Advances in the Analytical Determination and Toxicological Assessment of Dithiocarbamates and Their Hydrolysis Products in Fruits, Vegetables, and Cereals: Methodological Evolution, Challenges, and Future Directions
Abstract
1. Introduction
2. Material and Methods
3. Dithiocarbamates Toxicological Profile
4. Analytical Methods for Dithiocarbamates
4.1. Analytical Methods Involving the Development of CS2
4.2. Specific Analytical Methods for Single Dithiocarbamates Detection
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fanjul-Bolado, P.; Fogel, R.; Limson, J.; Purcarea, C.; Vasilescu, A. Advances in the Detection of Dithiocarbamate Fungicides: Opportunities for Biosensors. Biosensors 2020, 11, 12. [Google Scholar] [CrossRef]
- Chung, S.W.C.; Wong, W.W.K. Chromatographic Analysis of Dithiocarbamate Residues and Their Metabolites in Foods Employed in Dietary Exposure Studies—A Review. Food Addit. Contam. Part A 2022, 39, 1731–1743. [Google Scholar] [CrossRef]
- Tisdale, W.H.; Williams, I. Disinfectant and Fungicide. U.S. Patent 1972961A, 26 May 1931. [Google Scholar]
- Liu, K.; Li, Y.; Iqbal, M.; Tang, Z.; Zhang, H. Thiram Exposure in Environment: A Critical Review on Cytotoxicity. Chemosphere 2022, 295, 133928. [Google Scholar] [CrossRef]
- Jung, J.; Kiehs, K.; Zeeh, B.; Theobald, H. Salts of Phosphonic Acids. U.S. Patent 4135909 A, 29 December 1976. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2018/309 of 1 March 2018, Concerning the Non-Renewal of Approval of the Active Substance Propineb, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/309/oj (accessed on 12 August 2025).
- European Commission. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances/details/1008 (accessed on 12 August 2025).
- European Commission. Commission Implementing Regulation (EU) 2016/2035 of 21 November 2016 Amending Implementing Regulation (EU) No 540/2011 as Regards the Approval Periods of the Active Substances Fipronil and Maneb. Available online: https://eur-lex.europa.eu/eli/reg_impl/2016/2035/oj (accessed on 12 August 2025).
- European Commission. Commission Implementing Regulation (EU) 2020/2087 of 14 December 2020 Concerning the Non-Renewal of the Approval of the Active Substance Mancozeb, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/2087/oj (accessed on 12 August 2025).
- European Commission. Commission Implementing Regulation (EU) 2023/2455 of 7 November 2023 Concerning the Non-Renewal of the Approval of the Active Substance Metiram, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/2455/oj (accessed on 12 August 2025).
- European Commission. Commission Implementing Regulation (EU) 2018/1500 of 9 October 2018 Concerning the Non-Renewal of Approval of the Active Substance Thiram, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Thiram, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/eli/reg_impl/2018/1500/oj (accessed on 12 August 2025).
- Stuke, S.; Wirkner, H.; Safferling, M.; Gorgulu, N.; Zupanc, C.; Ertz, K.; Steinbeck, M.; Haack, K.J.; Jasak, J.; Diot, R. Stabilized Dithiocarbamate Formulations. EP20733398 B1, 15 June 2020. [Google Scholar]
- Bristow, J.T. Fungicidal Composition. Method and Use Thereof. WO2017041717 A, 16 March 2017. [Google Scholar]
- Van der Krieken, W.M.; Mazzitelli, S. Dithiocarbamate Fungicide Macromolecular Complexes. EP20730112 B1, 28 May 2020. [Google Scholar]
- Van der Krieken, W.M.; Stratmann, C. Aqueous Composition of Dithiocarbamate Fungicide. EP4156927 B1, 28 May 2021. [Google Scholar]
- Bellisai, G.; Bernasconi, G.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Del Aguila, M.; Ferreira, L.; Santonja, G.G.; Greco, L.; Jarrah, S.; et al. Review of the Existing Maximum Residue Levels for Dithiocarbamates according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2023, 21, 7987. [Google Scholar]
- Perz, R.C.; van Lishaut, H.; Schwack, W. CS2 Blinds in Brassica Crops: False Positive Results in the Dithiocarbamate Residue Analysis by the Acid Digestion Method. J. Agric. Food Chem. 2000, 48, 792–796. [Google Scholar] [CrossRef]
- Malik, A.K.; Faubel, W. Methods of Analysis of Dithiocarbamate Pesticides: A Review. Pestic. Sci. 1999, 55, 965–970. [Google Scholar] [CrossRef]
- Crnogorac, G.; Schwack, W. Residue Analysis of Dithiocarbamate Fungicides. TrAC Trends Anal. Chem. 2008, 28, 40–50. [Google Scholar] [CrossRef]
- Steidle Neto, J.A.; Lopes, D.C.; Silva, W.A. Feasibility of Vis/NIR Spectroscopy to Detect and Estimate Fungicide Residues on Intact Lettuces. Adv. Hortic. Sci. 2020, 34, 223–232. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Wickert, C.; Pizzutti, I.R.; de Kok, A. Clean-up Strategy for Dithiocarbamate Fungicide Determination in Soybean by GC-ITD-MS and GC-PFPD: Method Development and Validation. J. Agric. Food Chem. 2021, 69, 11485–11493. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Güler, A.; Güngör, N.; Dağaşan, Ö.; Yiğitkaya, S.; Kale, L.Y.; Numanoğlu, E.; Balaban, B.; Özaltın, K.E.; Merken, Ö.; et al. False Positive Effect of Sulfur Sources Used in Growing and Processing of Vine (Vitis vinifera L.) Leaves on the Results of Dithiocarbamate Analysis Based on Carbon Disulfide Measurement. Food Addit. Contam. Part A 2022, 39, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.B.; Kakkasery, J.T.; Arimboor, R.; Jacob, J.; Thankan, B. Development and Validation of a GC-MS Method for Analysis of Dithiocarbamate Fungicide Residues in the Spices Cardamom (Elettaria cardamomom) and Black Pepper (Piper nigrum). J. Food Sci. Technol. 2022, 59, 4097–4107. [Google Scholar] [CrossRef]
- da Silva, R.C.; Duarte, I.; Neu, J.P.; Wouters, R.D.; Zorzella, E.; Dotto, P.; Wagner, R.; Pizzutti, I.R. Commercial Yerba Mate (Ilex paraguariensis) Produced in South America: Determination of Dithiocarbamate Residues by Gas Chromatography-Mass Spectrometry. Food Chem. 2022, 394, 133513. [Google Scholar] [CrossRef]
- Tian, Q.; Li, H.; Chen, L.; Han, B. Microwave-Assisted “One-Pot” Acidolysis and Extraction for the Rapid Determination of Mancozeb in Fruit and Vegetable Samples. J. Food Qual. 2024, 2024, 2577585. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, M.; Li, H.; Huan, Z.; Wang, M.; Lin, J.; Li, B.; Han, B. Hyphenated Liquid Electrode Glow Discharge–Dielectric Barrier Discharge Molecular Emission Spectrometry for Determination of Dithiocarbamates. Food Chem. 2023, 429, 136884. [Google Scholar] [CrossRef]
- Tripathi, K.; Harshangkumar, T.; Narayanan, N.; Gupta, S.; Singh, S.B.; Banerjee, T. Development and Validation of a Sensitive Liquid Chromatography-Tandem Mass Spectrometry Method for the Analysis of Mancozeb Residues in Cauliflower: Risk Assessment of Real Samples. J. Chromatogr. Open 2025, 7, 100226. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L.; Ye, X.; Huang, C.; Yang, W.; Zhang, L.; Wu, Z.; Fu, F. Multicolor Visual Screening of Total Dithiocarbamate Pesticides in Foods Based on Sulfydryl-Mediated Growth of Gold Nanobipyramids. Anal. Chim. Acta 2020, 1139, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, L.; Song, C.; Yuan, H.; Li, X. Quantitative Detection of Dithiocarbamate Pesticides by Surface-Enhanced Raman Spectroscopy Combined with an Exhaustive Peak-Seeking Method. Anal. Methods 2021, 13, 1479–1488. [Google Scholar] [CrossRef]
- Tsen, C.-M.; Yu, C.-W.; Chen, S.-Y.; Lin, C.-L.; Chuang, C.-Y. Application of Surface-Enhanced Raman Scattering in Rapid Detection of Dithiocarbamate Pesticide Residues in Foods. Appl. Surf. Sci. 2021, 558, 149740. [Google Scholar] [CrossRef]
- Anh, N.H.; Doan, M.Q.; Dinh, N.X.; Huy, T.Q.; Tri, D.Q.; Le, A.-T. Gold Nanoparticles-Based SERS Nanosensor for Thiram and Chloramphenicol Monitoring in Food Samples: Insight into Effects of Analyte Molecular Structure on Their Sensing Performance and Signal Enhancement. Appl. Surf. Sci. 2022, 584, 152555. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; Xiang, G.; Chen, Y.; Yang, Z.; Li, Y.; Zhang, Y.; Lu, C.; Wang, X. Construction of Ratiometric Fluorescence Determination of Ethylene Thiourea in Foods Based on the Nanocomposite Combining with Sulfur Quantum Dots and Gold Clusters. Microchem. J. 2023, 189, 108549. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, W.; Li, H.; Liang, X.; Tong, F.; Sheng, X.; Hou, R.; Wan, X.; Liu, Y. Detection of Dithiocarbamate Pesticide Residues in Tea by Colorimetric and Fluorescent Double-Mode Probe Regulated by Tyrosinase. Sens. Actuators B Chem. 2024, 426, 137026. [Google Scholar] [CrossRef]
- Sayed, R.; Hussein, O.E.; Omran, A.A. Method Optimization and Validation for the Determination of Mancozeb in Chamomile by Modified QuEChERS and Liquid Chromatography–Tandem Mass Spectrometry. J. Food Compos. Anal. 2022, 111, 104646. [Google Scholar] [CrossRef]
- Bendhiab, I.; Dirtu, A.C.; Marchond, N.; Guérin, T.; Jitaru, P. A Novel Analytical Approach for the Determination of Ethylene-Thiourea and Propylene-Thiourea in Vegetal Foodstuffs by High-Performance Liquid Chromatography Hyphenated to Inductively Coupled Plasma-Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2023, 416, 431–438. [Google Scholar] [CrossRef]
- Zoratto, C.; Thais, D.; Aguera, R.G.; Sano Lini, R.; Pante, G.C.; Bueno, C.; Castro, J.C.; Aparecida, S.; Marchioni, C.; Machinski, M. Analytical and Toxicological Aspects of Dithiocarbamates: An Overview of the Last 10 Years. Toxicol. Mech. Methods 2022, 32, 637–649. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Use Pattern, Registered Uses of Thiram. Available online: https://www.fao.org/4/W5897E/w5897e5i.htm (accessed on 8 September 2025).
- National Pesticide Information Center (NPIC). EXTOXNET PIP—Thiram. Available online: https://extoxnet.orst.edu/pips/thiram.htm (accessed on 12 August 2025).
- New Jersey Department of Health. Hazardous Substance Fact Sheet, Thiram; New Jersey Department of Health: Trenton, NJ, USA, 2010. [Google Scholar]
- Edwards, I.R.; Ferry, D.G.; Temple, W.A. Fungicides & Related Compounds. In Handbook of Pesticide Toxicology; Volume 3: Classes of Pesticides; Academic Press, Inc.: New York, NY, USA, 1991; Chapter 21; pp. 1409–1470. [Google Scholar] [CrossRef]
- Roberts, J.R.; Reigart, J.R. Recognition and Management of Pesticide Poisonings; United States Environmental Protection Agency, Office of Pesticide Programs: Washington, DC, USA, 2013.
- World Health Organization (WHO). Dithiocarbamate Pesticides, Ethylenethiourea, and Propylenethiourea: A General Introduction/Published Under the Joint Sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization; WHO: Geneva, Switzerland, 1988; ISBN 9789241542784. [Google Scholar]
- National Pesticide Information Center (NPIC). EXTOXNET PIP—METIRAM. Available online: https://extoxnet.orst.edu/pips/metiram.htm (accessed on 12 August 2025).
- National Pesticide Information Center (NPIC). EXTOXNET PIP—ZINEB. Available online: https://extoxnet.orst.edu/pips/zineb.htm (accessed on 12 August 2025).
- International Agency for Research on Cancer (IARC). Ethylene Thiourea, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 79; International Agency for Research on Cancer: Lyon, France, 2001. [Google Scholar]
- National Pesticide Information Center (NPIC). EXTOXNET PIP—ZIRAM. Available online: https://extoxnet.orst.edu/pips/ziram.htm (accessed on 12 August 2025).
- Chou, A.P.; Maidment, N.; Klintenberg, R.; Casida, J.E.; Li, S.; Fitzmaurice, A.G.; Fernagut, P.-O.; Mortazavi, F.; Chesselet, M.-F.; Bronstein, J.M. Ziram Causes Dopaminergic Cell Damage by Inhibiting E1 Ligase of the Proteasome. J. Biol. Chem. 2008, 283, 34696–34703. [Google Scholar] [CrossRef]
- Chen, J.; Akhtar, M.; Hardej, D. Exposure to Dithiocarbamate Fungicide Ziram Results in Hepatic and Renal Toxicity in Long Evan Rats. Environ. Toxicol. Pharmacol. 2023, 99, 104116. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (EPA). Prevention, Pesticides and Toxic Substances, EPA-738-F-05-002, Ferbam; US EPA: Washington, DC, USA, 2005.
- Rasgele, P.G.; Muranli, F.D.; Kekeçoğlu, M. Assessment of the Genotoxicity of Propineb in Mice Bone Marrow Cells Using Micronucleus Assay. Tsitol. Genet. 2014, 48, 39–43. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Peer Review of the Pesticide Risk Assessment of the Active Substance Propineb. EFSA J. 2016, 14, e04605. [Google Scholar] [CrossRef]
- Kumar, K.; Sabarwal, A.; Singh, R.P. Mancozeb Selectively Induces Mitochondrial-Mediated Apoptosis in Human Gastric Carcinoma Cells through ROS Generation. Mitochondrion 2019, 48, 1–10. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Maneb-Developmental Toxicity Study in Rats; US EPA: Washington, DC, USA, 1993.
- Thiruchelvam, M.; Brockel, B.J.; Richfield, E.K.; Baggs, R.B.; Cory-Slechta, D.A. Potentiated and Preferential Effects of Combined Paraquat and Maneb on Nigrostriatal Dopamine Systems: Environmental Risk Factors for Parkinson’s Disease? Brain Res. 2000, 873, 225–234. [Google Scholar] [CrossRef]
- Vergova, M.; Jablonica, A.; Janota, S. Occupational Exposure to Mancozeb in Employees in the Production of Novozir Mn 80. Park. Lak 1988, 40, 425–430. [Google Scholar]
- International Agency for Research on Cancer (IARC). Overall Evaluations of Carcenogenicity. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7; International Agency for Research on Cancer, Ethylene thiourea: Lyon, France, 1987; pp. 207–208. [Google Scholar]
- Brent, G.A.; Koenig, R.J. Thyroid and Antithyroid Drugs. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (US). U.S. Department of Health and Human Services Toxicological Profile for Carbon Disulfide; ATSDR: Atlanta, GA, USA, 2000. [PubMed]
- European Commission. Commission Regulation (EU) 2017/171 of 30 January 2017 Amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Aminopyralid, Azoxystrobin, Cyantraniliprole, Cyflufenamid, Cyproconazole, Diethofencarb, Dithiocarbamates, Fluazifop-P, Fluopyram, Haloxyfop, Isofetamid, Metalaxyl, Prohexadione, Propaquizafop, Pyrimethanil, Trichoderma Atroviride Strain SC1 and Zoxamide in or on Certain Products. Available online: https://eur-lex.europa.eu/eli/reg/2017/171/oj/eng (accessed on 12 August 2025).
- Campanale, C.; Triozzi, M.; Ragonese, A.; Losacco, D.; Massarelli, C. Dithiocarbamates: Properties, Methodological Approaches and Challenges to Their Control. Toxics 2023, 11, 851. [Google Scholar] [CrossRef]
- EU Reference Laboratories for Residues of Pesticides (EURL). Analysis of Residues of Dithiocarbamate Fungicides in Low-Oil Content Food of Plant Origin Involving Cleavage into Carbon Disulfide, Partitioning into Isooctane and Measurement by GC-MS/MS or GC-ECD, Version 3.2, Updated December; EU Reference Laboratories for Residues of Pesticides (EURL): Freiburg, Germany, 2024. [Google Scholar]
- Clarke, D.; Baum, H.; Stanley, E.; Hester, W. Determination of Dithiocarbamates. Anal. Chem. 1951, 23, 1842–1846. [Google Scholar] [CrossRef]
- Crnogorac, G.; Schmauder, S.; Schwack, W. Trace Analysis of Dithiocarbamate Fungicide Residues on Fruits and Vegetables by Hydrophilic Interaction Liquid Chromatography/Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- U. S. Department of Health and Human Services, Public Health Service NTP (National Toxicology Program). Report on Carcinogens, 15th ed.; NTP: Research Triangle Park, NC, USA, 2021. [Google Scholar]
- Veiga-del-Baño, J.M.; Martínez-López, S.; Pérez-Lucas, G.; Cuenca-Martínez, J.J.; Andreo-Martínez, P. Trends in Dithiocarbamates Food Research: A Bibliometric Vision. Chemosphere 2023, 313, 137342. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.C.O.L.; Melchert, W.R. Trends in Determining Carbamates and Dithiocarbamates in Food Samples. J. Food Compos. Anal. 2025, 141, 107330. [Google Scholar] [CrossRef]
- Cullen, T.E. Spectrophotometric Determination of Dithiocarbamate Residues in Food Crops. Anal. Chem. 1964, 36, 221–224. [Google Scholar] [CrossRef]
- Keppel, G.E. Modification of the Carbon Disulfide Evolution Method for Dithiocarbamate Residues. J. AOAC Int. 1969, 52, 162–167. [Google Scholar] [CrossRef]
- Caldas, E.D.; Conceição, M.H.; Miranda, M.C.C.; de Souza, L.C.K.R.; Lima, J.F. Determination of Dithiocarbamate Fungicide Residues in Food by a Spectrophotometric Method Using a Vertical Disulfide Reaction System. J. Agric. Food Chem. 2001, 49, 4521–4525. [Google Scholar] [CrossRef]
- Heise, S.; Weber, H.; Alder, L. Reasons for the Decomposition of the Fungicide Thiram during Preparation of Fruit and Vegetable Samples and Consequences for Residue Analysis. Fresenius’ J. Anal. Chem. 2000, 366, 851–856. [Google Scholar] [CrossRef]
- Dogheim, S.M.; Gad Alla, S.A.; El-Marsafy, A.M. Monitoring of Pesticide Residues in Egyptian Fruits and Vegetables during 1996. J. AOAC Int. 2001, 84, 519–531. [Google Scholar] [CrossRef]
- Abbassy, M.S. Pesticide Residues in Selected Vegetables and Fruits in Alexandria City, Egypt, 1997–1998. Bull. Environ. Contam. Toxicol. 2001, 67, 0225–0232. [Google Scholar] [CrossRef]
- Andersen, J.H.; Poulsen, M.E. Results from the Monitoring of Pesticide Residues in Fruit and Vegetables on the Danish Market, 1998–1999. Food Addit. Contam. 2001, 18, 906–931. [Google Scholar] [CrossRef]
- Poulsen, M.E.; Andersen, J.H. Results from the Monitoring of Pesticide Residues in Fruit and Vegetables on the Danish Market, 2000–2001. Food Addit. Contam. 2003, 20, 742–757. [Google Scholar] [CrossRef]
- Elgueta, S.; Moyano, S.; Sepúlveda, P.; Quiroz, C.; Correa, A. Pesticide Residues in Leafy Vegetables and Human Health Risk Assessment in North Central Agricultural Areas of Chile. Food Addit. Contam. Part B Surveill. Commun. 2017, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D.; Tressou, J.; Boon, P.E. Dietary Exposure of Brazilian Consumers to Dithiocarbamate Pesticides—A Probabilistic Approach. Food Chem. Toxicol. 2006, 44, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Pastor Ciscato, C.H.; Bertoni Gebara, A.; Henrique Monteiro, S. Pesticide Residue Monitoring of Brazilian Fruit for Export 2006–2007. Food Addit. Contam. Part B-Surveill. 2009, 2, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Z.; DeBusk, S.M. GC–MS Analysis of Hydrogen Sulfide, Carbonyl Sulfide, Methanethiol, Carbon Disulfide, Methyl Thiocyanate and Methyl Disulfide in Mainstream Vapor Phase Cigarette Smoke. Chromatographia 2009, 71, 259–265. [Google Scholar] [CrossRef]
- Shao, H.; Liu, L.; Li, H.; Li, J.; Zhang, Y.-T.; Guo, Y.-Z. Determination of Amobam Residues in Vegetables and Cereals by Gas Chromatography-Mass Spectrometry with Solvent Absorption. J. Food Saf. Qual. 2017, 8, 2966–2972. [Google Scholar]
- Nguyen, N.V.; Le, T.T. Development of Analytical Method for Determination of Dithiocarbamate Resiudes, Expressed as CS2 in Fruits and Vegetables by GC-ECD. J. Sci. Technol.-IUH 2021, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Wade, K.L.; Prestera, T.; Talalay, P. Quantitative Determination of Isothiocyanates, Dithiocarbamates, Carbon Disulfide, and Related Thiocarbonyl Compounds by Cyclocondensation with 1,2-Benzenedithiol. Anal. Biochem. 1996, 239, 160–167. [Google Scholar] [CrossRef]
- Gonzálvez, A.; Garrigues, S.; Armenta, S.; De la Guardia, M. Miguel Determination at Low Ppm Levels of Dithiocarbamate Residues in Foodstuff by Vapour Phase-Liquid Phase Microextraction-Infrared Spectroscopy. Anal. Chim. Acta 2011, 688, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, Y.; Qian, B.; He, Y.; Peng, L.; Yu, H. A Novel Liquid Chromatography Detector Based on a Dielectric Barrier Discharge Molecular Emission Spectrometer with Online Microwave-Assisted Hydrolysis for Determination of Dithiocarbamates. Analyst 2018, 143, 2790–2798. [Google Scholar] [CrossRef]
- Ripley, B.D.; Lissermore, L.I.; Leishman, P.D.; Denommé, M.A. Pesticide Residues on Fruits and Vegetables from Ontario, Canada, 1991–1995. J. AOAC Int. 2000, 83, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Dogheim, S.M.; El-Marsafy, A.M.; Salama, E.Y.; Gadalla, S.A.; Nabil, Y.M. Monitoring of Pesticide Residues in Egyptian Fruits and Vegetables during 1997. Food Addit. Contam. 2002, 19, 1015–1027. [Google Scholar] [CrossRef]
- Morzycka, B.; Nowacka, A. Monitoring of Dithiocarbamate Fungicides in Polish Crops during 1999–2000. J. Plant Prot. Res. 2001, 42, 57–63. [Google Scholar]
- Vryzas, Z.; Papadakis, E.N.; Papadopoulou-Mourkidou, E. Microwave-Assisted Extraction (MAE)−Acid Hydrolysis of Dithiocarbamates for Trace Analysis in Tobacco and Peaches. J. Agric. Food Chem. 2002, 50, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Gad Alla, S.A. Monitoring of Dithiocarbamate (EBDC) Residues in Fruits and Vegetables throughout 1995–1999 and Estimation of Their Daily Intakes in Egypt. Egypt. J. Agric. Res. 2002, 80, 583–596. [Google Scholar]
- Nowacka, A. Polish Monitoring of Pesticide Residues in Crops. In Proceedings of the 6th Slovenian Conference on Plant Protection, Zreče, Slovenia, 4–6 March 2003; pp. 34–41. [Google Scholar]
- Caldas, E.D.; Miranda, M.C.C.; Conceição, M.H.; de Souza, L.C.K.R. Dithiocarbamates Residues in Brazilian Food and the Potential Risk for Consumers. Food Chem. Toxicol. 2004, 42, 1877–1883. [Google Scholar] [CrossRef]
- Guidotti, M.; Stella, D.; Blasi, G. Determination of Dithiocarbamate Residues as CS2 in Products of Vegetable Origin. In Proceedings of the 27th International Symposium on Capillary Chromatography, Riva del Garda, Italy, 31 May–4 June 2004. [Google Scholar]
- Kovacevic, D.; Jovanovic, A.; Pucarevic, M.; Rajkovic, M.B. Determination of Dithiocarbamates in Raspberries (Rubus idaeus L.) by Gas Chromatography via Head Space. Jugosl. Voćarstvo 2004, 38, 127–133. [Google Scholar]
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the Dithiocarbamate Pesticide Maneb in Tomato Homogenates during Cold Storage and Thermal Processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef]
- Chang, J.-M.; Chen, T.-H.; Fang, T.-J. Pesticide Residue Monitoring in Marketed Fresh Vegetables and Fruits in Central Taiwan (1999–2004) and an Introduction to the HACCP System. J. Food Drug Anal. 2005, 13, 5. [Google Scholar] [CrossRef]
- Česnik, H.B.; Gregorčič, A.; Bolta, Š.V.; Kmecl, V. Monitoring of Pesticide Residues in Apples, Lettuce and Potato of the Slovene Origin, 2001–2004. Food Addit. Contam. 2006, 23, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Česnik, H.B.; Gregorčič, A. Validation of the Method for the Determination of Dithiocarbamates and Thiuram Disulphide on Apple, Lettuce, Potato, Strawberry and Tomato Matrix. Acta Chim. Slov. 2006, 53, 100–104. [Google Scholar]
- Papadopoulou-Mourkidou, E.; Papadakis, E.N.; Vryzas, Z. Application of Microwave-Assisted Extraction for the Analysis of Dithiocarbamates in Food Matrices. Pestic. Protoc. 2006, 19, 319–329. [Google Scholar]
- Česnik, H.B.; Gregorčič, A.; Velikonja Bolta, S. Plant Protection Product Residues in Apples, Cauliflower, Cereals, Grapes, Lettuce, Peas, Peppers, Potatoes and Strawberries of the Slovene Origin. J. Cent. Eur. Agric. 2007, 10, 311–320. [Google Scholar]
- Bazzi, L.; Zougagh, M.; Salghi, R.; Hormatallah, A.; Lemerhyeratte, A.; Mihit, M.; Chakir, A. Pesticide Residue Monitoring in Green Beans from Souss-Massa (Morocco) and Half-Life Times of Dithiocarbamate Fungicide on Green Beans after Field Treatments by Mancozeb and Mefenoxam. Orient. J. Chem. 2009, 25, 461–470. [Google Scholar]
- Lazić, S.D.; Bursić, V.P.; Vuković, S.M.; Šunjka, D.B.; Pucarević, M.M. Pucarević Pesticide Residues in Vegetable Samples from the Market of the Republic of Serbia during 2007. Acta Hortic. 2009, 830, 569–576. [Google Scholar] [CrossRef]
- Berrada, H.; Fernández, M.; Ruiz, M.J.; Moltó, J.C.; Mañes, J.; Font, G. Surveillance of Pesticide Residues in Fruits from Valencia during Twenty Months (2004/05). Food Control 2010, 21, 36–44. [Google Scholar] [CrossRef]
- Khorshed, M.; Gomaa, A.; Attallah, E.; Amer, M. Ethylene-Bis- Dithiocarbamates Residues in Some Food and the Potential Risk for Egyptian Consumers. J. Plant Prot. Pathol. 2010, 1, 733–741. [Google Scholar] [CrossRef]
- El-Gohary, A.A.; Hassan, A. Method Validation of Dithiocarbamates Residues on Some Egyptian Commodieties Using GC-MS Instrument. J. Plant Prot. Pathol. 2011, 2, 59–65. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczynski, P. Pesticide Residues in Apples (2005–2010). Arch. Environ. Prot. 2011, 37, 43–54. [Google Scholar]
- Bempelou, E.; Liapis, K.S.; Miliadis, G.E. Validation of Single Residue Methods for the Determination of Dithiocarbamates and Inorganic Bromide Residues in Plant Products. Hell. Plant Prot. J. 2012, 5, 57–64. [Google Scholar]
- El-Sawi, S.; Khorshed, M.; Nabil, Y.; Mahmoud, A. Monitoring and Risk Exposure Studies of Some Pesticide Residues Detected in Egyptian Fruit and Vegetables. J. Plant Prot. Pathol. 2012, 3, 253–271. [Google Scholar] [CrossRef]
- Pucarević, M.; Bursić, V.; Lazić, S.; Radović, V.; Durović, R.; Grahovac, M. Trends of Dithiocarbamate Residues in Raspberries in the Republic of Serbia over the Period 2007/2010. Acta Hortic. 2012, 946, 327–332. [Google Scholar] [CrossRef]
- Kostik, V. Determination of Pesticide Residues in Plant-Based Foods from the Republic of Macedonia. J. Food Nutr. Sci. 2014, 2, 124. [Google Scholar] [CrossRef]
- Mujawar, S.; Utture, S.C.; Fonseca, E.; Matarrita, J.; Banerjee, K. Validation of a GC–MS Method for the Estimation of Dithiocarbamate Fungicide Residues and Safety Evaluation of Mancozeb in Fruits and Vegetables. Food Chem. 2014, 150, 175–181. [Google Scholar] [CrossRef]
- Szpyrka, E.; Kurdziel, A.; Rupar, J.; Słowik-Boroweic, M. Pesticide Residues in Fruit and Vegetable Crops from the Central and Eastern Region of Poland. Rocz. Państwowego Zakładu Hig. 2015, 66, 107–113. [Google Scholar]
- Łozowicka, B.; Hrynko, I.; Kaczyński, P.; Rutkowska, E.; Jankowska, M.; Mojsak, P. Occurrence of Pesticide Residues in Fruit from Podlasie (Poland) in 2012. J. Plant Prot. Res. 2015, 55, 142–150. [Google Scholar] [CrossRef]
- Szpyrka, E.; Slowik-Borowiec, M.; Matyaszek, A.; Podbielska, M.; Rupar, J. Pesticide Residues in Raw Agricultural Products from the South Eastern Region of Poland and the Acute Risk Assessment. Rocz. Państwowego Zakładu Hig. 2016, 67, 237–245. [Google Scholar]
- Addi, E.H.A. Dissipation Behavior of a Mancozeb Residue (Dithiocarbamate Fungicide) in Tomato under South Moroccan Climatic Condition. IRA-Int. J. Appl. Sci. 2017, 7, 62. [Google Scholar] [CrossRef]
- Atuhaire, A.; Kaye, E.; Mutambuze, I.L.; Matthews, G.; Friedrich, T.; Jørs, E. Assessment of Dithiocarbamate Residues on Tomatoes Conventionally Grown in Uganda and the Effect of Simple Washing to Reduce Exposure Risk to Consumers. Environ. Health Insights 2017, 11, 117863021771221. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.; de Kok, A.; da Silva, R.; Rohers, G. Comparison between Three Chromatographic (GC-ECD, GC-PFPD and GC-ITD-MS) Methods and a UV-Vis Spectrophotometric Method for the Determination of Dithiocarbamates in Lettuce. J. Braz. Chem. Soc. 2017, 28, 775–781. [Google Scholar] [CrossRef]
- Song, S.; Wei, J.; Chen, Z.; Lei, Y.; Zhang, Y.; Deng, C.; Tan, H.; Li, X. Determination of Propineb and Its Metabolites Propylenethiourea and Propylenediamine in Banana and Soil Using Gas Chromatography with Flame Photometric Detection and LC–MS/MS Analysis. J. Environ. Sci. Health Part B 2017, 53, 153–160. [Google Scholar] [CrossRef]
- Arslan, S.; Mert, I.D.; Yiğitkaya, S.; Dagaşan, O.; Sakallı, F.N.; Oztürk, S. The False Positive Effect of Residue of Sulphur Sources on Dithiocarbamate Analysis Based on CS2 Measurement. Food Addit. Contam. Part A 2019, 36, 131–140. [Google Scholar] [CrossRef]
- Mozzaquatro, J.; Mello, D.; Oliveira, R.; Rosa, R.; Costa, A.; Caldas, E. Dithiocarbamate Residues in Fruits and Leaves of Passion Fruit (Passiflora edulis) from Different Brazilian Regions. J. Braz. Chem. Soc. 2019, 30, 1834–1840. [Google Scholar] [CrossRef]
- Patil, C.; Deore, B.; Saindane, Y. Residues Dnd Dissipation of Mancozeb 75% WP In/on Onion. Int. J. Chem. Stud. 2020, 8, 2433–2437. [Google Scholar] [CrossRef]
- Rahman, A.A. Pesticides Residue Analysis of Fruits for Farm Accreditation Schemes in Sabah, Malysia. Int. Symp. Trop. Fruits 2019, 123, 28–31. [Google Scholar]
- de Araujo, F.J.M.; Mello, D.C.; Junqueira, A.M.R.; Caldas, E.D. Análise de Resíduos de Fungicidas Ditiocarbamatos Em Hortaliças Produzidas Na Região de Vargem Bonita, Distrito Federal. Hortic. Bras. 2022, 40, 226–230. [Google Scholar] [CrossRef]
- Kontou, S.; Tsipi, D.; Oreopoulou, V.; Tzia, C. Determination of ETU in Tomatoes and Tomato Products by HPLC-PDA. Evaluation of Cleanup Procedures. J. Agric. Food Chem. 2001, 49, 1090–1097. [Google Scholar] [CrossRef]
- Bonnechère, A.; Hanot, V.; Van Loco, J. A Rapid and Environmental Friendly Determination of the Dithiocarbamate Metabolites Ethylenethiourea and Propylenethiourea in Fruit and Vegetables by Ultra High Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 4627–4631. [Google Scholar] [CrossRef]
- Peruga, A.; Grimalt, S.; López, F.J.; Sancho, J.V.; Hernández, F. Optimisation and Validation of a Specific Analytical Method for the Determination of Thiram Residues in Fruits and Vegetables by LC–MS/MS. Food Chem. 2012, 135, 186–192. [Google Scholar] [CrossRef]
- Bansal, O.P. Health Impacts of the Carbamate and Dithiocarbamate Pesticides: A Review. Int. J. Sci. Res. Publ. (IJSRP) 2022, 12, 366. [Google Scholar] [CrossRef]
- Saad, B.; Chandran, M.; Saleh, M. Flow-Injection Spectrophotometric Method for the Determination of Ziram (Dithiocarbamate Fungicide). Malays. J. Anal. Sci. 2001, 7, 103–107. [Google Scholar]
- Nakazawa, H.; Tsuda, Y.; Ito, K.; Yoshimura, Y.; Kubo, H.; Homma, H. Determination of Dithiocarbamate Fungicides by Reversed-Phase Ion-Pair Liquid Chromatography with Chemiluminescence Detection. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 705–713. [Google Scholar] [CrossRef]
- Turker, A.R.; Sezer, B. Indirect Determination of Maneb (Manganese Ethylenebisdithiocarbamate) in Some Foods by Flame Atomic Absorption Spectrometry. Gazi Univ. J. Sci. 2004, 18, 93–101. [Google Scholar]
- Cajka, T.; Riddellova, K.; Zomer, P.; Mol, H.; Hajslova, J. Direct Analysis of Dithiocarbamate Fungicides in Fruit by Ambient Mass Spectrometry. Food Addit. Contam. Part A 2011, 28, 1372–1382. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Batista, A.D.; Melchert, W.R. Current Overview and Perspectives in Environmentally Friendly Microextractions of Carbamates and Dithiocarbamates. Compr. Rev. Food Sci. Food Saf. 2021, 20, 6116–6145. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Melchert, W.R. Solid-Liquid Phase Microextraction Coupled to Digital Images for Determination of Dithiocarbamates in Food Samples. Microchem. J. 2025, 213, 113614. [Google Scholar] [CrossRef]
- Blasco, C.; Font, G.; Picó, Y. Determination of Dithiocarbamates and Metabolites in Plants by Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2004, 1028, 267–276. [Google Scholar] [CrossRef]
- Crnogorac, G.; Schwack, W. Determination of Dithiocarbamate Fungicide Residues by Liquid Chromatography/Mass Spectrometry and Stable Isotope Dilution Assay. Rapid Commun. Mass Spectrom. 2007, 21, 4009–4016. [Google Scholar] [CrossRef]
- Hayama, T.; Takada, M. Simple and Rapid Method for the Determination of Ethylenebisdithiocarbamate Fungicides in Fruits and Vegetables Using Liquid Chromatography with Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2008, 392, 969–976. [Google Scholar] [CrossRef]
- Qiu, P.; Ni, Y.N. Determination of Ziram in Vegetable Samples by Square Wave Voltammetry. Chin. Chem. Lett. 2008, 19, 1337–1340. [Google Scholar] [CrossRef]
- Nakamura, M.; Noda, S.; Kosugi, M.; Ishiduka, N.; Mizukoshi, K.; Taniguchi, M.; Nemoto, S. Determination of Dithiocarbamates and Milneb Residues in Foods by Gas Chromatography-Mass Spectrometry. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Jafari, A.; Shoeibi, S.; Amini, M.; Amirahmadi, M.; Rastegar, H.; Ghaffarian, A.; Ghazi-Khansari, M. Monitoring Dithiocarbamate Fungicide Residues in Greenhouse and Non-Greenhouse Tomatoes in Iran by HPLC-UV. Food Addit. Contam. Part B 2012, 5, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C.; Lam, C.H. Development and Validation of a Method for Determination of Residues of 15 Pyrethroids and Two Metabolites of Dithiocarbamates in Foods by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2012, 403, 885–896. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Rial-Otero, R.; González-Barreiro, C.; Simal-Gándara, J. Surveillance of Fungicidal Dithiocarbamate Residues in Fruits and Vegetables. Food Chem. 2012, 134, 366–374. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. Solid-State UV–MALDI–MS Assay of Transition Metal Dithiocarbamate Fungicides. Environ. Sci. Pollut. Res. 2013, 21, 1163–1177. [Google Scholar] [CrossRef]
- Ringli, D.; Schwack, W. Selective Determination of Thiram Residues in Fruit and Vegetables by Hydrophilic Interaction LC-MS. Food Addit. Contam. Part A 2013, 30, 1909–1917. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S. Micro-Determination of Dithiocarbamates in Pesticide Formulations Using Voltammetry. Microchem. J. 2013, 110, 334–339. [Google Scholar] [CrossRef]
- Rastegarzadeh, S.; Pourreza, N.; Larki, A. Dispersive Liquid–Liquid Microextraction of Thiram Followed by Microvolume UV–Vis Spectrophotometric Determination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 46–50. [Google Scholar] [CrossRef]
- Schmidt, B.; Christensen, H.B.; Petersen, A.; Sloth, J.J.; Poulsen, M.E. Method Validation and Analysis of Nine Dithiocarbamates in Fruits and Vegetables by LC-MS/MS. Food Addit. Contam. Part A 2013, 30, 1287–1298. [Google Scholar] [CrossRef]
- Rohit, J.V.; Solanki, J.N.; Kailasa, S.K. Surface Modification of Silver Nanoparticles with Dopamine Dithiocarbamate for Selective Colorimetric Sensing of Mancozeb in Environmental Samples. Sens. Actuators B Chem. 2014, 200, 219–226. [Google Scholar] [CrossRef]
- Rossi Lemes, V.R.; Martins-Júnior, H.A.; Carvalho de Souza, S.V.; Colacioppo, S. Ethylenethiourea in Fruits: Optimization and In-House Validation of a Method by Liquid Chromatography Tandem Mass Spectrometry, Occurrence and Dietary Exposure Assessment. Food Control 2014, 42, 321–328. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Chailapakul, O.; Siangproh, W. Development of Gold Nanoparticles Modified Screen-Printed Carbon Electrode for the Analysis of Thiram, Disulfiram and Their Derivative in Food Using Ultra-High Performance Liquid Chromatography. Talanta 2015, 132, 416–423. [Google Scholar] [CrossRef]
- Al-Alam, J.; Bom, L.; Chbani, A.; Fajloun, Z.; Millet, M. Analysis of Dithiocarbamate Fungicides in Vegetable Matrices Using HPLC-UV Followed by Atomic Absorption Spectrometry. J. Chromatogr. Sci. 2017, 55, 429–435. [Google Scholar] [CrossRef]
- Kakitani, A.; Yoshioka, T.; Nagatomi, Y.; Harayama, K. A Rapid and Sensitive Analysis of Dithiocarbamate Fungicides Using Modified QuEChERS Method and Liquid Chromatography–Tandem Mass Spectrometry. J. Pestic. Sci. 2017, 42, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, W.; Liu, Q.; Hao, N.; Zhu, Y.; Liu, M.; Wang, L.; Yang, H.; Chen, X. Simultaneous in Situ Extraction and Fabrication of Surface-Enhanced Raman Scattering Substrate for Reliable Detection of Thiram Residue. Anal. Chem. 2018, 90, 13647–13654. [Google Scholar] [CrossRef] [PubMed]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M. Silver Nanoparticles with Sodium Dodecyl Sulfate as a Colorimetric Probe for the Detection of Dithiocarbamate Pesticides in Environmental Samples. Anal. Sci. 2019, 35, 631–637. [Google Scholar] [CrossRef]
- Ghoto, S.A.; Khuhawar, M.Y.; Jahangir, T.M.; Mangi, J.U.D. Applications of Copper Nanoparticles for Colorimetric Detection of Dithiocarbamate Pesticides. J. Nanostruct. Chem. 2019, 9, 77–93. [Google Scholar] [CrossRef]
- Bodur, S.; Erarpat, S.; Günkara, Ö.T.; Chormey, D.S.; Bakırdere, S. A New Derivatization Method for the Determination of Propineb in Black Tea and Infant Formula Samples Using Dispersive Liquid-Liquid Microextraction Followed by Gas Chromatography-Mass Spectrometry. Talanta 2020, 213, 120846. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.G.; Mamat, M.H.; Vasimalai, N. Spectrophotometric Detection of Thiram Pesticide in Vegetables, Fruits, Soil and Water Samples Using Silane-Modified Silver Nanoparticles. J. Food Compos. Anal. 2023, 120, 105327. [Google Scholar] [CrossRef]
- Wei, L.; Gan, W.; Cai, M.; Cai, H.; Zhang, G.; Cheng, X. Development of a Novel HPLC-CDCL Method Utilizing Nitrogen-Doped Carbon Dots for Sensitive and Selective Detection of Dithiocarbamate Pesticides in Tea. Food Chem. 2024, 458, 140237. [Google Scholar] [CrossRef] [PubMed]


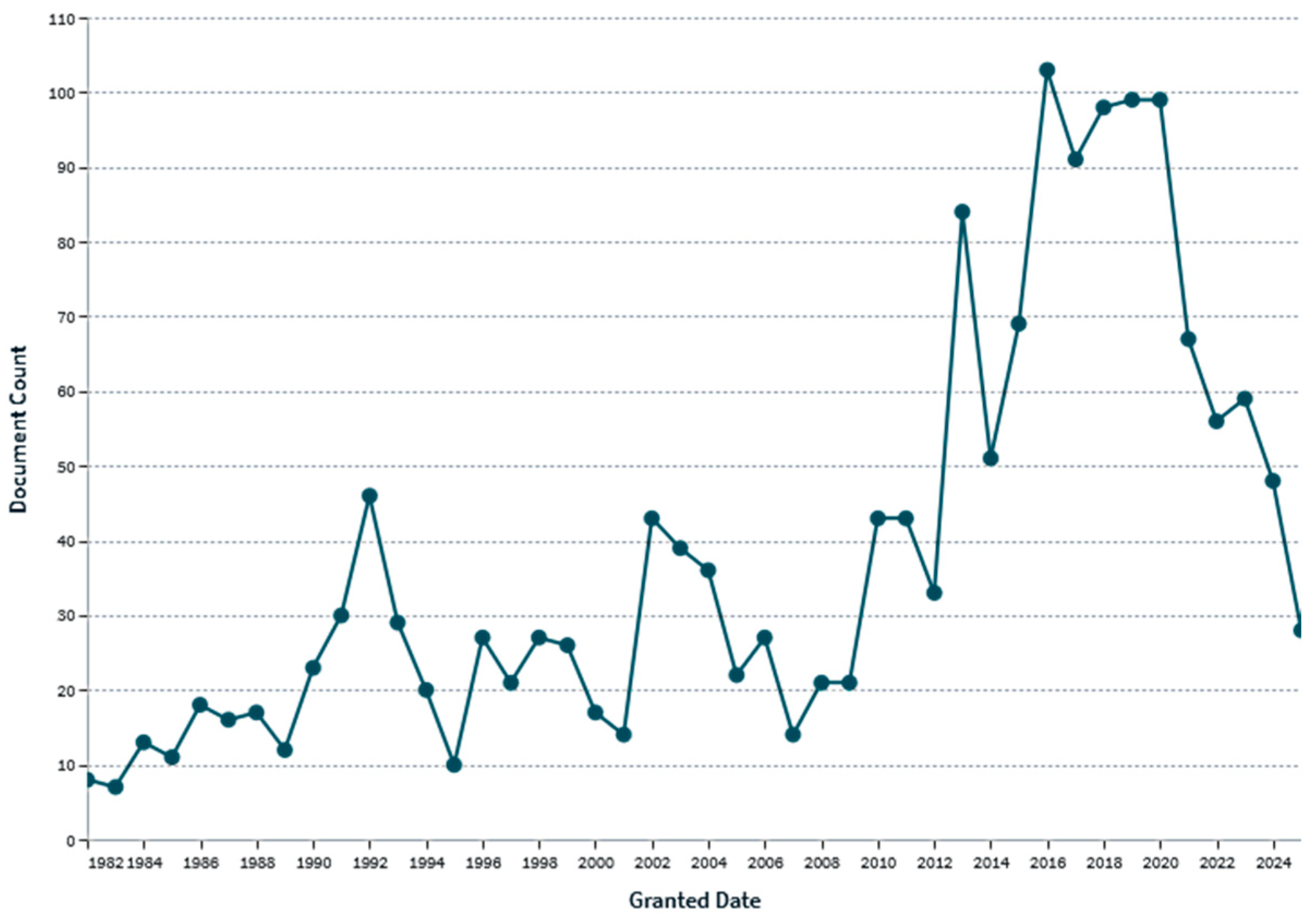
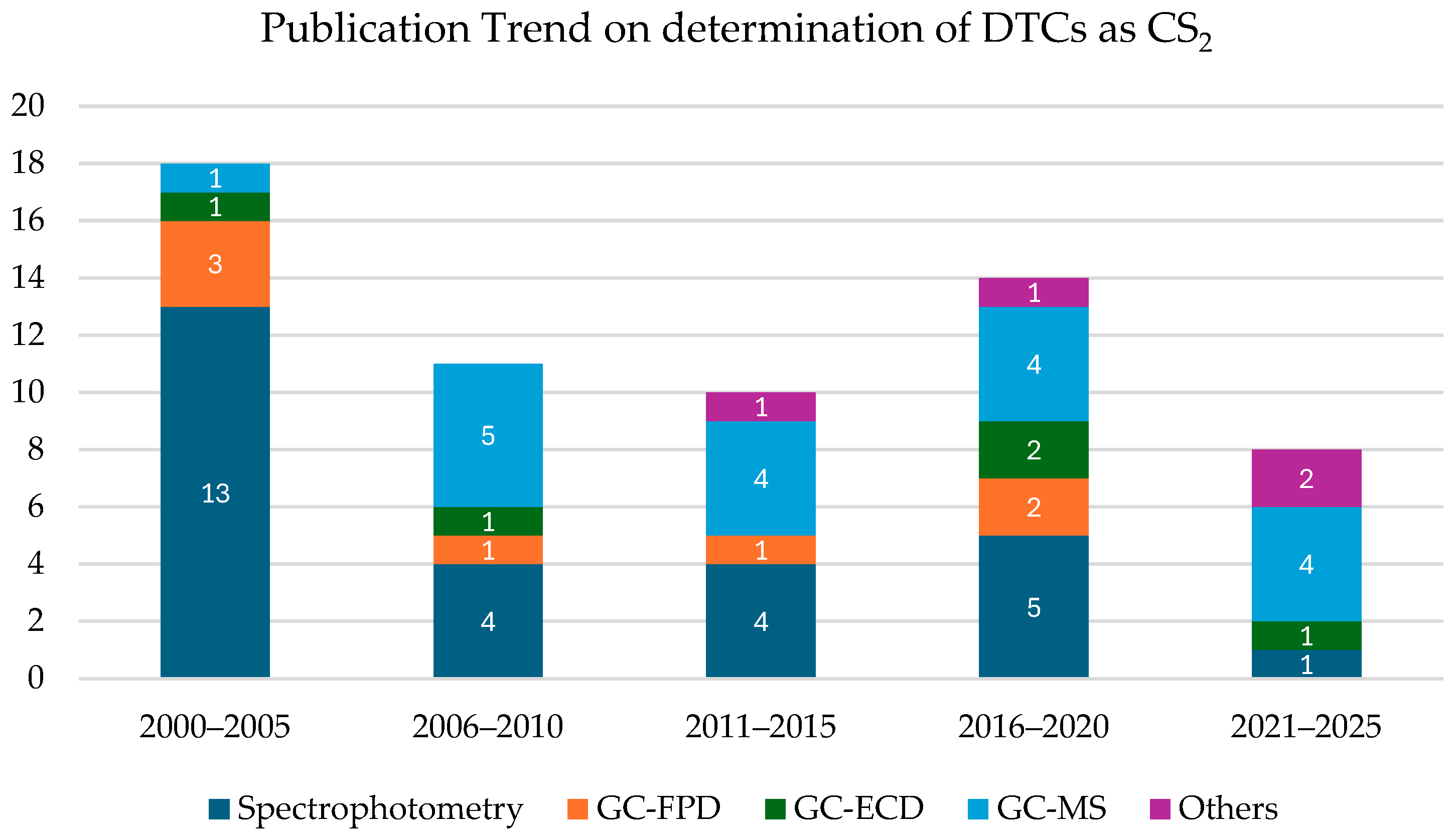
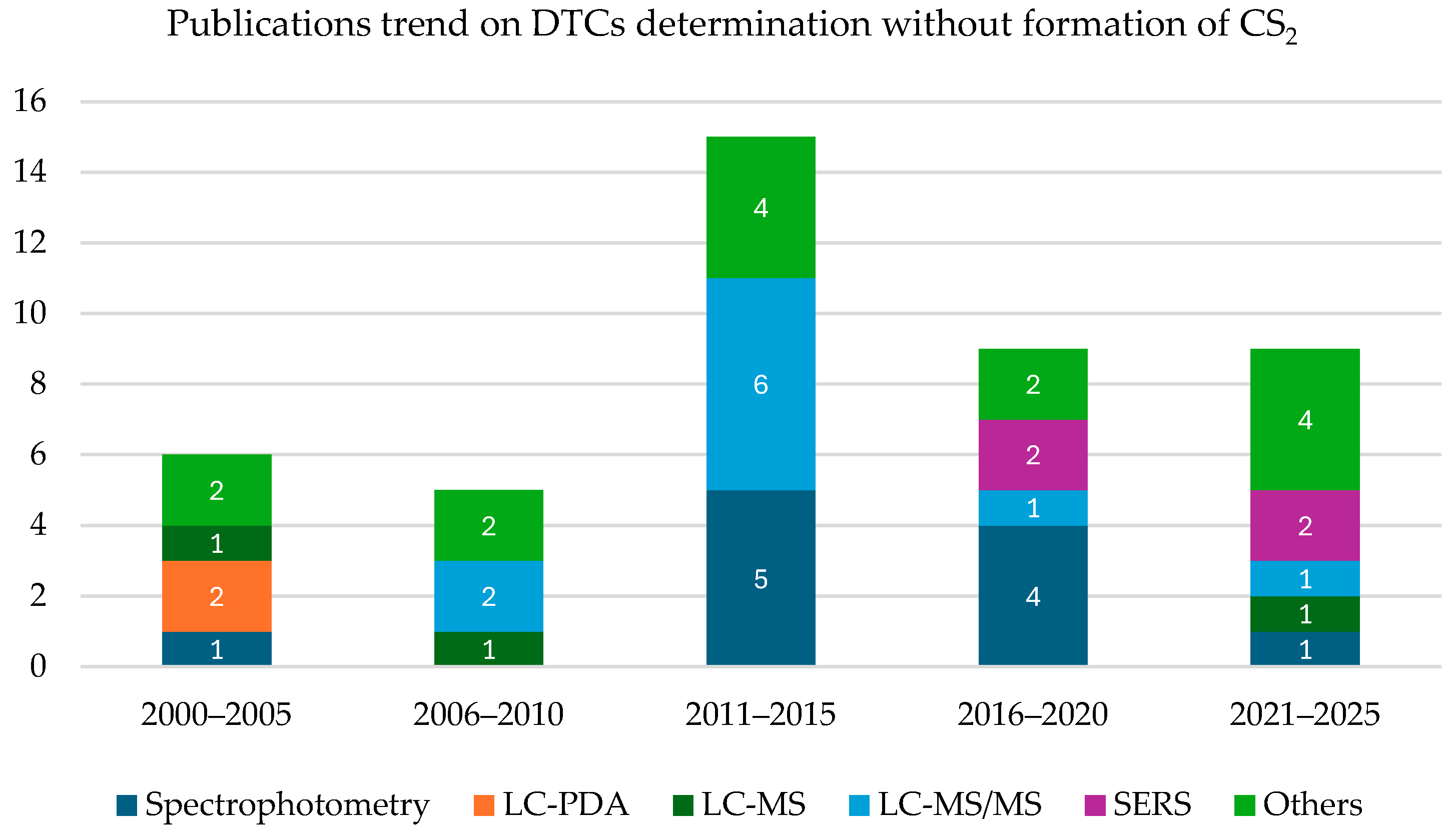
| Substance | Main Endpoints | Human Effects | Main Metabolites | Oral LD50 mg/kg (Rat) | References |
|---|---|---|---|---|---|
| Ferbam | Hepatotoxicity, Nephrotoxicity, Skin/Eye Irritation | Nausea, Headache, Respiratory Irritation, Chronic Liver and Thyroid Alterations | CS2, ETU | ~3300 | [42,45,49] |
| Mancozeb | Thyroid Alterations, Reproductive/Developmental Toxicity, Neurotoxicity | Acute Irritation, Subclinical Hypothyroidism, Neurobehavioral Changes | CS2, ETU | 8000–10,000 | [42,45,52] |
| Maneb | Thyroid Alterations, Neurotoxicity (Experimental Parkinsonism) | Tremors, Cognitive and Thyroid Disturbances in Chronic Exposure | CS2, ETU | 5000–8000 | [42,45,53,54] |
| Metiram | Thyroid Toxicity, Reproductive Toxicity (Through ETU Formation) | Endocrine and Developmental Effects | CS2, ETU | >6000 | [42,43,45] |
| Propineb | Skeletal Abnormalities, Thyrostatic Effect, Hepatotoxicity (Through PTU Formation) | No Direct Human Clinical Studies | CS2, PTU | >5000 | [42,50,51] |
| Thiram | Potential Neurotoxicity, Hepatoxicity, Dermal Irritation, Nephrotoxicity | Drowsiness, Confusion, Loss of Sex Drive, Incoordination, Slurred Speech, Weakness, Dermatitis, Conjunctivitis, Nausea, Cardiovascular Disturbances | CS2 | 210–2000 | [38,39,41,42] |
| Zineb | Thyroid Toxicity, Reproductive Toxicity (Through ETU Formation) | Tiredness, Dizziness, Weakness, Headache, Nausea, Fatigue | CS2, ETU | 1850–8900 | [42,44,45] |
| Ziram | Potential Neurotoxicity, Dopaminergic Neuron Toxicity | Reproductive Effects | CS2 | 400–480 | [46,47,48] |
| ETU (Ethylene thiourea) | Thyroid toxicity, Goitrogenic, Experimental Carcinogenicity, Reproductive Toxicity | Hypothyroidism, Thyroid Hormone Disruption | — | 1800–2000 | [42,45] |
| PTU (Propylene thiourea/Propylthiouracil) | Thyrostatic Effect, Hepatotoxicity, Potential Mutagenicity | Hypothyroidism, Goiter, Hepatotoxicity | — | 1300–1500 | [42,57] |
| CS2 (Carbon disulfide) | Neurotoxicity (CNS and PNS), Cardiovascular Effects, Reproductive Toxicity | Peripheral Neuropathy, Cognitive/Mood Disorders, Accelerated Atherosclerosis, Acute Headache/Dizziness | Thiocarbonates, Protein-bound Compounds | 1200–3000 | [42,58] |
| Method | Standard Used | Matrix | Chromatographic Method | Detection Method | Year | Author [Ref] |
|---|---|---|---|---|---|---|
| 1 | Thiram | Tree Nuts, Celeriac, Endive, Carrot, Radish, Onion, Garlic, Shallot, Cucumber, Cherries, Plums, Cauliflower, Apple, Lettuce | None | UV (435 nm) | 2000 | Heise et al. [70] |
| 2 | Carbon Disulfide | Savoy Cabbage, Red Cabbage, Turnip-Rooted Cabbage, Cauliflower, Leek, Table Mustard | None | UV-Vis (240–360 nm) | 2000 | Perz et al. [17] |
| 3 | Zineb | Apple, Apricot, Blueberry, Cherry, Grape, Nectarine, Peach, Pear, Plum, Raspberry, Rhubarb, Strawberry, Asparagus, Beans, Broccoli, Cabbage, Carrot, Cauliflower, Celery, Cucumber, Lettuce, Mushroom, Onion, Parsnip, Pepper, Potato, Radish, Tomato, Specialty | None | UV (435 nm) | 2000 | Ripley et al. [84] |
| 4 | Thiram | Apple, Papaya, Orange, Banana, Dry Beans, Polished Rice, Potato, Tomato, Cucumber | None | UV (435 nm) | 2001 | Caldas et al. [69] |
| Mancozeb | ||||||
| Ziram | ||||||
| 5 | Sodium Diethyldithiocarbamate | Grape leaf, Lettuce, Cantaloupe, Cucumber, Eggplant, Green Beans, Green Peas, Pepper, Tomato, Apple, Grape, Peach, Strawberry | None | UV (435 nm) | 2001 | Dogheim et al. [71] |
| 6 | None | Apple, Grape, Orange, Tomato, Eggplant, Cucumber, Potato | None | UV (435 nm) | 2001 | Abbassy et al. [72] |
| 7 | Carbon Disulfide | Apple, Apricot, Aubergine, Beans, Carrot, Cherry, Cucumber, Black Currant, Red Currant, Dill, Gooseberry, Grape, Kumquat, Leek, Lettuce, Melon, Nectarine, Okra, Oregano, Papaya, Parsley, Passion Fruit, Peas, Peach, Pear, Pepper, Plum, Raspberry, Spinach, Onion, Strawberry, Tomato, Watermelon | None | UV (372, 430 nm) | 2001 | Andersen et al. [73] |
| 8 | Sodium Diethyldithiocarbamate | Cucumber, Green Peas, Pepper, Tomato, Grape, Peach, Strawberry | None | UV (435 nm) | 2002 | Dogheim et al. [85] |
| 9 | Carbon Disulfide | Cucumber, Lettuce, Tomato, Mushroom, Red beet, Sugar beet, Cherry, Currant, Plum, Raspberry, Strawberry, | None | UV (662 nm) | 2002 | Morzycka et al. [86] |
| 10 | Zineb | Tobacco (T), Peach (P) | GC | FPD | 2002 | Vryzas et al. [87] |
| Mancozeb | ||||||
| Ziram | ||||||
| Maneb | ||||||
| Thiram | ||||||
| 11 | Carbon Disulfide | Grape Leaf, Lettuce, Molokhia, Spinach, Cantaloupe, Cucumber, Eggplant, Green Beans, Green Peas, Pepper, Tomato, Apple, Grape, Lemon, Lime, Peach, Pear, Strawberry | None | UV (435 nm) | 2002 | Gad Alla et al. [88] |
| 12 | Carbon Disulfide | Apple, Apricot, Asparagus, Avocado, Banana, Basil, Beans, Beetroot, Blackberry, Broccoli, Carambola, Carrot, Celery, Cherry, Chili, Cucumber, Black Currant, Red Currant, Dandelion leaves, Fennel, Fig, Grapefruit, Kaki, Kale, Kiwi, Kumquat, Lemon, Lettuce, Mandarin, Mango, Melon, Orange, Papaya, Passion Fruit, Peach, Pear, Pepper, Pineapple, Pomegranate, Potato, Radish, Rambutan,, Raspberry, Spinach, Onion, Strawberry, Tomato, Watercress | None | UV (372, 430 nm) | 2003 | Poulsen et al. [74] |
| 13 | None | Tomato, Plum, Strawberry, Currant, Lettuce, Mushrooms, Cherry, Cucumber, Apple | None | UV (662 nm) | 2003 | Nowacka A. [89] |
| 14 | Mancozeb | Apple, Banana, Orange, Papaya, Strawberry, Potato, Tomato, Dry Beans, Rice | None | UV (435 nm) | 2004 | Caldas et al. [90] |
| Ziram | ||||||
| Thiram | ||||||
| 15 | Ziram | Courgette | GC | MS | 2004 | Guidotti M. et al. [91] |
| 16 | Carbon Disulfide | Raspberry | GC | ECD | 2004 | Kovacevic et al. [92] |
| 17 | Maneb | Tomato | GC | FPD | 2004 | Kontou et al. [93] |
| 18 | Carbon Disulfide | Chinese Chive, Carrot, Spinach, Lettuce, Grape, Chinese Mustard, Celery, Radish, Ching Geeng, Wax apples, Cabbage, Bitter melon, Green Pepper | GC | FPD | 2005 | Chang et al. [94] |
| 19 | N.R. | Apple, Lettuce, Potato | GC | MS | 2006 | Česnik et al. [95] |
| 20 | None | Apple, Tomato, Papaya, Lettuce, Strawberry, Banana, Orange, Carrot, Potato, Beans, Rice | None | UV (435 nm) | 2006 | Caldas et al. [76] |
| 21 | Thiram | Apple, Lettuce, Potato, Strawberry, Tomato | GC | MS | 2006 | Česnik et al. [96] |
| 22 | Thiram | Peach, Green Beans, Apple, Tomato, Green Pepper, Potato, Fruit Cream Powder, Dehydrated vegetable cub | GC | FPD | 2006 | Papadopoulou-Mourkidou et al. [97] |
| Mancozeb | ||||||
| 23 | None | Apples, Cauliflower, Cereals, Grapes, Lettuce, Peas, Peeper, Potato, Strawberries | GC | MS | 2007 | Česnik et al. [98] |
| 24 | Ziram | Apple, Pear, Cherry, Grape, Tomato, Cucumber, Tamarillos, Papaya, Broccoli | GC | MS | 2008 | Crnogorac et al. [63] |
| Carbon Disulfide | ||||||
| Dithiane | ||||||
| Antracol | ||||||
| 25 | Mancozeb | Green Beans | None | UV (435 nm) | 2009 | Bazzi et al. [99] |
| Mefenoxam | ||||||
| 26 | None | Fig, Mango, Papaya, Persimmon | None | UV (435 nm) | 2009 | Pastor Ciscato et al. [77] |
| 27 | None | Tomato, Cabbage, Lettuce, Cucumber, Carrot, Spinach, Potato, Onion, Pepper | GC | ECD | 2009 | Lazic et al. [100] |
| 28 | Thiram | Tangerine, Clementine, Orange, Peach, Nectarine, Khakis | GC | MS | 2009 | Berrada et al. [101] |
| Zineb | ||||||
| Ziram | ||||||
| 29 | Maneb | Grape, Strawberry, Carrot, Lettuce, Corn | VP-LPME | IR | 2010 | Gonzalvez et al. [82] |
| Ziram | GC | MS | ||||
| Mancozeb | ||||||
| 30 | Carbon Disulfide | Grape Leaf, Lettuce, Cantaloupe, Cucumber, Eggplant, Green Beans, Green Peas, Pepper, Tomato, Apple, Grape, Peach, Strawberry, Squash, Broccoli, Potato, Apricot, Orange, Plum, Watermelon | None | UV (435 nm) | 2010 | Khorshed et al. [102] |
| Sodium Diethyldithiocarbamate | ||||||
| 31 | Carbon Disulfide | Green Beans, Green Peas, Broccoli, Green Onion, Potato Leaves, Peanut | GC | MS | 2011 | El-Gohary et al. [103] |
| Sodium Diethyldithiocarbamate | ||||||
| 32 | None | Apple | None | UV (435 nm) | 2011 | Łozowicka et al. [104] |
| 33 | Thiram | Apple, Leek, Potato, Strawberry, Wheat, Tomato, Lettuce, Rice | GC | FPD | 2012 | Bempelou et al. [105] |
| 34 | Carbon Disulfide | Watermelon, Banana, Mango, Cauliflower, Potato, Apricot, Grape, Green Peas, Lettuce, Molokhia, Watercress, Cucumber, Eggplant, Squash, Tomato, Cantaloupe, Guava, Strawberry, Spinach, Grape Leaf | None | UV (435 nm) | 2012 | El-Sawi Sanaa A. et al. [106] |
| Sodium Diethyldithiocarbamate | ||||||
| 35 | Mancozeb | Raspberry | GC | MS | 2012 | Pucarevic et al. [107] |
| 36 | Carbon Disulfide | Tomato, Paprika, Cucumber, Potato, Onion, Carrot, Cabbage, Ketchup, Apple, Cherry, Grape, Wine | GC | MS | 2014 | Kostik et al. [108] |
| 37 | Mancozeb | Grape, Green Chili, Tomato, Potato, Brinjal, Pineapple, Chayote | GC | MS | 2014 | Mujawar et al. [109] |
| 38 | None | Apple, Blueberry, Currant, Raspberry, Tomato, Broccoli, Parsley, Cucumber, Cabbage | None | UV (435 nm) | 2015 | Szpykra E. et al. [110] |
| 39 | None | Apple, Black Berries, Chokeberries, Blueberries, Currants, Elderberries, Gooseberries, Pears, Plums, Raspberries, Sea Sallowthorns, Cherries, Strawberries | None | UV (662 nm) | 2015 | Łozowicka et al. [111] |
| 40 | None | Apple, Apricot, Black Currant, Gooseberry, Grape, Peach, Raspberry, Red Currant, Strawberry, Sweet Cherry, Broccoli, Brussels Sprout, Carrot, Celeriac, Dill, Lettuce, Parsley, Peaking Cabbage, Spinach, Tomato, Wheat | None | UV (435 nm) | 2016 | Szpykra E. et al. [112] |
| 41 | Mancozeb | Tomato | None | UV (435 nm) | 2017 | El Habib Ait Addi et al. [113] |
| 42 | Mancozeb | Tomato | GC | MS | 2017 | Atuhaire et al. [114] |
| 43 | Mancozeb | Leafy Vegetables (Lettuce, Chard, Spinach) | None | UV-Vis (240–360 nm) | 2017 | Elgueta et al. [75] |
| 44 | Thiram | Lettuce | GC | ECD | 2017 | Pizzutti et al. [115] |
| GC | PFPD | |||||
| GC | MS | |||||
| None | UV (435 nm) | |||||
| 45 | Mancozeb | Rice, Corn, Cabbage | GC | MS | 2017 | Shao et al. [79] |
| 46 | Propineb and metabolites (PDA-PTU) | Banana | GC | FPD | 2017 | Song et al. [116] |
| Carbon Disulfide | ||||||
| 47 | Carbon Disulfide | Apricot | GC | MS | 2018 | Arslan et al. [117] |
| 48 | Zineb | Eggplant, Broccoli, Potato, Pear, Onion, Cabbage, Lettuce, Spinach, Lettuce, Ginger, Pepper, Cucumber, Cowpea, Tomato, Orange, Pumpkin, Strawberry, Banana, Papaya, Guava, Star Fruit, Watermelon, Apple, Radish, Carrot | LC | DBD-MES | 2018 | Han et al. [83] |
| 49 | Carbon Disulfide | Tomato, Mango, Cabbage, Grape | GC | ECD | 2018 | Nguyen et al. [80] |
| 50 | Thiram | Passion Fruit | None | UV (435 nm) | 2019 | Mozzaquatro et al. [118] |
| 51 | Mancozeb | Onion, Onion Leaves | GC | MS | 2019 | Patil et al. [119] |
| 52 | Carbon Disulfide | Apple, Avocado, Papaya, Durian, Soursop, Lemon, Guava, Mango, Orange, Mangosteen, Passion Fruit, Pineapple, Pomelo, Banana, Pitaya, Rambutan, Rockmelon, Salacca, Watermelon, Jackfruit | GC | FPD | 2019 | Rahman Alinah A. [120] |
| 53 | Mancozeb | Lettuce | None | Vis-NIR (600 µm) | 2020 | Steidle Neto et al. [20] |
| 54 | Thiram | Soybean | GC | ITD-MS | 2021 | da Silva et al. [21] |
| PFPD | ||||||
| 55 | None | Arugula, Bean pod, Bean root, Carrot, Chayote, Chicory, Chili, Coriander, Chive, Eggplant, Ginger Leek, Lettuce, Parsley, Pumpkin, Scarlet eggplant, Spinach, Sweet pepper, Sweet potato, Tomato, Watercress, Yam, Zucchini | None | UV (435 nm) | 2022 | de Araujo et al. [121] |
| 56 | Carbon Disulfide | Vine Leaves | GC | MS | 2022 | Arslan et al. [22] |
| 57 | Carbon Disulfide | Cardamom, Black Pepper | GC | MS | 2022 | Natarajan et al. [23] |
| 58 | Thiram | Yerba Mate | GC | MS | 2022 | Da Silva [24] |
| 59 | Mancozeb | Mango, Banana, Rice, Cowpea, Lychee, Cabbage | LC | LEGD-DBD-MES (257.94 nm) | 2023 | Tian et al. [26] |
| Metiram | ||||||
| Thiram | ||||||
| Propineb | ||||||
| 60 | Mancozeb | Banana, Mango, Pineapple, Cowpea, Dragon Fruit, Lychee, Apple, Eggplant, Peanuts | GC | ECD | 2024 | Tian et al. [25] |
| 61 | Mancozeb | Cauliflower | HPLC | MS-MS | 2025 | Tripathi et al. [27] |
| Method | Target Compounds | Matrix | Chromatographic Method | Detection Method | Year | Author [Ref] |
|---|---|---|---|---|---|---|
| 1 | Ziram | Potato, Cabbage, Tomato, Cucumber | None | UV (590 nm) | 2001 | Saad et al. [126] |
| 2 | Ethylene thiourea | Tomato, Tomato Juice, Tomato Paste | HPLC | PDA | 2001 | Kontou et al. [122] |
| 3 | Dazomet | Avocado, Cherry, Lemon, Nuts, Oat, Orange, Peach, Rice, Tomato | LC | APCI-MS | 2003 | Blasco et al. [132] |
| Disulfiram | ||||||
| Thiram | ||||||
| Ethylene thiourea | ||||||
| Propylene thiourea | ||||||
| 4 | Ethylene thiourea | Tomato | HPLC | PDA | 2004 | Kontou et al. [93] |
| 5 | Mancozeb | Cucumber, Apple | HPLC | CL | 2004 | Nakazawa et al. [127] |
| Propineb | ||||||
| 6 | Maneb | Tomato, Wheat Grain, Water | None | FAAS | 2005 | Turker et al. [128] |
| 7 | Ziram | Grapes, Cucumbers, Tomatoes, Rucola | HPLC | MS | 2007 | Crnogorac et al. [133] |
| Dithiane | ||||||
| Antracol | ||||||
| Zineb | ||||||
| Propineb | ||||||
| 8 | Ziram | Apple, Pear, Cherry, Grape, Tomato, Cucumber, Tamarillos, Papaya, Broccoli | HPLC | MS-MS | 2008 | Crnogorac et al. [63] |
| Dithiane | ||||||
| Antracol | ||||||
| 9 | Mancozeb | Persimmons, Pears, Strawberries, Cabbage, Lettuce, Spinach | HPLC | MS-MS | 2008 | Hayama et al. [134] |
| Maneb | ||||||
| Zineb | ||||||
| 10 | Ziram | Potato, Cabbage, Tomato | None | Square Wave Voltammetry (SWV) | 2008 | Qiu et al. [135] |
| 11 | Ziram | Cacao, Spinach, Potato, Brown Rice, Pumpkin, Orange, Soybean, Cabbage, Apple, Green Tea | GC | MS | 2010 | Nakamura et al. [136] |
| Thiram | ||||||
| Ferbam | ||||||
| Nickel bis(dithiocarbamate) | ||||||
| Propineb | ||||||
| Maneb | ||||||
| Zineb | ||||||
| Mancozeb | ||||||
| Polycarbamate | ||||||
| Milneb | ||||||
| 12 | Ethylene thiourea | Celery, Melon, Spinach | UHPLC | MS-MS | 2011 | Bonnechere et al. [123] |
| Propylene thiourea | ||||||
| 13 | Thiram | Apple, Pear, Strawberry, Lettuce | DART | MS | 2011 | Cajka et al. [129] |
| Ziram | DESI | MS-MS | ||||
| 14 | Thiram | Tomato | HPLC | UV (272 nm) | 2012 | Jafari et al. [137] |
| Mancozeb | ||||||
| Propineb | ||||||
| 15 | Thiram | Eggplant, Lettuce, Strawberry, Apple | HPLC | MS-MS | 2012 | Peruga et al. [124] |
| 16 | Ethylene thiourea | Rice, Leaf Mustard | UPLC | MS-MS | 2012 | Chung et al. [138] |
| Propylene thiourea | ||||||
| 17 | Mancozeb | Apple, Wine Grape, Lettuce, Pepper, Tomato, Strawberry | HPLC | UV (270 nm) | 2012 | Lopez-Fernandez et al. [139] |
| Maneb | ||||||
| Propineb | ||||||
| 18 | Antracol | N.A. | MALDI | MS (Orbitrap) | 2013 | Ivanova et al. [140] |
| Ferbam | ||||||
| Maneb | ||||||
| Mancozeb | ||||||
| Propineb | ||||||
| Thiram | ||||||
| Ziram | ||||||
| 19 | Thiram | Tomato, Grape, Sweet Peppers, Nectarine, Peach | HPLC | MS-MS | 2013 | Ringli et al. [141] |
| 20 | Mancozeb | N.A. | None | Adsorptive Stripping Voltammetry (AdSV) | 2013 | Amorello et al. [142] |
| Maneb | ||||||
| Propineb | ||||||
| Nabam | ||||||
| Na (CH3)2DTC | ||||||
| Zineb | ||||||
| Ziram | ||||||
| Ferbam | ||||||
| Thiram | ||||||
| 21 | Thiram | Tomato, Cucumber, Watermelon | None | UV (430 nm) | 2013 | Rastegarzadeh et al. [143] |
| 22 | Ferbam | Apple, Pear, Plum, Grape, Papaya, Broccoli, Tomato | HPLC | MS-MS | 2013 | Schmidt et al. [144] |
| Mancozeb | ||||||
| Maneb | ||||||
| Metiram | ||||||
| Nabam | ||||||
| Propineb | ||||||
| Thiram | ||||||
| Zineb | ||||||
| Ziram | ||||||
| 23 | Mancozeb | Fruit Juice, Water | None | UV (620 nm) | 2014 | Rohit et al. [145] |
| HNMR | ||||||
| FT-IR | ||||||
| TEM | ||||||
| 24 | Ethylene thiourea | Apple, Papaya, Strawberry | HPLC | MS-MS | 2014 | Rossi Lemes et al. [146] |
| 25 | Disulfiram | Apple, Grape, Lettuce | UHPLC | ED | 2015 | Charoenkitamorn et al. [147] |
| Thiram | ||||||
| N,N-diethyl-N′,N′-dimethyl thiuram disulfide | ||||||
| 26 | Dazomet | Apple, Leek, Tomato, Pine needles | HPLC | UV (272 nm) AAS | 2016 | Al-Alam et al. [148] |
| Metam Sodium | ||||||
| Ferbam | ||||||
| Ziram | ||||||
| Zineb | ||||||
| Maneb | ||||||
| Mancozeb | ||||||
| Metiram | ||||||
| Nabam | ||||||
| Propineb | ||||||
| 27 | Propineb | Beer, Fruit Juice, Malt | HPLC | MS-MS | 2017 | Kakitani et al. [149] |
| Mancozeb | ||||||
| Maneb | ||||||
| Zineb | ||||||
| Polycarbamate as EB | ||||||
| Milneb | ||||||
| Thiuram | ||||||
| Nickel diethyldithiocarbamate | ||||||
| Polycarbamate as DD | ||||||
| Ferbam | ||||||
| Ziram | ||||||
| 28 | Propineb | Banana | GC | FPD | 2017 | Song et al. [116] |
| Propylene thiourea | HPLC | MS-MS | ||||
| Propylene diamine | ||||||
| 29 | Thiram | Strawberry, Cucumber | None | SERS | 2018 | Chen et al. [150] |
| 30 | Ziram | Water, Tomato, Mango Beverage | None | UV-Vis (400–570 nm) | 2019 | Ghoto et al. [151] |
| Zineb | ||||||
| Maneb | ||||||
| 31 | Ziram | Water, Tomato, Mango Beverage | None | UV-Vis (490–570 nm) | 2019 | Ghoto et al. [152] |
| Zineb | ||||||
| Maneb | ||||||
| 32 | Ziram | Apple Black Tea | None | UV-Vis (525–683 nm) | 2020 | Wang et al. [28] |
| Thiram | ||||||
| Zineb | ||||||
| 33 | Propineb | Infant Formula, Black tea | GC | MS | 2020 | Bodur et al. [153] |
| 34 | Ziram | Apple Juice | None | SERS | 2020 | Wei et al. [29] |
| Thiram | ||||||
| 35 | Thiram | Lettuce, Broccoli | None | SERS | 2021 | Tsen et al. [30] |
| Mancozeb | ||||||
| Propineb | ||||||
| 36 | Thiram | Tap Water, Orange Juice | None | SERS | 2022 | Ahn et al. [31] |
| 37 | Mancozeb | Chamomile | HPLC | MS-MS | 2022 | Sayed et al. [34] |
| 38 | Ethylene thiourea | Grape, Cherry Tomato, Strawberry | HPLC | ICP-MS | 2023 | Bendhiab et al. [35] |
| Propylene thiourea | ||||||
| 39 | Thiram | Water, Apple, Guava, Broad Beans, Green Beans | None | UV-Vis (420 nm) | 2023 | Eswaran et al. [154] |
| 40 | Ethylene thiourea | Cucumber, Celery, Tomato, Green pepper, Potato, Citrus, Apple, Jujube, Raisins, Grape | None | Fluorescence (450–570 nm) | 2023 | Han et al. [32] |
| 41 | Maneb | Tomato, Rice, Papaya | None | Digital Images | 2023 | Martins et al. [131] |
| 42 | Ziram | Green tea, Flower tea, Red tea, Black tea | HPLC | CDCL | 2024 | Wei et al. [155] |
| Zineb | ||||||
| Propineb | ||||||
| 43 | Zineb | Green Tea, White Tea, Black Tea | None | Fluorescence (500–650 nm) | 2025 | Feng et al. [33] |
| UV (365 nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacini, T.; Orsini, S.; Verdini, E.; Cristofani, E.; Pelliccia, A.; Sdogati, S.; Colosio, C.; Pecorelli, I. Advances in the Analytical Determination and Toxicological Assessment of Dithiocarbamates and Their Hydrolysis Products in Fruits, Vegetables, and Cereals: Methodological Evolution, Challenges, and Future Directions. Toxics 2025, 13, 819. https://doi.org/10.3390/toxics13100819
Pacini T, Orsini S, Verdini E, Cristofani E, Pelliccia A, Sdogati S, Colosio C, Pecorelli I. Advances in the Analytical Determination and Toxicological Assessment of Dithiocarbamates and Their Hydrolysis Products in Fruits, Vegetables, and Cereals: Methodological Evolution, Challenges, and Future Directions. Toxics. 2025; 13(10):819. https://doi.org/10.3390/toxics13100819
Chicago/Turabian StylePacini, Tommaso, Serenella Orsini, Emanuela Verdini, Elisa Cristofani, Alessandro Pelliccia, Stefano Sdogati, Claudio Colosio, and Ivan Pecorelli. 2025. "Advances in the Analytical Determination and Toxicological Assessment of Dithiocarbamates and Their Hydrolysis Products in Fruits, Vegetables, and Cereals: Methodological Evolution, Challenges, and Future Directions" Toxics 13, no. 10: 819. https://doi.org/10.3390/toxics13100819
APA StylePacini, T., Orsini, S., Verdini, E., Cristofani, E., Pelliccia, A., Sdogati, S., Colosio, C., & Pecorelli, I. (2025). Advances in the Analytical Determination and Toxicological Assessment of Dithiocarbamates and Their Hydrolysis Products in Fruits, Vegetables, and Cereals: Methodological Evolution, Challenges, and Future Directions. Toxics, 13(10), 819. https://doi.org/10.3390/toxics13100819









