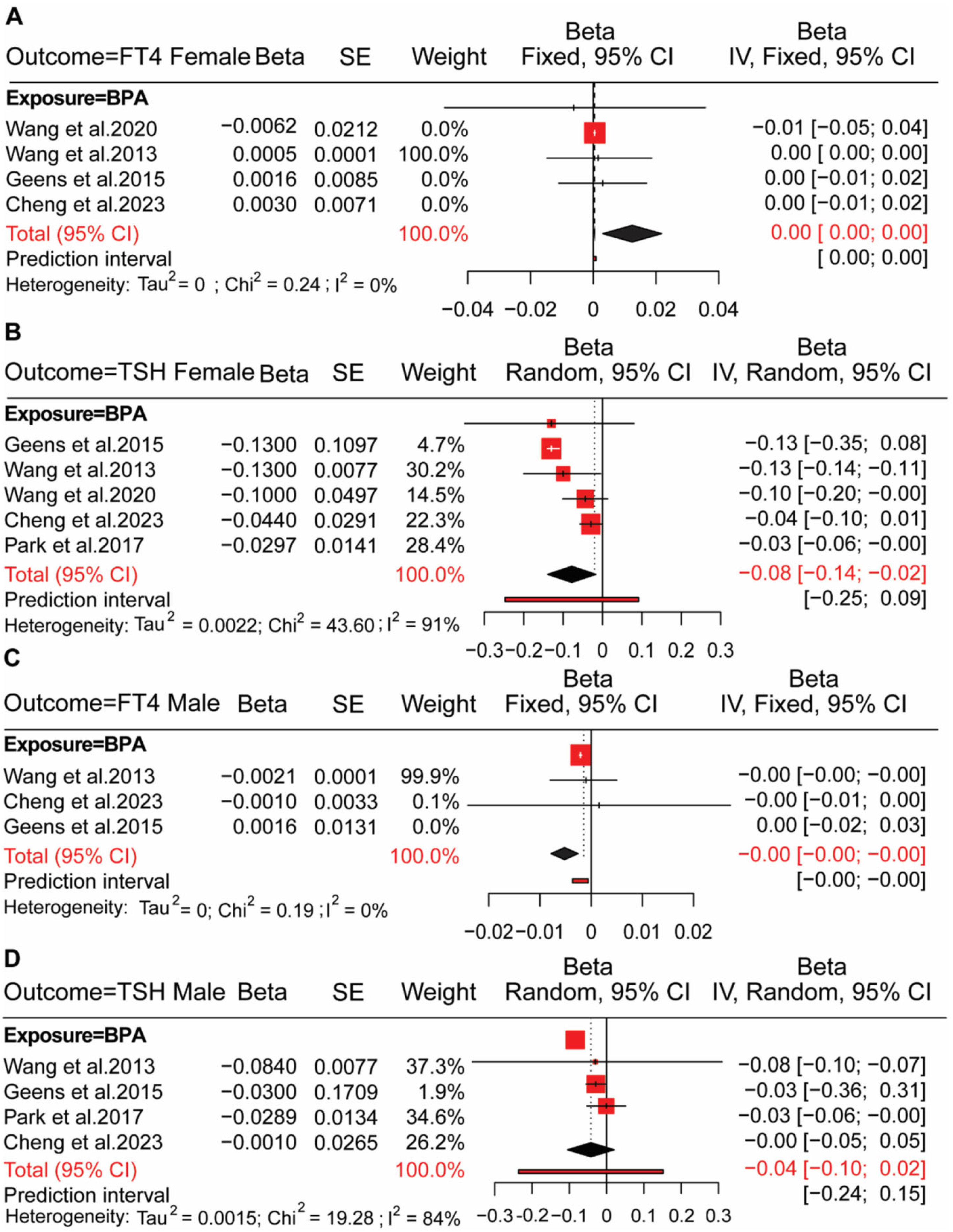
4.2. BPs Exposure in Adults and HPT Axis Hormones
4.3. BPs Exposure and HPT Axis Hormones in Populations from Different Regions
4.4. Small Study Effect and Sensitivity Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Bernot, M.J.; Bernot, R.J. The influence of TCS on the growth and behavior of the freshwater snail, Physa acuta. J. Environ. Sci. Health Part A 2012, 47, 1626–1630. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Fennix-Agudelo, M.; Miranda-Castro, W. Occurrence of personal care products as emerging chemicals of concern in water resources: A review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Jala, A.; Varghese, B.; Dutta, R.; Adela, R.; Borkar, R.M. Levels of parabens and bisphenols in personal care products and urinary concentrations in Indian young adult women: Implications for human exposure and health risk assessment. Chemosphere 2022, 297, 134028. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wong, C.K.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef]
- Moriyama, K.; Tagami, T.; Akamizu, T.; Usui, T.; Saijo, M.; Kanamoto, N.; Hataya, Y.; Shimatsu, A.; Kuzuya, H.; Nakao, K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002, 87, 5185–5190. [Google Scholar] [CrossRef]
- Heimeier, R.A.; Das, B.; Buchholz, D.R.; Shi, Y.B. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology 2009, 150, 2964–2973. [Google Scholar] [CrossRef]
- Aung, M.T.; Johns, L.E.; Ferguson, K.K.; Mukherjee, B.; McElrath, T.F.; Meeker, J.D. Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: A repeated measures study. Environ. Int. 2017, 104, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, J.; Gunier, R.B.; Bradman, A.; Holland, N.T.; Calafat, A.M.; Eskenazi, B.; Harley, K.G. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ. Health Perspect. 2013, 121, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.E.; Webster, G.M.; Vuong, A.M.; Thomas Zoeller, R.; Chen, A.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Lanphear, B.P.; et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environ. Res. 2015, 138, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, Y.J. Bisphenols and Thyroid Hormone. Endocrinol. Metab. 2019, 34, 340–348. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Chaker, L.; Baumgartner, C.; den Elzen, W.P.; Ikram, M.A.; Blum, M.R.; Collet, T.H.; Bakker, S.J.; Dehghan, A.; Drechsler, C.; Luben, R.N.; et al. Subclinical Hypothyroidism and the Risk of Stroke Events and Fatal Stroke: An Individual Participant Data Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2181–2191. [Google Scholar] [CrossRef]
- Chaker, L.; Baumgartner, C.; den Elzen, W.P.; Collet, T.H.; Ikram, M.A.; Blum, M.R.; Dehghan, A.; Drechsler, C.; Luben, R.N.; Portegies, M.L.; et al. Thyroid Function Within the Reference Range and the Risk of Stroke: An Individual Participant Data Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 4270–4282. [Google Scholar] [CrossRef]
- Milczarek-Banach, J.; Miśkiewicz, P. The role of bisphenol A and its analogues as endocrine disruptors influencing the thyroid gland: A short review. J. Med. Sci. 2020, 89, 1–7. [Google Scholar] [CrossRef]
- Negro, R. Levothyroxine before conception in women with thyroid antibodies: A step forward in the management of thyroid disease in pregnancy. Thyroid. Res. 2019, 12, 5. [Google Scholar] [CrossRef]
- Huang, H.; Liang, J.; Tang, P.; Yu, C.; Fan, H.; Liao, Q.; Long, J.; Pan, D.; Zeng, X.; Liu, S.; et al. Associations of bisphenol exposure with thyroid hormones in pregnant women: A prospective birth cohort study in China. Environ. Sci. Pollut. Res. Int. 2022, 29, 87170–87183. [Google Scholar] [CrossRef]
- Park, C.; Choi, W.; Hwang, M.; Lee, Y.; Kim, S.; Yu, S.; Lee, I.; Paek, D.; Choi, K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012-2014. Sci. Total Environ. 2017, 584–585, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, J.; Xu, M.; Xu, Y.; Li, M.; Liu, Y.; Tian, X.; Chen, Y.; Dai, M.; Wang, W.; et al. Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology 2013, 24, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ferguson, K.K. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ. Health Perspect. 2011, 119, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid.-Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- UnitsLab. Online Converter from Conventional to SI Units. Available online: https://unitslab.com/node/135 (accessed on 20 March 2025).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Jpttj, H.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Updated August 2023; Cochrane: London, UK, 2024. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. BMJ Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Watkins, D.J.; Johns, L.E.; Ferguson, K.K.; Soldin, O.P.; Del Toro, L.V.A.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ. Res. 2016, 151, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.M.; Johns, L.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int. 2018, 113, 341–349. [Google Scholar] [CrossRef]
- Aker, A.M.; Ferguson, K.K.; Rosario, Z.Y.; Mukherjee, B.; Alshawabkeh, A.N.; Calafat, A.M.; Cordero, J.F.; Meeker, J.D. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ. Health A Glob. Access Sci. Source 2019, 18, 28. [Google Scholar] [CrossRef]
- Derakhshan, A.; Shu, H.; Peeters, R.P.; Kortenkamp, A.; Lindh, C.H.; Demeneix, B.; Bornehag, C.-G.; Korevaar, T.I.M. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ. Int. 2019, 133, 105123. [Google Scholar] [CrossRef]
- Derakhshan, A.; Philips, E.M.; Ghassabian, A.; Santos, S.; Asimakopoulos, A.G.; Kannan, K.; Kortenkamp, A.; Jaddoe, V.W.V.; Trasande, L.; Peeters, R.P.; et al. Association of urinary bisphenols during pregnancy with maternal, cord blood and childhood thyroid function. Environ. Int. 2021, 146, 106160. [Google Scholar] [CrossRef]
- Ryva, B.A.; Pacyga, D.C.; Anderson, K.Y.; Calafat, A.M.; Whalen, J.; Aung, M.T.; Gardiner, J.C.; Braun, J.M.; Schantz, S.L.; Strakovsky, R.S. Associations of urinary non-persistent endocrine disrupting chemical biomarkers with early-to-mid pregnancy plasma sex-steroid and thyroid hormones. Environ. Int. 2024, 183, 108433. [Google Scholar] [CrossRef]
- Geens, T.; Dirtu, A.C.; Dirinck, E.; Malarvannan, G.; Van Gaal, L.; Jorens, P.G.; Covaci, A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ. Int. 2015, 76, 98–105. [Google Scholar] [CrossRef]
- Kwon, J.A.; Shin, B.; Kim, B. Urinary bisphenol A and thyroid function by BMI in the Korean National Environmental Health Survey (KoNEHS) 2012-2014. Chemosphere 2020, 240, 124918. [Google Scholar] [CrossRef]
- Wang, X.; Tang, N.; Nakayama, S.F.; Fan, P.; Liu, Z.; Zhang, J.; Ouyang, F. Maternal urinary bisphenol A concentration and thyroid hormone levels of Chinese mothers and newborns by maternal body mass index. Environ. Sci. Pollut. Res. Int. 2020, 27, 10939–10949. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, X.; Fan, J.; Qiao, J.; Jia, H. Sex-specific association of exposure to a mixture of phenols, parabens, and phthalates with thyroid hormone and antibody levels in US adolescents and adults. Env. Sci. Pollut. Res. Int. 2023, 30, 121207–121223. [Google Scholar] [CrossRef]
- Hu, Y.; Lai, S.; Li, Y.; Wu, X.; Xing, M.; Li, X.; Xu, D.; Chen, Y.; Xiang, J.; Cheng, P.; et al. Association of urinary bisphenols with thyroid function in the general population: A cross-sectional study of an industrial park in China. Environ. Sci. Pollut. Res. Int. 2023, 30, 107517–107532. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Ning, S.; Miao, H.; Fang, C.; Li, J.; Zhang, L.; Bao, Y.; Fan, S.; Zhao, Y.; Wu, Y. Human exposure to a mixture of endocrine disruptors and serum levels of thyroid hormones: A cross-sectional study. J. Environ. Sci. 2023, 125, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Song, Y.; Jia, Z.; Huan, C.; Cao, Q.; Wang, C.; Mao, Z.; Huo, W. Association of exposure to a mixture of phenols, parabens, and phthalates with altered serum thyroid hormone levels and the roles of iodine status and thyroid autoantibody status: A study among American adults. Ecotoxicol. Environ. Saf. 2024, 282, 116754. [Google Scholar] [CrossRef] [PubMed]
- Sriphrapradang, C.; Chailurkit, L.-o.; Aekplakorn, W.; Ongphiphadhanakul, B. Association between bisphenol A and abnormal free thyroxine level in men. Endocrine 2013, 44, 441–447. [Google Scholar] [CrossRef]
- Yuan, S.; Du, X.; Liu, H.; Guo, X.; Zhang, B.; Wang, Y.; Wang, B.; Zhang, H.; Guo, H. Association between bisphenol A exposure and thyroid dysfunction in adults: A systematic review and meta-analysis. Toxicol. Ind. Health 2023, 39, 188–203. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Cao, J.; Guo, L.H.; Wan, B.; Wei, Y. In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A. J. Environ. Sci. 2011, 23, 315–321. [Google Scholar] [CrossRef]
- Marchesini, G.R.; Meimaridou, A.; Haasnoot, W.; Meulenberg, E.; Albertus, F.; Mizuguchi, M.; Takeuchi, M.; Irth, H.; Murk, A.J. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol. Appl. Pharmacol. 2008, 232, 150–160. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Bansal, R.; Parris, C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 2005, 146, 607–612. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Fu, C.; Wang, H.; Huang, P.; Wang, B.; Su, M.; Jiang, F.; Fang, H.; Zhao, Q.; et al. Influence of Bisphenol A on Thyroid Volume and Structure Independent of Iodine in School Children. PLoS ONE 2015, 10, e0141248. [Google Scholar] [CrossRef]

| Author Name | Year | Research Design | Sample Size | Country | Stage/Gender | Bisphenol Type (BP) | Sample | Detection Method | Outcome | Adjusted Variables |
|---|---|---|---|---|---|---|---|---|---|---|
| Aker et al. [37] | 2019 | Cohort | 602 | United States | 2nd trimester | A, S, F | Urine | HPLC-ID-MS/MS | TSH, FT4, T4 | Maternal age (MA), study visit (SV), Specific gravity (SG), BMI at 1st visit, the passive smoking exposure (PS) in hours, and a socio-economic variable (SEV) |
| Derakhshan et al. [38] | 2019 | Cohort | 1996 | Sweden | 1st trimester | A, S, F | Urine | LC-MS/MS | TSH, FT4, FT3, TT4, TT3 | MA, thyroid peroxidase antibodies (TPa), thyroglobulin antibodies (Tba), gestational age (GA), human chorionic gonadotropin, urinary creatinine (UC), smoking, BMI and SEV |
| Derakhshan et al. [39] | 2021 | Cohort | 1267 | The Netherlands | 1st trimester | A, S | Urine | HPLC-ESI-MS/MS | TSH, FT4, TT4 | GA, MA, TPa, human chorionic gonadotropin, UC, BMI, education level, ethnicity, smoking status, and parity |
| Aker et al. [36] | 2018 | Cohort | 439 | Boston, MA | 1st, 2nd, 3rd trimesters | S | Urine | ID-LC-MS/MS | TSH, FT4, T4, T3 | SG, SV, BMI, MA and GA, and insurance provider. |
| Kwon et al. [42] | 2020 | Cross-Sectional | 5108 | Korea | Adults | A | Urine | UPLC-MS/MS | TSH, T4, T3 | Smoking status, alcohol consumption, exercise, SV, MEHHP, MEOHP, MECPP, MnBP, MBzP, age and SEV. |
| Chevrier et al. [13] | 2013 | Cohort | 335 | United States | 1st, 2nd trimester | A | Urine | HPLC- ID-MS/MS | TSH, FT4, TT4 | MA, iodine intake, and hexachlorobenzene and polychlorinated biphenyl, SEV, drugs in pregnancy |
| Geens et al. [41] | 2015 | Cohort | 151 | Belgium | Male, female, and Total adults | A | Urine | GC–MS system | TSH, FT4 | Age and weight loss |
| Yue et al. [46] | 2023 | Cross-Sectional | 177 | China | Adults | A, S, F | Urine | UPLC-MS/MS | TSH, FT4, FT3, TT4, TT3 | Age, sex, BMI, smoking, urinary iodine, and SEV. |
| Wang et al. [43] | 2020 | Cohort | 555 | China | Female | A | Urine | HPLC-MS/MS | TSH, FT4 | UC, MA, PS, GDM (yes/no), GA and SEV |
| Aker et al. [35] | 2016 | Cohort | 106 | United States | 2nd trimester | A | Urine | HPLC-ID-MS/MS | TSH, FT4, FT3 | SG, SV, MA, BMI, and SEV |
| Aung et al. [12] | 2017 | Cohort | 439 | Boston, MA | 1st, 2nd, 3rd trimester | A | Urine | ID-LC-MS/MS | TSH, FT4, FT3, TT4, TT3 | SG, GA, MA, BMI and SEV |
| Park et al. [23] | 2017 | Cross-Sectional | 5870 | Korea | Male, female, and total adults | A | Urine | LLE-UPLC-MS/MS | TSH, TT4, TT3 | Age, BMI, sex, PS, UC, and SEV |
| Meeker & Ferguson [25] | 2011 | Cross-Sectional | 1675 | United States | Adults | A | Urine | HPLC-ID-MS/MS | TSH, FT4, FT3, TT4, TT3 | Age, BMI, sex, ethnicity, BMI, UC, and iodine |
| Wang et al. [24] | 2013 | Cross-Sectional | 3394 | China | Male and female | A | Urine | HPLC-MS/MS | TSH, FT4, FT3 | UC, age, BMI, SEV, alcohol, triglycerides, HDLC, LDL-C, Tba, and TPa |
| Cheng et al. [44] | 2023 | Cross-Sectional | 2911 | United States | Male and female | A | Urine | SPE-HPLC-MS/MS | TSH, FT4, FT3, TT4, TT3 | Age, sex, race, education, poverty level, BMI, serum cotinine, UI, hypertension, DM, and UC |
| Gao et al. [47] | 2024 | Cross-Sectional | 2385 | United States | Adults | A | Urine | SPE-HPLC-MS/MS | TSH, FT4, FT3, T4, T3 | Age, BMI, gender, smoking, drinking, race, education, medication history, marital status, PIR, and UC level |
| Hu et al. [45] | 2023 | Cross-Sectional | 150 | China | Adults | A | Urine | UPLC-MS/MS | TSH, FT4, FT3, TT4, TT3 | Age, BMI, gender, education levels, smoking, alcohol, UC, occupation |
| Ryva et al. [40] | 2024 | Cohort | 302 | Chicago | 2nd trimester | A & S | Urine | ID-MS/MS | TSH, FT4, TT4 | Age, diet, pre-pregnancy BMI, stress, smoking status, parity, race, GA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, M.; Ma, X.; Yu, Q.; Bigambo, F.M.; Tang, Y.; Chitakwa, N.; Kafauit, F.; Chen, Q.; Guan, Q.; Xia, Y. Association of Bisphenol Exposure and Serum Hypothalamic–Pituitary–Thyroid Axis Hormone Levels in Adults and Pregnant Women: A Systematic Review and Meta-Analysis. Toxics 2025, 13, 836. https://doi.org/10.3390/toxics13100836
Sultan M, Ma X, Yu Q, Bigambo FM, Tang Y, Chitakwa N, Kafauit F, Chen Q, Guan Q, Xia Y. Association of Bisphenol Exposure and Serum Hypothalamic–Pituitary–Thyroid Axis Hormone Levels in Adults and Pregnant Women: A Systematic Review and Meta-Analysis. Toxics. 2025; 13(10):836. https://doi.org/10.3390/toxics13100836
Chicago/Turabian StyleSultan, Mazhar, Xuan Ma, Qiurun Yu, Francis Manyori Bigambo, Yufeng Tang, Natasha Chitakwa, Farah Kafauit, Qinrou Chen, Quanquan Guan, and Yankai Xia. 2025. "Association of Bisphenol Exposure and Serum Hypothalamic–Pituitary–Thyroid Axis Hormone Levels in Adults and Pregnant Women: A Systematic Review and Meta-Analysis" Toxics 13, no. 10: 836. https://doi.org/10.3390/toxics13100836
APA StyleSultan, M., Ma, X., Yu, Q., Bigambo, F. M., Tang, Y., Chitakwa, N., Kafauit, F., Chen, Q., Guan, Q., & Xia, Y. (2025). Association of Bisphenol Exposure and Serum Hypothalamic–Pituitary–Thyroid Axis Hormone Levels in Adults and Pregnant Women: A Systematic Review and Meta-Analysis. Toxics, 13(10), 836. https://doi.org/10.3390/toxics13100836








