Cerebellar Mechanisms Underlying Autism-like Cognitive Deficits in Mouse Offspring with Prenatal Valproic Acid Exposure
Highlights
- VPA exposure induced significant ASD-like cognitive impairment, anxiety-like behaviors, and pathological cerebellar alterations.
- VPA caused cerebellar impairment through the dysfunction of glutamate receptor signaling and disruptions in axon-guidance signaling.
- Cerebellar impairments constitute a core component of ASD pathophysiology.
- Grin2b represents a potential therapeutic target for addressing cognitive impairment in ASD.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
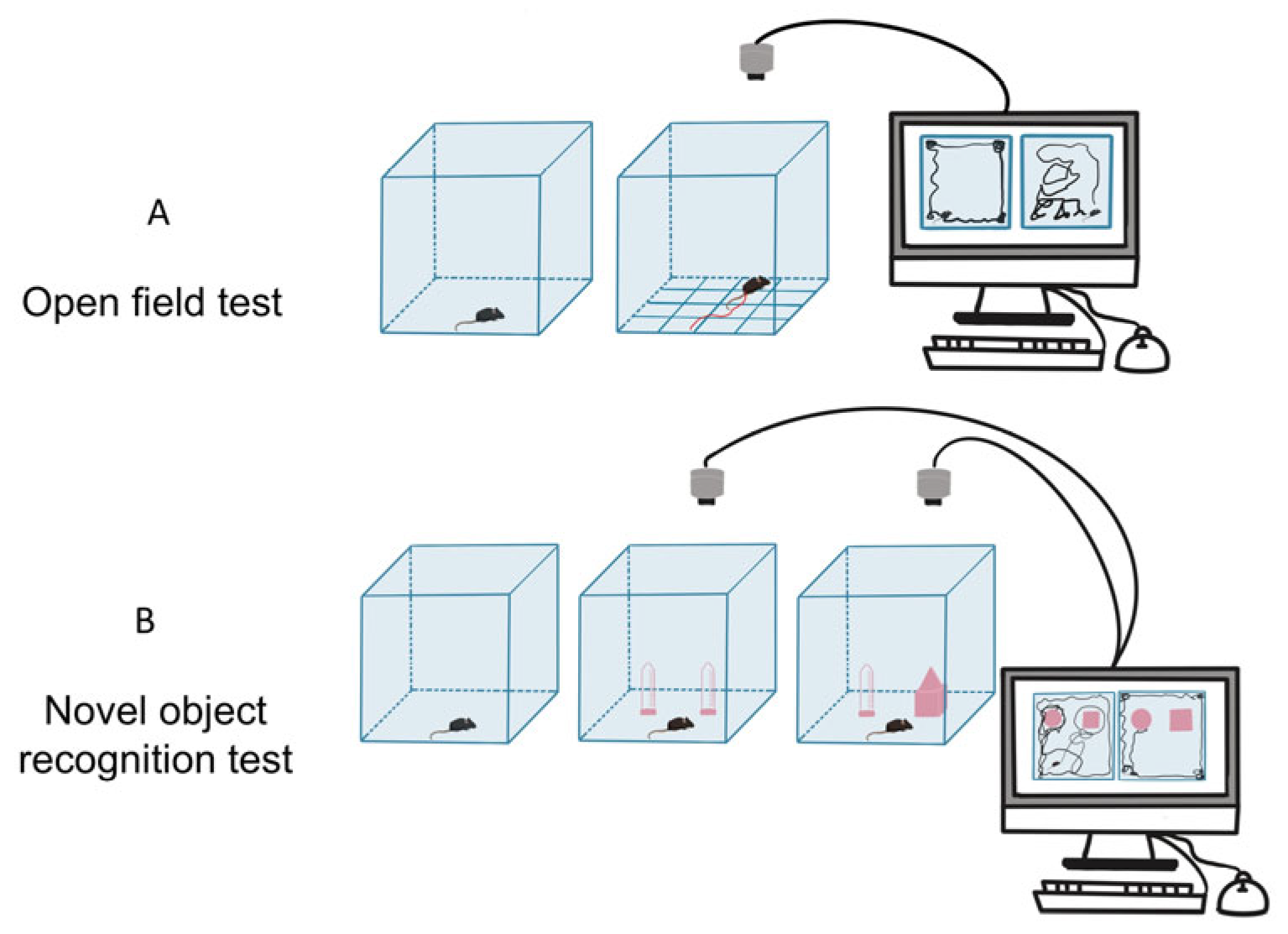
2.2. Behavioral Tests
2.3. Hematoxylin and Eosin (H&E) Staining
2.4. DIA-Based Quantitative Proteomics Analysis
2.4.1. Protein Extraction and Digestion
2.4.2. DIA Analysis
2.4.3. Data Analysis
2.5. Western Blotting Analysis
2.6. Statistical Analysis
3. Results
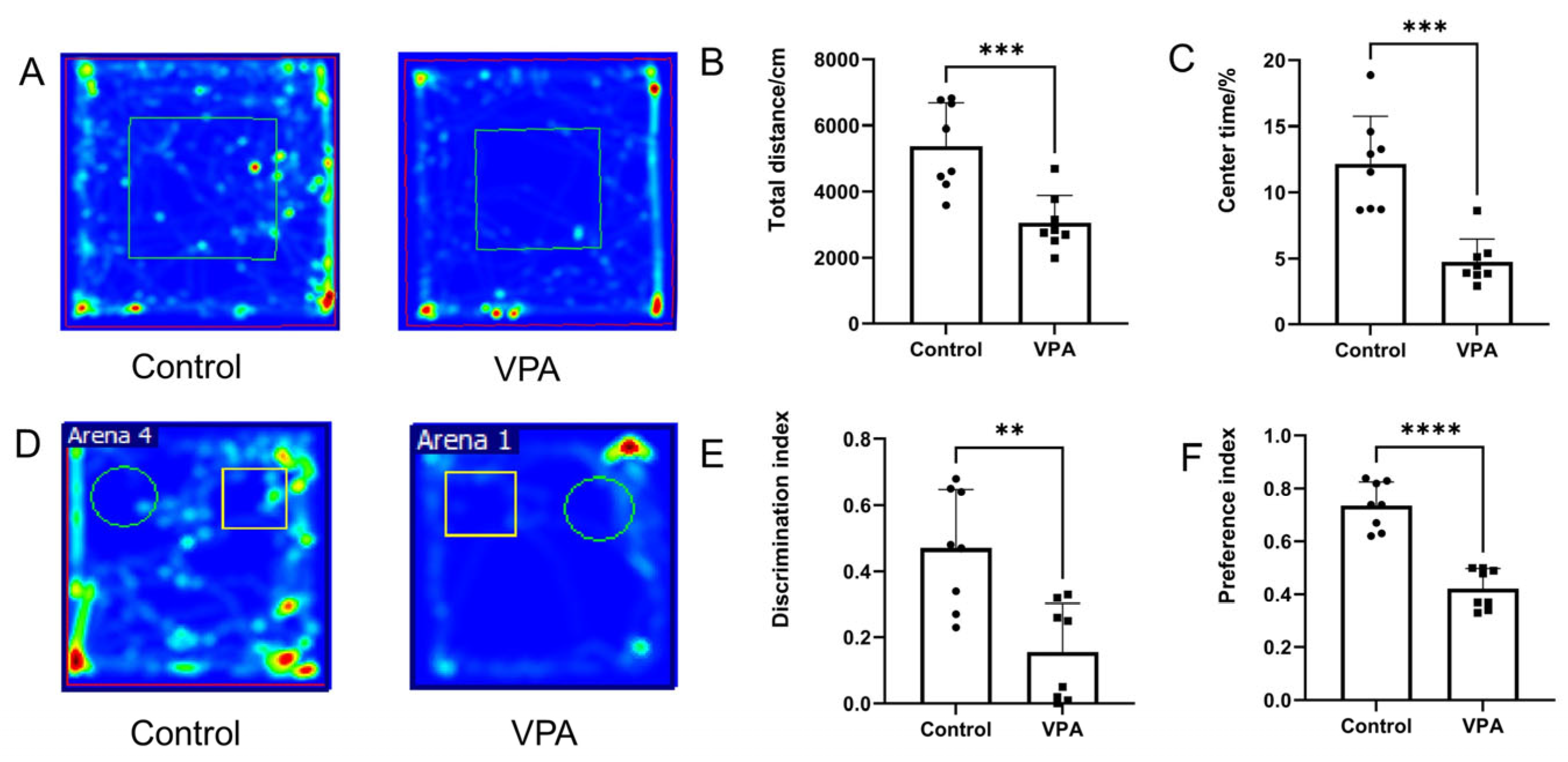
3.1. Prenatal Exposure to VPA Induced Social Cognitive and Anxiety-like Behaviors
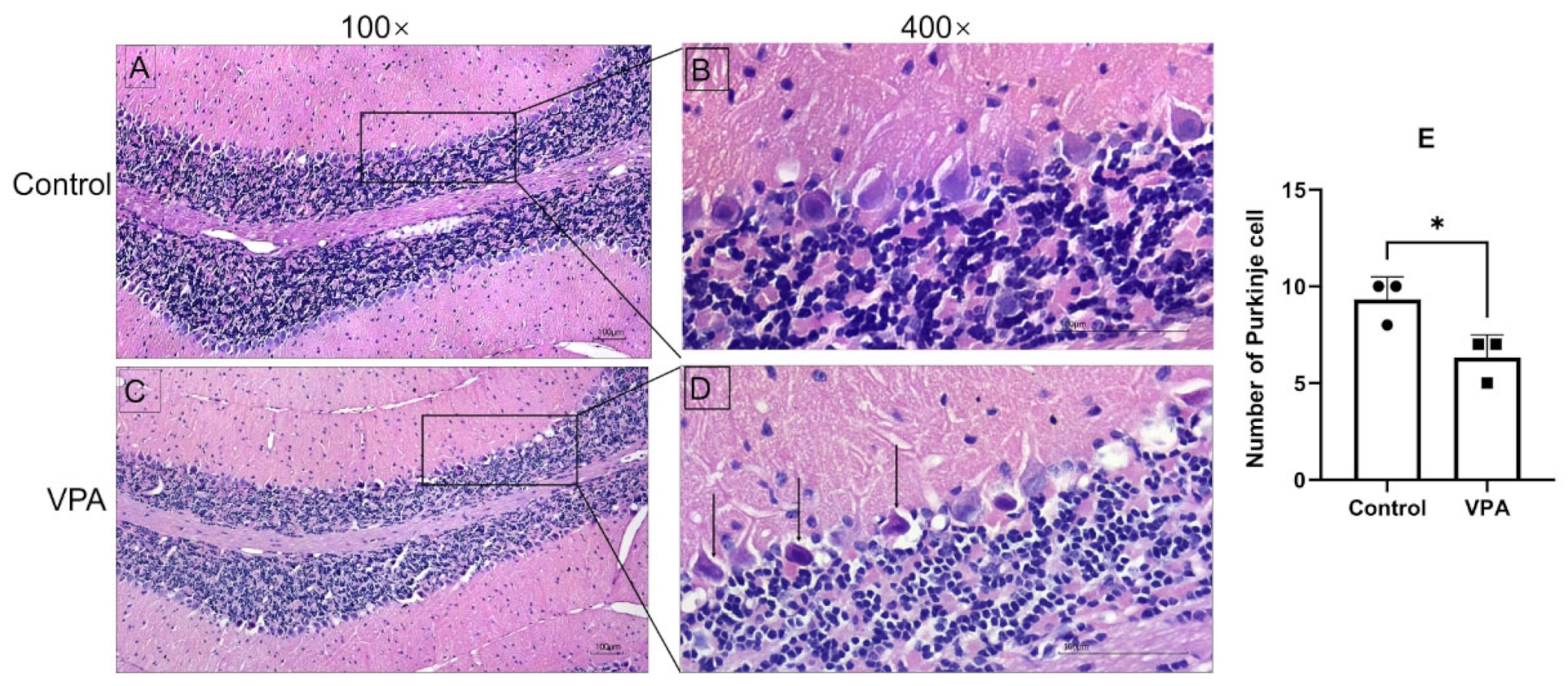
3.2. Prenatal Exposure to VPA Induced Significant Cerebellar Defects
3.3. Quantification of Proteomic Profiling of Prenatal Exposure to VPA and Control
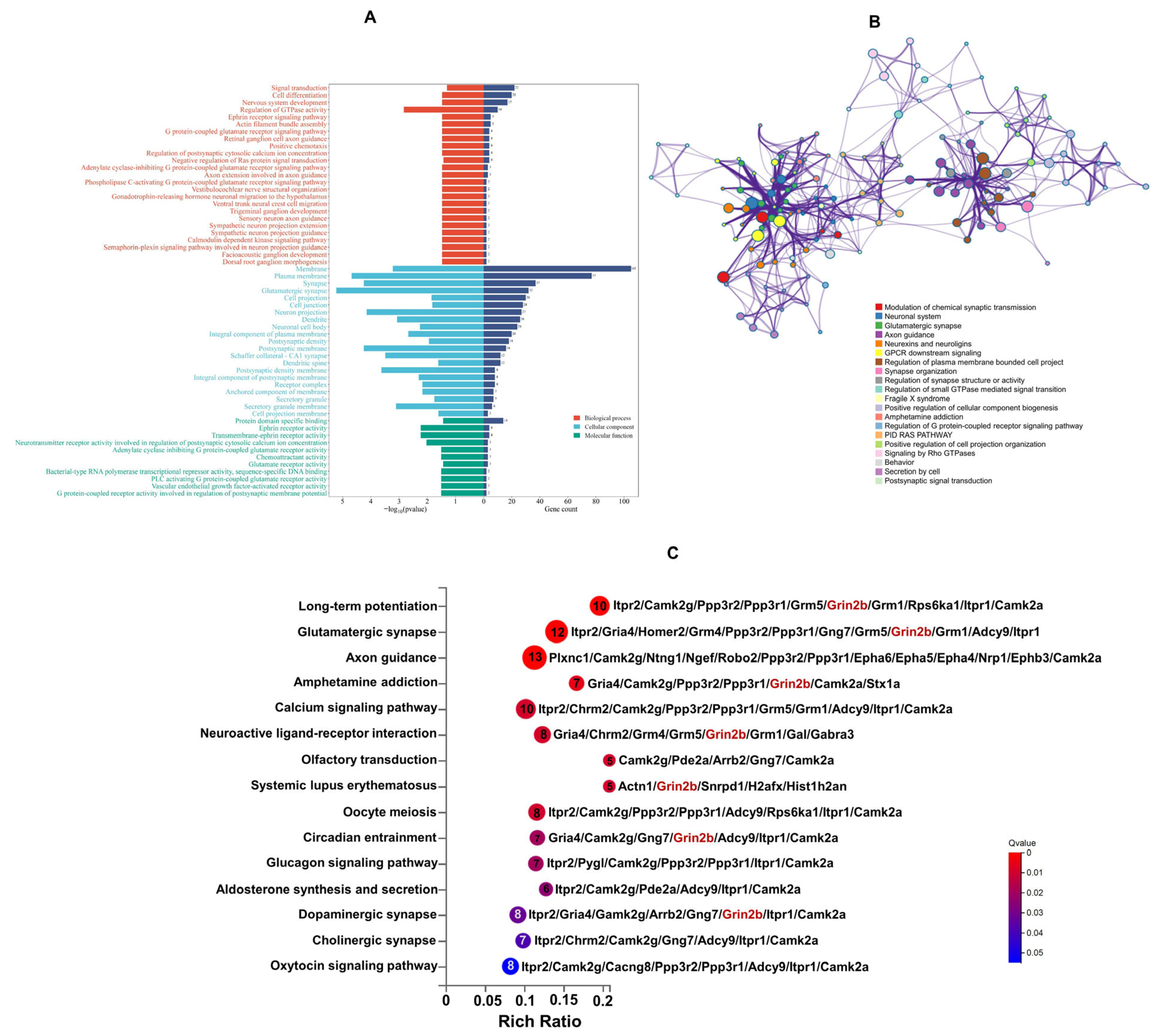
3.4. Functional Enrichment Analysis of DEPs
3.5. Protein–Protein Interaction (PPI) Network Analysis of DEPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| DEPs | Differentially expressed proteins |
| VPA | Valproic acid |
| DIA | Data-independent acquisition |
| DDA | Data-dependent acquisition |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NMDAR | N-methyl-d-aspartate receptor |
| AMPAR | 2-amino-3-propionate receptor |
References
- Yuan, J.J.; Zhao, Y.N.; Lan, X.Y.; Zhang, Y.; Zhang, R. Prenatal, perinatal and parental risk factors for autism spectrum disorder in China: A case- control study. BMC Psychiatry 2024, 24, 219. [Google Scholar] [CrossRef]
- Yu, X.; Rahman, M.M.; Carter, S.A.; Lin, J.C.; Chow, T.; Lurmann, F.W.; Chen, J.C.; Martinez, M.P.; Schwartz, J.; Eckel, S.P.; et al. Neighborhood Disadvantage and Autism Spectrum Disorder in a Population With Health Insurance. JAMA Psychiatry 2024, 81, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Hirota, T.; Sakamoto, Y.; Adachi, M.; Takahashi, M.; Osato-Kaneda, A.; Kim, Y.S.; Leventhal, B.; Shui, A.; Kato, S.; et al. Prevalence and cumulative incidence of autism spectrum disorders and the patterns of co-occurring neurodevelopmental disorders in a total population sample of 5-year-old children. Mol. Autism 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Williams, S.; Patrick, M.E.; Valencia-Prado, M.; Durkin, M.S.; Howerton, E.M.; Ladd-Acosta, C.M.; Pas, E.T.; Bakian, A.V.; Bartholomew, P.; et al. Prevalence and Early Identification of Autism Spectrum Disorder Among Children Aged 4 and 8 Years—Autism and Developmental Disabilities Monitoring Network, 16 Sites, United States, 2022. MMWR Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2021 Autism Spectrum Collaborators. The global epidemiology and health burden of the autism spectrum: Findings from the Global Burden of Disease Study 2021. Lancet Psychiatry 2025, 12, 111–121. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Gong, H.; Liu, T.; Li, X.; Fan, X. Implication of Hippocampal Neurogenesis in Autism Spectrum Disorder: Pathogenesis and Therapeutic Implications. Curr. Neuropharmacol. 2023, 21, 2266–2282. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Li, Q.; Yang, S.; Li, W.; Lai, Y.; Lv, Z. Research progress on hippocampal neurogenesis in autism spectrum disorder. Pediatr. Investig. 2024, 8, 215–223. [Google Scholar] [CrossRef]
- Santos-Terra, J.; Deckmann, I.; Schwingel, G.B.; Paz, A.V.C.; Gama, C.S.; Bambini-Junior, V.; Fontes-Dutra, M.; Gottfried, C. Resveratrol prevents long-term structural hippocampal alterations and modulates interneuron organization in an animal model of ASD. Brain Res. 2021, 1768, 147593. [Google Scholar] [CrossRef]
- Cortes, B.I.; Meza, R.C.; Ancaten-Gonzalez, C.; Ardiles, N.M.; Aranguiz, M.I.; Tomita, H.; Kaplan, D.R.; Cornejo, F.; Nunez-Parra, A.; Moya, P.R.; et al. Loss of protein tyrosine phosphatase receptor delta PTPRD increases the number of cortical neurons, impairs synaptic function and induces autistic-like behaviors in adult mice. Biol. Res. 2024, 57, 40. [Google Scholar] [CrossRef]
- Khaliulin, I.; Hamoudi, W.; Amal, H. The multifaceted role of mitochondria in autism spectrum disorder. Mol. Psychiatry 2025, 30, 629–650. [Google Scholar] [CrossRef]
- Sabaie, H.; Dehghani, H.; Shiva, S.; Asadi, M.R.; Rezaei, O.; Taheri, M.; Rezazadeh, M. Mechanistic Insight Into the Regulation of Immune-Related Genes Expression in Autism Spectrum Disorder. Front. Mol. Biosci. 2021, 8, 754296. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Yamasaki, R.; Saitoh, B.Y.; Abdelhadi, A.; Nagata, S.; Yoshidomi, S.; Inoue, Y.; Matsumoto, K.; Kira, J.I.; Isobe, N. Postnatal Allergic Inhalation Induces Glial Inflammation in the Olfactory Bulb and Leads to Autism-Like Traits in Mice. Int. J. Mol. Sci. 2024, 25, 10464. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Bowirrat, A.; Sunder, K.; Thanos, P.K.; Hanna, C.; Gold, M.S.; Dennen, C.A.; Elman, I.; Murphy, K.T.; Makale, M.T. Dopamine Dysregulation in Reward and Autism Spectrum Disorder. Brain Sci. 2024, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, A.M.; Pandian, A. Cerebellar Roles in Motor and Social Functions and Implications for ASD. Cerebellum 2024, 23, 2564–2574. [Google Scholar] [CrossRef]
- Biswas, M.S.; Roy, S.K.; Hasan, R.; Uddin, P. The crucial role of the cerebellum in autism spectrum disorder: Neuroimaging, neurobiological, and anatomical insights. Health Sci. Rep. 2024, 7, e2233. [Google Scholar] [CrossRef]
- Guerra, M.; Medici, V.; Weatheritt, R.; Corvino, V.; Palacios, D.; Geloso, M.C.; Farini, D.; Sette, C. Fetal exposure to valproic acid dysregulates the expression of autism-linked genes in the developing cerebellum. Transl. Psychiatry 2023, 13, 114. [Google Scholar] [CrossRef]
- Frosch, I.R.; Mittal, V.A.; D’Mello, A.M. Cerebellar Contributions to Social Cognition in ASD: A Predictive Processing Framework. Front. Integr. Neurosci. 2022, 16, 810425. [Google Scholar] [CrossRef]
- Van Overwalle, F. Social and emotional learning in the cerebellum. Nat. Rev. Neurosci. 2024, 25, 776–791. [Google Scholar] [CrossRef]
- Katz, D.B.; Steinmetz, J.E. Psychological functions of the cerebellum. Behav. Cogn. Neurosci. Rev. 2002, 1, 229–241. [Google Scholar] [CrossRef]
- Kakei, S.; Tanaka, H.; Mitoma, H.; Manto, M. The Cerebellum as a Predictor: Recent Insights Into Cognitive Control and Extension of Kalman Filter Theory to Cognition. Discov Med. 2025, 37, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Yeungcourchesne, R.; Press, G.A.; Hesselink, J.R.; Jernigan, T.L. Hypoplasia of cerebellar vermal lobule-VI and lobule-VII in autism. N. Engl. J. Med. 1988, 318, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Clausi, S.; Olivito, G.; Lupo, M.; Siciliano, L.; Bozzali, M.; Leggio, M. The Cerebellar Predictions for Social Interactions: Theory of Mind Abilities in Patients With Degenerative Cerebellar Atrophy. Front. Cell. Neurosci. 2019, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Palmen, S.; van Engeland, H.; Hof, P.R.; Schmitz, C. Neuropathological findings in autism. Brain 2004, 127, 2572–2583. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Bassan, H.; Gauvreau, K.; Robertson, R.L.; Sullivan, N.R.; Benson, C.B.; Avery, L.; Stewart, J.; Soul, J.S.; Ringer, S.A.; et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007, 120, 584–593. [Google Scholar] [CrossRef]
- Fan, K.Y.; Wang, J.; Zhu, W.Q.; Zhang, X.A.; Deng, F.; Zhang, Y.; Zou, S.; Kong, L.J.; Shi, H.; Li, Z.L.; et al. Urinary proteomics for noninvasive monitoring of biomarkers of chronic mountain sickness in a young adult population using data-independent acquisition (DIA)-based mass spectrometry. J. Proteom. 2024, 302, 105195. [Google Scholar] [CrossRef]
- Liu, W.L.; Li, L.M.; Xia, X.C.; Zhou, X.L.; Du, Y.K.; Yin, Z.Q.; Wang, J. Integration of Urine Proteomic and Metabolomic Profiling Reveals Novel Insights Into Neuroinflammation in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 780747. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef]
- Maisterrena, A.; Matas, E.; Mirfendereski, H.; Balbous, A.; Marchand, S.; Jaber, M. The State of the Dopaminergic and Glutamatergic Systems in the Valproic Acid Mouse Model of Autism Spectrum Disorder. Biomolecules 2022, 12, 1691. [Google Scholar] [CrossRef]
- Deng, W.L.; Ke, H.R.; Wang, S.Q.; Li, Z.T.; Li, S.T.; Lv, P.J.; Li, F.; Chen, Y. Metformin Alleviates Autistic-Like Behaviors Elicited by High-Fat Diet Consumption and Modulates the Crosstalk Between Serotonin and Gut Microbiota in Mice. Behav. Neurol. 2022, 2022, 6711160. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Q.; Chen, Y.; Wu, L.; Liu, X.; Liang, D. Behavioral and Gene Expression Analysis of Stxbp6-Knockout Mice. Brain Sci. 2021, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Alterations in the intrinsic discharge activity of CA1 pyramidal neurons associated with possible changes in the NADPH diaphorase activity in a rat model of autism induced by prenatal exposure to valproic acid. Brain Res. 2022, 1792, 148013. [CrossRef]
- Liu, L.; Zhou, X.L.; Ma, Z.H.; Liu, R.M.; Zhang, Y.H.; Wang, Y.Q.; Liu, Y.W.; Xia, X.C.; Wang, J. Hippocampal Proteomics Reveals the Novel Molecular Profiling of Postnatal Lead (Pb) Exposure on Autism-like Behaviors. Toxics 2025, 13, 465. [Google Scholar] [CrossRef]
- Tang, D.D.; Chen, M.J.; Huang, X.H.; Zhang, G.C.; Zeng, L.; Zhang, G.S.; Wu, S.J.; Wang, Y.W. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Rodrigues, B.A.; Silva, J.D.; Kawamoto, E.M. Cerebellar Alterations in Autism Spectrum Disorder: A Mini-Review. Cerebellum 2025, 24, 52. [Google Scholar] [CrossRef]
- Zhou, P.L.; Peng, S.Y.; Wen, S.Z.; Lan, Q.H.; Zhuang, Y.Y.; Li, X.Y.; Shi, M.L.; Zhang, C.Z. The Cerebellum-Ventral Tegmental Area Microcircuit and Its Implications for Autism Spectrum Disorder: A Narrative Review. Neuropsychiatr. Dis. Treat. 2024, 20, 2039–2048. [Google Scholar] [CrossRef]
- Wang, R.N.; Tan, J.H.; Guo, J.X.; Zheng, Y.H.; Han, Q.; So, K.F.; Yu, J.D.; Zhang, L. Aberrant Development and Synaptic Transmission of Cerebellar Cortex in a VPA Induced Mouse Autism Model. Front. Cell. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef]
- Hansel, C. Deregulation of synaptic plasticity in autism. Neurosci. Lett. 2019, 688, 58–61. [Google Scholar] [CrossRef]
- Ebrahimi-Fakhari, D.; Sahin, M. Autism and the synapse: Emerging mechanisms and mechanism-based therapies. Curr. Opin. Neurol. 2015, 28, 91–102. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, K.; Cao, X.; Zhao, Y.; Ullah Khan, N.; Liu, X.; Tang, X.; Chen, M.; Zhang, H.; Shen, L. iTRAQ-Based Proteomics Analysis of Rat Cerebral Cortex Exposed to Valproic Acid before Delivery. ACS Chem. Neurosci. 2022, 13, 648–663. [Google Scholar] [CrossRef]
- Nisar, S.; Bhat, A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Sabo, S.L.; Lahr, J.M.; Offer, M.; Weekes, A.; Sceniak, M.P. GRIN2B-related neurodevelopmental disorder: Current understanding of pathophysiological mechanisms. Front. Synaptic Neurosci. 2022, 14, 1090865. [Google Scholar] [CrossRef]
- den Hollander, B.; Veenvliet, A.R.J.; Rothuizen-Lindenschot, M.; van Essen, P.; Peters, G.; Santos-Gomez, A.; Olivella, M.; Altafaj, X.; Brands, M.M.; Jacobs, B.A.W.; et al. Evidence for effect of l-serine, a novel therapy for GRIN2B-related neurodevelopmental disorder. Mol. Genet. Metab. 2023, 138, 107523. [Google Scholar] [CrossRef]
- Santos-Gómez, A.; Juliá-Palacios, N.; Rejano-Bosch, A.; Marí-Vico, R.; Miguez-Cabello, F.; Masana, M.; Soto, D.; Olivella, M.; García-Cazorla, A.; Altafaj, X. Spermidine Treatment Improves GRIN2B Loss-Of-Function, A Primary Disorder of Glutamatergic Neurotransmission. J. Inherit. Metab. Dis. 2025, 48, e70015. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Guo, H.; Ou, J.; Peng, Y.; Liu, Q.; Shen, Y.; Shi, L.; Liu, Y.; Xiong, Z.; et al. Association of genetic variants of GRIN2B with autism. Sci. Rep. 2015, 5, 8296. [Google Scholar] [CrossRef] [PubMed]
- Zoodsma, J.D.; Keegan, E.J.; Moody, G.R.; Bhandiwad, A.A.; Napoli, A.J.; Burgess, H.A.; Wollmuth, L.P.; Sirotkin, H.I. Disruption of grin2B, an ASD-associated gene, produces social deficits in Zebrafish. Mol. Autism 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, S.; Umino, A.; Balu, D.; Coyle, J.; Nishikawa, T. Neuronal serine racemase regulates extracellular d-serine levels in the adult mouse hippocampus. J. Neural Transm. 2015, 122, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cheng, Z.; Bass, N.; Krystal, J.H.; Farrer, L.A.; Kranzler, H.R.; Gelernter, J. Genome-wide association study identifies glutamate ionotropic receptor GRIA4 as a risk gene for comorbid nicotine dependence and major depression. Transl. Psychiatry 2018, 8, 208. [Google Scholar] [CrossRef]
- Martin, S.; Chamberlin, A.; Shinde, D.N.; Hempel, M.; Strom, T.M.; Schreiber, A.; Johannsen, J.; Ousager, L.B.; Larsen, M.J.; Hansen, L.K.; et al. De Novo Variants in GRIA4 Lead to Intellectual Disability with or without Seizures and Gait Abnormalities. Am. J. Hum. Genet. 2017, 101, 1013–1020. [Google Scholar] [CrossRef]
- Xue, S.; He, J.; Lu, L.; Song, S.; Chen, M.; Wang, F.; Chen, J. Enhanced TARP-γ8-PSD-95 coupling in excitatory neurons contributes to the rapid antidepressant-like action of ketamine in male mice. Nat. Commun. 2023, 14, 7971. [Google Scholar] [CrossRef]
- Lee, L.; Su, M.; Huang, H.; Cho, Y.; Yeh, T.; Chang, C. Association of CaMK2A and MeCP2 signaling pathways with cognitive ability in adolescents. Mol. Brain 2021, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Suutari, B.; He, X.; Wang, Y.; Sanchez, S.; Tirko, N.; Mandelberg, N.; Mullins, C.; Zhou, G.; Wang, S.; et al. Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat. Commun. 2018, 9, 2451. [Google Scholar] [CrossRef] [PubMed]
- Rigter, P.; de Konink, C.; van Woerden, G. Loss of CAMK2G affects intrinsic and motor behavior but has minimal impact on cognitive behavior. Front. Neurosci. 2023, 16, 1086994. [Google Scholar] [CrossRef] [PubMed]
- Chana, G.; Laskaris, L.; Pantelis, C.; Gillett, P.; Testa, R.; Zantomio, D.; Burrows, E.; Hannan, A.; Everall, I.; Skafidas, E. Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain Behav. Immun. 2015, 49, 197–205. [Google Scholar] [CrossRef]
- Brasic, J.; Nandi, A.; Russell, D.; Jennings, D.; Barret, O.; Martin, S.; Slifer, K.; Seibyl, J.; Berry-Kravis, E.; Wong, D.; et al. Cerebral expression of metabotropic glutamate receptor subtype 5 in autism spectrum disorder and fragile X syndrome: A Pilot Study. Int J Mol Sci. 2021, 22, 2863. [Google Scholar] [CrossRef]
- Eshraghi, A.; Memis, I.; Wang, F.; White, I.; Furar, E.; Mittal, J.; Moosa, M.; Atkins, C.; Mittal, R. Genetic ablation of metabotropic glutamate receptor 5 in rats results in an autism-like behavioral phenotype. PLoS ONE 2022, 17, e0275937. [Google Scholar] [CrossRef]
- Peralta, F.; Fuentealba, C.; Fiedler, J.; Aliaga, E. Prenatal valproate treatment produces autistic-like behavior and increases metabotropic glutamate receptor 1A-immunoreactivity in the hippocampus of juvenile rats. Mol. Med. Rep. 2016, 14, 2807–2814. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Gholipour, P.; Mohammadkhani, R.; Ghahremani, R.; Sarihi, A.; Komaki, A.; Salehi, I.; Karimi, S. Effect of intrahippocampal microinjection of VU0155041, a positive allosteric modulator of mGluR4, on long term potentiation in a valproic acid-induced autistic male rat model. IBRO Neurosci. Rep. 2024, 16, 629–634. [Google Scholar] [CrossRef]
- Suda, S.; Iwata, K.; Shimmura, C.; Kameno, Y.; Anitha, A.; Thanseem, I.; Nakamura, K.; Matsuzaki, H.; Tsuchiya, K.; Sugihara, G.; et al. Decreased expression of axon-guidance receptors in the anterior cingulate cortex in autism. Mol. Autism 2011, 2, 14. [Google Scholar] [CrossRef]
- Anitha, A.; Nakamura, K.; Yamada, K.; Suda, S.; Thanseem, I.; Tsujii, M.; Iwayama, Y.; Hattori, E.; Toyota, T.; Miyachi, T.; et al. Genetic Analyses of Roundabout (ROBO) Axon Guidance Receptors in Autism. Am. J. Med. Genet. B 2008, 147B, 1019–1027. [Google Scholar] [CrossRef]
- Gong, J.; Körner, R.; Gaitanos, L.; Klein, R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 2016, 214, 35–44. [Google Scholar] [CrossRef]
- Lai, K.; Ip, N. Synapse development and plasticity: Roles of ephrin/Eph receptor signaling. Curr. Opin. Neurobiol. 2009, 19, 275–283. [Google Scholar] [CrossRef]
- Carlezon, W.A., Jr.; Kim, W.; Missig, G.; Finger, B.C.; Landino, S.M.; Alexander, A.J.; Mokler, E.L.; Robbins, J.O.; Li, Y.; Bolshakov, V.Y.; et al. Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Sci. Rep. 2019, 9, 16928. [Google Scholar] [CrossRef]
- Ornoy, A.; Weinstein-Fudim, L.; Tfilin, M.; Ergaz, Z.; Yanai, J.; Szyf, M.; Turgeman, G. S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicol. Teratol. 2019, 71, 64–74. [Google Scholar] [CrossRef]
- Wu, J.; Lin, X.; Wu, D.; Yan, B.; Bao, M.; Zheng, P.; Wang, J.; Yang, C.; Li, Z.; Jin, X.; et al. Poly(I:C)-exposed Zebrafish shows autism-like behaviors which are ameliorated by fabp2 gene knockout. Front. Mol. Neurosci. 2022, 15, 1068019. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, X.-L.; Ma, Z.-H.; Liu, L.; Zhou, Q.; Wen, J.-W.; Wen, J.-H.; Su, H.; Zhang, Y.-H.; Xia, X.-C. Cerebellar Mechanisms Underlying Autism-like Cognitive Deficits in Mouse Offspring with Prenatal Valproic Acid Exposure. Toxics 2025, 13, 833. https://doi.org/10.3390/toxics13100833
Wang J, Zhou X-L, Ma Z-H, Liu L, Zhou Q, Wen J-W, Wen J-H, Su H, Zhang Y-H, Xia X-C. Cerebellar Mechanisms Underlying Autism-like Cognitive Deficits in Mouse Offspring with Prenatal Valproic Acid Exposure. Toxics. 2025; 13(10):833. https://doi.org/10.3390/toxics13100833
Chicago/Turabian StyleWang, Juan, Xu-Lan Zhou, Zi-Han Ma, Li Liu, Qian Zhou, Jia-Wei Wen, Jia-Hui Wen, Hui Su, Yu-Han Zhang, and Xiao-Chun Xia. 2025. "Cerebellar Mechanisms Underlying Autism-like Cognitive Deficits in Mouse Offspring with Prenatal Valproic Acid Exposure" Toxics 13, no. 10: 833. https://doi.org/10.3390/toxics13100833
APA StyleWang, J., Zhou, X.-L., Ma, Z.-H., Liu, L., Zhou, Q., Wen, J.-W., Wen, J.-H., Su, H., Zhang, Y.-H., & Xia, X.-C. (2025). Cerebellar Mechanisms Underlying Autism-like Cognitive Deficits in Mouse Offspring with Prenatal Valproic Acid Exposure. Toxics, 13(10), 833. https://doi.org/10.3390/toxics13100833











