Abstract
Objective: To synthesize the current evidence on the cardiovascular effects of electronic cigarettes (ECs) in young adults (18–30 years), distinguishing between acute and chronic exposure, and comparing their effects to conventional tobacco (CT) use. Methods: A systematic review and meta-analysis (PROSPERO: CRD420251072847) was conducted following PRISMA guidelines. A total of 21 studies (12 RCTs, 8 case–control, 1 cohort) involving 17241 participants were included. Results: Acute EC use, particularly with nicotine, significantly increased systolic blood pressure (SBP: MD = 3.14 mmHg, 95% CI: 0.76 to 5.52), diastolic blood pressure (DBP: MD = 2.05 mmHg, 95% CI: 0.85 to 3.25), and heart rate (HR: MD = 4.23 bpm, 95% CI: 2.10 to 6.37), with effects most pronounced at 0 min post-exposure and dissipating within 1 h. Chronic EC use was associated with reduced endothelium-dependent vasodilation and early atherosclerotic changes. Nicotine-free ECs induced fewer cardiovascular alterations. Comparisons with CT revealed less severe cardiovascular damage with ECs, though still significant when compared to non-smokers. Conclusion: Nicotine-containing EC use in young individuals is associated with modest, predominantly acute and dose-dependent, cardiovascular effects, including transient increases in BP and HR. While initially less harmful than CT, the evidence is largely from cross-sectional studies and acute use, so ECs cannot be considered safe and their use warrants caution in youth.
1. Introduction
Electronic cigarettes (ECs) are devices classified within the group known as “electronic nicotine delivery systems” (ENDSs). Their mechanism of action is based on heating a liquid to generate an inhalable aerosol. This liquid typically contains nicotine, flavoring agents, and either propylene glycol or glycerin [1]. ECs are composed of two primary components [2], i.e., a “mod”, which is the battery that supplies the electrical current, and an atomizer, where the liquid is stored and vaporized upon contact with the current. The resulting aerosol has a white appearance similar to water vapor, which is why these devices are commonly referred to as “vapes”. It is important to distinguish ECs from so-called “heated tobacco products”, which, although they also generate aerosols, do so by heating processed tobacco leaves at high temperatures rather than by vaporizing a liquid solution [3].
ECs, with or without nicotine, were initially designed as substitutes for conventional combustion tobacco (CT) products, simulating their mode of use through inhalation and vapor formation, with the aim of serving as a less harmful alternative to support smoking cessation [4]. In fact, in such countries as the United Kingdom, ECs are one of the tools employed to aid smoking cessation. Some authors have reported greater effectiveness of ECs compared to nicotine replacement therapy in adults, provided their use is accompanied by behavioral support [5]. However, although several studies have demonstrated that the levels of toxicants and carcinogens in ECs are lower than those found in CT products, current knowledge regarding the potential health effects of inhaling the aerosol generated from e-liquids remains limited [6].
Most studies conducted to date on EC toxicity have compared with the harms associated with tobacco combustion, a process that differs fundamentally from the one used by electronic devices. In fact, CO-oximetry measurements in EC users show levels of exhaled carbon monoxide comparable to those of non-smokers.
On the other hand, the recent introduction of these products to the market, combined with intensive advertising on the Internet and promotion by social media influencers, has facilitated their widespread reach, particularly among adolescents and young adults. This demographic group is a primary target for the tobacco industry due to their potential for long-term use, thereby increasing the risk of smoking initiation within this age group [7]. According to the Spanish “ESTUDES 2023” survey, more than half of students aged 14 to 18 (54.6%) admit to having used e-cigarettes at least once in their lives. This represents an increase of 10.3 percentage points compared to 2021, placing the use of these devices at its highest point in the historical series [8]. Globally, the prevalence of EC use among young people is 16.8%, with the United States and Taiwan reporting the highest rates [9].
The growing use of ECs among youth in recent years is a concerning trend, particularly given the potential health risks and the increasing evidence that ECs are not as harmless as initially assumed [4]. In this regard, the scientific community raised alarms about the condition known as “E-cigarette or Vaping-Associated Lung Injury” (EVALI), a lung disease linked to EC use that has resulted in numerous deaths among young individuals in the United States [10]. Therefore, the exposure of young people to these devices is especially troubling, not only because of their increased susceptibility to the addictive potential of nicotine, but also due to the toxic potential of other substances contained in these products, such as propylene glycol, glycerin, various flavorings and scents, volatile organic compounds, like benzene, heavy metals, and other chemicals that have already been associated with damage to the respiratory and cardiovascular systems [11]. Most studies on the effects of components, such as glycerol, flavorings, or polyethylene glycol in the aerosol, have focused on oral, respiratory, or dermal exposure in humans. The potential harmful effects resulting specifically from the inhalation of these substances remain unknown [4]. Siddiqi et al. (2023) [12] showed in their meta-analysis that ECS is associated with an increase in cardiovascular hemodynamic measures and biomarkers, although it was conducted in a general population neither focused on young people nor on time-dependent effects.
Therefore, the primary objective of this systematic review and meta-analysis was to synthesize the most recent evidence on the cardiovascular effects of EC use in young populations. Specifically, the study sought to evaluate whether these effects are more consistently observed after acute or chronic exposure, and to compare the magnitude of cardiovascular effects associated with ECs. An additional aim was to explore the potential influence of nicotine content on acute cardiovascular effects in EC users and the time- and dose-dependent cardiovascular effects.
2. Materials and Methods
2.1. Design
A systematic review and meta-analysis was conducted following the recommendation from the Preferred Reported Items for Systematic Review and Meta-analysis (PRISMA) statement [13]. The systematic review protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews), with the registration number CRD420251072847.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria for the studies were defined according to the Population, Intervention, Comparator, Outcome, and Study Design (PICOs) framework [14]. Accordingly, the population (P) consisted of young adults, and studies were included if the mean age of participants ranged between 18 and 30 years, regardless of whether some individual participants fell outside this range; the intervention (I) involved either acute or chronic exposure to nicotine-containing ENDSs; the comparator (C) included non-smoking young adults or young smokers using CT or non-nicotine ENDSs; the outcome (O) referred to cardiovascular effects resulting from such exposure; and the study design (S) comprised cross-sectional studies, cohort studies or crossover interventions.
The exclusion criteria applied were as follows: (1) studies conducted in animal models; (2) studies analyzing the effectiveness of strategies aimed at promoting cessation of ENDS use; (3) studies addressing marketing and/or advertising strategies related to ENDS consumption; (4) qualitative studies focused on perceptions, usage patterns, associated behaviors, or consumption patterns of ENDSs; (5) case reports, systematic reviews or meta-analysis; (6) studies examining heated tobacco products rather than ENDSs; (7) studies comparing different types of ENDSs without analyzing their harmful effects.
2.3. Search Strategy
The search was conducted by two independent researchers (CRL and DCR) in the databases Medline (PudMed), Scopus, and Web of Science (WoS) until June of 2025. The search was carried out by combining the mesh terms “electronic nicotine delivery system”, “young adults”, “health”, or “prevalence” with Boolean operators (Supplementary Material S1), and the Rayyan web app [15] was used throughout the screening phase before the actual review process.
2.4. Selection Criteria and Data Extraction
The review process consisted of three individual phases, namely a screening of the databases, a review of the databases, and data extraction.
In the screening phase, two individual screeners (CRL and DCR) performed the initial analysis of study titles and abstract. If the studies fit the inclusion criteria and were relevant to the review or no agreement was achieved in this phase, the full text was reviewed.
In the review phase, the full text was assessed to determine whether it met the inclusion criteria by the same screeners as the first phase. If after the full text assessment any discrepancies existed, they were resolved by consensus with a third reviewer (ARS) if necessary [13].
Finally, the data extraction phase was conducted to extract any essential information from the selected studies. Data from the included studies were systematically extracted and tabulated using a standardized table capturing the following information: author and year of publication, study design, study groups, sample size, mean age, sex distribution (female/male), nicotine concentration in electronic cigarettes (mg/mL), measured effects, assessed variables, and main results. Specifically, data on blood pressure and heart rate were extracted for subsequent meta-analysis.
Inter-rater agreement was assessed using Cohen’s kappa coefficient [16] and was interpreted as follows: negligible if <0.2, fair if 0.21–0.4, moderate if 0.41–0.6, substantial if 0.61–0.8, and almost perfect if 0.81–1, according to the criteria described by Landis and Koch (1977) [17].
2.5. Quality and Risk of Bias Evaluation
Firstly, to evaluate the quality of the included studies two independent tools were used, namely the STROBE declaration for the observational studies and the CONSORT declaration for the crossover interventions.
Secondly, the risk of bias of the included crossover interventions was assessed with the Cochrane Risk of Bias 2 (RoB 2) [18] tool. This tool evaluates bias derived from the randomization process, deviation from intended intervention, missing outcome data, measurement of the outcome, and selection of the reported results, which were evaluated as having a low risk of bias, some concerns, or a high risk of bias.
2.6. Statistical Analysis
All statistical analyses were conducted using Review Manager (RevMan) version 5.4.1, following a random effects model to account for potential between-study heterogeneity. Continuous outcomes were summarized using mean differences (MDs) and 95% confidence intervals (CIs). Separate meta-analyses were performed for systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR), comparing nicotine exposure with nicotine-free conditions. When available, subgroup analyses were conducted based on the timing of post-exposure assessment (e.g., 0 min, 30 min, and ≥1 h) and nicotine concentration (e.g., 3 mg/mL, 18 mg/mL).
Statistical heterogeneity was assessed using the chi-squared test (χ2) and quantified with the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively [19]. A significance level of p < 0.05 was considered for all comparisons. Where applicable, differences between subgroups were tested to explore time-dependent effects and nicotine-dependent effects.
3. Results
3.1. Studies Selected
A total of 2256 records were identified through the initial database search, of which 255 were removed as duplicates. The remaining 2001 records were screened based on title and abstract, resulting in the exclusion of 1859 records. Subsequently, 142 full-text articles were sought for retrieval, but 4 could not be obtained. A total of 138 full-text articles were assessed for eligibility, and 21 studies met the inclusion criteria. These included 12 crossover interventions, 8 cross-sectional studies, and 1 cohort study (Figure 1). Inter-rater agreement for the inclusion of articles was almost perfect, with a Cohen’s kappa coefficient of 0.9, indicating a high level of consistency between reviewers.
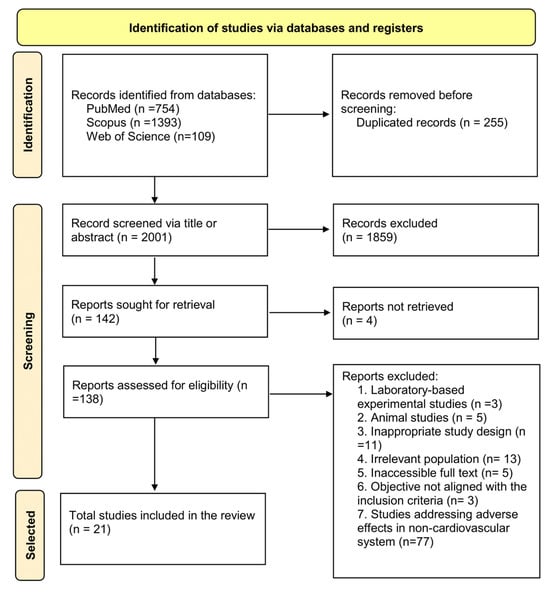
Figure 1.
Flowchart for systematic reviews recommended by PRISMA 2020 [13].
3.2. Description and Characteristics of the Studies
The total sample comprised 17,241 youths (1258 EC users including dual consumers), with a mean age ranging from 18 to 30 years. All studies were published between 2015 and 2024.
The included studies focused on regular users of ENDSs and compared them with CT users and non-smokers. The average duration of ENDS use varied across studies, ranging from approximately 3 months [20,21,22], to over 6 months [23] and more than one year [24,25,26,27,28].
Sample sizes ranged from 10 to 30 participants in most studies. An exception was the study by Shi et al., (2023) [22], a cohort study with a sample with 372 EC users, 573 dual users, 4933 CT users, and 10,486 non-smokers which investigated chronic effects, particularly hypertension, among ENDS users, CT users, and dual users. Apart from the study by Shi et al., only Kelesidis et al. (2023) [25] and Sahota et al. (2021) [21] also assessed chronic health effects, whereas the remaining studies primarily focused on acute outcomes (Table 1). Specific characteristics about the study design of every individual study are presented in Supplementary Material S2.

Table 1.
Characteristics of the included studies.
3.3. Quality of the Included Studies
Most of the studies met at least 50% of the assessed quality criteria. The quality scores of the cross-sectional studies ranged from 45.45% to 84.5%, with an average quality rating of 71.40%. The cohort study conducted by Shi et al. (2023) [22] achieved the highest quality score, at 94.11% (Supplementary Material S3). In contrast, the crossover interventions demonstrated quality scores between 54.5% and 72.73%, with a mean score of 63.26% (Supplementary Material S4).
3.4. Risk of Bias
Only six studies were classified as having a low risk of bias [29,32,35,36,37,40], with four studies assessed as having some concerns regarding bias [27,30,33,34] and only two studies being judged to have a high risk of bias [31,38] (Figure 2).
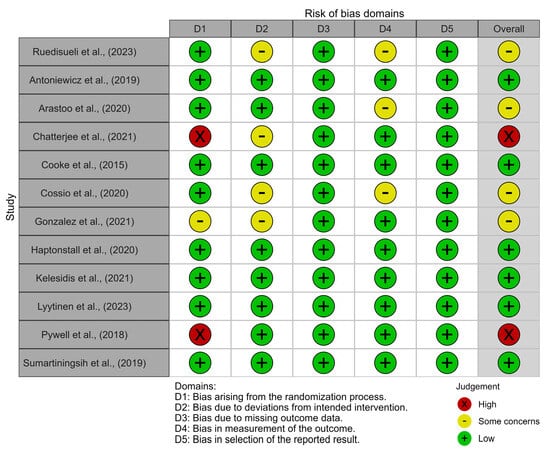
Figure 2.
Risk of bias traffic light plot [27,29,30,31,32,33,34,35,36,37,38,40].
The primary source of bias was related to the randomization process, with some bias in the deviation from intervention and measurement of outcome domains. In contrast, the domains of outcome selection and data reporting exhibited the lowest risk of bias (Figure 3).
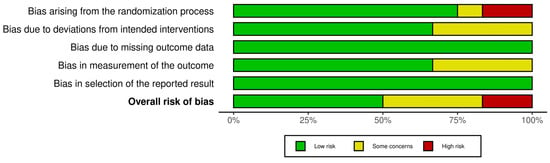
Figure 3.
Risk of bias summary plot.
3.5. Narrative Synthesis of Individual Study Results
Of the included studies, 10 reported adverse effects associated with the use of ECs [20,21,23,26,27,28,29,31,32,34]. Two studies specifically attributed these adverse effects to the presence of nicotine in EC components [37,38]. Conversely, two studies found no significant differences when compared with CT use or to a control group [27,33]. Finally, seven studies indicated that EC use was associated with fewer harmful effects compared to CT [22,24,25,30,35,40].
3.5.1. Dysfunction of Endothelial Vasodilatation
Three of the studies assessed endothelial function in regular EC users and reported impairments in nitric oxide-dependent vasodilatory function [20,23,34]. In contrast, the study by Haptonstall et al. (2020) [35] found no evidence of endothelial dysfunction in an acute comparison between ECs and CT. Additionally, Pywell et al. (2018) [38] concluded that the observed vasodilatory impairment was attributable to the effect of nicotine.
3.5.2. Increase in Blood Pressure and Heart Rate
In the studies conducted by Cooke et al. (2015) [32] and Gonzalez and Cooke (2021) [34], exposure of healthy young non-smokers to a single session of EC inhalation resulted in elevated blood pressure and HR. In contrast, Sumartiningsih et al. (2019) [40] reported that such increases were observed with CT use, but not with ECs. Similarly, Shi et al. (2023) [22] found that elevated blood pressure was associated with CT smoking, but not with EC use or among non-smokers. Finally, the study by Cossio et al. (2020) [33] found no significant differences in blood pressure or HR between groups.
3.5.3. Increase in Cellular Oxidative Stress
Moheimani et al. (2017) [26] reported that EC users exhibited higher plasma levels of biomarkers associated with cellular oxidative stress compared to non-smokers. Similarly, Kelesidis et al. (2021) [36] observed an increase in these oxidative stress markers following ENDS inhalation; however, the magnitude of this increase was lower than that observed in CT smokers.
3.5.4. Increase in Systemic Inflammatory Activity
The studies by Boas et al. (2017) [24] and Chatterjee et al. (2021) [31] reported elevated levels of inflammatory and metabolic activity markers, as assessed through imaging techniques and biochemical biomarkers. These increases were more pronounced in CT smokers than in ECs users. In contrast, Ruedisueli et al. (2022) [39] found no significant differences in inflammatory activity between groups.
3.5.5. Pro-Atherogenic and Pro-Thrombotic Effects
Kelesidis et al. (2023) [25] observed an increase in cells involved in atherogenesis among ENDS users, although the effect was more pronounced in CT smokers. Similarly, Lyytinen et al. (2023) [37] reported enhanced platelet activation and thrombus formation, following exposure to nicotine-containing ECs. These pro-thrombotic effects were attributed primarily to the action of nicotine and may contribute to the overall pro-atherogenic risk profile associated with ECs use.
3.5.6. Prolongation of Ventricular Repolarization Time
Ruedisueli et al. (2023) [27] found, through electrocardiographic analysis, that acute ENDSs exposure in chronic CT smokers led (only in men) to a greater prolongation of ventricular repolarization times, a recognized risk factor for sudden cardiac death.
3.5.7. Heart Rate Variability
Moheimani et al. (2017) [26] found a shift toward sympathetic dominance and reduced vagal tone in habitual ENDS users, a pattern linked to increase cardiovascular risk. Similarly, Sumartiningsih et al. (2019) [40] observed that acute nicotine exposure from ENDSs and CT increased HR variability parameters during exercise, indicating heightened autonomic modulation.
3.6. Meta-Analysis
3.6.1. SBP (Nicotine vs. No Nicotine)
A meta-analysis comparing SBP responses between nicotine and non-nicotine conditions showed that, overall, nicotine exposure significantly increased SBP compared to non-nicotine ECs (MD = 3.14 mmHg; 95% CI: 5.52 to 0.76; p = 0.010).
This effect was most evident immediately after acute exposure (0 min), with a significant increment in the nicotine condition (MD = 3.67 mmHg; 95% CI: 0.95 to 6.39; p = 0.008). No significant differences were found at 30 min (MD = 4.58 mmHg; 95% CI: −4.57 to 13.73; p = 0.33) or ≥1 h (MD = 0.16 mmHg; 95% CI: −5.65 to 5.96; p = 0.96) post-exposure. There was no evidence of heterogeneity across studies (I2 = 0%), and subgroup analysis revealed no significant differences between time points (Figure 4).
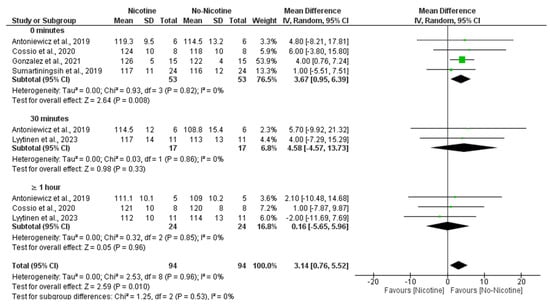
Figure 4.
Forest plot of the effects of nicotine versus no nicotine on systolic blood pressure at different post-exposure time points [29,33,34,37,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
Moreover, subgroup analyses indicated that the nicotine concentration within e-cigarettes also played a significant role, with higher doses being associated with greater increases in SBP, particularly at 59 mg/mL (MD = 4.00 mmHg; 95% CI: 0.76 to 7.24), contributing to the overall significant effect (MD = 3.90 mmHg; 95% CI: 1.37 to 6.43; p = 0.003) with negligible heterogeneity (I2 = 0%) (Figure 5).
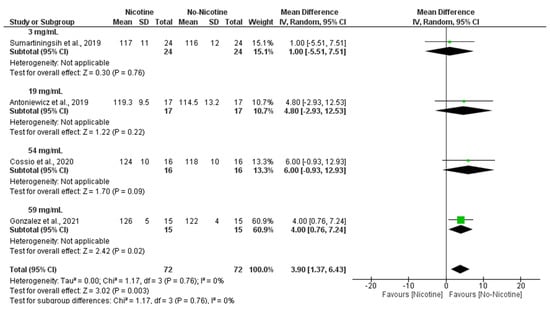
Figure 5.
Forest plot of the acute effects of nicotine versus no nicotine on systolic blood pressure at different nicotine concentrations [29,33,34,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
3.6.2. DBP (Nicotine vs. No Nicotine)
A meta-analysis examining DBP revealed that nicotine exposure significantly increased DBP compared to non-nicotine ECs (MD = 2.05 mmHg; 95% CI: 0.85 to 3.25; p = 0.0008). This effect was primarily observed immediately after exposure (0 min), where the nicotine group showed significantly higher DBP values (MD = 2.29 mmHg; 95% CI: 0.98 to 3.60; p = 0.0006). No significant differences were found at 30 min (MD = 1.71 mmHg; 95% CI: −3.40 to 6.81; p = 0.51) or ≥1 h post-exposure (MD = 0.31 mmHg; 95% CI: −3.36 to 3.98; p = 0.87). Heterogeneity was negligible across studies (I2 = 0%), and no significant subgroup differences were detected across time points (Figure 6).
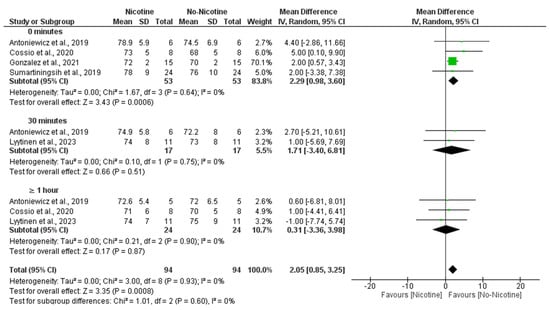
Figure 6.
Forest plot of the effects of nicotine versus no nicotine on diastolic blood pressure at different post-exposure time points [29,33,34,37,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
Similarly, subgroup analyses demonstrated that nicotine concentration was a significant determinant of DBP responses, with the strongest effects observed at 54 mg/mL (MD = 5.00 mmHg; 95% CI: 1.54 to 8.46; p = 0.005) and 59 mg/mL (MD = 2.00 mmHg; 95% CI: 0.57 to 3.43; p = 0.006), leading to an overall significant increase in DBP following nicotine exposure (MD = 2.70 mmHg; 95% CI: 1.3 to 4.10; p = 0.0002) with very low heterogeneity (I2 = 7%) (Figure 7).
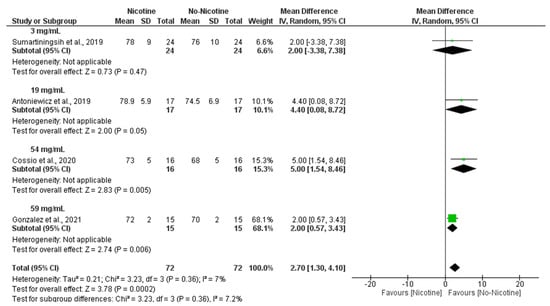
Figure 7.
Forest plot of the acute effects of nicotine versus no nicotine on diastolic blood pressure at different nicotine concentrations [29,33,34,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
3.6.3. Heart Rate (Nicotine vs. No Nicotine)
The meta-analysis demonstrated that nicotine exposure significantly increased HR compared to non-nicotine ECs (MD = 4.23 bpm; 95% CI: 2.10 to 6.37; p < 0.0001). The most prominent effect was observed immediately after exposure (0 min), with significantly higher HR in the nicotine condition (MD = 3.83 bpm; 95% CI: 1.49 to 6.16; p = 0.001). A significant difference was also observed at 30 min (MD = 7.32 bpm; 95% CI: 0.12 to 14.52; p = 0.05), although the confidence interval was wide and nearly included the null. At ≥1 h post-exposure, no significant difference was found (MD = 5.14 bpm; 95% CI: −2.57 to 12.84; p = 0.19). There was no heterogeneity across studies (I2 = 0%), and subgroup analysis showed no statistically significant differences between time points (Figure 8).
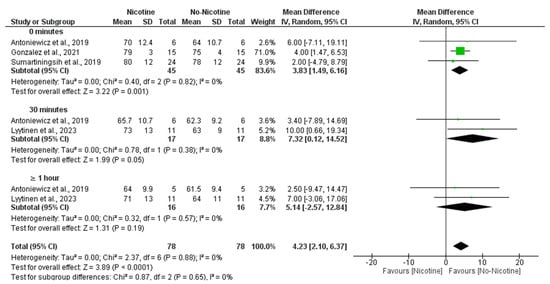
Figure 8.
Forest plot of the effects of no nicotine versus control on heart rate at different post-exposure time points [29,34,37,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
In line with these findings, subgroup analyses revealed that nicotine concentration also influenced HR responses, with 59 mg/mL nicotine conditions producing a significant increase compared to non-nicotine conditions (MD = 4.00 bpm; 95% CI: 1.47 to 6.53; p = 0.002), while 19 mg/mL nicotine conditions showed the larger MD, with its confidence interval nearly including the null hypothesis (MD = 7.70 bpm; 95% CI: 0.30 to 15.10; p = 0.04). Altogether, this resulted in an overall significant increase in HR in the nicotine group compared with the non-nicotine group (MD = 4.12 bpm; 95% CI: 1.87 to 6.38; p < 0.001) with no heterogeneity (I2 = 0%) (Figure 9).
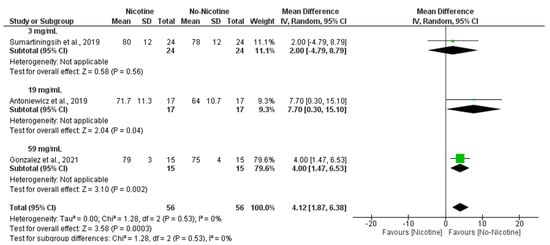
Figure 9.
Forest plot of the acute effects of nicotine versus no nicotine on heart rate at different nicotine concentrations [29,34,40]. Green squares show each study’s weighted mean difference, and black diamonds show the pooled mean difference with its 95% confidence interval.
4. Discussion
The main objective of this systematic review and meta-analysis was to compile the most recent evidence on the cardiovascular effects of EC use in young populations. The findings indicate that, although most of the included studies reported cardiovascular effects associated with EC use, some discrepancies were observed across studies. Overall, cardiovascular effects were more consistently observed and of greater magnitude with CT consumption compared to ECs.
Additionally, the study aimed to assess whether the effects resulting from EC inhalation were linked to acute use, chronic use, or both and the time- and dose-dependent cardiovascular effects. The findings derived from the reviewed literature indicate that acute exposure to these devices was associated with the following cardiovascular effects: activation of the sympathetic nervous system and reduced vagal tone [26], heightened autonomic modulation during physical exercise [40], impaired endothelium-dependent vasodilatation mediated by nitric oxide [20,23,28], increased blood pressure and HR [26,29,30,32,40], elevated cellular oxidative stress [26], enhanced inflammatory activity [21,31] and increased thrombotic and platelet activity at the microcirculatory level [37]. In the narrative synthesis, some discrepancies were noted regarding the influence of nicotine on the increases in blood pressure and HR. However, the meta-analysis indicated that these cardiovascular changes were primarily attributed to the nicotine content in these ENDSs. Most of these acute effects are consistent with those reported by Siddiqi et al. (2023) [12] in their 2023 meta-analysis conducted in populations of varying ages.
Regarding chronic exposure to ENDS vaping, the most consistently reported findings associated with months-long use of ECs included a reduction in endothelium-dependent vasodilatation mediated by nitric oxide [20,23,28], and the presence of atherosclerotic plaque formation [36]. It could be hypothesized that acute increase in BP, if sustained over time, may contribute to the development of hypertension in the future. Additionally, a prolongation of ventricular repolarization has been observed in EC vapers [27]. However, despite the documented increases in inflammatory, thrombotic, and/or platelet activity, as well as the prolongation of ventricular repolarization, which is known to promote arrhythmias, this systematic review did not identify any scientific literature reporting clusters of serious and/or fatal cardiovascular events in adolescents or young adults who use ECs. This stands in contrast to the pulmonary damage observed in the so-called EVALI syndrome, first reported by the US Centers for Disease Control and Prevention (CDC) in 2019 [41]. In this regard, Adkins et al., in their 2019 study, included hospitalized or deceased adolescents and concluded that the use of any EC or vaping product is unsafe [42].
The rationale for investigating acute exposure lies in the increasing trend of “weekend” or occasional use of e-cigarettes during social events among many contemporary European and North American youth. A striking finding from the meta-analysis was the time- and dose-dependent effects of ENDSs on the increases in blood pressure (both systolic and diastolic) and HR after vaping, compared to nicotine-free devices. These elevations tend to normalize within 30 to 60 min post-inhalation. While this transient effect might appear to trivialize ENDS use or even promote it, it is crucial to emphasize that smoking behaviour also involves elements of “orality” and “gesturally” that can reinforce or introduce active CT smoking habits. Consequently, dual use alongside CT, or even initiation into CT use, cannot be ruled out, particularly in young individuals who, due to their age, represent long-term target population for the tobacco industry. In this regard, even Hajek et al. (2019) concluded that ECs may hold greater potential for leading to regular use among non-smokers [5]. The seemingly lower harm profile of e-cigarettes compared to combustible tobacco is one of the key arguments used by proponents who advocate for their use as a smoking cessation aid, a practice permitted in such countries as the United Kingdom, Norway, and Belgium [43]. In relation to this, Pearson et al. (2020), in a cohort study conducted among American youth, found that EC use was neither associated with an increase nor a reduction in CT consumption after one year [44]. This is perhaps because most adolescents and young smokers are in the pre-contemplative stage of change phase [45]. In contrast, EC use is prohibited by law in some countries, like Spain, where these devices are not considered medical products [46]. In this context, legislation is increasingly being developed to regulate ECs with the aim of ensuring their safe use [43] or even prohibiting their use.
A third objective of this study was to compare the cardiovascular damage associated with EC use to that caused by CT consumption, in order to determine whether the impact on the cardiovascular system was greater than, lesser than, or comparable to that of CT. Most studies comparing both types of cigarettes reported greater cardiovascular damage associated with CT use [22,24,25,30,35,36,40] while only three studies associated greater damage with EC use [21,27,28]. Ruediseli et al. (2022) did not observe differences in metabolic activity, underscoring the role of the sympathetic nervous system in provoking inflammatory monocyte proliferation, thus instigating atherosclerotic development [39].
Notably, the study of Shi et al. stands out for two reasons. First, it was the only cohort study to analyze the effects retrospectively. Secondly, it had a large sample size and high-quality score. It is noteworthy that in this study, neither EC use nor dual use were associated with an increased risk of hypertension in either men or women, but CT use was associated with a higher risk of hypertension in female smokers, as a relevant result from a gender perspective. The hypertension effect was also seen in EC users [22], although this information on clinically relevant adverse effects of EC users is weak, mostly because of a lack of powerful studies. Further exploring this gender perspective, Halstead et al. reported endothelial-dependent VD dysfunction due to an excess of reactive oxygen species in plasma, which was more pronounced in women compared to men [23]. Conversely, the prolongation of ventricular repolarization indices, highlighted in the study by Ruedisueli et al. (2023) [27], was observed only in men vaping with ECs.
Regarding the non-nicotine components of ECs, the study by Chatterjee S et al. focused on assessing the effects of acute inhalation of nicotine-free EC aerosol, with the aim of determining whether other EC constituents exert toxic effects [31]. This study found an increase in inflammatory markers, that impaired endothelial response to increased flow reduced hyperemic response following vascular occlusion, and additional metabolic disturbances, including reduced venous oxygen saturation. The damage caused by these non-nicotine components, present in EC liquids, has been analyzed in previous studies, which demonstrated their toxicity through the exposure of cultured cells to EC liquid in vitro [47,48,49]. Among these components, propylene glycol and glycerol stand out, as they can induce apoptosis of the cells they contact and cause other adverse effects, such as mitochondrial dysfunction, particularly when combined with other compounds in flavored EC liquids, such as certain aldehydes [47,48,49]. Nonetheless, it is important to consider users’ health habits and to clarify whether the observed adverse effects are attributable solely to nicotine or also to other excipients, or even to the contact of the generated aerosol with the body.
Despite the rigorous methodological approach, this meta-analysis has several limitations. The number of studies included in certain comparisons was limited, potentially reducing the statistical power and the precision of the pooled estimates, and precluding the exploration of heterogeneity through meta-regression. This may be because additional databases, such as EMBASE or CINHAL, as well as the gray literature, were not searched. Moreover, individual studies often relied on small, non-representative samples, with minimal stratification by sex, ethnicity, or other relevant demographic variables. Among the main ones is the heterogeneity observed in the comparison groups across the included studies. Not all selected studies compared young CT smokers, EC users, and non-smokers simultaneously, which may affect the interpretation of the findings. Additionally, while some studies focused exclusively on healthy non-smokers acutely exposed to ECs, others evaluated chronic users. There was also significant variability in the cardiovascular outcomes and measurements techniques used across studies. Future studies employing more standardized and comparable variables are needed to enable more precise conclusions regarding the cardiovascular effects of EC use in young adults. Moreover, this review did not specifically examine gender-related differences, which may influence the observed effects and limit the generalizability of the findings; future meta-analysis should assess sex differences in EC use and cardiovascular related effects.
5. Conclusions
The evidence synthesized in this systematic review and meta-analysis suggests that EC use among young individuals is associated with modest cardiovascular effects, which appear more consistently following acute exposure. These effects, including transient increases in BP and HR, were more pronounced in nicotine-containing ECs as the nicotine dose increased. However, the absolute effect sizes were generally small.
Importantly, the available literature is largely based on acute and cross-sectional data, with a scarcity of robust longitudinal studies evaluating long-term cardiovascular outcomes in young populations. Therefore, the observed associations should not be interpreted as causal.
Based on the current evidence, both ECs and CTs should be considered unsafe from a cardiovascular health perspective, and their use should be approached with caution, especially in young populations. These findings may contribute to the development of future public health policies and preventive strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics13100831/s1, Supplementary material S1: Search Engine; Supplementary material S2: Specific Design Characteristics; Supplementary material S3: Strobe Scale; Supplementary material S4: CONSORT scale.
Author Contributions
Conceptualization, A.R.-S. and J.M.J.-C.; methodology, M.J.D.L.T.-A. and J.M.J.-C.; software, C.R.-L.; validation, C.R.-L., D.C.-R., A.R.-S. and J.M.J.-C.; formal analysis, D.C.-R. and J.M.J.-C.; investigation, C.R.-L.; data curation, C.R.-L. and D.C.-R.; writing—original draft preparation, C.R.-L.; writing—review and editing, C.R.-L., D.C.-R., M.J.D.L.T.-A., A.R.-S. and J.M.J.-C.; visualization, C.R.-L.; supervision, A.R.-S. and M.J.D.L.T.-A.; project administration, A.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
Department of Nursing, Pharmacology, and Physiotherapy, Faculty of Medicine and Nursing, University of Cordoba, Cordoba, Spain for open-access publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| µM | Micromolar |
| ANSM | Autonomic nervous system modulation |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| bpm | Beats per minute |
| CRP | C-reactive protein |
| CT | Conventional tobacco |
| CVC | Cutaneous vascular conductance |
| DBP | Diastolic blood pressure |
| EC | Electronic cigarette |
| ENDS | Electronic nicotine delivery system |
| EVALI | E-cigarette or vaping-associated lung injury |
| FMD | Flow-mediated dilation |
| HMGB1 | High-mobility Group Box 1 Protein |
| HR | Heart rate |
| ICAM | Intercellular adhesion molecule |
| MAP | Mean arterial pressure |
| mmHg | Millimeters of mercury |
| MRI | Magnetic resonance imaging |
| ng/mL | Nanograms per milliliter |
| nmol | Nanomole |
| NO | Nitric oxide |
| PET | Positron emission tomography |
| PU | Perfusion unit |
| PWV | Pulse wave velocity |
| QT | Electrocardiogram QT interval |
| QTc | Corrected QT interval |
| RCT | Randomized controlled trial |
| SBP | Systolic blood pressure |
| SUVmax | Maximum standardized uptake value |
| Tp-e | T-wave peak to end interval |
References
- Orellana-Barrios, M.A.; Payne, D.; Mulkey, Z.; Nugent, K. Electronic Cigarettes—A Narrative Review for Clinicians. Am. J. Med. 2015, 128, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Salud Pública. Informe Sobre Los Cigarrillos Electrónicos: Situación Actual, Evidencia Disponible y Regulación; Ministerio de Sanidad: Madrid, Spain, 2024. [Google Scholar]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Hawk, E.T.; Colbert Maresso, K. E-Cigarettes: Unstandardized, Under-Regulated, Understudied, and Unknown Health and Cancer Risks. Cancer Res. 2019, 79, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Myers Smith, K.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 2019, 380, 629–637. [Google Scholar] [CrossRef]
- Baker, R.R. Smoke Generation inside a Burning Cigarette: Modifying Combustion to Develop Cigarettes That May Be Less Hazardous to Health. Prog. Energy Combust. Sci. 2006, 32, 373–385. [Google Scholar] [CrossRef]
- Thirión-Romero, I.; Pérez-Padilla, R.; Zabert, G.; Barrientos-Gutiérrez, I. Respiratory impact of electronic cigarettes and “low-risk” tobacco. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2019, 71, 17–27. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Encuesta Sobre Uso de Drogas en Enseñanzas Secundarias en España, ESTUDES 2023; Ministerio de Sanidad: Madrid, Spain, 2023. [Google Scholar]
- Salari, N.; Rahimi, S.; Darvishi, N.; Abdolmaleki, A.; Mohammadi, M. The Global Prevalence of E-Cigarettes in Youth: A Comprehensive Systematic Review and Meta-Analysis. Public Health Pract. Oxf. Engl. 2024, 7, 100506. [Google Scholar] [CrossRef]
- Sund, L.J.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. E-Cigarette or Vaping-Associated Lung Injury (EVALI): A Review of International Case Reports from Outside the United States of America. Clin. Toxicol. Phila. Pa 2023, 61, 91–97. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Stylianou, M.; Agapiou, A. Main and Side Stream Effects of Electronic Cigarettes. J. Environ. Manag. 2019, 238, 10–17. [Google Scholar] [CrossRef]
- Siddiqi, T.J.; Rashid, A.M.; Siddiqi, A.K.; Anwer, A.; Usman, M.S.; Sakhi, H.; Bhatnagar, A.; Hamburg, N.M.; Hirsch, G.A.; Rodriguez, C.J.; et al. Association of Electronic Cigarette Exposure on Cardiovascular Health: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101748. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46, Original work published 1960. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Matheson, C.; Simovic, T.; Heefner, A.; Colon, M.; Tunon, E.; Cobb, K.; Thode, C.; Breland, A.; Cobb, C.O.; Nana-Sinkam, P.; et al. Evidence of Premature Vascular Dysfunction in Young Adults Who Regularly Use E-Cigarettes and the Impact of Usage Length. Angiogenesis 2024, 27, 229–243. [Google Scholar] [CrossRef]
- Sahota, A.; Naidu, S.; Jacobi, A.; Giannarelli, C.; Woodward, M.; Fayad, Z.A.; Mani, V. Atherosclerosis Inflammation and Burden in Young Adult Smokers and Vapers Measured by PET/MR. Atherosclerosis 2021, 325, 110–116. [Google Scholar] [CrossRef]
- Shi, H.; Leventhal, A.M.; Wen, Q.; Ossip, D.J.; Li, D. Sex Differences in the Association of E-Cigarette and Cigarette Use and Dual Use With Self-Reported Hypertension Incidence in US Adults. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2023, 25, 478–485. [Google Scholar] [CrossRef]
- Halstead, K.M.; Wetzel, E.M.; Cho, J.L.; Stanhewicz, A.E. Sex Differences in Oxidative Stress-Mediated Reductions in Microvascular Endothelial Function in Young Adult e-Cigarette Users. Hypertension 2023, 80, 2641–2649. [Google Scholar] [CrossRef]
- Boas, Z.; Gupta, P.; Moheimani, R.S.; Bhetraratana, M.; Yin, F.; Peters, K.M.; Gornbein, J.; Araujo, J.A.; Czernin, J.; Middlekauff, H.R. Activation of the “Splenocardiac Axis” by Electronic and Tobacco Cigarettes in Otherwise Healthy Young Adults. Physiol. Rep. 2017, 5, e13393. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Sharma, M.; Sharma, E.; Ruedisueli, I.; Tran, E.; Middlekauff, H.R. Chronic Electronic Cigarette Use and Atherosclerosis Risk in Young People: A Cross-Sectional Study-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Moheimani, R.S.; Bhetraratana, M.; Yin, F.; Peters, K.M.; Gornbein, J.; Araujo, J.A.; Middlekauff, H.R. Increased Cardiac Sympathetic Activity and Oxidative Stress in Habitual Electronic Cigarette Users: Implications for Cardiovascular Risk. JAMA Cardiol. 2017, 2, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ruedisueli, I.; Lakhani, K.; Nguyen, R.; Gornbein, J.; Middlekauff, H.R. Electronic Cigarettes Prolong Ventricular Repolarization in People Who Smoke Tobacco Cigarettes: Implications for Harm Reduction. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H821–H832. [Google Scholar] [CrossRef]
- Youn, J.Y.; Middlekauff, H.R.; Reudiseuli, I.; Huang, K.; Cai, H. Endothelial Damage in Young Adult E-Cigarette Users. Redox Biol. 2023, 62, 102688. [Google Scholar] [CrossRef]
- Antoniewicz, L.; Brynedal, A.; Hedman, L.; Lundbäck, M.; Bosson, J.A. Acute Effects of Electronic Cigarette Inhalation on the Vasculature and the Conducting Airways. Cardiovasc. Toxicol. 2019, 19, 441–450. [Google Scholar] [CrossRef]
- Arastoo, S.; Haptonstall, K.P.; Choroomi, Y.; Moheimani, R.; Nguyen, K.; Tran, E.; Gornbein, J.; Middlekauff, H.R. Acute and Chronic Sympathomimetic Effects of E-Cigarette and Tobacco Cigarette Smoking: Role of Nicotine and Non-Nicotine Constituents. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H262–H270. [Google Scholar] [CrossRef]
- Chatterjee, S.; Caporale, A.; Tao, J.Q.; Guo, W.; Johncola, A.; Strasser, A.A.; Leone, F.T.; Langham, M.C.; Wehrli, F.W. Acute E-Cig Inhalation Impacts Vascular Health: A Study in Smoking Naïve Subjects. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H144–H158. [Google Scholar] [CrossRef]
- Cooke, W.H.; Pokhrel, A.; Dowling, C.; Fogt, D.L.; Rickards, C.A. Acute Inhalation of Vaporized Nicotine Increases Arterial Pressure in Young Non-Smokers: A Pilot Study. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2015, 25, 267–270. [Google Scholar] [CrossRef]
- Cossio, R.; Cerra, Z.A.; Tanaka, H. Vascular Effects of a Single Bout of Electronic Cigarette Use. Clin. Exp. Pharmacol. Physiol. 2020, 47, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.E.; Cooke, W.H. Acute Effects of Electronic Cigarettes on Arterial Pressure and Peripheral Sympathetic Activity in Young Nonsmokers. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H248–H255. [Google Scholar] [CrossRef] [PubMed]
- Haptonstall, K.P.; Choroomi, Y.; Moheimani, R.; Nguyen, K.; Tran, E.; Lakhani, K.; Ruedisueli, I.; Gornbein, J.; Middlekauff, H.R. Differential Effects of Tobacco Cigarettes and Electronic Cigarettes on Endothelial Function in Healthy Young People. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H547–H556. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Tran, E.; Nguyen, R.; Zhang, Y.; Sosa, G.; Middlekauff, H.R. Association of 1 Vaping Session With Cellular Oxidative Stress in Otherwise Healthy Young People With No History of Smoking or Vaping: A Randomized Clinical Crossover Trial. JAMA Pediatr. 2021, 175, 1174–1176. [Google Scholar] [CrossRef]
- Lyytinen, G.; Brynedal, A.; Anesäter, E.; Antoniewicz, L.; Blomberg, A.; Wallén, H.; Bosson, J.A.; Hedman, L.; Mobarrez, F.; Tehrani, S.; et al. Electronic Cigarette Vaping with Nicotine Causes Increased Thrombogenicity and Impaired Microvascular Function in Healthy Volunteers: A Randomised Clinical Trial. Cardiovasc. Toxicol. 2023, 23, 255–264. [Google Scholar] [CrossRef]
- Pywell, M.J.; Wordsworth, M.; Kwasnicki, R.M.; Chadha, P.; Hettiaratchy, S.; Halsey, T. The Effect of Electronic Cigarettes on Hand Microcirculation. J. Hand Surg. 2018, 43, 432–438. [Google Scholar] [CrossRef]
- Ruedisueli, I.; Arastoo, S.; Gupta, P.K.; Gornbein, J.; Middlekauff, H.R. Neural-Hematopoietic-Inflammatory Axis in Nonsmokers, Electronic Cigarette Users, and Tobacco Smokers. Physiol. Rep. 2022, 10, e15412. [Google Scholar] [CrossRef]
- Sumartiningsih, S.; Lin, H.-F.; Lin, J.-C. Cigarette Smoking Blunts Exercise-Induced Heart Rate Response among Young Adult Male Smokers. Int. J. Environ. Res. Public. Health 2019, 16, 1032. [Google Scholar] [CrossRef]
- Evans, M.E. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-Cigarette, or Vaping, Product Use–Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 68, 1189–1194. [Google Scholar] [CrossRef]
- Adkins, S.H.; Anderson, K.N.; Goodman, A.B.; Twentyman, E.; Danielson, M.L.; Kimball, A.; Click, E.S.; Ko, J.Y.; Evans, M.E.; Weissman, D.N.; et al. Demographics, Substance Use Behaviors, and Clinical Characteristics of Adolescents With e-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) in the United States in 2019. JAMA Pediatr. 2020, 174, e200756. [Google Scholar] [CrossRef]
- Campus, B.; Fafard, P.; St Pierre, J.; Hoffman, S.J. Comparing the Regulation and Incentivization of E-Cigarettes across 97 Countries. Soc. Sci. Med. 2021, 291, 114187. [Google Scholar] [CrossRef]
- Pearson, J.L.; Sharma, E.; Rui, N.; Halenar, M.J.; Johnson, A.L.; Cummings, K.M.; Hammad, H.T.; Kaufman, A.R.; Tworek, C.; Goniewicz, M.L.; et al. Association of Electronic Nicotine Delivery System Use With Cigarette Smoking Progression or Reduction Among Young Adults. JAMA Netw. Open 2020, 3, e2015893. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Norcross, J.C. Stages of Change. Psychother. Theory Res. Pract. Train. 2001, 38, 443–448. [Google Scholar] [CrossRef]
- Ministerio de la Presidencia y Para las Administraciones Territoriales Real Decreto 579/2017, de 9 de Junio, por el que se Regulan Determinados Aspectos Relativos a la Fabricación, Presentación y Comercialización de los Productos del Tabaco y los Productos Relacionados. 2017. Available online: https://www.boe.es/eli/es/rd/2017/06/09/579/con (accessed on 29 July 2025).
- El Hajj Moussa, F.; Hayeck, N.; Hajir, S.; El Hage, R.; Salman, R.; Karaoghlanian, N.; Saliba, N.A. Enhancement of Benzene Emissions in Special Combinations of Electronic Nicotine Delivery System Liquid Mixtures. Chem. Res. Toxicol. 2024, 37, 227–233. [Google Scholar] [CrossRef]
- Komura, M.; Sato, T.; Yoshikawa, H.; Nitta, N.A.; Suzuki, Y.; Koike, K.; Kodama, Y.; Seyama, K.; Takahashi, K. Propylene Glycol, a Component of Electronic Cigarette Liquid, Damages Epithelial Cells in Human Small Airways. Respir. Res. 2022, 23, 216. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of E-Liquid Toxicity Using an Open-Source High-Throughput Screening Assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).