1. Introduction
The urinary bladder is responsible for storing and voiding urine [
1]. The urinary bladder wall consists of four distinct layers of tissue structure: the mucosa, the submucosa, the muscularis, and the parietal peritoneum. The mucosal layer, also known as the urothelium, lines the urinary bladder and is composed of three layers of specialized epithelial cells: an apical layer of umbrella-shaped cells, a middle intermediate layer of epithelial cells that can be several cells thick and a basal stem cell layer. The stem cell layer supports regeneration of the upper epithelial cells in response to injury and also provides injury induced signals to activate underlying stromal fibroblasts [
2]. The submucosa contains urinary bladder fibroblasts, which provide both physical support and nutrition to the overlying epithelial cells. These fibroblasts engage in the synthesis of extracellular matrix and play a crucial role in the maturation and homeostasis of the urothelium [
2]. The main role of the mucosal layers is to function as an osmotic and chemical barrier, protecting the underlying tissues from potential damage caused by urine [
3]. Urine in the bladder accumulates the terminal products of the body’s metabolism, leading to frequent exposure of the urinary bladder epithelium to various harmful factors, including carcinogens and toxins. In addition, a wide range of pathogens cause urinary tract infections. Therefore, most bladder diseases are closely linked to damage to the bladder epithelium.
Generally, two-dimensional (2D) cell culture has been used to study the physiopathological mechanisms of bladder-related diseases. However, 2D cell culture cannot accurately mimic the in vivo condition of the urinary bladder epithelium. In our body, the urinary bladder epithelium is closely connected to the underlying connective tissue containing bladder fibroblast cells, and this unique microenvironment influences cell behavior, including proliferation, differentiation, and apoptosis [
4]. In recent years, the widespread use of three-dimensional (3D) cell models of tumor and skin epithelium has also provided valuable insights into the study of bladder-related diseases [
5,
6]. One of the main advantages of 3D models is that they provide a cellular model more in accordance with the physiological structure of the tissue. Moreover, the emergence of diseases is often a consequence of complex cellular interactions. Chen’s research indicated that inflammation in bladder fibroblast cells could impact overlying urothelial cells, thereby playing a pivotal role in bladder carcinogenesis [
7]. Therefore, when investigating the mechanisms of bladder-related diseases in vitro, 3D models are preferable to 2D cell culture systems.
Inorganic arsenic is a known human carcinogen, linked to the development of urinary bladder, lung, and skin cancers. The International Agency for Research on Cancer (IARC) categorizes arsenic and inorganic arsenic compounds as Group 1 carcinogens (carcinogenic to humans) [
8]. Chronic arsenic exposure remains a significant public health issue in many countries. Human exposure to arsenic primarily occurs through drinking contaminated water, as well as through inhalation, food and skin contact [
9]. When arsenic-containing water is consumed, it is metabolized in the body into two forms, inorganic arsenic and organic arsenic, both of which are closely related to the incidence of urinary bladder cancer [
10]. Inorganic arsenic has two main forms in the body, pentavalent (iAs
V) and trivalent (iAs
III). iAs
III is more rapidly absorbed than iAs
V and exhibits significant cytotoxicity even at low concentrations. Its quick uptake and high reactivity trigger cellular oxidative stress and inflammation in the urinary bladder epithelium, fostering DNA damage and irregular cell growth, potentially resulting in carcinogenesis.
The organic arsenical Dimethylarsenic acid (DMA
V) is classified as a Group 2B compound, possibly carcinogenic in humans, by IARC [
8]. DMA
V is easily absorbed through the intestines, subsequently distributing to organs such as the kidneys, urinary bladder, and skin [
11]. DMA
V is a urinary metabolite of various inorganic arsenic compounds, including iAs
III, and has been shown to be closely associated with the development of urinary bladder cancer in rats, mainly through cytotoxicity, oxidative stress, inflammation, and other pathways that lead to abnormal proliferation of urothelial cells [
12,
13]. However, Cohen et al. report that metabolism of DMA
V resulting in production of high levels of Trimethylarsine oxide (TMAO) is unique to rats [
14]. They show that in rats administered DMA
V, the levels of TMAO in the urine are markedly higher than in mice and humans. They also note that during the formation of TMAO the highly reactive arsenical DMA
III is formed and reviews by Cohen et al. state that the mode of action of DMA
V-induced bladder carcinogenesis in the rat involves the generation of DMA
III [
14,
15]. Cohen et al. also report that in all tested animal species, including humans, DMA
V does not readily translocate into intact cells and consequently DMA
V metabolites, including DMA
III, are produced at low levels [
14]. However, Cohen et al. also report that in the rat there is higher cellular uptake and metabolism of DMA
V, and the rat is the only species in which administration of DMA
V results in urinary concentrations of DMA
III that are cytotoxic to urothelial cells in vitro [
14]. In agreement with these reports, Arnold et al. found that administration of DMA
V caused bladder cancer in rats but did not induce bladder hyperplasia or bladder cancer in mice [
16]. This suggests that in this study the low translocation of DMA
V into cells in mice resulted in low metabolism of DMA
V to DMA
III, resulting in urinary concentrations of DMA
III that did not reach cytotoxic levels in DMA
V-treated mice. In addition, Dodmane et al. reported that administration of DMA
III to wild-type mice resulted in urothelial cytotoxicity and regenerative proliferation [
17]. Overall, current data indicates that in mice and humans the cytotoxic potential of DMA
V is less than that of iAs
III.
Despite extensive animal experiments to explore the mechanisms of organic and inorganic arsenic toxicity, there is only a limited understanding of the mechanisms of toxicity and carcinogenicity in the human urinary bladder. To address this gap, we utilized primary human bladder urothelial cells and fibroblast cells to construct a 3D urinary bladder mucosa model that incorporated an overlying epithelium and a supporting subepithelial layer, referred to as the 3D-UBMM, that closely resembles the characteristics of the human urinary bladder mucosa. We also tested the applicability of our 3D-UBMM by assessing the cytotoxicity of an arsenical with stronger in vivo cytotoxicity, iAsIII, and an arsenical with weaker in vivo cytotoxicity, DMAV.
2. Materials and Methods
2.1. Chemicals
Sodium arsenite (iAsIII, purity > 90%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). DMAV [(CH3)2AsO(OH)] (purity > 99%) was purchased from Fluka (Steinheim, Germany).
2.2. Human Urinary Bladder Tissue
Normal human urinary bladder tissue was obtained from an autopsy case at Osaka Metropolitan University Hospital, Osaka, Japan. The Ethical Committee of the Osaka Metropolitan University Graduate School of Medicine approved the use of this human specimen and clinical data (Approval number #2021-047) in accordance with the Declaration of Helsinki and the guidelines of the Osaka Metropolitan University Graduate School of Medicine.
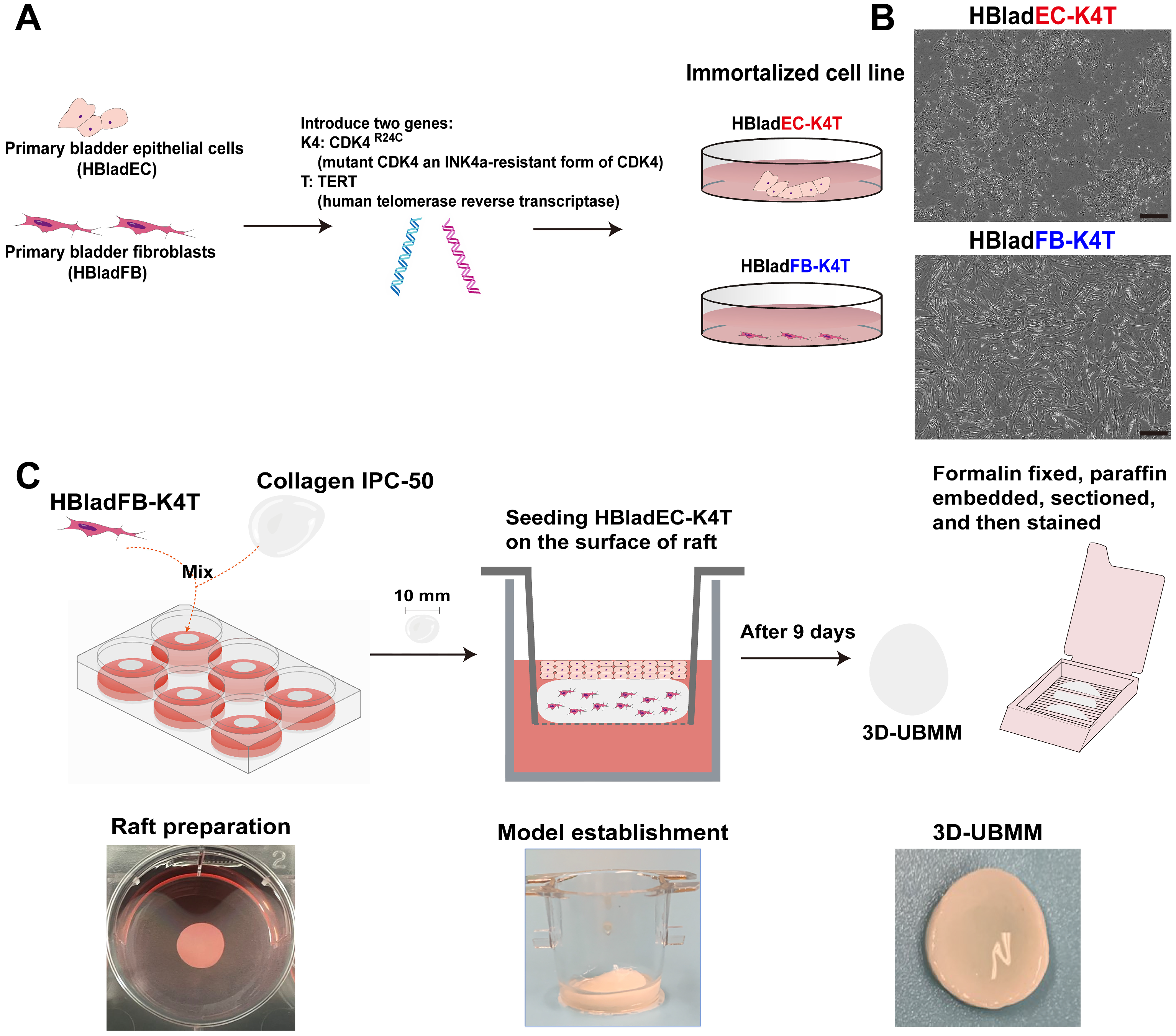
2.3. Establishment of Immortalized Urinary Bladder Fibroblast and Epithelial Cell Lines
Primary human urinary bladder fibroblast cells (HBladFB) were purchased from ATCC
® (ATCC
® PCS-420-013TM, Manassas, VA, USA),and primary human urinary bladder epithelial cells (male Caucasian, 19 years old) (HBladEC) were purchased from KURABO (KP-4109, Osaka, Japan). To immortalize the HBladFB and HBladEC cells, lentivirus vectors CSII-CMV-TERT and CSII-CMV-CDK4
R24C, expressing human telomerase reverse transcriptase (TERT) and CDK4
R24C (mutant CDK4 an INK4a-resistant form of CDK4), were introduced into each cell line using methods described previously [
18,
19]. The resulting immortalized cell lines were designated HBladFB-K4T and HBladEC-K4T and expressed both TERT and CDK4
R24C.
HBladEC-K4T has been confirmed to express As3MT by Western blot analysis (
Supplementary Figure S1) and to be negative for P53 by immunochemistry [
20] Whole-exome sequencing using the SureSelect V8-Post kit on the Illumina platform, conducted by Cell Innovator Inc. (Fukuoka, Japan), identified a single nucleotide substitution at chr17:7676154 within exon 4 of the TP53 gene, resulting in an amino acid change from proline to arginine. Functional prediction using the SIFT algorithm (
https://sift.bii.a-star.edu.sg/index.html, accessed on 17 September 2025) classified this variant as “tolerated,” suggesting that it is unlikely to have a significant impact on TP53 protein function (data not shown).
In addition, although the arsenic metabolic capacity of HBladEC-K4T cells was not directly examined in the present study, our previous work demonstrated that HBladEC-T cells, which are immortalized with TERT alone, are capable of metabolizing various arsenicals, including iAs
III and DMA
V, via pathways consistent with known arsenic metabolism [
20]. In preliminary experiments, HBladEC-T cells also exhibited stratification and urothelial differentiation; however, the multilayer structure was thinner than that observed in HBladEC-K4T cells. This difference is likely due to the absence of mutant CDK4 (K4) in HBladEC-T cells. Since K4 is primarily involved in enhancing proliferative capacity and is not known to influence arsenic metabolism, we expect that the arsenic-metabolizing capacity of HBladEC-K4T cells is comparable to that of HBladEC-T cells.
The HBladFB-K4T cells were cultured in DMEM culture medium (Nacalai Tesque Inc., Kyoto, Japan) supplemented with 2% FBS, 5 ng/mL basic FGF, 50 mg/mL ascorbic acid, 1 mg/mL hydrocortisone hemisuccinate, 5 mg/mL recombinant human insulin, and 100 u/mL penicillin and 100 mg/mL streptomycin, referred to as HBladFB-K4T medium. The HBladEC-K4T cells were cultured in F medium (DMEM/Ham’s F-12 culture medium (Nacalai Tesque Inc., Kyoto, Japan) containing FBS, hEGF, adenine-HCl, insulin, hydrocortisone, cholera toxin, and penicillin-streptomycin) [
21] supplemented with 10 mM Y-27632 (Y), 5% (
v/
v) conditioned medium from Wnt-3A cells expressing human RSPO-1 and human Noggin (purchased from ATCC (CRL-2647)), 50 nM A-83-01 (A), and 50 nM DMH1 (D), collectively referred to as FYWRAD medium.
2.4. Evaluation of Cytotoxicity of iAsIII and DMAV in 2D Culture
The cytotoxicity of iAs
III and DMA
V to HBladEC-K4T epithelial cells was investigated by evaluating cell viability. Stock solutions of 10 mM iAs
III and 5 M DMA
V were prepared in 0.5 M HEPES (pH 7.2, Thermo Fisher Scientific, Waltham, MA, USA) and subsequently diluted to the appropriate working concentrations using FYWRAD medium. HBladEC-K4T epithelial cells were seeded at a density of 5 × 10
3 cells/well into 96-well plates and incubated for 24 h. Subsequently, the FYWRAD medium was replaced with FYWRAD medium containing different concentrations of iAs
III and DMA
V, and the cells were incubated for 24 h. After incubation with the arsenicals, cell viability was determined by Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). The FYWRAD medium containing the iAs
III and DMA
V was removed, and fresh medium was added to each well. After adding CCK-8 solution into each well, the 96-well plates were incubated at 37 °C in an incubator with 5% CO
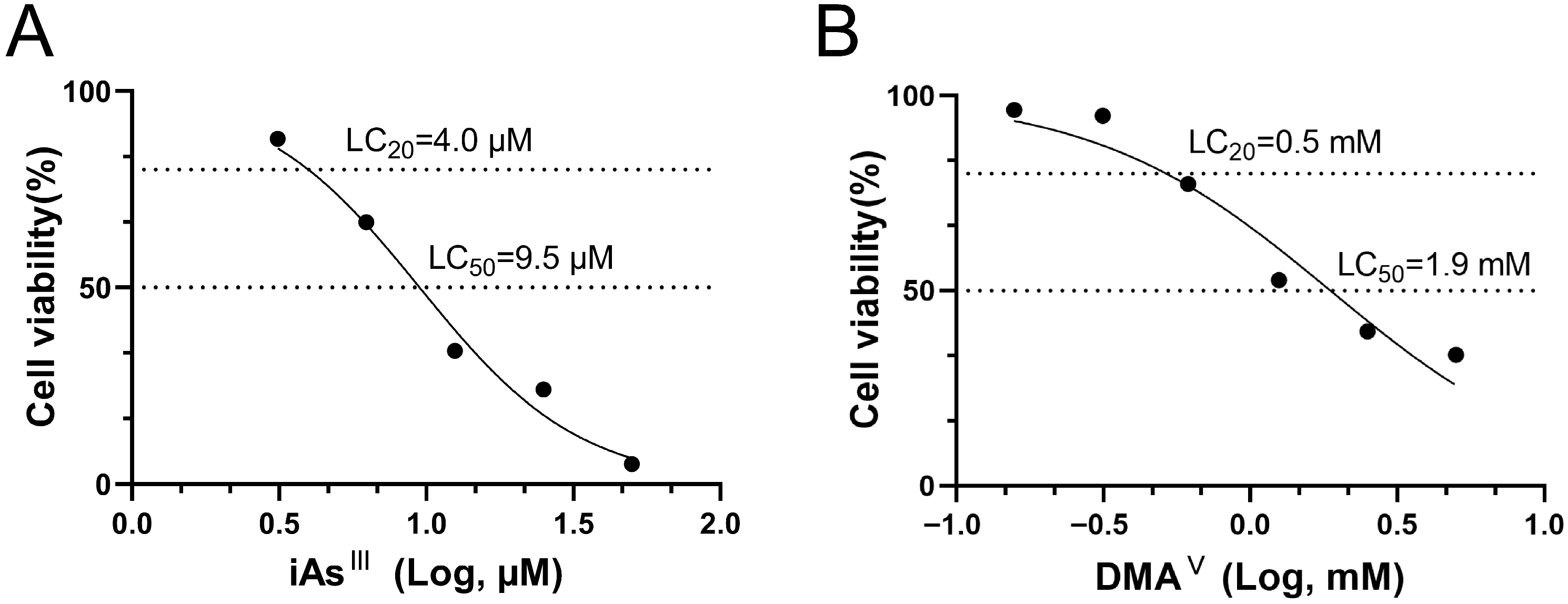
2 for 4 h. The absorbance was measured at 450 nm using a Model 680 Microplate Reader (Bio-Rad Laboratories, Hercules, CA, USA). The optical density (OD) of all control and treated wells was corrected by subtracting the average OD of the background wells. The percent viability of each well was calculated as the ratio of the absorbance of the treated wells to the mean absorbance of the control wells without test chemicals. The LC
20 and LC
50 doses were separately determined for the iAs
III and DMA
V and calculated by nonlinear regression analysis using GraphPad Prism 8 [
22].
2.5. Construction of the 3D-UBMM
To establish the 3D-UBMM, we first performed a series of optimization experiments to determine the most suitable conditions for supporting epithelial differentiation and maintaining the 3D-UBMM integrity. Various combinations of HBladEC-K4T epithelial cells (1 × 10
5, 1.5 × 10
5, 3 × 10
5, 5 × 10
5) and HBladFB-K4T fibroblast cells (0, 1 × 10
5, 3 × 10
5, 5 × 10
5, 1 × 10
6) were tested, along with different ratios of HBladFB-K4T medium to FWR medium (F medium supplemented with WR, see
Section 2.3) at 3:1, 1:1, and 1:3. These experiments showed that HBladEC-K4T cells failed to differentiate and maintain normal morphology in the absence of fibroblasts, indicating that matrix cellularity is critical for establishing a stable and functional 3D model. As differences in matrix cellularity can influence not only tissue homeostasis but also toxicological outcomes—such as the epithelial response to arsenic exposure—by altering the microenvironmental support, we fixed the optimized fibroblast density to maintain consistency across all toxicological evaluations. Based on these results, we selected the optimized condition of 5 × 10
5 fibroblasts embedded in a collagen matrix, 1.5 × 10
5 epithelial cells seeded on top, and a 3:1 mixture of HBladFB-K4T medium and FWR medium.
We also compared the impact of submerged versus air-liquid interface culture conditions on epithelial differentiation. Despite testing various culture media, including artificial urine solution (#900489945, Isekyu Co., Ltd., Nagoya, Japan), under submerged conditions, HBladEC-K4T epithelial cells consistently formed only a monolayer and failed to differentiate into uroplakin-positive umbrella cells (data not shown). Although the underlying mechanism remains unclear, our findings suggest that the air-liquid interface is essential for promoting stratification and urothelial differentiation in vitro.
To construct the model under these optimized conditions, 5 mL of HBladFB-K4T medium containing 5 × 105 HBladFB-K4T fibroblasts, 1 mL of 0.5% Collagen IPC-50 (AteloCell, Japan), and 0.05 mL of 1M HEPES (Gibco, Grand Island, NY, USA) were mixed and poured into one well of a 6-well plate (Sumitomo Bakelite. Tokyo, Japan). The 6-well plate was then placed in a 37 °C incubator to allow the mixture to solidify. This solid fibroblast-containing material is referred to as a collagen raft.
After 4–5 days, the collagen rafts shrunk to about 10 mm in diameter and were transferred to Falcon Cell Culture Inserts with 0.4 μm pores and a 10.5 mm diameter (Corning, New York, NY, USA, #353493) in a 12-well plate. Then, 0.1 mL of FYWRAD medium containing 1.5 × 105 HBladEC-K4T epithelial cells were seeded onto the surface of the collagen rafts. An additional 750 μL of HBladFB-K4T medium was added to the bottom of the well.
After 24 h, the HBladFB-K4T medium in the insert was removed, and the medium in the bottom of the well was replaced with 750 μL of a 3:1 mix of HBladFB-K4T medium and FWR medium to maintain HBladEC-K4T cells at the air-liquid interface. After nine days the cells had achieved the 3D-UBMM configuration. On day 10, arsenicals were added into the 3D-UBMM to evaluate their cytotoxicity.
2.6. Evaluation of the Cytotoxicity of iAsIII and DMAV Using the 3D-UBMM
To assess the cytotoxicities of iAsIII and DMAV, 100 μL of FYWRAD medium containing targeted concentrations of iAsIII or DMAV was added into the insert containing the 3D-UBMM, and 750 μL of the 3:1 mix of HBladFB-K4T and FWR medium was added into the bottom of the wells, and the cells were incubated at 37 °C in an incubator with 5% CO2. Twenty-four hours after treatment, the 3D-UBMM was fixed in 10% phosphate-buffered formalin for one day and then cut into 3 strips, embedded in paraffin, and processed for hematoxylin/eosin and immunohistochemical staining.
2.7. Immunohistochemical Staining
Paraffin-embedded sections of the human urinary bladder (
Section 2.2) and the 3D-UBMM were stained using the avidin-biotin-peroxidase complex (ABC) method. Briefly, the section was deparaffinized, dehydrated, and the antigen was retrieved by microwaving at 98 °C for 20 min in 0.01 M citrate buffer (pH 6.0). Next, the endogenous peroxidase activity was blocked with 3% H
2O
2 in distilled water for 5 min, followed by blocking of non-specific binding with 10% goat or horse serum at room temperature for 20 min. Sections were then incubated with primary antibodies overnight at 4 °C. The primary antibodies and dilutions were as follows: Uroplakin 1b (UPK1B; ab263454, Abcam, Cambridge, MA, USA, mouse monoclonal, 1:200), P63 (ab735, Abcam, USA, rabbit monoclonal, 1:1000), cytokeratin-5 (CK5; ab53121, Abcam, USA, rabbit polyclonal, 1:1000), γ-H2AX (9718S, CST, USA, rabbit monoclonal, 1:500). Reactivity with the primary antibody was detected by incubating the sections with biotin-labeled goat anti-rabbit IgG or biotin-labeled horse anti-mouse IgG using the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA, USA) and diaminobenzidine tetrahydrochloride. Tissue sections were counterstained with hematoxylin.
2.8. Statistical Analysis
Data are reported as mean ± standard deviation (mean ± SD). Statistical analyses were performed using Prism 8 software (GraphPad Software, Inc., San Diego, CA, USA). One-way ANOVA, followed Dunnett’s multiple comparison test, was used to compare differences comparison between multiple groups. p values less than 0.05 were considered statistically significant.
4. Discussion
Arsenic is a well-established human bladder carcinogen, with cytotoxicity playing a pivotal role in its carcinogenicity. However, a comprehensive understanding of the cytotoxic effects of arsenic on the human urinary bladder epithelium remains elusive. Therefore, we developed a novel 3D urinary bladder mucosa model (3D-UBMM) using immortalized human bladder fibroblast cells and urothelial cells. The applicability of our 3D-UBMM was tested by evaluating the cytotoxicity of two in vivo carcinogens, a stronger carcinogen iAsIII and a weaker carcinogen DMAV.
The human urinary bladder mucosal layer consists of an overlying urothelium composed of three layers of specialized epithelial cells and a lower connective tissue layer. Our 3D-UBMM also has a bilayer structure with an upper layer of human urothelial cells and a lower layer composed of a collagen raft containing human urinary bladder fibroblast cells. The epithelial cell layers of the urothelium consist of apical umbrella cells, an intermediate layer of epithelial cells, and a basal stem cell layer. UPK1B, CK5, and P63 were selected to evaluate the histology of the 3D-UBMM. UPK1B is expressed almost exclusively in the urinary bladder epithelium [
25]. UPK1B is normally expressed in apical umbrella cells and intermediate cells of the urothelium. CK5, a member of the cytoskeletal protein family exhibits high expression in the basal layer of the urothelium and lower expression in the intermediate layer [
26]. It contributes to the formation of intermediate filaments, thereby supporting cellular structural stability and morphology [
27]. P63 is a basal layer cell marker within the human urinary bladder epithelium. P63-expressing cells are situated at the base of the bladder epithelium and adhere firmly to the connective tissue layer, playing an essential role in the structural integrity and regenerative capacity of the urinary bladder epithelium [
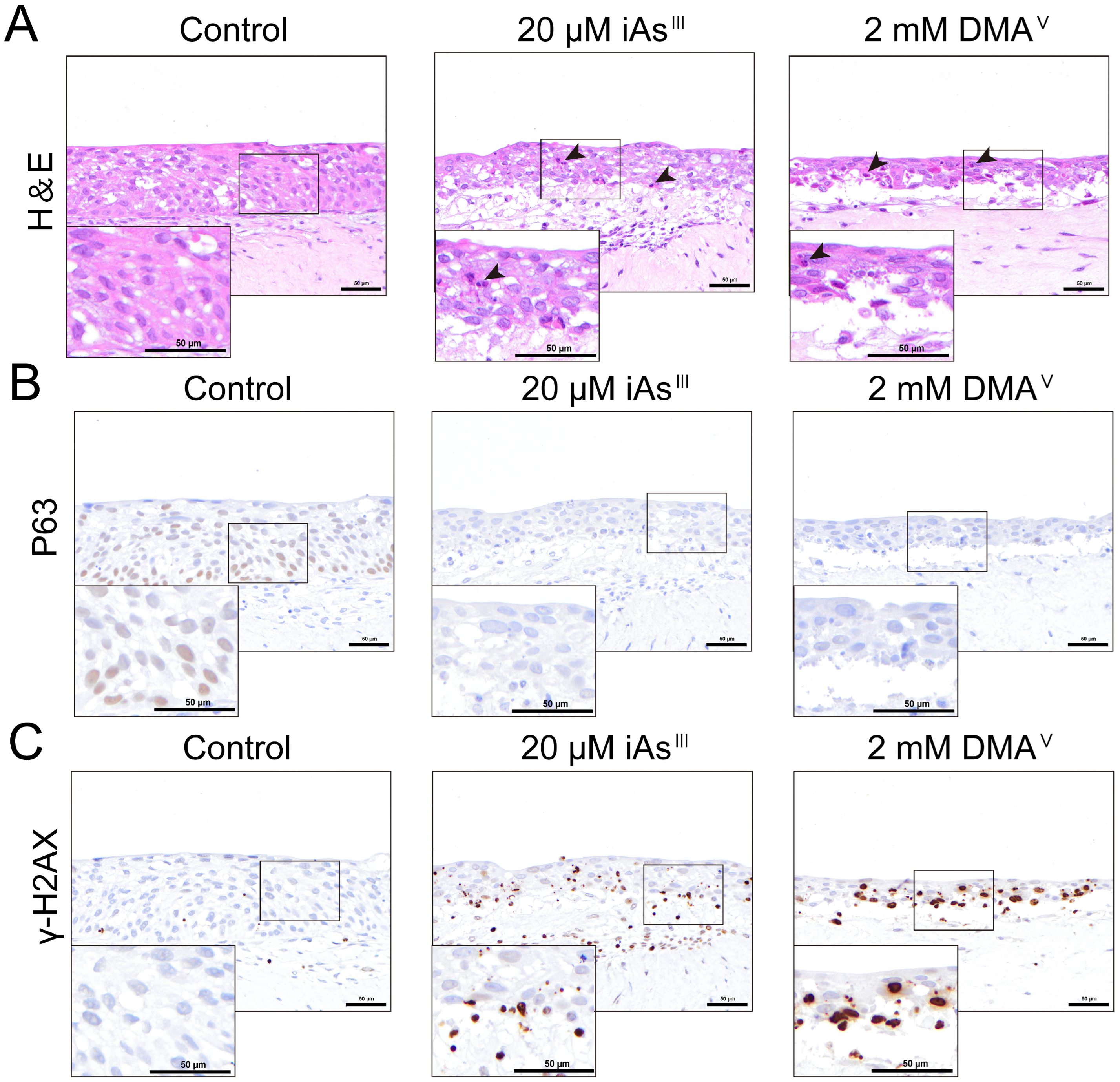
28]. As can be seen from the results of the present study, the 3D-UBMM closely resembles the human urinary bladder epithelium in terms of morphology and expression of the marker proteins UPK1B, CK5, and P63.
Although arsenicals do not directly interact with DNA, and consequently, are not direct-acting genotoxicants, oxidative stress has been proposed as an indirect mechanism contributing to their genotoxic potential [
13,
20,
29]. Reactive oxygen species (ROS), which are generated during arsenic metabolism in the cell, can cause oxidative damage to DNA, proteins, and lipids. Excessive ROS generation—whether arising as a consequence of cytotoxicity or contributing to it—can exacerbate cellular injury, creating a feedback loop that amplifies both cytotoxic and genotoxic stress [
11,
20]. In our study, administration of iAs
III and DMA
V resulted in a dose-dependent increase in γ-H2AX-positive cells, a well-recognized marker of DNA double-strand breaks. While γ-H2AX does not indicate direct genotoxicity, it serves as a sensitive biomarker of DNA damage potentially resulting from sustained oxidative stress or other indirect mechanisms [
30,
31]. We also observed a dose-dependent decrease in P63-positive cells in the 3D-UBMM. Given that basal cells function as a stem cell reservoir in the bladder urothelium, their depletion may compromise epithelial integrity and regenerative capacity, potentially contributing to carcinogenic progression over time.
It is now widely recognized that 3D cell models may more accurately reflect the in vivo situation compared to 2D cell cultures. In a study by Carrie J. Lovitt, it was demonstrated that breast cancer cells cultured in a 3D in vitro model can form an extracellular matrix, which is closely related to both drug delivery and tumor cell growth. In contrast, breast cancer cells in 2D culture struggled to establish an extracellular matrix [
32]. Marta Nowacka’s research also showed that the 3D ovarian cancer cell model exhibited greater drug resistance compared to ovarian cancer cells in 2D culture [
23]. In our study, no necrotic cells or γ-H2AX-positive cells were observed in the 3D-UBMM treated with 5 μM iAs
III or 0.5 mM DMA
V, while the same concentrations in 2D-cultured HBladEC-K4T resulted in about 20% cell death. Importantly, during proliferation and cell differentiation of basal layer stem cells, some cells migrate upwards and differentiate into umbrella cells, forming the apical layer of the urinary bladder epithelium. This umbrella structure plays an important role in protecting the urinary bladder epithelium from irritation and chemicals in the urine [
25,
33]. While this structure is difficult to organize in 2D cell cultures of bladder urothelial cells, the 3D-UBMM does allow for the formation of an apical umbrella structure. Therefore, the tolerance of the 3D-UBMM to iAs
III and 0.5 mM DMA
V is higher than that of 2D-cultures.
In conclusion, we have constructed a novel 3D-UBMM with characteristics similar to human urinary bladder epithelium. The cytotoxicity and DNA damage induced by iAs
III and DMA
V were tested in the 3D-UBMM and reflected the stronger cytotoxicity of iAs
III compared to DMA
V in mice and humans. Suzuki et al. and Tokar et al. [
34,
35] reported that mice exposed to Sodium arsenite (iAs
III) developed urinary bladder hyperplasia while Arnold et al. [
16] reported that no hyperplastic effects, or tumors, were observed in mice exposed to DMA
V. In humans, there is sufficient evidence that iAs
III is a urinary bladder carcinogen, while DMA
V is classified as possibly carcinogenic in humans, but this classification is based on animal studies and no studies on the effect of DMA
V in humans are presented [
8]. Thus, the IARC report strongly suggests that DMA
V is less carcinogenic in humans than iAs
III. Importantly, while the cytotoxicity of iAs
III and DMA
V in 2D-cultures of HBladEC-K4T cells differed by approximately 125 to 200-fold, exposure of 3D-UBMM to approximately 2D-culture LD50 levels of these arsenicals induced significant formation of γ-H2AX, suggesting that their mode of action was similar. These findings indicate the effectiveness of the 3D-UBMM in evaluating arsenic toxicity. Importantly, one of the inconsistencies in the in vivo data is that while iAs
V and iAs
III are known human urinary bladder carcinogens and also induce urinary bladder cancer in rats, there are no studies demonstrating that iAs
V or iAs
III induce urinary bladder cancer in mice. Future studies may provide information helping to resolve this inconsistency and increase our understanding of arsenic cytotoxicity. In addition, the 3D-UBMM can be used to investigate the carcinogenicity of DMA
V. DMA
V is metabolized differently by rats than by mice and humans, and while it is a rat urinary bladder carcinogen, it does not induce urinary bladder cancer in mice, and its effect in humans is currently unknown. As this proof-of-concept model was established from a single donor, future work using cells from multiple donors will be needed to improve its generalizability. Finally, future studies should employ the 3D-UBMM to investigate the role of cancer-related genes in bladder carcinogenesis, particularly focusing on aromatic amines and occupational bladder cancer.