Inhibition of Muscle-Specific Protein Kinase (MuSK) Releases Organophosphate-Aged Acetylcholinesterase (AChE) from C2C12 Cells
Abstract
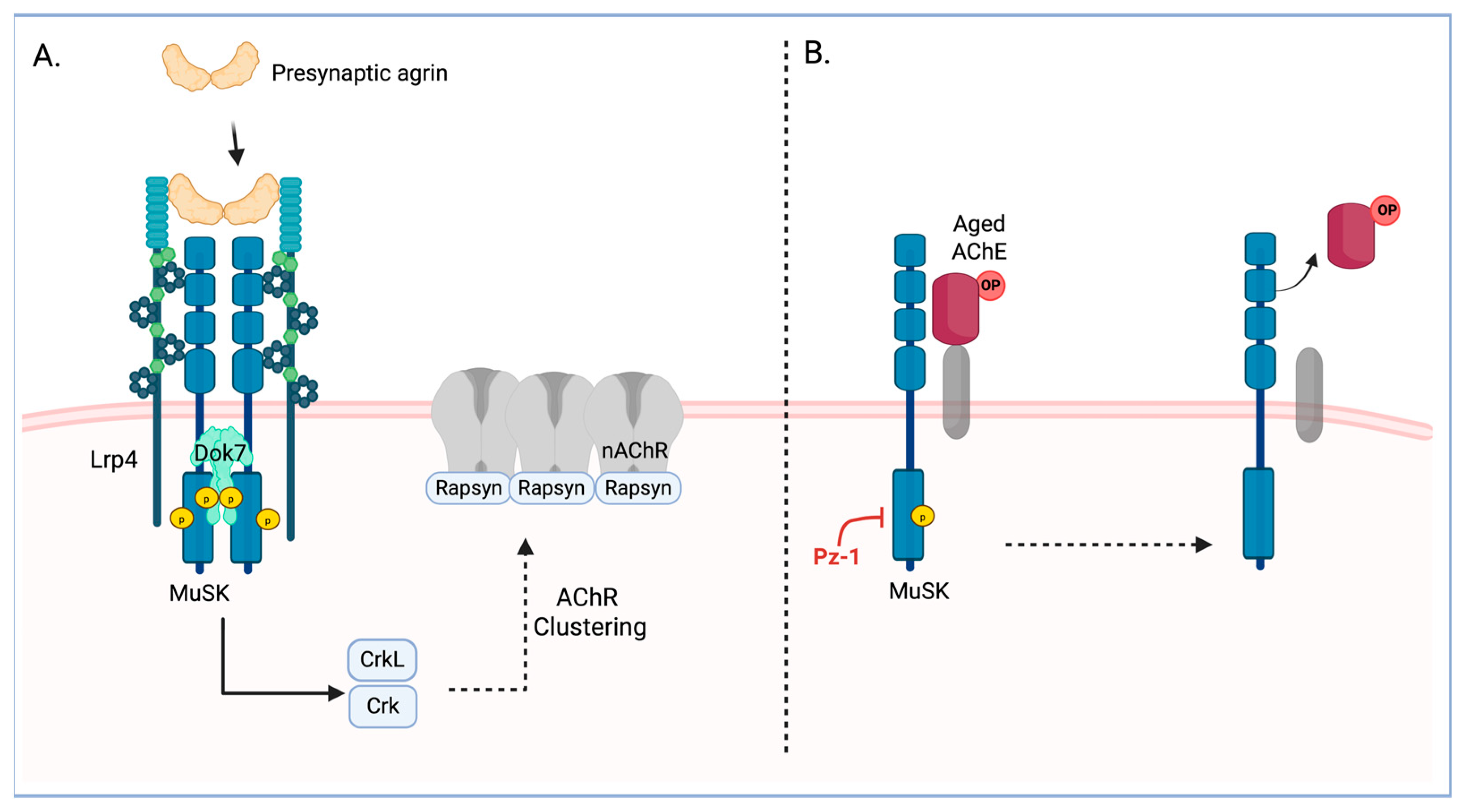
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Transfection
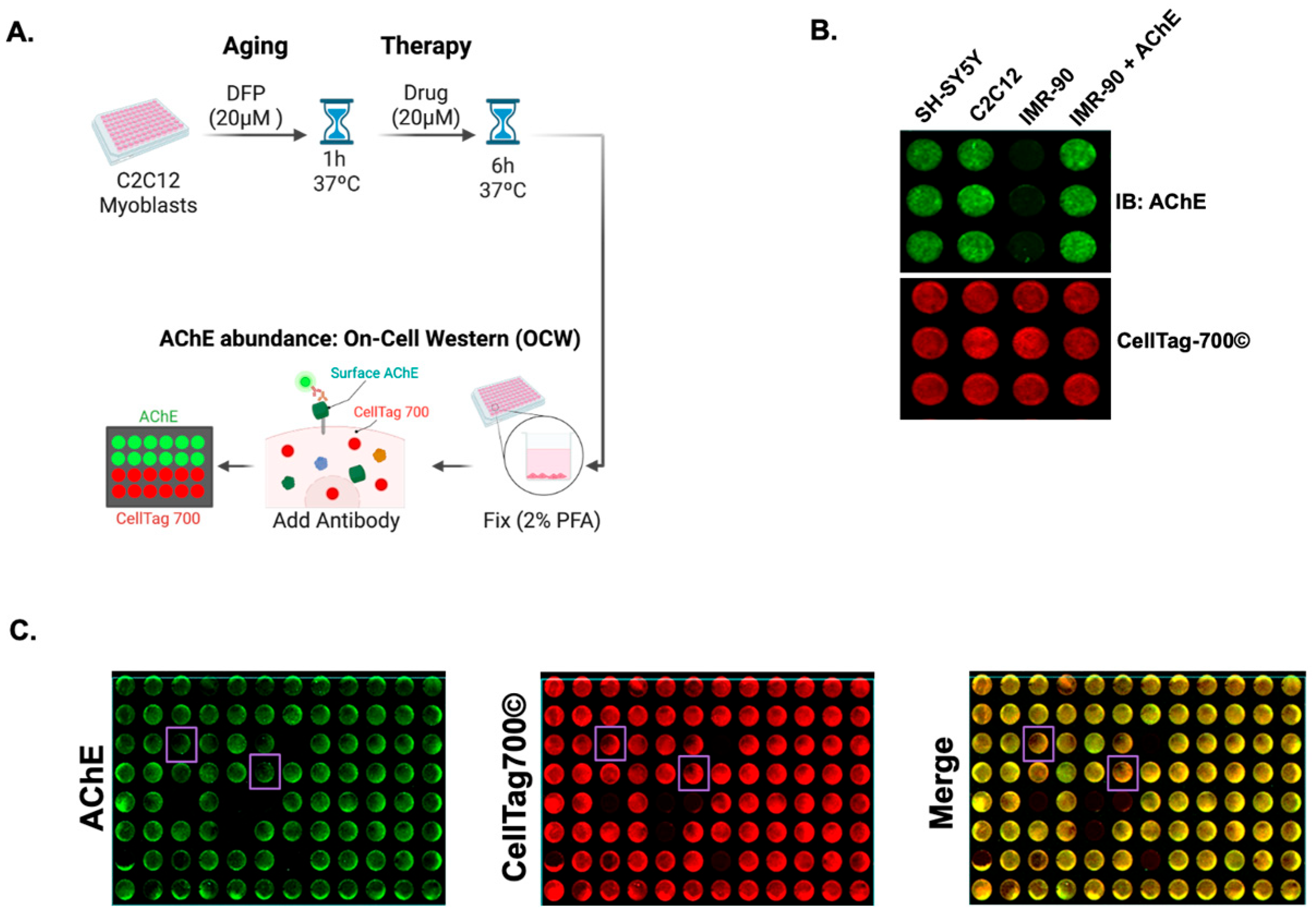
2.4. On-Cell Western (OCW) for Surface AChE Detection
2.5. Screening of Chemical Library
2.6. AChE Activity Assay
2.7. Surface AChE Activity Assay
2.8. Western Blotting Analyses
2.9. In Vitro MuSK Kinase Assay
2.10. Statistical Analysis
3. Results
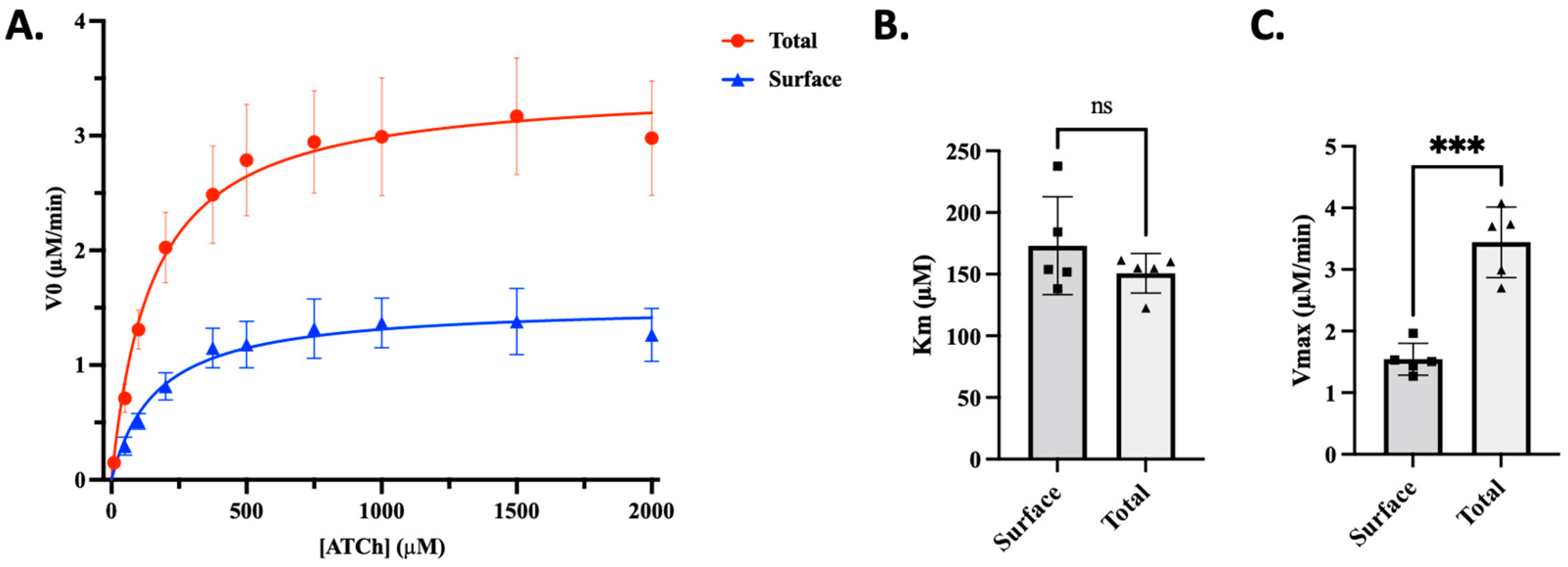
3.1. Surface AChE Contributes to Significantly Less Total AChE Activity than Intracellular Pools in C2C12 Cells
3.2. AChE On-Cell Western Assay Identifies Chemicals Capable of Reducing Surface AChE Levels in C2C12 Myoblast-like Cells
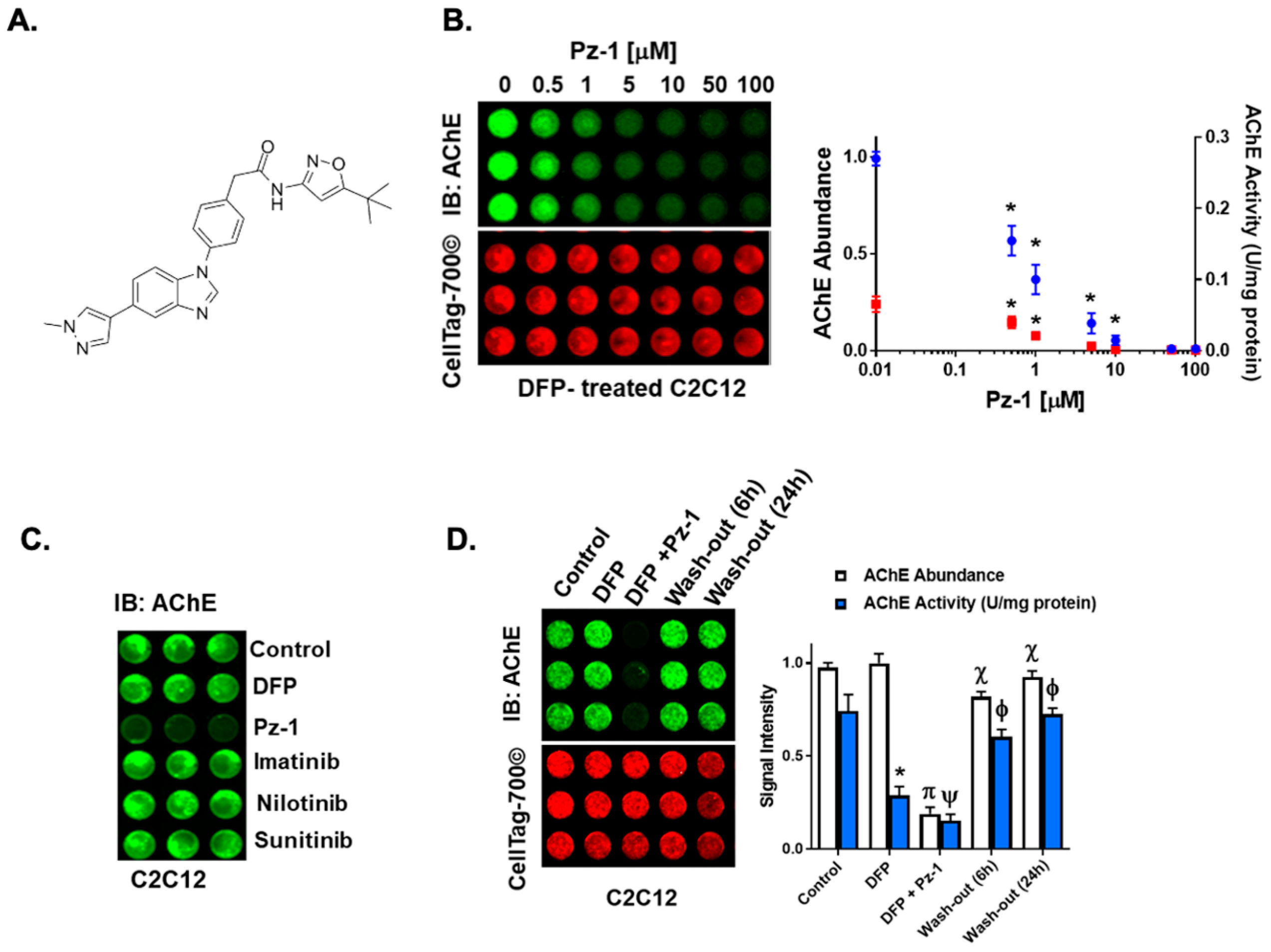
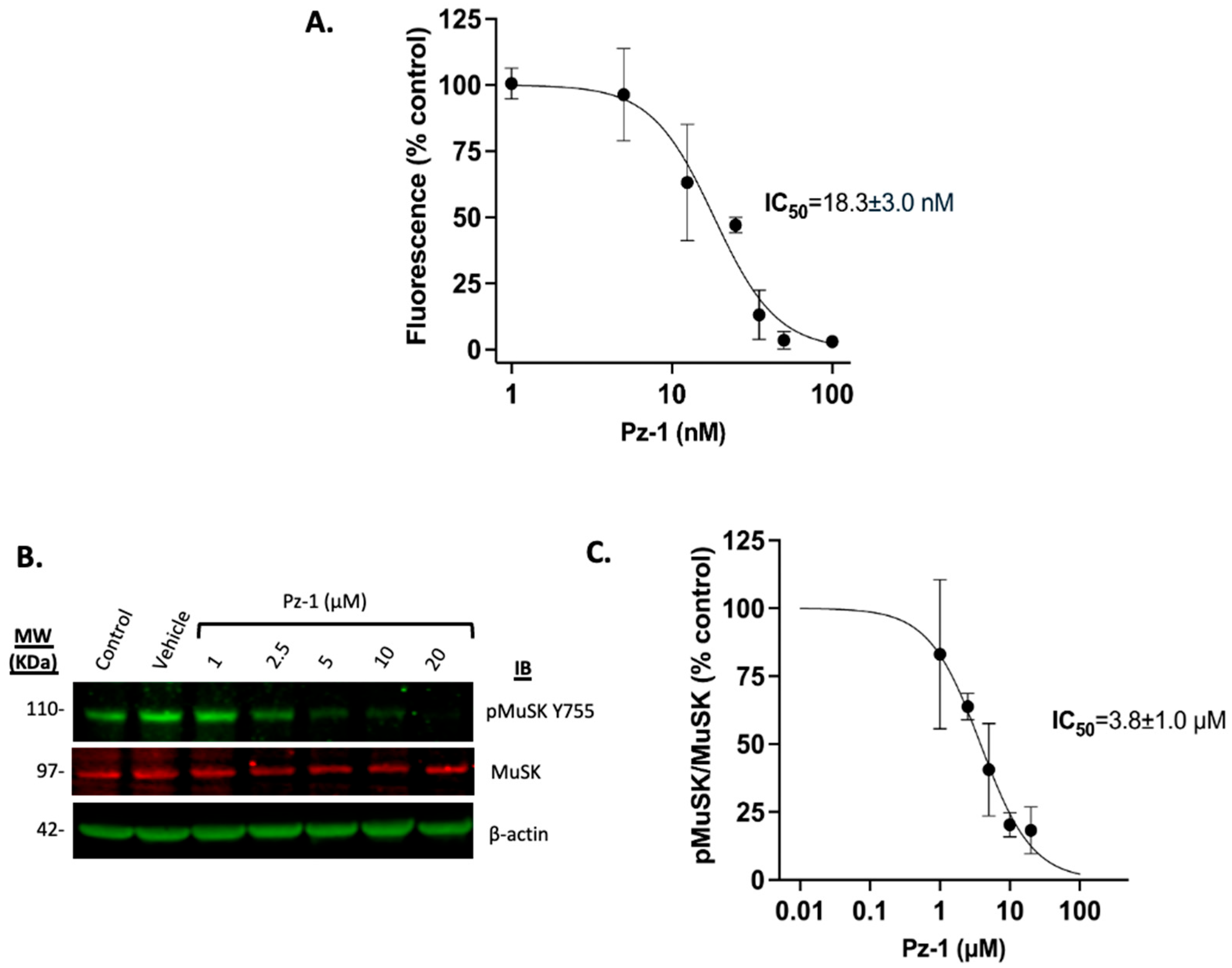
3.3. Pz-1 Decreases AChE Surface Levels in a Dose-Dependent Manner
3.4. Exchanging Culture Media Restores AChE in DFP-Exposed Myoblasts Treated with Pz-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-PAM | Pralidoxime |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChR | Acetylcholine Receptor |
| ATCC | American Type Culture Collection |
| ATCh | Acetylthiocholine |
| BSA | Bovine Serum Albumin |
| DFP | Diisopropyl Fluorophosphate |
| DM | Differentiation Medium |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| DTNB | 5,5′-Dithiobis(2-nitrobenzoic acid) |
| EDTA | Ethylenediaminetetraacetic Acid |
| FBS | Fetal Bovine Serum |
| FDA | Food and Drug Administration |
| HBSS | Hanks’ Balanced Salt Solution |
| HBSS-CM | HBSS containing 0.4926 mM of CaCl2 and 0.9009 mM of MgCl2 |
| HS | Horse Serum |
| LrP4 | Low-Density Lipoprotein Receptor-Related Protein 4 |
| MuSK | Muscle-Specific Protein Kinase |
| OP | Organophosphate |
| OCW | On-Cell Western |
| RET | Rearranged during transfection |
| TBS | Tris-Buffered Saline |
| TCh | Thiocholine |
| TNB2− | 5-Thio-2-nitrobenzoate |
| V0 | Initial Reaction Velocity |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| Vmax | Maximum Reaction Velocity |
References
- Watson, A.; Opresko, D.; Young, R.; Hauschild, V.; King, J.; Bakshi, K. CHAPTER 6—Organophosphate Nerve Agents. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 43–67. [Google Scholar]
- John, H.; Balszuweit, F.; Kehe, K.; Worek, F.; Thiermann, H. CHAPTER 50—Toxicokinetics of Chemical Warfare Agents: Nerve Agents and Vesicants. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 755–790. [Google Scholar]
- Soltaninejad, K.; Shadnia, S. History of the Use and Epidemiology of Organophosphorus Poisoning. In Basic and Clinical Toxicology of Organophosphorus Compounds; Balali-Mood, M., Abdollahi, M., Eds.; Springer: London, UK, 2014; pp. 25–43. [Google Scholar]
- World Health Organization; United Nations Environment Programme. Public Health Impact of Pesticides Used in Agriculture; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Dolgin, E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013, 19, 1194. [Google Scholar] [CrossRef]
- Sezigen, S.; Ivelik, K.; Ortatatli, M.; Almacioglu, M.; Demirkasimoglu, M.; Eyison, R.K.; Kunak, Z.I.; Kenar, L. Victims of chemical terrorism, a family of four who were exposed to sulfur mustard. Toxicol. Lett. 2019, 303, 9–15. [Google Scholar] [CrossRef]
- Swenson, K. A gruesome North Korean murder plot: Trial sheds new light on assassination of Kim Jong Un’s brother. The Washington Post, 17 October 2017. [Google Scholar]
- Eddleston, M.; Chowdhury, F.R. Pharmacological treatment of organophosphorus insecticide poisoning: The old and the (possible) new. Br. J. Clin. Pharmacol. 2016, 81, 462–470. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.H.; Jr Dochterman, L.W.; Smith, C.D.; Shih, T.M. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology 1995, 16, 123–132. [Google Scholar]
- Thiermann, H.; Szinicz, L.; Eyer, F.; Worek, F.; Eyer, P.; Felgenhauer, N.; Zilker, T. Modern strategies in therapy of organophosphate poisoning. Toxicol. Lett. 1999, 107, 233–239. [Google Scholar] [CrossRef]
- Volans, A.P. Sarin: Guidelines on the management of victims of a nerve gas attack. J. Accid. Amp; Emerg. Med. 1996, 13, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Blumenberg, A.; Benabbas, R.; de Souza, I.S.; Conigliaro, A.; Paladino, L.; Warman, E.; Sinert, R.; Wiener, S.W. Utility of 2-Pyridine Aldoxime Methyl Chloride (2-PAM) for Acute Organophosphate Poisoning: A Systematic Review and Meta-Analysis. J. Med. Toxicol. 2018, 14, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Eyer, P.; Worek, F.; Juszczak, E.; Alder, N.; Mohamed, F.; Senarathna, L.; Hittarage, A.; Azher, S.; Jeganathan, K.; et al. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS Med. 2009, 6, e1000104. [Google Scholar] [CrossRef]
- Kharel, H.; Pokhrel, N.B.; Ghimire, R.; Kharel, Z. The Efficacy of Pralidoxime in the Treatment of Organophosphate Poisoning in Humans: A Systematic Review and Meta-analysis of Randomized Trials. Cureus 2020, 12, e7174. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.P.; Meek, E.C.; Chambers, J.E. Reactivation by novel pyridinium oximes of rat serum and skeletal muscle acetylcholinesterase inhibited by organophosphates. J. Biochem. Mol. Toxicol. 2024, 38, e23750. [Google Scholar] [CrossRef]
- Peter, J.V.; Moran, J.L.; Graham, P. Oxime therapy and outcomes in human organophosphate poisoning: An evaluation using meta-analytic techniques. Critical Care Medicine 2006, 34, 502–510. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Increased morbidity and mortality in acute human organophosphate-poisoned patients treated by oximes: A meta-analysis of clinical trials. Human & Experimental Toxicology 2006, 25, 157–162. [Google Scholar]
- Quinn, D.M.; Topczewski, J.; Yasapala, N.; Lodge, A. Why is Aged Acetylcholinesterase So Difficult to Reactivate? Molecules 2017, 22, 1464. [Google Scholar] [CrossRef]
- Franjesevic, A.J.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and Reactivation of Acetylcholinesterase and Butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef]
- Zhuang, Q.; Young, A.; Callam, C.S.; McElroy, C.A.; Ekici, O.D.; Yoder, R.J.; Hadad, C.M. Efforts toward treatments against aging of organophosphorus-inhibited acetylcholinesterase. Ann. N. Y. Acad. Sci. 2016, 1374, 94–104. [Google Scholar] [CrossRef]
- Chambers, J.E.; Meek, E.C. Novel centrally active oxime reactivators of acetylcholinesterase inhibited by surrogates of sarin and VX. Neurobiol. Dis. 2020, 133, 104487. [Google Scholar] [CrossRef]
- Zhuang, Q.; Franjesevic, A.J.; Corrigan, T.S.; Coldren, W.H.; Dicken, R.; Sillart, S.; DeYong, A.; Yoshino, N.; Smith, J.; Fabry, S.; et al. Demonstration of In Vitro Resurrection of Aged Acetylcholinesterase after Exposure to Organophosphorus Chemical Nerve Agents. J. Med. Chem. 2018, 61, 7034–7042. [Google Scholar] [CrossRef]
- Shi, L.; Fu, A.K.; Ip, N.Y. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012, 35, 441–453. [Google Scholar] [CrossRef]
- Cartaud, A.; Strochlic, L.; Guerra, M.; Blanchard, B.; Lambergeon, M.; Krejci, E.; Cartaud, J.; Legay, C. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J. Cell Biol. 2004, 165, 505–515. [Google Scholar] [CrossRef]
- Burden, S.J.; Yumoto, N.; Zhang, W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a009167. [Google Scholar] [CrossRef]
- Ueta, R.; Tezuka, T.; Izawa, Y.; Miyoshi, S.; Nagatoishi, S.; Tsumoto, K.; Yamanashi, Y. The carboxyl-terminal region of Dok-7 plays a key, but not essential, role in activation of muscle-specific receptor kinase MuSK and neuromuscular synapse formation. J. Biochem. 2017, 161, 269–277. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Messéant, J.; Dobbertin, A.; Girard, E.; Delers, P.; Manuel, M.; Mangione, F.; Schmitt, A.; Le Denmat, D.; Molgó, J.; Zytnicki, D.; et al. MuSK frizzled-like domain is critical for mammalian neuromuscular junction formation and maintenance. J. Neurosci. 2015, 35, 4926–4941. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, A.L.; Burden, S.J.; Hubbard, S.R. Crystal structure of the frizzled-like cysteine-rich domain of the receptor tyrosine kinase MuSK. J. Mol. Biol. 2009, 393, 1–9. [Google Scholar] [CrossRef]
- Sigoillot, S.M.; Bourgeois, F.; Karmouch, J.; Molgo, J.; Dobbertin, A.; Chevalier, C.; Houlgatte, R.; Leger, J.; Legay, C. Neuromuscular junction immaturity and muscle atrophy are hallmarks of the ColQ-deficient mouse, a model of congenital myasthenic syndrome with acetylcholinesterase deficiency. FASEB J. 2016, 30, 2382–2399. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Kaczowka, S.J.; Payne, S.; Wang, F.; Gawdi, G.; Pizzo, S.V. Plasminogen Structural Domains Exhibit Different Functions When Associated with Cell Surface GRP78 or the Voltage-dependent Anion Channel*. J. Biol. Chem. 2007, 282, 32811–32820. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Takahashi, Y.; Kishi, H.; Taguchi, Y.; Takashima, S.; Tanaka, K.; Muraguchi, A.; Mori, H. Detection of autoantibody against extracellular epitopes of N-methyl-d-aspartate receptor by cell-based assay. Neurosci. Res. 2011, 71, 294–302. [Google Scholar] [CrossRef]
- Worek, F.; Eyer, P.; Thiermann, H. Determination of acetylcholinesterase activity by the Ellman assay: A versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal. 2012, 4, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Siow, N.; Choi, R.; Cheng, A.; Jiang, J.; Wan, D.; Zhu, S. A Cyclic AMP-dependent Pathway Regulates the Expression of Acetylcholinesterase during Myogenic Differentiation of C2C12 Cells. J. Biol. Chem. 2002, 277, 36129–36136. [Google Scholar] [CrossRef]
- Adeyinka, A.; Muco, E.; Regina, A.C.; Pierre, L. Organophosphates. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Blau, H.M.; Pavlath, G.K.; Hardeman, E.C.; Chiu, C.-P.; Silberstein, L.; Webster, S.G.; Miller, S.C.; Webster, C. Plasticity of the Differentiated State. Science 1985, 230, 758–766. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Frett, B.; Carlomagno, F.; Moccia, M.L.; Brescia, A.; Federico, G.; De Falco, V.; Admire, B.; Chen, Z.; Qi, W.; Santoro, M.; et al. Fragment-based discovery of a dual pan-RET/VEGFR2 kinase inhibitor optimized for single-agent polypharmacology. Angew. Chem. (Int. Ed. Engl.) 2015, 54, 8717–8721. [Google Scholar] [CrossRef]
- Rotundo, R.L. Biogenesis, assembly and trafficking of acetylcholinesterase. J. Neurochem. 2017, 142 (Suppl. 2), 52–58. [Google Scholar] [CrossRef]
- Timchalk, C. Chapter 66—Organophosphorus Insecticide Pharmacokinetics. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 1409–1433. [Google Scholar]
- Papaetis, G.S.; Syrigos, K.N. Sunitinib. BioDrugs 2009, 23, 377–389. [Google Scholar] [CrossRef]
- de Groot, J.W.; Plaza Menacho, I.; Schepers, H.; Drenth-Diephuis, L.J.; Osinga, J.; Plukker, J.T.; Links, T.P.; Eggen, B.J.; Hofstra, R.M. Cellular effects of imatinib on medullary thyroid cancer cells harboring multiple endocrine neoplasia Type 2A and 2B associated RET mutations. Surgery 2006, 139, 806–814. [Google Scholar] [CrossRef]
- Oury, J.; Zhang, W.; Leloup, N.; Koide, A.; Corrado, A.D.; Ketavarapu, G.; Hattori, T.; Koide, S.; Burden, S.J. Mechanism of disease and therapeutic rescue of Dok7 congenital myasthenia. Nature 2021, 595, 404–408. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Karachaliou, E.; Chroni, E.; Zouvelou, V.; Tzanetakos, D.; Salakou, S.; Papadopoulou, M.; Tzartos, S.; Voumvourakis, K.; Kilidireas, C.; et al. Immunotherapies in MuSK-positive Myasthenia Gravis; an IgG4 antibody-mediated disease. Front. Immunol. 2023, 14, 1212757. [Google Scholar] [CrossRef]
- Rotundo, R.L.; Fambrough, D.M. Synthesis, transport and fate of acetylcholinesterase in cultured chick embryo muscle cells. Cell 1980, 22, 583–594. [Google Scholar] [CrossRef]
- Verdín-Betancourt, F.A.; Figueroa, M.; Soto-Ramos, A.G.; de Lourdes López-González, M.; Castañeda-Hernández, G.; Bernal-Hernández, Y.Y.; Rojas-García, A.E.; Sierra-Santoyo, A. Toxicokinetics of temephos after oral administration to adult male rats. Arch. Toxicol. 2021, 95, 935–947. [Google Scholar] [CrossRef]
- Akgür, S.; Öztürk, P.; Yemişcigil, A.; Ege, B. Rapid Communication: Postmortem Distribution of Organophosphate Insecticides in Human Autopsy Tissues Following Suicide. J. Toxicol. Environ. Health Part A 2003, 66, 2187–2191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncada-Restrepo, M.; Eysoldt, S.; Medina, J.; Di Guida, V.; Chambers, J.W. Inhibition of Muscle-Specific Protein Kinase (MuSK) Releases Organophosphate-Aged Acetylcholinesterase (AChE) from C2C12 Cells. Toxics 2025, 13, 829. https://doi.org/10.3390/toxics13100829
Moncada-Restrepo M, Eysoldt S, Medina J, Di Guida V, Chambers JW. Inhibition of Muscle-Specific Protein Kinase (MuSK) Releases Organophosphate-Aged Acetylcholinesterase (AChE) from C2C12 Cells. Toxics. 2025; 13(10):829. https://doi.org/10.3390/toxics13100829
Chicago/Turabian StyleMoncada-Restrepo, Monica, Sarah Eysoldt, Jeronimo Medina, Valentina Di Guida, and Jeremy W. Chambers. 2025. "Inhibition of Muscle-Specific Protein Kinase (MuSK) Releases Organophosphate-Aged Acetylcholinesterase (AChE) from C2C12 Cells" Toxics 13, no. 10: 829. https://doi.org/10.3390/toxics13100829
APA StyleMoncada-Restrepo, M., Eysoldt, S., Medina, J., Di Guida, V., & Chambers, J. W. (2025). Inhibition of Muscle-Specific Protein Kinase (MuSK) Releases Organophosphate-Aged Acetylcholinesterase (AChE) from C2C12 Cells. Toxics, 13(10), 829. https://doi.org/10.3390/toxics13100829






