Enhanced Toxicity of Polymethylmethacrylate Microparticles on Cells and Tissue of the Marine Mussel Mytilus trossulus After UV Irradiation
Highlights
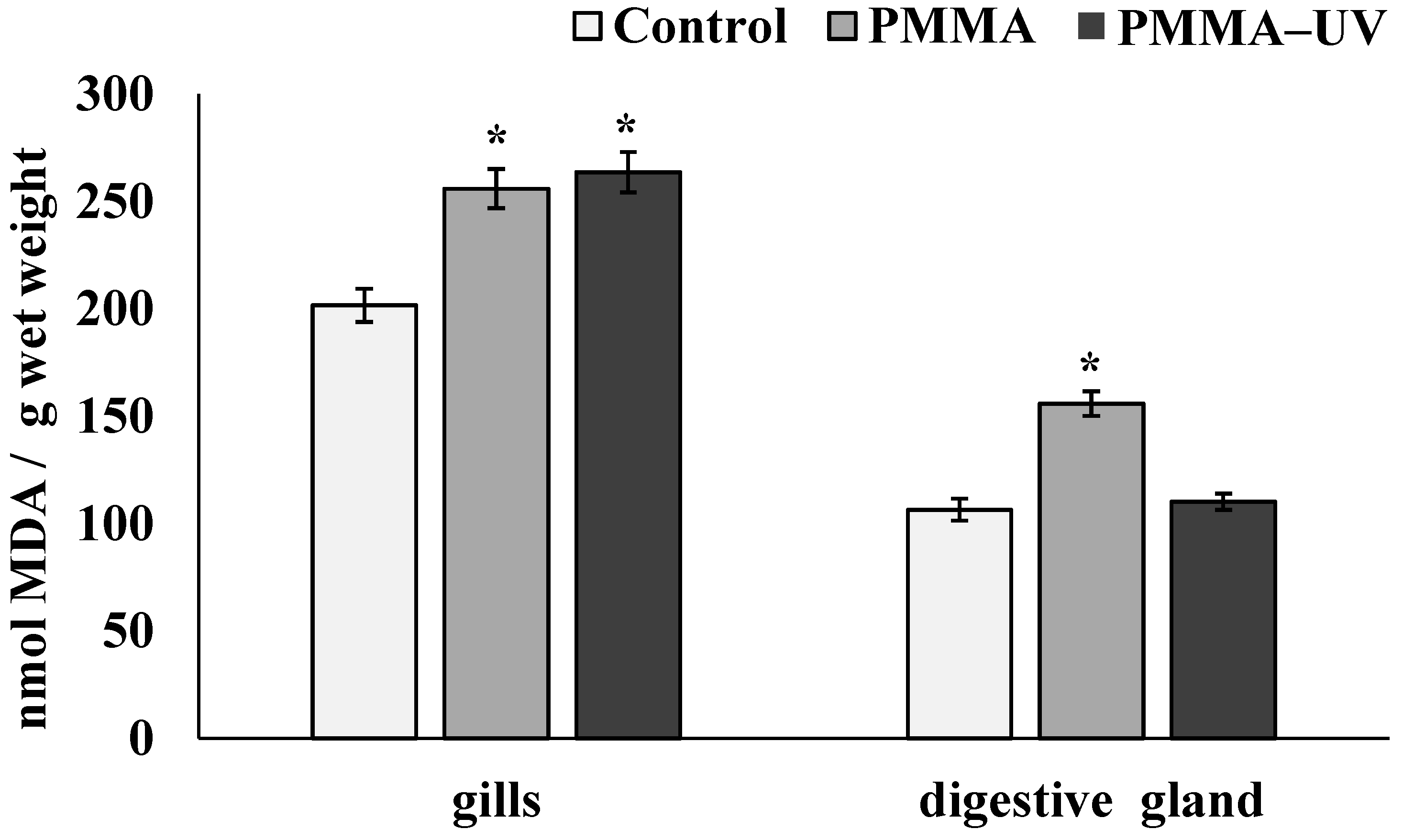
- Both PMMA and UV-aged PMMA reduce lysosomal membrane stability, causing oxidative stress in the gills, while only PMMA induces oxidative stress in the digestive gland.
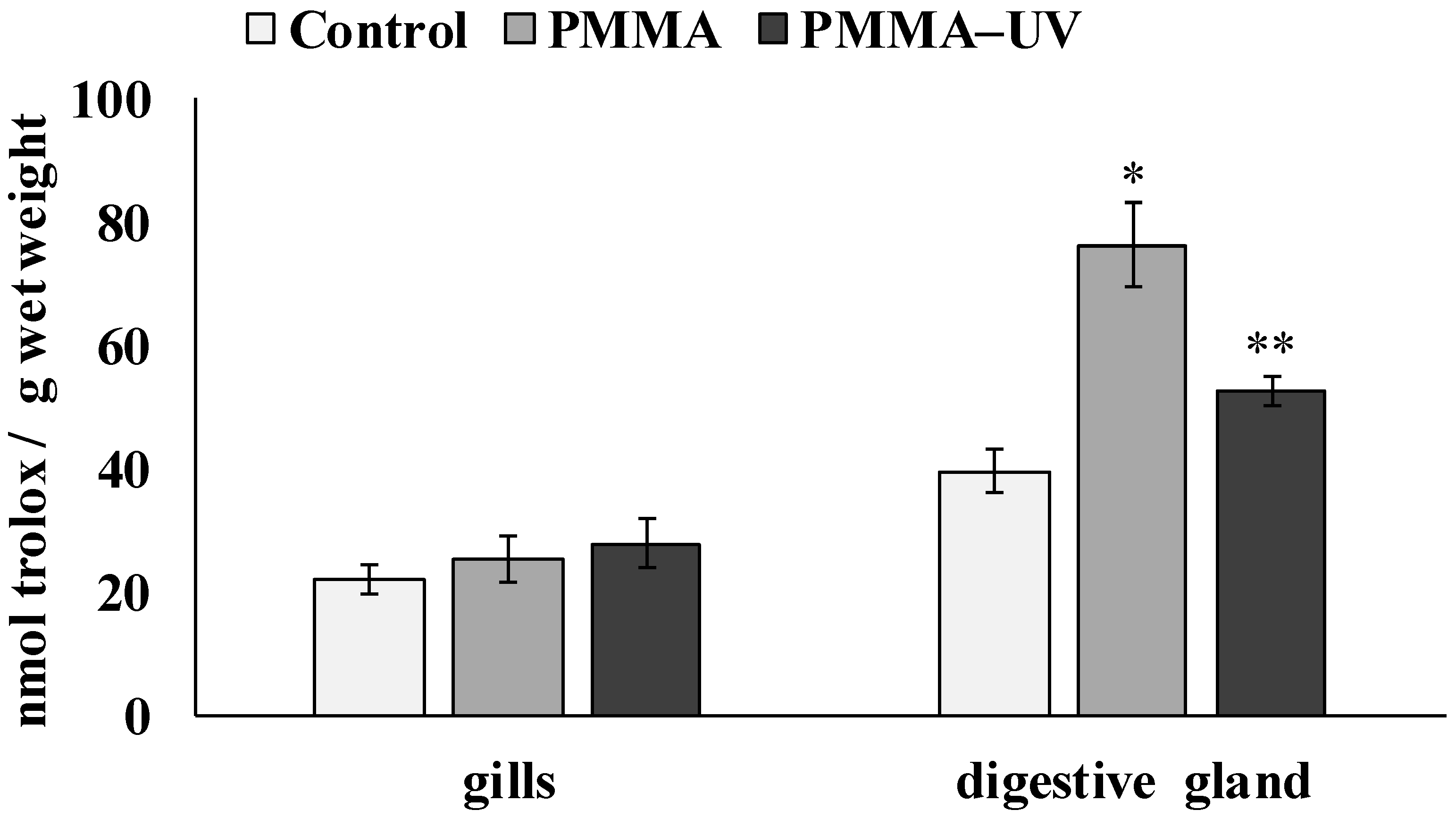
- Biochemical responses were tissue-specific, with antioxidant activity increasing mainly in digestive gland cells exposed to PMMA.
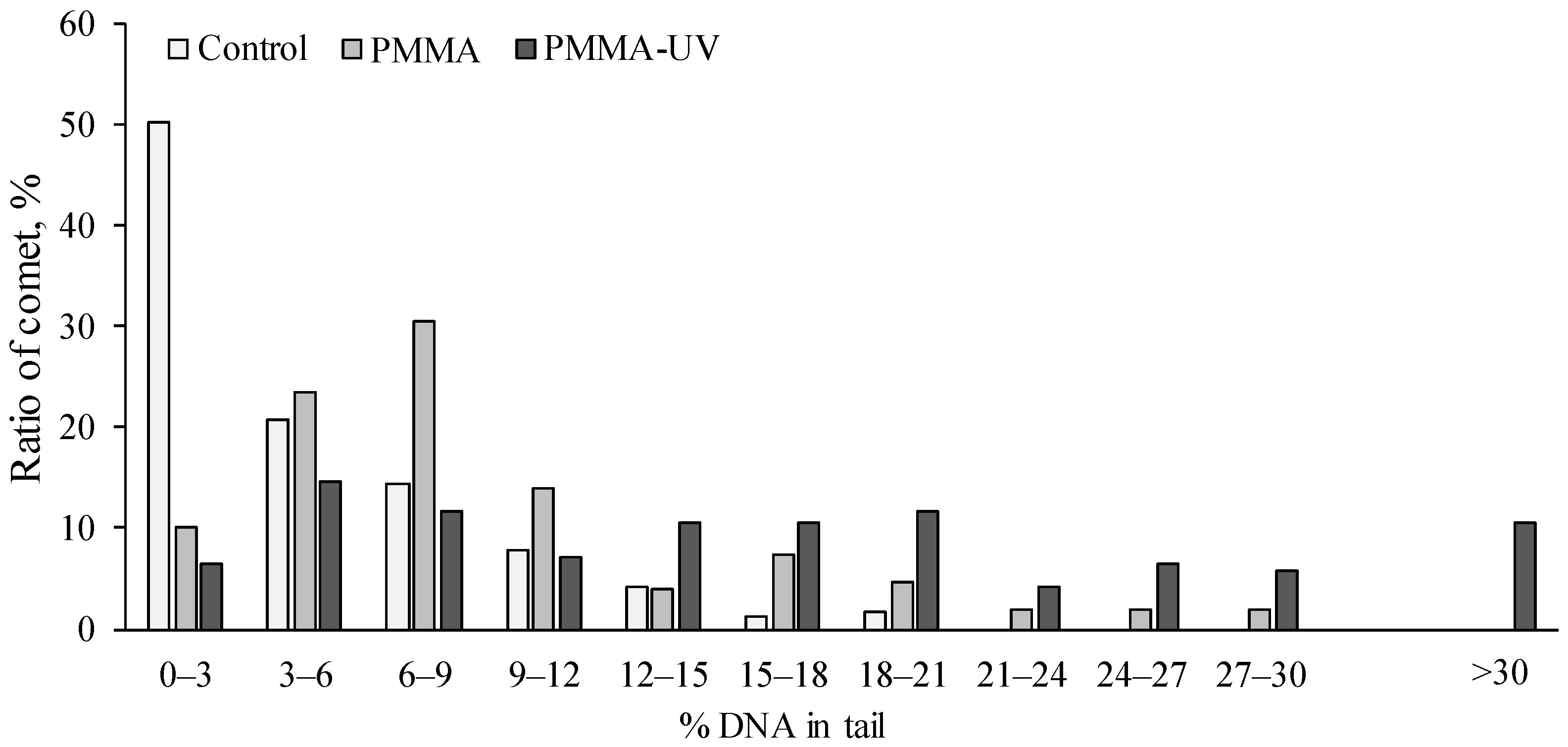
- PMMA and especially PMMA-UV exposure increased DNA.
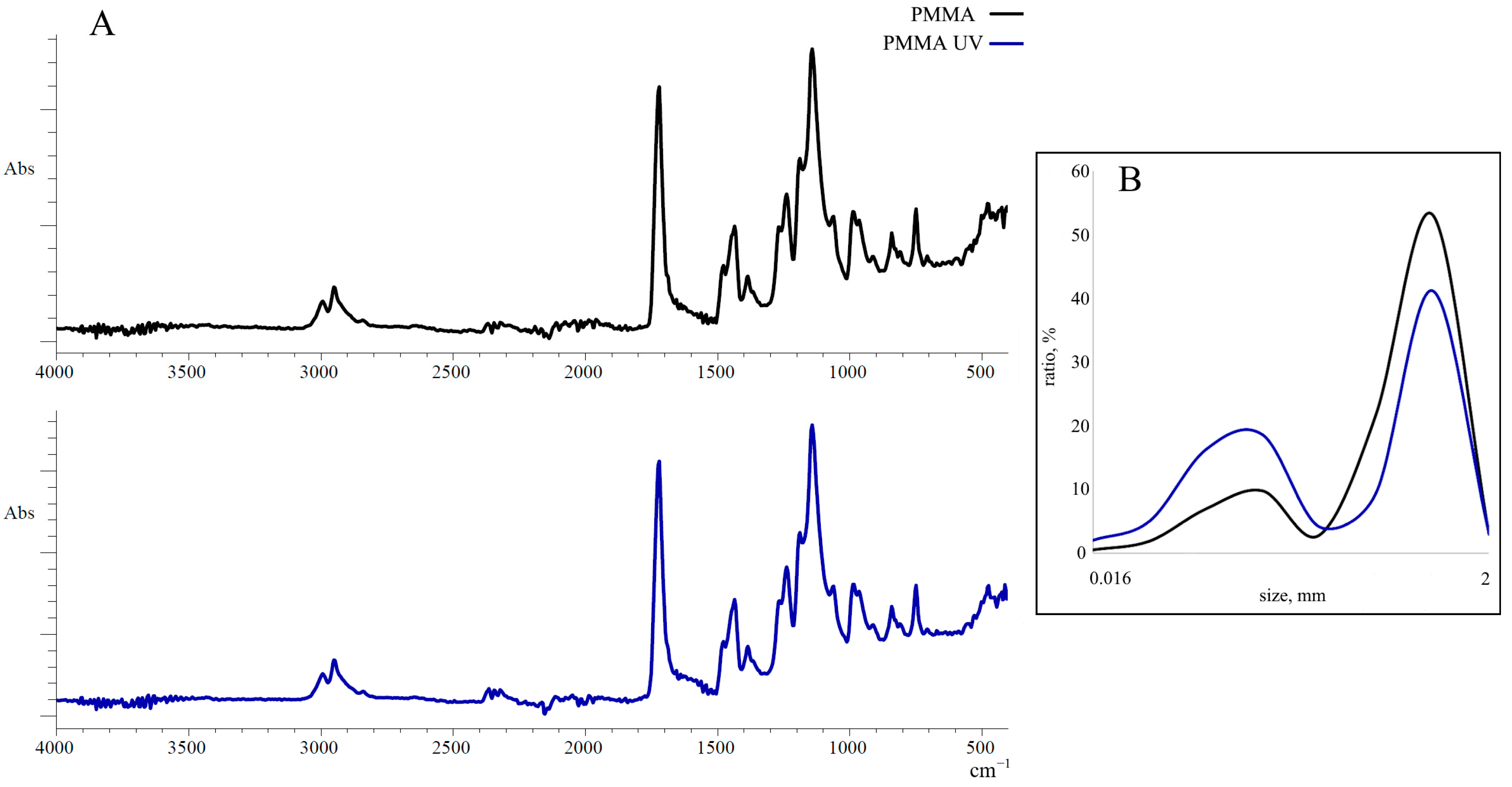
- UV aging of PMMA alters its particle size distribution, resulting in a higher proportion of small microparticles (50–125 μm)
- PMMA and its UV-aged form trigger distinct and tissue-specific stress pathways, suggesting differential toxicokinetics and cellular sensitivity.
Abstract
1. Introduction
2. Materials and Methods
2.1. PMMA and PMMA-UV Preparation and Characterization
2.2. Experiment Design
2.3. Cytochemical Assays
2.4. Biochemical Assays
2.5. Statistical Processing
3. Results
3.1. The Effect of UV on the Composition and Size of PMMA Microparticles
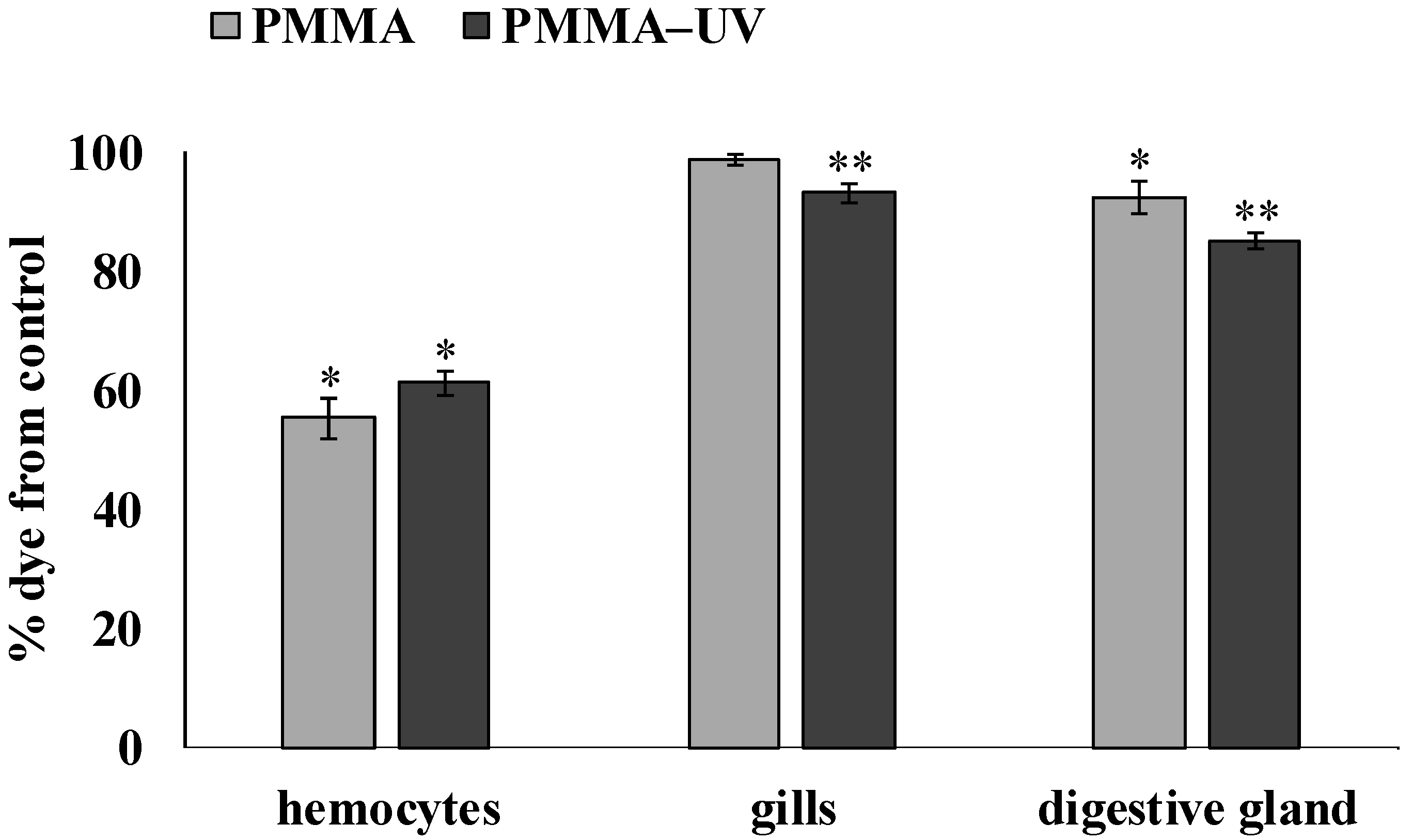
3.2. Cell Viability Analysis
3.3. Pro-Oxidative Processes Indicators
3.4. Genotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, M.; Shim, W.J.; Han, G.M.; Rani, M.; Song, Y.K.; Hong, S.H. Widespread detection of a brominated flame retardant, hexabromocyclododecane, in expanded polystyrene marine debris and microplastics from South Korea and the Asia-Pacific coastal region. Environ. Pollut. 2017, 231, 785–794. [Google Scholar] [CrossRef]
- Kako, S.; Isobe, A.; Kataoka, T.; Hinata, H. A decadal prediction of the quantity of plastic marine debris littered on beaches of the East Asian marginal seas. Mar. Pollut. Bull. 2014, 81, 174–184. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, K.N.; Woo, J.R. Post-consumer plastic packaging waste from online food delivery services in South Korea. Waste Manag. 2023, 156, 177–186. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513. [Google Scholar] [CrossRef]
- Song, J.A.; Choi, C.Y.; Park, H.S. Exposure of bay scallop Argopecten irradians to micro-polystyrene: Bioaccumulation and toxicity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 236, 108801. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Surface properties of beached plastics. Environ. Sci. Pollut. R. 2015, 22, 11022–11032. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Rubin, A.E.; Zucker, I. Engineered Polystyrene-Based Microplastics of High Environmental Relevance. Environ. Sci. Technol. 2021, 55, 10491–10501. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, X.; Jiang, R.; You, J.; Ouyang, G. New insights into the photo-degraded polystyrene microplastic: Effect on the release of volatile organic compounds. J. Hazard. Mater. 2022, 431, 128523. [Google Scholar] [CrossRef]
- Zha, F.; Dai, J.; Han, Y.; Liu, P.; Wang, M.; Liu, H.; Guo, X. Release of millions of micro(nano)plastic fragments from photoox-idation of disposable plastic boxes. Sci. Total Environ. 2023, 858 Pt 3, 160044. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Dovzhenko, N.V.; Mazur, A.A.; Kukla, S.P. Photoaging elevated the genotoxicity of polystyrene microplastics to marine mussel Mytilus trossulus (Gould, 1850). Int. J. Mol. Sci. 2024, 25, 5740. [Google Scholar] [CrossRef] [PubMed]
- Monsores, K.G.D.C.; Oliveira, S.D.S.A.; Oliveira, S.D.S.; Rodrigues, J.G.P.; Weber, R.P. Influence of ultraviolet radiation on polymethylmethacrylate (PMMA). J. Mater. Res. Technol. 2019, 8, 3713–3718. [Google Scholar] [CrossRef]
- Cao, H.; Ding, P.; Li, X.; Huang, C.; Li, X.; Chen, X.; Zhang, L.; Qi, J. Environmentally persistent free radicals on photoaged microplastics from disposable plastic cups induce the oxidative stress associated toxicity. J. Hazard. Mater. 2024, 464, 132990. [Google Scholar] [CrossRef]
- Suhrhoff, T.J.; Scholz-Böttcher, B.M. Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment. Mar. Pollut. Bull. 2016, 102, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, R. Solar radiation stimulates release of semi-labile dissolved organic matter from microplastics. Front. Mar. Sci. 2023, 10, 1284280. [Google Scholar] [CrossRef]
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Marine debris occurrence and treatment: A review. Renew. Sustain. Energy Rev. 2016, 64, 394–402. [Google Scholar] [CrossRef]
- Fernandes, S.; Farzaneh, S.; Bendell, L.I. Abundance and distribution of beach litter with acutely toxic metal concentrations. Mar. Pollut. Bull. 2020, 159, 111479. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Kukla, S.P.; Mazur, A.A.; Dovzhenko, N.V.; Zhukovskaya, A.F.; Karpenko, A.A.; Karpenko, M.A.; Odintsov, V.S. Dietary Exposure to Particles of Polytetrafluoroethylene (PTFE) and Polymethylmethacrylate (PMMA) Induces Different Responses in Periwinkles Littorina brevicula. Int. J. Mol. Sci. 2023, 24, 8243. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Tvarijonaviciute, A.; Pereira, M.L.; Martins, M.A.; Tort, L.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on Dicentrarchus labrax. Genomics 2018, 110, 435–441. [Google Scholar] [CrossRef]
- Kokalj, A.; Kuehnel, D.; Puntar, B.; Žgajnar Gotvajn, A.; Kalčikova, G. An exploratory ecotoxicity study of primary microplastics versus aged in natural waters and wastewaters. Environ. Pollut. 2019, 254, 112980. [Google Scholar] [CrossRef]
- Prokic, M.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Gewert, B.; MacLeod, M.; Breitholtz, M. Variability in Toxicity of Plastic Leachates as a Function of Weathering and Polymer Type: A Screening Study with the Copepod Nitocra spinipes. Biol. Bull. 2021, 240, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Z.; Wu, Y.; Li, H.; Jiang, J.; Yang, Y.; Xu, L.; Shi, H. Insight into chemical features of migrated additives from plastics and associated risks to estuarine ecosystem. J. Hazard. Mater. 2023, 448, 130861. [Google Scholar] [CrossRef]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Accumulation and ecotoxicological risk of weathered polyethylene (wPE) microplastics on green mussel (Perna viridis). Ecotoxicol. Environ. Saf. 2021, 208, 111765. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Purvaja, R.; Anandavelu, I.; Robin, R.S.; Ramesh, R. Ingestion and toxic impacts of weathered polyethylene (wPE) microplastics and stress defensive responses in whiteleg shrimp (Penaeus vannamei). Chemosphere 2022, 300, 134487. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Sun, J.; Li, J.; Pan, X. Effects of aging on environmental behavior of plastic additives: Migration, leaching, and ecotoxicity. Sci. Total Environ. 2022, 849, 157951. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A.; et al. Adverse effects polystyrene microplastics exert on zebrafish heart—Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Zhou, L.; Mao, Z.; Zhang, X.; Cai, J.; Huang, P. Enhanced hepatic metabolic perturbation of polystyrene nanoplastics by UV irradiation-induced hydroxyl radical generation. J. Environ. Sci. 2024, 142, 259–268. [Google Scholar] [CrossRef]
- Dovzhenko, N.V.; Slobodskova, V.V.; Mazur, A.A.; Kukla, S.P.; Istomina, A.A.; Chelomin, V.P.; Beskhmelnov, D.D. Oxidative Stress in Mussel Mytilus trossulus Induced by Different-Sized Plastics. J. Xenobiot. 2024, 14, 1826–1835. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in vitro toxicity of plastic leachates after UV irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef]
- Pandi, P.; Madhuvandhi, J.; Priya, K.K.; Thiagarajan, R.; Gopalakrishnan, S.; Elumalai, S.; Thilagam, H. Weathered polyethylene microplastics exposure leads to modulations in glutathione-S-transferase activity in fish. Front. Mar. Sci. 2022, 9, 990351. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Dovzhenko, N.V.; Kukla, S.P. Leachate from Weathered Face Masks Increases DNA Damage to Sperm of Sand Dollars Scaphechinus mirabilis. Toxics 2025, 13, 372. [Google Scholar] [CrossRef]
- Albertini, R.J. The lower alkyl methacrylates: Genotoxic profile of non-carcinogenic compounds. Regul. Toxicol. Pharmacol. 2017, 84, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.J.; Carlin, J.P.; Hammerschmidt, J.A.; Buck, R.C.; Buxton, L.W.; Fiedler, H.; Seed, J.; Hernandez, O. A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers. Integr. Environ. Assess. Manag. 2018, 14, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Almeida, A.C.; Georgantzopoulou, A. Characterization of cell responses in Rhodomonas baltica exposed to PMMA nanoplastics. Sci. Total Environ. 2020, 726, 138547. [Google Scholar] [CrossRef]
- Venâncio, C.; Melnic, I.; Tamayo-Belda, M.; Oliveira, M.; Martins, M.A.; Lopes, I. Polymethylmethacrylate nanoplastics can cause developmental malformations in early life stages of Xenopus laevis. Sci. Total. Environ. 2022, 806, 150491. [Google Scholar] [CrossRef]
- Biba, R.; Cvjetko, P.; Jakopčić, M.; Komazec, B.; Tkalec, M.; Dimitrov, N.; Begović, T.; Balen, B. Phytotoxic Effects of Polystyrene and Polymethyl Methacrylate Microplastics on Allium cepa Roots. Plants 2023, 12, 747. [Google Scholar] [CrossRef]
- Silva, M.S.S.; Pires, A.; Vethaak, D.; Martínez-Gómez, C.; Almeida, M.; Pinto, R.; Figueira, E.; Oliveira, M. Effects of polymethylmethacrylate nanoplastics on the polychaete Hediste diversicolor: Behavioural, regenerative, and biochemical responses. Aquat. Toxicol. 2023, 265, 106743. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson ADave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, D.; Zhao, Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Oral, R.; Pagano, G.; Tez, S.; Toscanesi, M.; Ranieri, P.; Trifuoggi, M.; Lyons, D.M. Mild toxicity of polystyrene and polymethylmethacrylate microplastics in Paracentrotus lividus early life stages. Mar. Environ. Res. 2020, 161, 105132. [Google Scholar] [CrossRef]
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L). Environ. Sci. Technol. 2008, 42, 5026–5031. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265 Pt B, 114864. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Jin, S.; Yang, F.; Hui, Y.; Xu, Y.; Lu, Y.; Liu, J. Cytotoxicity and apoptosis induction on RTG-2 cells of 2,2′,4,4′-tetrabromidiphenyl ether (BDE-47) and decarbominated diphenyl ether (BDE-209). Toxicol. In Vitro 2010, 24, 1190–1196. [Google Scholar] [CrossRef]
- Zuang, V. The Neutral Red Release Assay: A Review. Altern. Lab. Anim. 2001, 29, 575–599. [Google Scholar] [CrossRef]
- Bartosz, G.; Janaszewska, A.; Ertel, D.; Bartosz, M. Simple determination of peroxyl radical-trapping capacity. Biochem. Mol. Biol. Int. 1998, 46, 519–528. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzym. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.C.; Marques, J.C.; Gonçalves, A.M.M. Microplastics in commercial marine bivalves: Abundance, characterization and main effects of single and combined exposure. Aquat. Toxicol. 2025, 279, 107227. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Yang, H.; Du, Y.; Li, X. Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms. Toxics 2025, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.; Oliveira, M.; Frazão, C.; Almeida, M.; Pinto, R.J.B.; Figueira, E.; Pires, A. The Role of Life Stages in the Sensitivity of Hediste diversicolor to Nanoplastics: A Case Study with Poly(Methyl)Methacrylate (PMMA). Toxics 2024, 12, 352. [Google Scholar] [CrossRef]
- Barkhau, J.; Sanchez, A.; Lenz, M.; Thiel, M. Effects of microplastics (PVC, PMMA) on the mussel Semimytilus algosus differ only at high concentrations from those of natural microparticles (clay, celite). Mar. Pollut. Bull. 2022, 177, 113414. [Google Scholar] [CrossRef]
- Pavičić-Hamer, D.; Kovačić, I.; Sović, T.; Marelja, M.; Lyons, D.M. Exposure to Polymethylmethacrylate Microplastics Induces a Particle Size-Dependent Immune Response in Mediterranean Mussel Mytilus galloprovincialis. Fishes 2022, 7, 307. [Google Scholar] [CrossRef]
- Gao, C.; Wu, Z.; Liang, B.; Lu, J.; Fu, G.; Sun, M.; Yu, W.; Zhang, S.; Gao, S. Toxic effects of exposure to polymethyl methacrylate and polyvinyl chloride microplastics in Pacific oysters (Crassostrea gigas). Environ. Pollut. 2025, 366, 125484. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Barría, C.; Martins, M.A.; Franco-Martínez, L.; Barreto, A.; Tvarijonaviciute, A.; Tort, L.; Oliveira, M.; Teles, M. Waterborne exposure of gilthead seabream (Sparus aurata) to polymethylmethacrylate nanoplastics causes effects at cellular and molecular levels. J. Hazard. Mater. 2021, 403, 123590. [Google Scholar] [CrossRef]
- Manuel, P.; Almeida, M.; Martins, M.; Oliveira, M. Effects of nanoplastics on zebrafish embryo-larval stages: A case study with polystyrene (PS) and polymethylmethacrylate (PMMA) particles. Environ. Res. 2022, 213, 113584. [Google Scholar] [CrossRef]
- Zheng, P.C.; Pan, X.Q.; Zhou, Y.J.; Lai, K.P.; Li, R.; Zhang, X.X. Unraveling the Impact of Micro- and Nano-sized Polymethyl methacrylate on Gut Microbiota and Liver Lipid Metabolism: Insights from Oral Exposure Studies. Environ. Pollut. 2025, 373, 126157. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Techn. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Chronic toxicity of polystyrene nanoparticles in the marine mussel Mytilus galloprovincialis. Chemosphere 2022, 287, 132356. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Auguste, M.; Balbi, T.; Ciacci, C.; Canonico, B.; Papa, S.; Borello, A.; Vezzulli, L.; Canesi, L. Shift in Immune Parameters after Repeated Exposure to Nanoplastics in the Marine Bivalve Mytilus. Front. Immunol. 2020, 11, 426. [Google Scholar] [CrossRef]
- Gonzalez, P.; Dominique, Y.; Massabuau, J.C.; Boudou, A.; Bourdineaud, J.P. Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ. Sci. Technol. 2005, 39, 3972–3980. [Google Scholar] [CrossRef]
- Impellitteri, F.; Curpăn, A.S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public. Health 2022, 19, 16830. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Rouillon, C.; Bussiere, P.O.; Desnoux, E.; Collin, S.; Vial, C.; Therias, S.; Gardette, J.L. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polym. Degrad. Stabil. 2016, 128, 200–208. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier transform infrared spectroscopy to assess the degree of al-teration of artificially aged and environmentally weathered microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- De Bomfim, A.S.C.; Maciel, M.M.D.; Voorwald, H.J.C.; Benini, K.C.C.; de Oliveira, D.M.; Cioffi, M.O.H. Effect of different degradation types on properties of plastic waste obtained from espresso coffee capsules. Waste Manag. 2019, 83, 123–130. [Google Scholar] [CrossRef]
- Kavda, S.; Golfomitsou, S.; Richardson, E. Effects of selected solvents on PMMA after prolonged exposure: Unilateral NMR and ATR-FTIR investigations. Herit. Sc. 2023, 11, 63. [Google Scholar] [CrossRef]
- Rummel, C.D.; Escher, B.I.; Sandblom, O.; Plassmann, M.M.; Arp, H.P.H.; MacLeod, M.; Jahnke, A. Effects of Leachates from UV-Weathered Microplastic in Cell-Based Bioassays. Environ. Sci. Technol. 2019, 53, 9214–9223. [Google Scholar] [CrossRef]
- Shi, Y.; Qin, J.; Tao, Y.; Jie, G.; Wang, J. Natural weathering severity of typical coastal environment on polystyrene: Experiment and modeling. Polym. Test. 2019, 76, 138–145. [Google Scholar] [CrossRef]
- Hüffer, T.; Weniger, A.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 236, 218–225. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Wang, H.; Huang, H.; Shi, Y.; Yang, C.; Gao, S. Photo aging of polypropylene microplastics in estuary water and coastal seawater: Important role of chlorine ion. Water Res. 2021, 202, 117396. [Google Scholar] [CrossRef] [PubMed]
- Prinz, N.; Korez, Š. Understanding How Microplastics Affect Marine Biota on the Cellular Level Is Important for Assessing Ecosystem Function: A Review. In OUMARES 9—The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 101–120. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dovzhenko, N.V.; Chelomin, V.P.; Kukla, S.P.; Slobodskova, V.V.; Mazur, A.A. Enhanced Toxicity of Polymethylmethacrylate Microparticles on Cells and Tissue of the Marine Mussel Mytilus trossulus After UV Irradiation. Toxics 2025, 13, 818. https://doi.org/10.3390/toxics13100818
Dovzhenko NV, Chelomin VP, Kukla SP, Slobodskova VV, Mazur AA. Enhanced Toxicity of Polymethylmethacrylate Microparticles on Cells and Tissue of the Marine Mussel Mytilus trossulus After UV Irradiation. Toxics. 2025; 13(10):818. https://doi.org/10.3390/toxics13100818
Chicago/Turabian StyleDovzhenko, Nadezhda Vladimirovna, Victor Pavlovich Chelomin, Sergey Petrovich Kukla, Valentina Vladimirovna Slobodskova, and Andrey Alexandrovich Mazur. 2025. "Enhanced Toxicity of Polymethylmethacrylate Microparticles on Cells and Tissue of the Marine Mussel Mytilus trossulus After UV Irradiation" Toxics 13, no. 10: 818. https://doi.org/10.3390/toxics13100818
APA StyleDovzhenko, N. V., Chelomin, V. P., Kukla, S. P., Slobodskova, V. V., & Mazur, A. A. (2025). Enhanced Toxicity of Polymethylmethacrylate Microparticles on Cells and Tissue of the Marine Mussel Mytilus trossulus After UV Irradiation. Toxics, 13(10), 818. https://doi.org/10.3390/toxics13100818





