The Contamination of Microplastic Debris in Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) from Artisanal Fisheries in the Eastern Gulf of Thailand
Abstract
1. Introduction
2. Materials and Methods
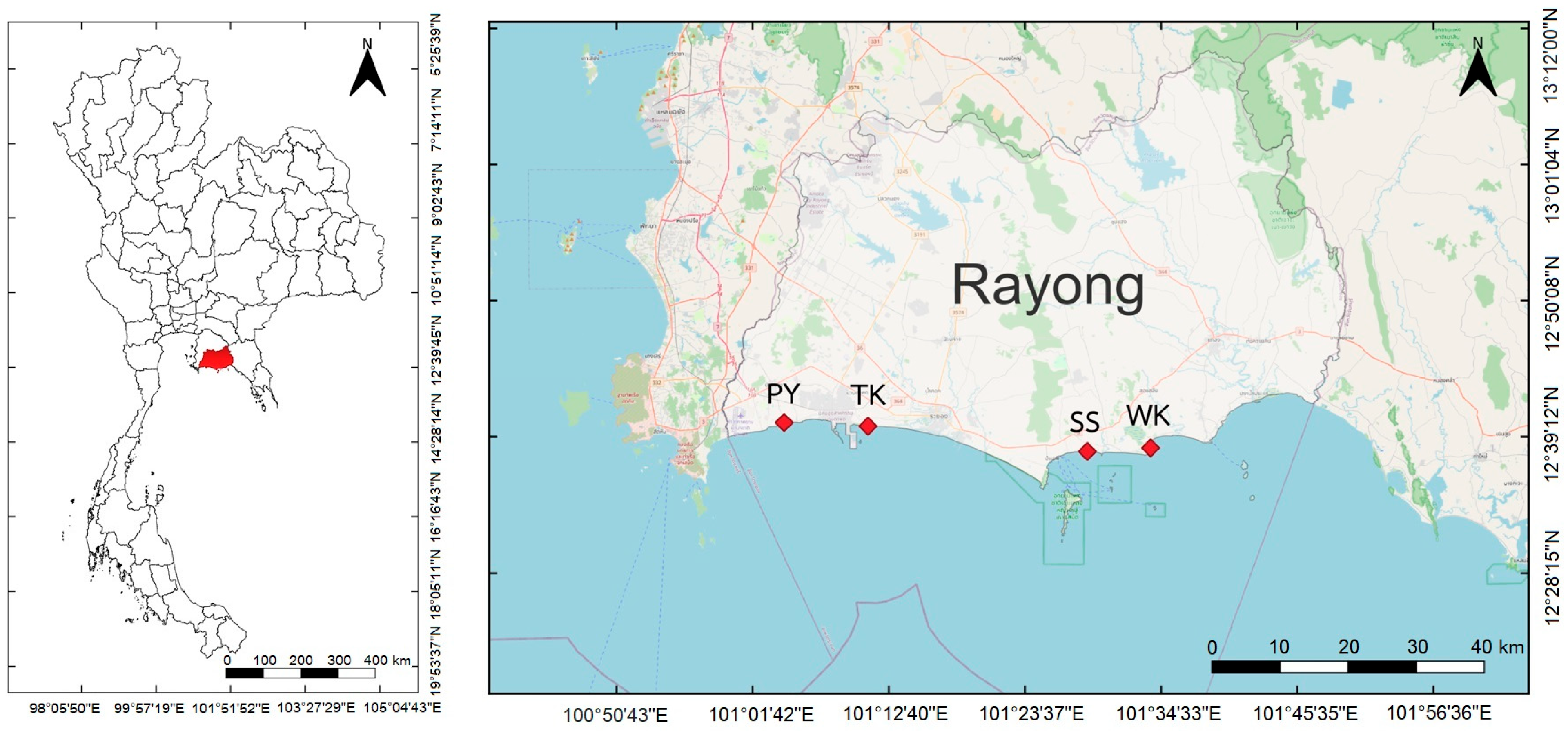
2.1. Study Area and Sampling Sites
2.2. Fisheries Data Gathering
2.3. Sampling Method
2.4. Microplastics Digestion and Separation
2.5. Microplastics Visualization and Identification
2.6. Data Analysis
2.7. Quality Control
3. Results and Discussion
3.1. Blue Swimming Crab Fishing Ground
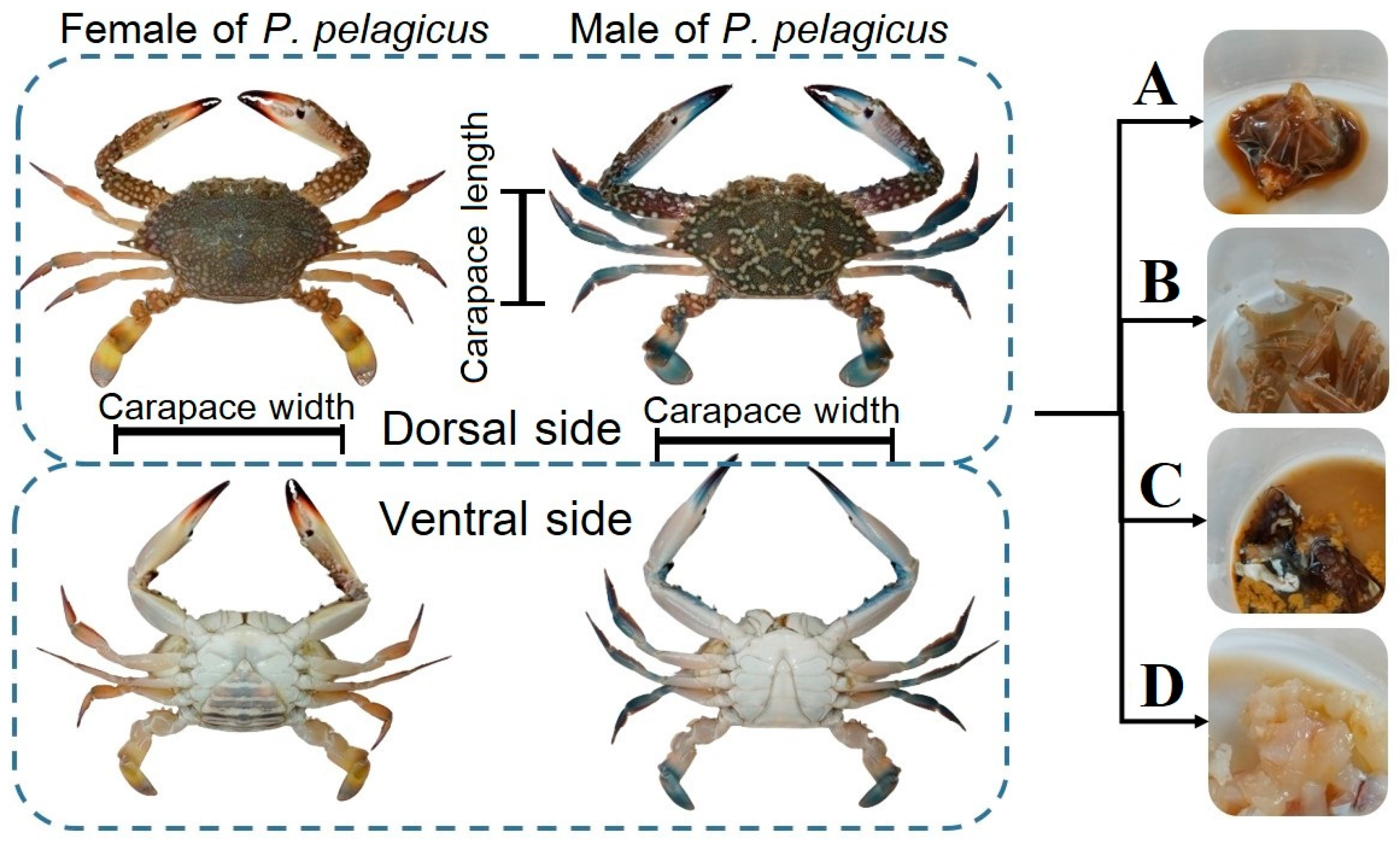
3.2. Crab Characteristics
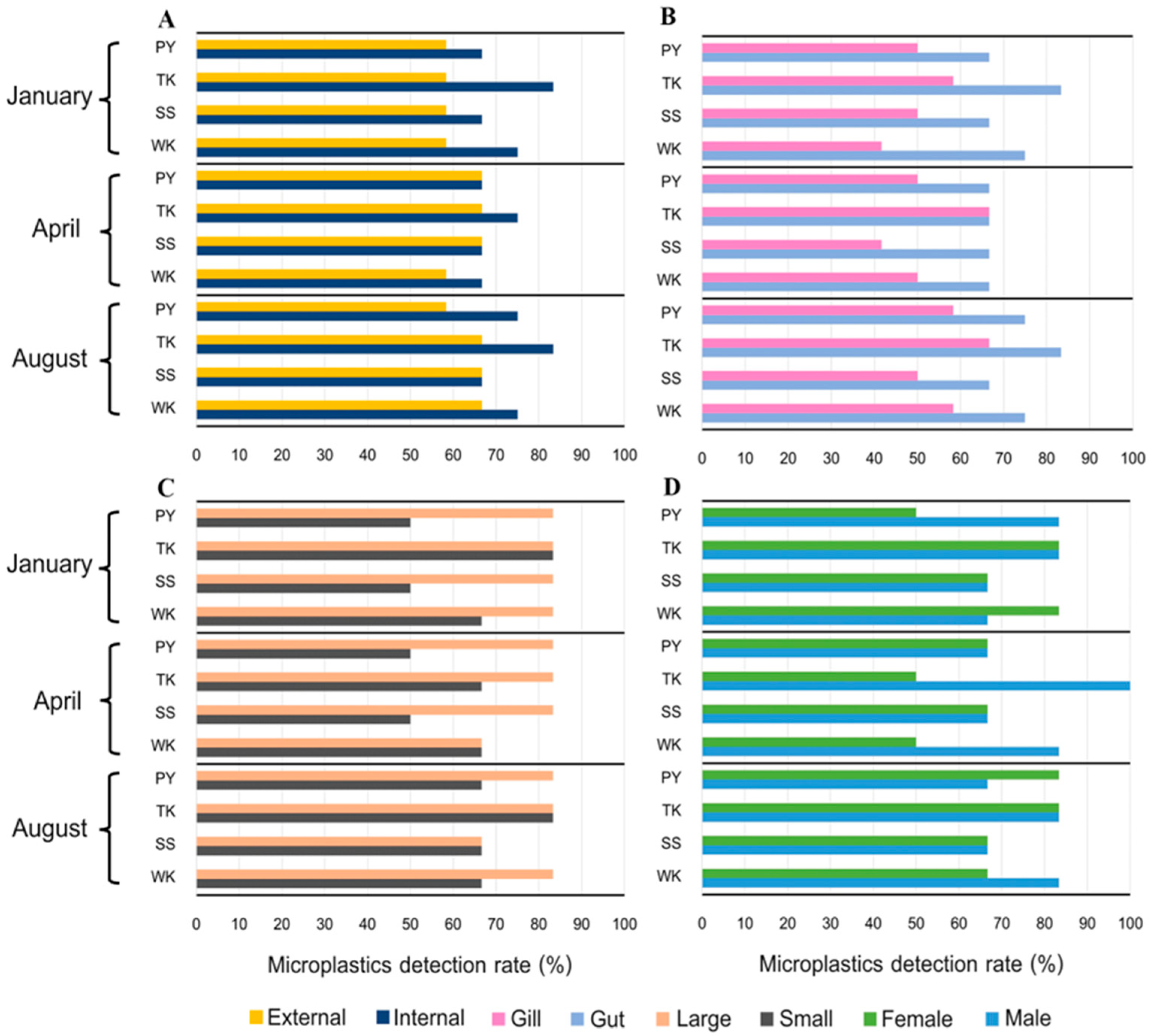
3.3. Microplastics Detection Rate
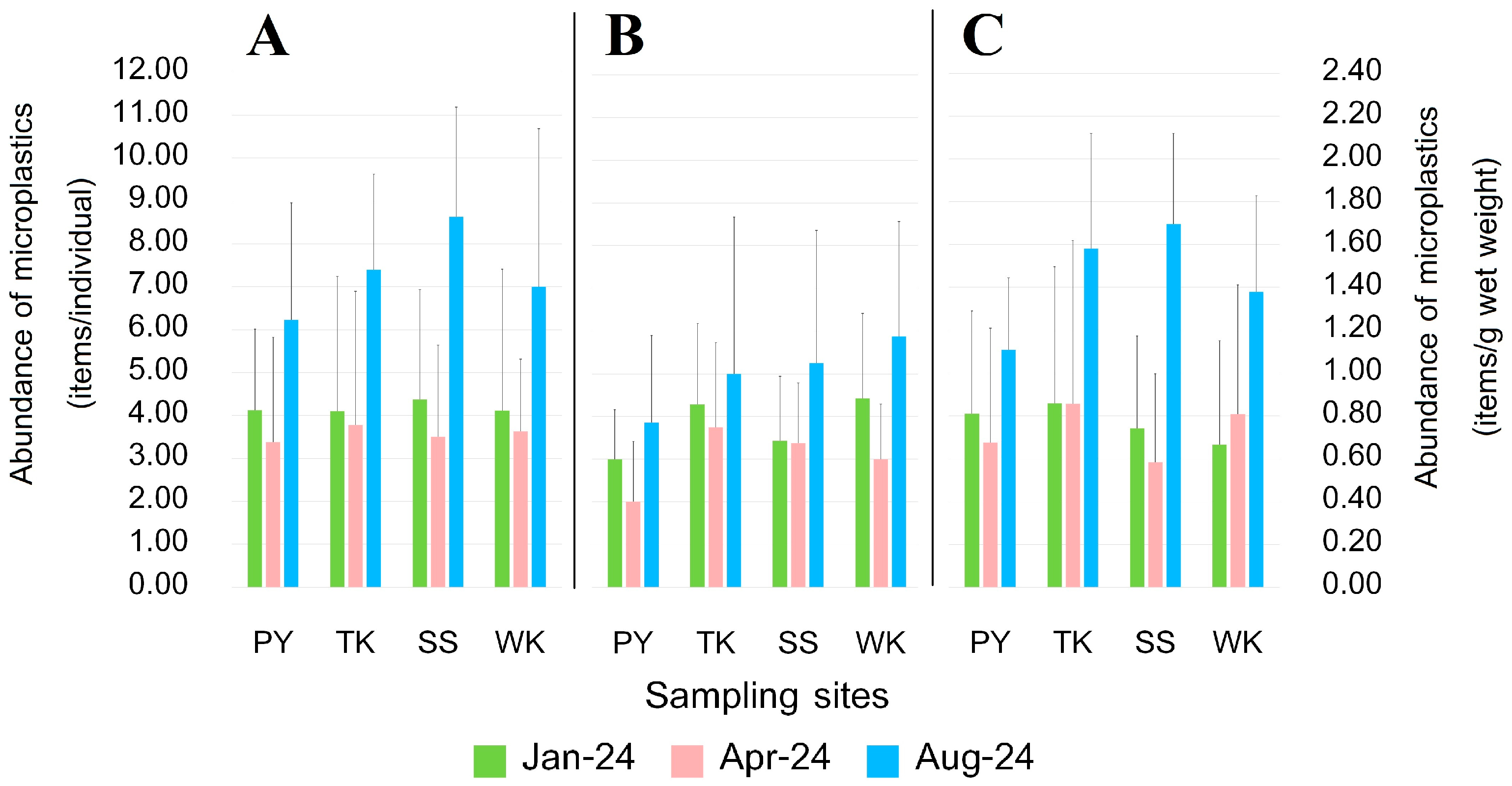
3.4. Abundance of MPs
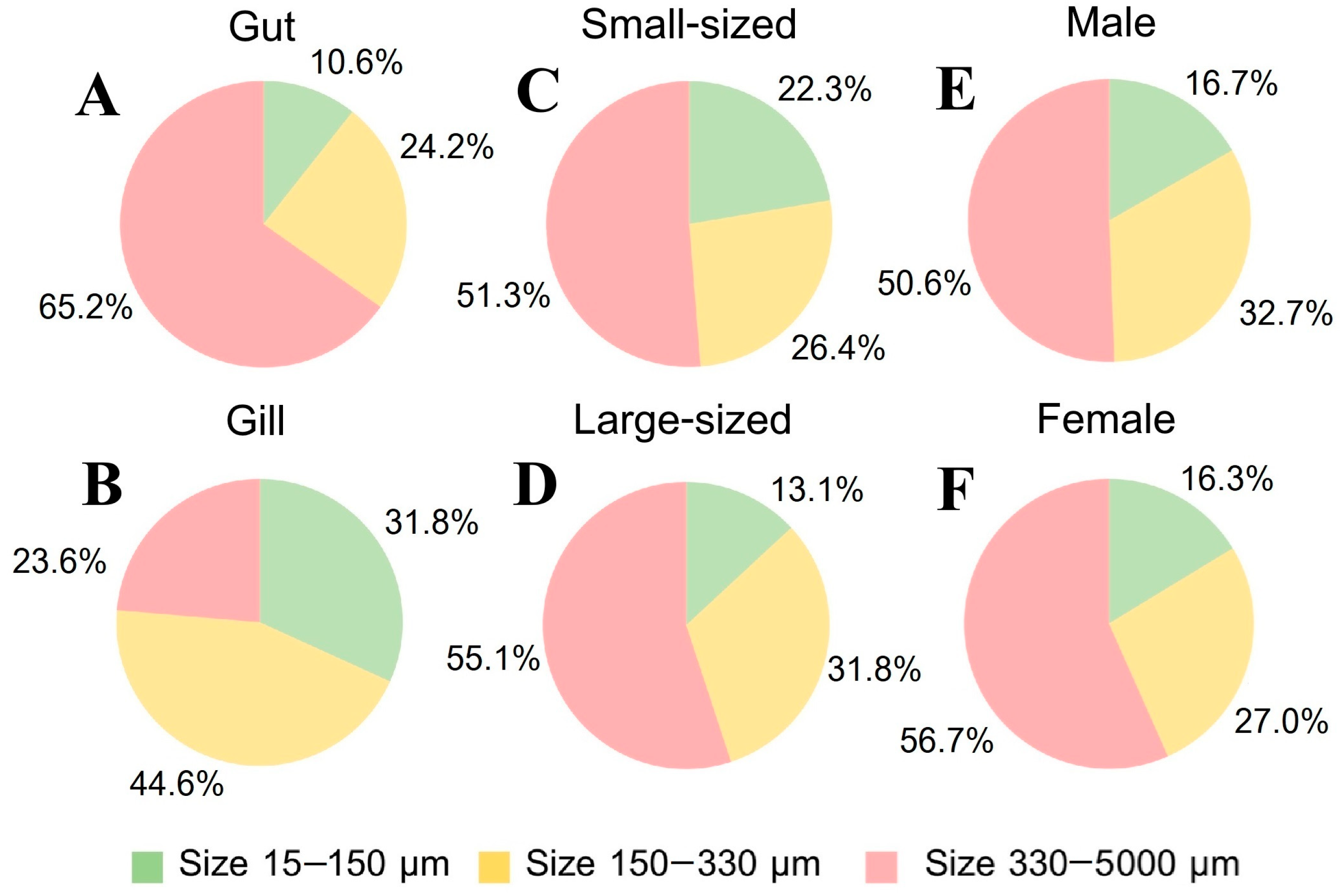
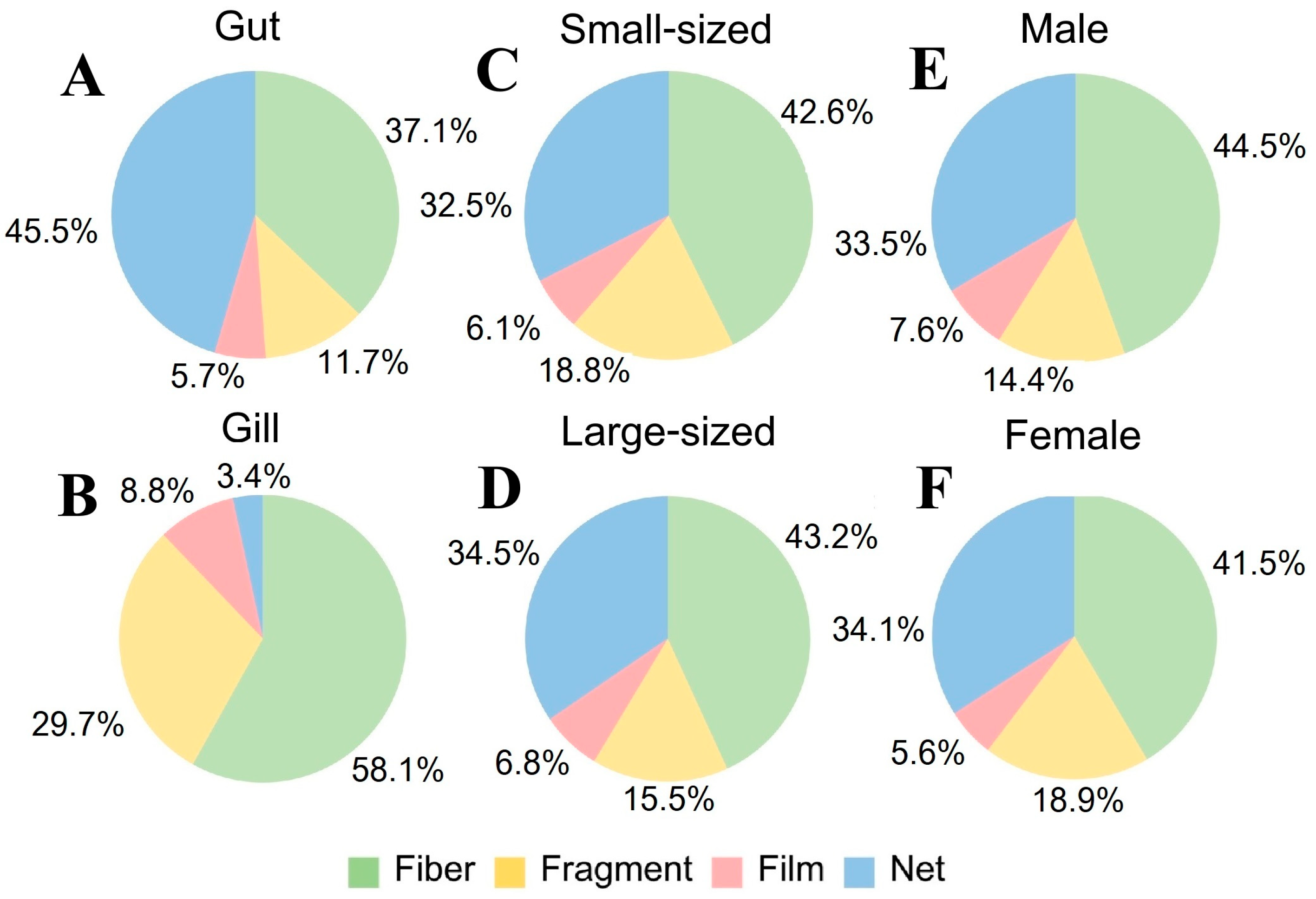
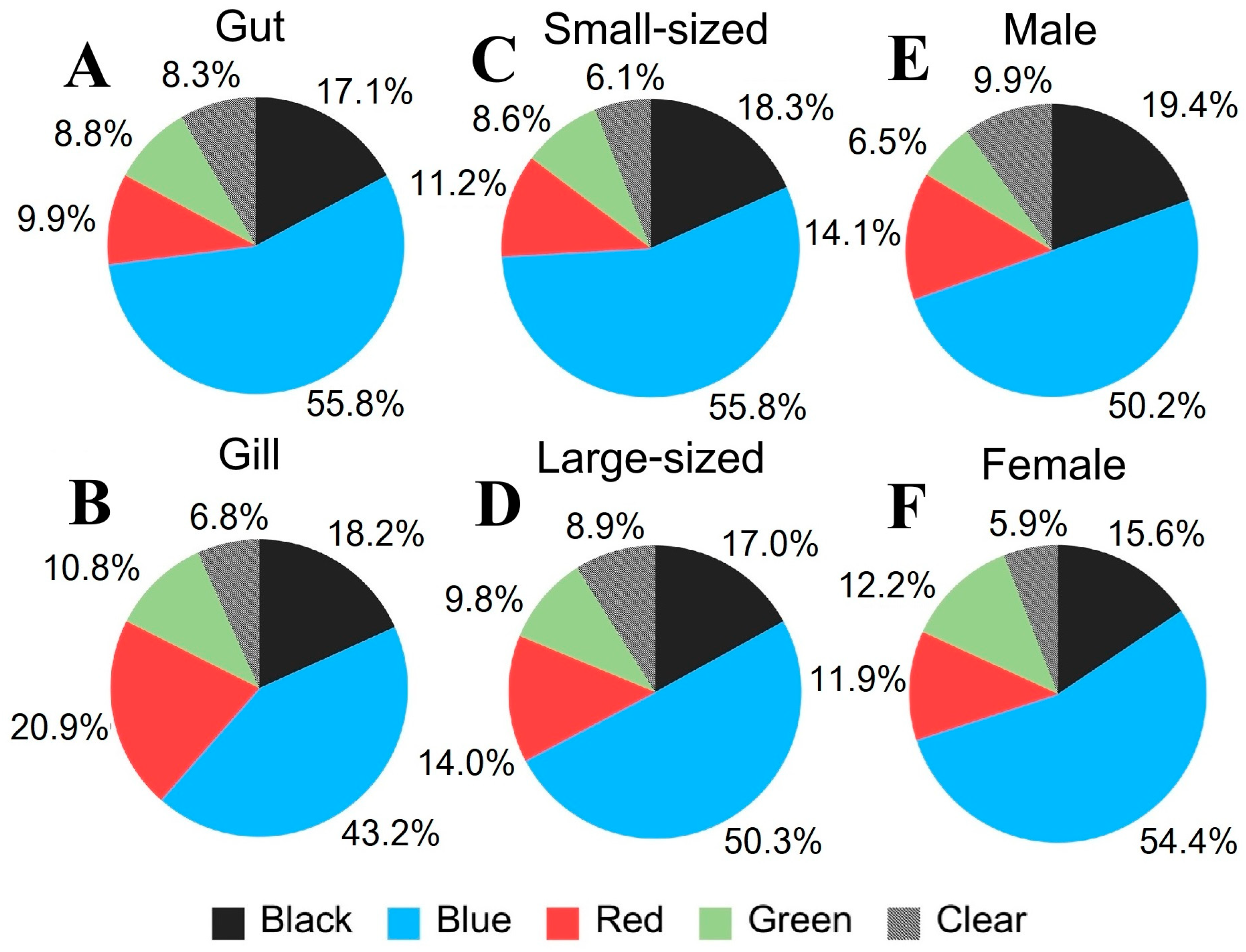
3.4.1. Crab Tissue
3.4.2. Crab Size
3.4.3. Crab Sex
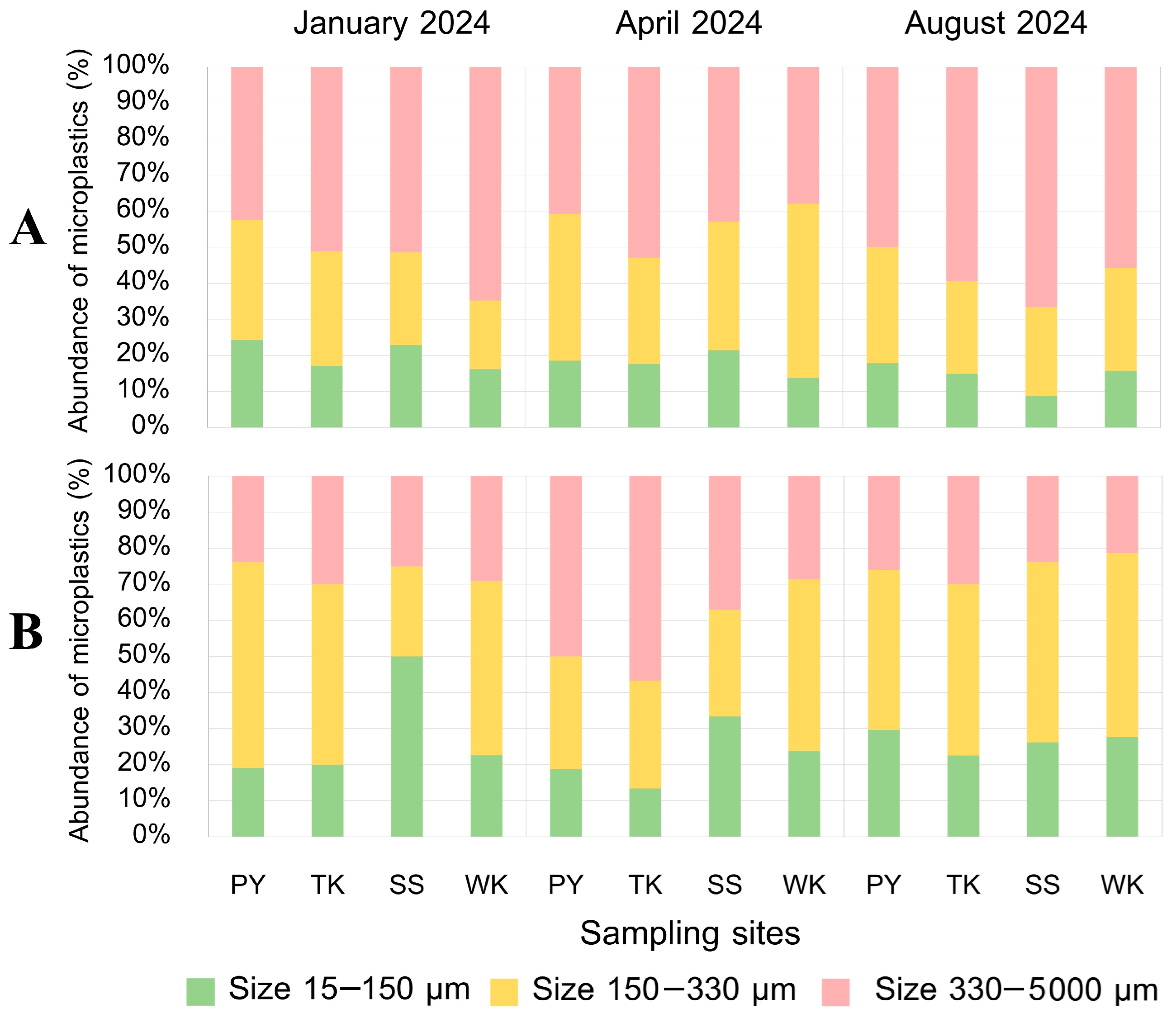
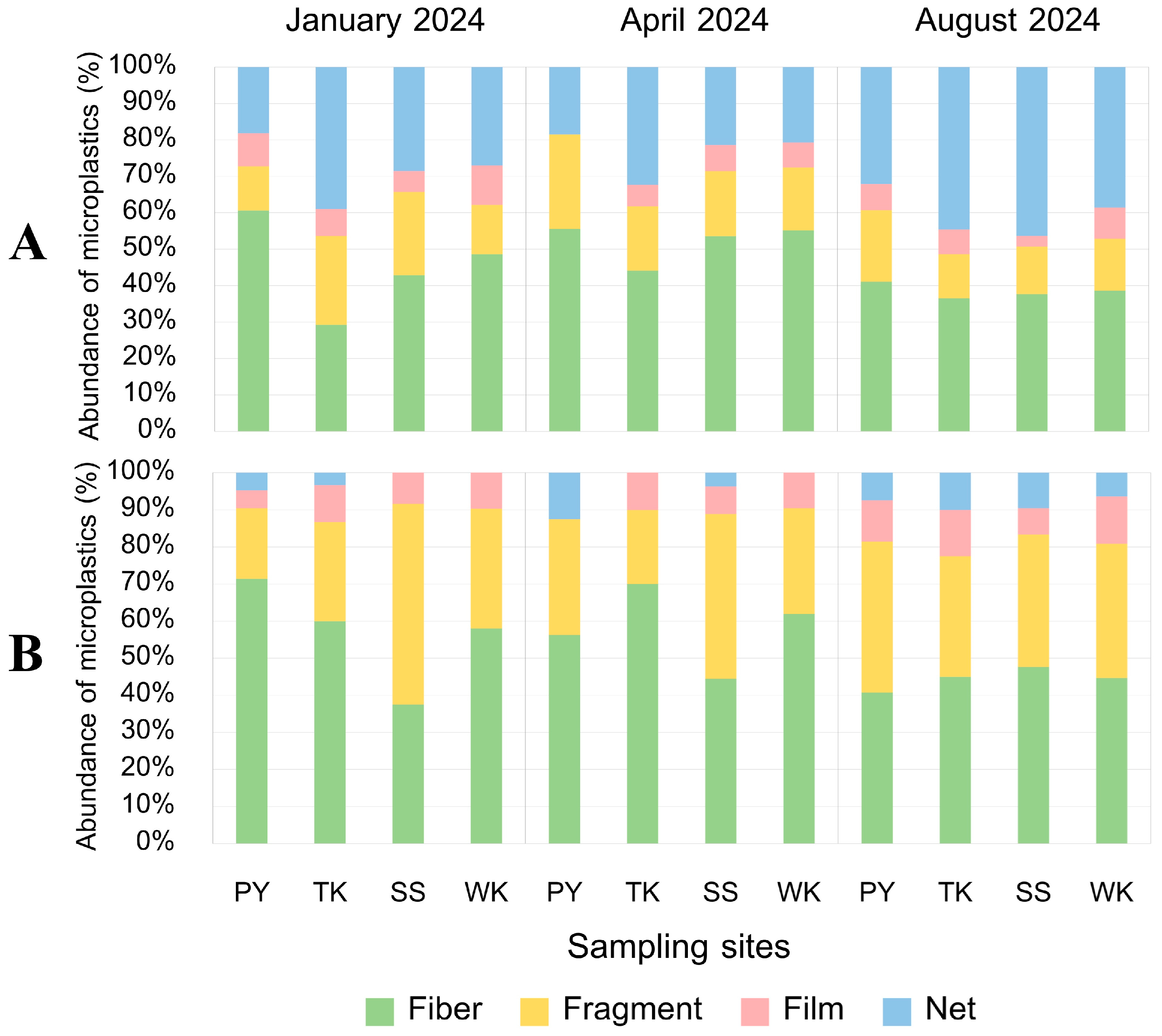
3.5. Characteristics of MPs
3.5.1. MP Size
3.5.2. MP Shape
3.5.3. MP Color
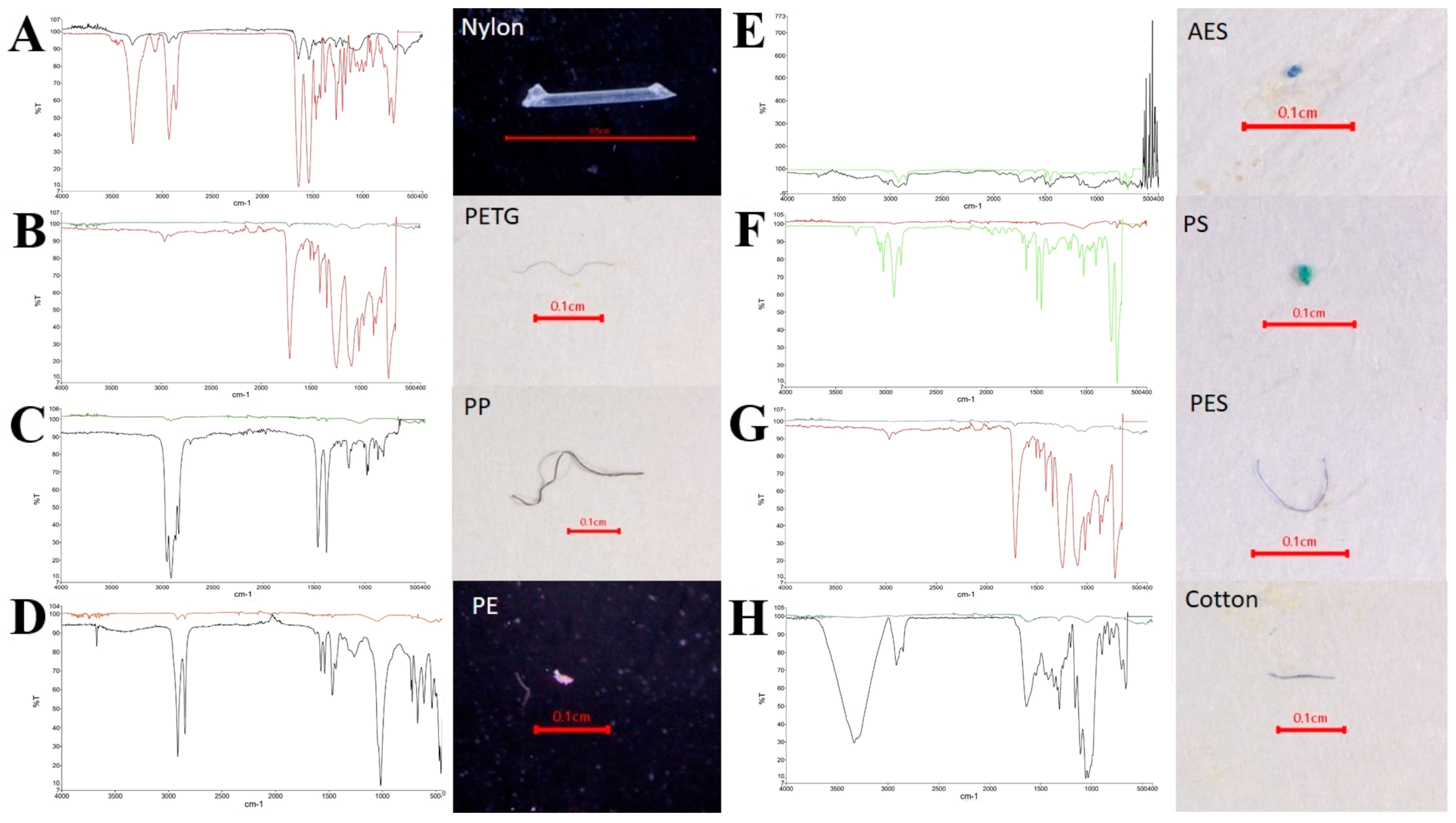
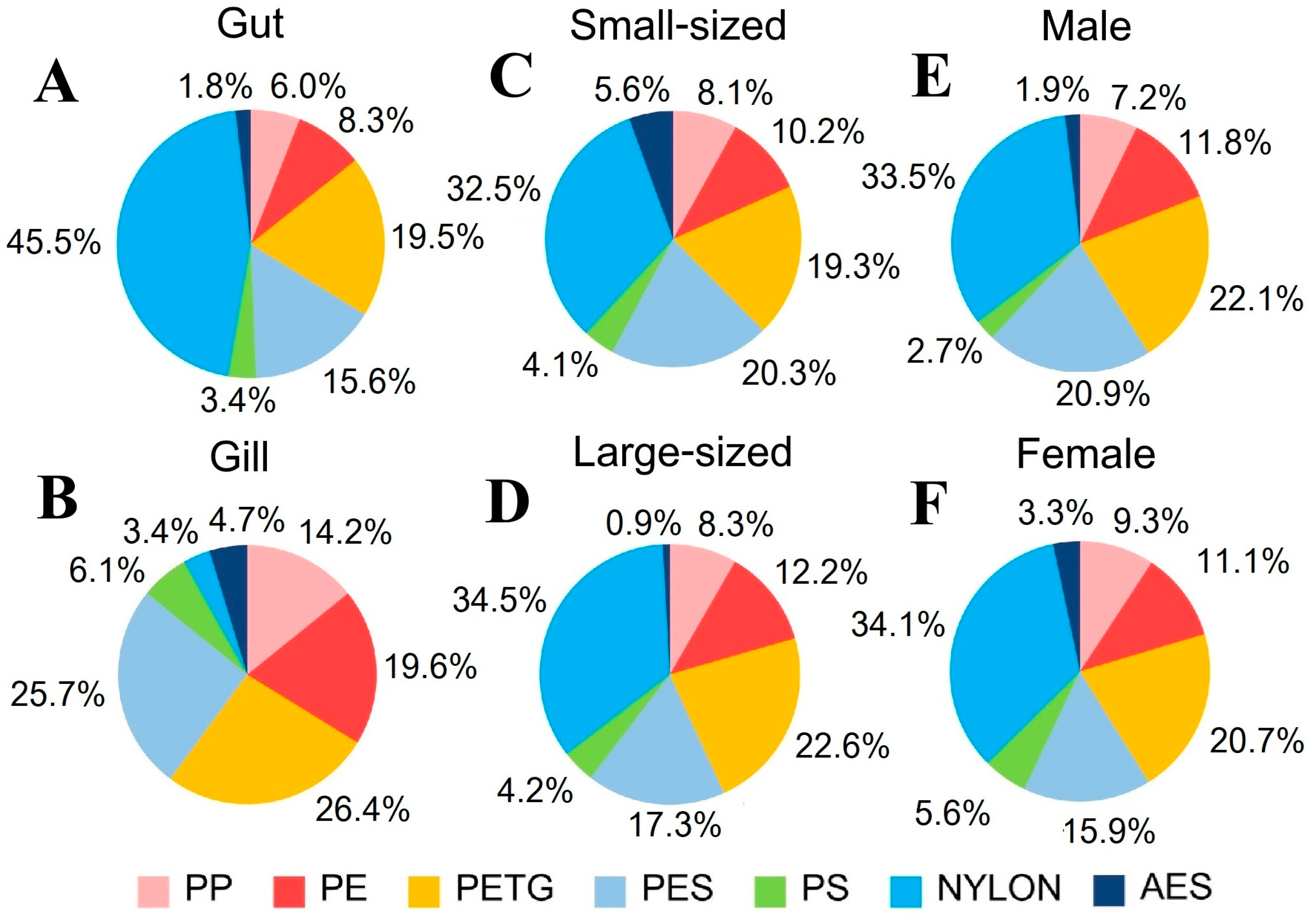
3.5.4. MP Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| PY | The station is called “Payoon” |
| TK | The station is called “Takuan” |
| SS | The station is called “Suanson” |
| WK | The station is called “Wangkaew” |
| PP | Polypropylene |
| PE | Polyethylene |
| PETG | Polyethylene Terephthalate Glycol |
| PES | Polyester |
| PS | Polystyrene |
| AES | poly (Acrylonitrile Ethylene Styrene) |
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–772. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Chavanich, S.; Yakupitiyage, A. Coastal debris analysis in beaches of Chonburi Province, eastern of Thailand as implications for coastal conservation. Mar. Pollut. Bull. 2017, 116, 121–129. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Reinert, T.R.; Spellman, A.C.; Bassett, B.L. Entanglement in and ingestion of fishing gear and other marine debris by Florida manatees, 1993 to 2012. Endanger. Species Res. 2017, 32, 415–427. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Lee, J.; Hong, S.; Mok, J.Y.; Kim, K.S.; Lee, Y.J.; Choi, H.-W.; Kang, H.; Lee, S. Estimation of the annual flow and stock of marine debris in South Korea for management purposes. Mar. Pollut. Bull. 2014, 86, 505–511. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Sterl, M.F.; Delandmeter, P.; van Sebille, E. Influence of barotropic tidal currents on transport and accumulation of floating microplastics in the global open ocean. J. Geophys. Res. Oceans 2020, 125, e2019JC015583. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Marine Litter: A Global Challenge; United Nations Environment Programme: Washington, DC, USA, 2009; 232p, Available online: https://www.unep.org/resources/report/marine-litter-global-challenge (accessed on 17 December 2024).
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Lusher, A.; Bråte, I.L.N.; Hurley, R.; Iversen, K.; Olsen, M. Testing of Methodology for Measuring Microplastics in Blue Mussels (Mytilus spp.) and Sediments, and Recommendations for Future Monitoring of Microplastics (R & D-Project); Norsk Institutt for Vannforskning: Oslo, Norwegen, 2017; 87p, Available online: https://www.researchgate.net/publication/321724113_Testing_of_methodology_for_measuring_microplasti… (accessed on 8 October 2024).
- Thompson, R.; Olsen, Y.; Mitchell, R.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Jendanklang, P.; Meksumpun, S.; Pokavanich, T.; Ruengsorn, C.; Kasamesiri, P. Distribution and flux assessment of microplastic debris in the middle and lower Chao Phraya River, Thailand. J. Water Health 2023, 21, 771–788. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P. Microplastic pollution in surface seawater and beach sand from the shore of Rayong province, Thailand: Distribution, characterization, and ecological risk assessment. Mar. Pollut. Bull. 2022, 174, 113200. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P.; Chouychai, B. Microplastic occurrence in surface sediments from coastal mangroves in Eastern Thailand: Abundance, characteristics, and ecological risk implications. Reg. Stud. Mar. Sci. 2024, 71, 103389. [Google Scholar] [CrossRef]
- Chaisanguansuk, P.; Ploenbuppa, S.; Assawincharoenkij, T.; Phantuwongraj, S.; Jiraphinyakul, A. Microplastic contamination in the coastal environment: A case study from the Mae Klong River, Samut Songkhram. App. Environ. Res. 2023, 45, 2. [Google Scholar] [CrossRef]
- Kashfi, F.S.; Ramavandi, B.; Arfaeinia, H.; Mohammadi, A.; Saeedi, R.; De-la-Torre, G.E.; Dobaradaran, S. Occurrence and exposure assessment of microplastics in indoor dusts of buildings with different applications in Bushehr and Shiraz cities, Iran. Sci. Total Environ. 2022, 829, 154651. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Amouei Torkmahalleh, M.; Saeedi, R.; Aibaghi, R.; Faraji Ghasemi, F. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environ. Res. 2021, 192, 110339. [Google Scholar] [CrossRef]
- Yamchomchuen, S.; Meksumpun, S.; Ruengsorn, C.; Phaksopa, J. Contamination of microplastics in zooplankton: A case study in the coastal area Chonburi province. In Proceedings of 61st Kasetsart University Annual Conference; Kasetsart University Research and Development Institute: Bangkok, Thailand, 2023; pp. 154–163. Available online: https://annualconference.ku.ac.th/61/eproc/E-ProceedingKUConf61-1.pdf (accessed on 20 November 2024). (In Thai)
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Hongsawat, P.; Thinjong, W.; Chouychai, B.; Punyapalakul, P.; Prarat, P. Microplastics in retail shellfish from a seafood market in eastern Thailand: Occurrence and risks to human food safety. Mar. Pollut. Bull. 2024, 201, 116228. [Google Scholar] [CrossRef] [PubMed]
- Phaksopa, J.; Sukhsangchan, R.; Keawsang, R.; Tanapivattanakul, K.; Thamrongnawasawat, T.; Worachananant, S.; Sreesamran, P. Presence and characterization of microplastics in coastal fish around the Eastern Coast of Thailand. Sustainability 2021, 13, 13110. [Google Scholar] [CrossRef]
- Kimura, R.; Inoguchi, E.; Kitayama, C.; Michishita, M.; Fujinuma, R. Microplastic ingestion by sea turtles around Tokyo Bay: Level of water pollution influences ingestion amounts. Mar. Pollut. Bull. 2024, 206, 116673. [Google Scholar] [CrossRef]
- van Franeker, J.A.; Blaize, C.; Danielsen, J.; Fairclough, K.; Gollan, J.; Guse, N.; Hansen, P.L.; Heubeck, M.; Jensen, J.-K.; Le Guillou, G.; et al. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ. Pollut. 2011, 159, 2609–2615. [Google Scholar] [CrossRef]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in beluga whales (Delphinapterus leucas) from the eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Jeong, E.; Lee, J.; Redwan, M. Animal exposure to microplastics and health effects: A review. Emerg. Contam. 2024, 10, 100369. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Macku’ak, T.; Žemlička, L.; Bírošová, L. Review microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Kunsook, C.; Gajaseni, N.; Paphavasit, N. The feeding ecology of the blue swimming crab, Portunus pelagicus (Linnaeus, 1758), at Kung Krabaen Bay, Chanthaburi Province, Thailand. Trop. Life Sci. Res. 2014, 25, 13–27. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4156471/pdf/tlsr-25-1-13.pdf (accessed on 17 November 2024). [PubMed]
- Lai, J.C.Y.; Ng, P.K.L.; Davie, P.J.F. A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. Raffles Bull. Zool. 2010, 58, 199–237. [Google Scholar] [CrossRef]
- DOF. Statistics of Marine Capture of Artisanal Fisheries 2023; Department of Fisheries, Ministry of Agriculture and Cooperatives: Bangkok, Thailand, 2024; 143p. Available online: https://www4.fisheries.go.th/local/file_document/20240614145556_new.pdf (accessed on 20 October 2024).
- Chanchiem, T.; Boutson, A.; Kaewnern, M.; Ebata, K.; Arimoto, T. Study on Bycatch and Discards of Bottom Crab Gillnet Targeting Blue Swimming Crab (Portunus pelagicus) in Rayong Province, Thailand. SEAFDEC Technical Seminar 2015. Available online: https://repository.seafdec.or.th/bitstream/handle/20.500.12067/1383/4_Thaweesak%28KU%29-Study%20of%20bycatch_crab%20gillnet.pdf?sequence=1&isAllowed=y (accessed on 20 December 2024).
- Kleawkla, N. Microplastic Fragments in Stomach Content of Blue Swimming Crab, Portunus pelagicus from Wonnapha Coastal Wetland, Chonburi Province, Thailand. Ramkhamhaeng Int. J. Sci. Technol. 2019, 2, 7–16. Available online: https://ph02.tci-thaijo.org/index.php/RIST/article/view/244995 (accessed on 15 December 2024).
- Jittalerk, R.; Babel, S. Microplastic Contamination in Thai Vinegar Crabs (Episesarma mederi), Giant Mudskippers (Periophthalmodon schlosseri), and Their Surrounding Environment from the Bang Pu Mangrove Forests, Samut Prakan Province, Thailand. Mar. Pollut. Bull. 2024, 198, 115849. [Google Scholar] [CrossRef] [PubMed]
- Bissen, R.; Chawchai, S. Microplastics on Beaches along the Eastern Gulf of Thailand—A Preliminary Study. Mar. Pollut. Bull. 2020, 157, 111345. [Google Scholar] [CrossRef]
- Boutson, A.; Ebata, K.; Ishikawa, S.; Watanabe, K.; Arimoto, T. Field Guides on Small-Scale Fisheries in Rayong, Thailand; Research Institute for Humanity and Nature: Kyoto, Japan, 2016; 73p, Available online: https://www.chikyu.ac.jp/rihn/publicity/detail/184/ (accessed on 16 October 2024).
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Technical Memorandum NOS-OR&R-48; NOAA Institutional Repository: Silver Spring, MD, USA, 2015; 31p. [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality Assessment of the Blue Mussel (Mytilus edulis): Comparison Between Commercial and Wild Types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Song, K.; Du, W.; Huang, W.; Gu, Z.; Feng, Z. Microplastics in Different Tissues of Wild Crabs at Three Important Fishing Grounds in China. Chemosphere 2021, 271, 129479. [Google Scholar] [CrossRef]
- Cordova, M.R.; Purwiyanto, A.I.S.; Suteja, Y. Abundance and Characteristics of Microplastics in the Northern Coastal Waters of Surabaya, Indonesia. Mar. Pollut. Bull. 2019, 142, 183–188. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Wu, W.; Wang, L.; Liu, J.; An, L.; Xu, Q. Microplastic Characteristics in Organisms of Different Trophic Levels from Liaohe Estuary, China. Sci. Total Environ. 2021, 789, 148027. [Google Scholar] [CrossRef]
- Waite, H.R.; Donnelly, M.J.; Walters, L.J. Quantity and Types of Microplastics in the Organic Tissues of the Eastern Oyster Crassostrea virginica and Atlantic Mud Crab Panopeus herbstii from a Florida Estuary. Mar. Pollut. Bull. 2018, 129, 179–185. [Google Scholar] [CrossRef]
- Piarulli, S.; Scapinello, S.; Comandini, P.; Magnusson, K.; Granberg, M.; Wong, J.X.; Sciutto, G.; Prati, S.; Mazzeo, R.; Booth, A.M.; et al. Microplastic in Wild Populations of the Omnivorous Crab Carcinus aestuarii: A Review and a Regional-Scale Test of Extraction Methods, Including Microfibres. Environ. Pollut. 2019, 251, 117–127. [Google Scholar] [CrossRef]
- Horn, D.; Miller, M.; Anderson, S.; Steele, C. Microplastics are Ubiquitous on California Beaches and Enter the Coastal Food Web through Consumption by Pacific Mole Crabs. Mar. Pollut. Bull. 2019, 139, 231–237. [Google Scholar] [CrossRef]
- Rabari, V.; Patel, H.; Ali, D.; Yadav, V.K.; Patel, A.; Sahoo, D.K.; Trivedi, J. Ingestion and Polymeric Risk Assessment of Microplastic Contamination in Commercially Important Brachyuran Crab Portunus sanguinolentus. Front. Mar. Sci. 2023, 10, 1286782. [Google Scholar] [CrossRef]
- Sucharitakul, P.; Wu, W.M.; Zhang, Y.; Peng, B.Y.; Gao, J.; Wang, L.; Hou, D. Exposure Pathways and Toxicity of Microplastics in Terrestrial Insects. Environ. Sci. Technol. 2024, 58, 11887–11900. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.J.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R. Uptake and Retention of Microplastics by the Shore Crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Astutik, S.; Abida, I.W. Microplastics in the Gastrointestinal Tract of Blue Crab (Portunus pelagicus) Caught by Bandaran, Bangkalan Fishermen at Different Sizes. Symp. Biol. Educ. 2023, 3, 87–94. [Google Scholar] [CrossRef]
- Griffen, B.D. Metabolic Costs of Capital Energy Storage in a Small-Bodied Ectotherm. Ecol. Evol. 2017, 7, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.T. Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance. In Functional Morphology of Feeding and Grooming in Crustaceans; Felgenhaur, B.E., Walting, L., Thistle, A.B., Eds.; A.A. Balkema: Rotterdam, The Netherlands, 1989; pp. 49–58. Available online: https://research.nhm.org/pdfs/31534/31534.pdf (accessed on 12 December 2024).
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, Characteristics, and Seasonal Variation of Microplastics in Indian White Shrimps (Fenneropenaeus indicus) from Coastal Waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [CrossRef]
- Kasamesiri, P.; Meksumpun, C.; Meksumpun, S.; Ruengsorn, C. Assessment on Microplastics Contamination in Freshwater Fish: A Case Study of the Ubolratana Reservoir, Thailand. GEOMATE J. 2021, 20, 62–68. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M. The Contribution of Washing Processes of Synthetic Clothes to Microplastic Pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Sathish, N.; Jeyasanta, K.I.; Patterson, J. Abundance, Characteristics and Surface Degradation Features of Microplastics in Beach Sediments of Five Coastal Areas in Tamil Nadu, India. Mar. Pollut. Bull. 2019, 142, 112–118. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating Microplastics Bioaccumulation and Biomagnification in Seafood from the Persian Gulf: A Threat to Human Health? Food Addit. Contam. 2019, 36, 1696–1708. [Google Scholar] [CrossRef]
- Piskuła, P.; Astel, A.M. Microplastics in Commercial Fishes and By-Catch from Selected FAO Major Fishing Areas of the Southern Baltic Sea. Animals 2023, 13, 458. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Cocca, M.; Dorigato, G.; Giaccone, V.; Colavita, G. Impact of Fibrous Microplastic Pollution on Commercial Seafood and Consumer Health: A Review. Animals 2023, 13, 1736. [Google Scholar] [CrossRef]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, Composition, and Fate of Microplastics in Water, Sediment, and Shellfish in the Tapi-Phumduang River System and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef] [PubMed]
- Cerbule, K.; Herrmann, B.; Grimaldo, E.; Brinkhof, J.; Manu, B.S.; Larsen, R.B.; Bak-Jensen, Z. Ghost Fishing Efficiency by Lost, Abandoned or Discarded Pots in Snow Crab (Chionoecetes opilio) Fishery. Mar. Pollut. Bull. 2023, 193, 115249. [Google Scholar] [CrossRef] [PubMed]
- Orasutthikul, S.; Unno, D.; Yokota, H. Effectiveness of Recycled Nylon Fiber from Waste Fishing Net with Respect to Fiber Reinforced Mortar. Constr. Build. Mater. 2017, 146, 594–602. [Google Scholar] [CrossRef]
- Masthawee, P.; Chokesanguan, B.; Pornpatimakorn, S. Study on Monofilament and Multifilament Crab Bottom Gill Net; Southeast Asian Fisheries Development Center, Training Department: Bangkok, Thailand, 1986; Available online: https://repository.seafdec.or.th/bitstream/handle/20.500.12067/223/TDRES10_Crab_BGN.PDF?sequence=1&isAllowed=y (accessed on 11 November 2024).
- Sipe, J.M.; Bossa, N.; Berger, W.; von Windheim, N.; Gall, K.; Wiesner, M.R. From Bottle to Microplastics: Can We Estimate How Our Plastic Products Are Breaking Down? Sci. Total Environ. 2022, 814, 152460. [Google Scholar] [CrossRef] [PubMed]
- Guessasma, S.; Belhabib, S.; Nouri, H. Printability and Tensile Performance of 3D Printed Polyethylene Terephthalate Glycol Using Fused Deposition Modelling. Polymers 2019, 11, 1220. [Google Scholar] [CrossRef]
- Yang, T.; Luo, J.; Nowack, B. Characterization of Nanoplastics, Fibrils, and Microplastics Released During Washing and Abrasion of Polyester Textiles. Environ. Sci. Technol. 2021, 55, 15873–15881. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical Scavenging Polyethylene Films as Antioxidant Active Packaging Materials. Food Control 2020, 109, 106946. [Google Scholar] [CrossRef]
- Ronca, S. Polyethylene. Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 247–278. [Google Scholar] [CrossRef]
- Deshpande, P.C.; Philis, G.; Brattebø, H.; Fet, A.M. Using Material Flow Analysis (MFA) to generate the evidence on plastic waste management from commercial fishing gears in Norway. Resour. Conserv. Recycl. Adv. 2020, 5, 100024. [Google Scholar] [CrossRef]
- Arfin, T.; Mohammad, F.; Yusof, N.A. Applications of polystyrene and its role as a base in industrial chemistry. In Polystyrene: Synthesis, Characteristics and Applications; Lynwood, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 269–280. Available online: http://repo.upertis.ac.id/1928/1/%28Chemistry%20Research%20and%20Applications%29%20Cole%20Lynwood-Polystyrene_%20Synthesis%2C%20Characteristics%20and%20Applications-Nova%20Science%20Pub%20Inc%20%282014%29.pdf (accessed on 22 December 2024).
- Wang, J.; Lee, J.; Kwon, E.E.; Jeong, S. Quantitative analysis of polystyrene microplastic and styrene monomer released from plastic food containers. Heliyon 2023, 9, e15787. [Google Scholar] [CrossRef] [PubMed]



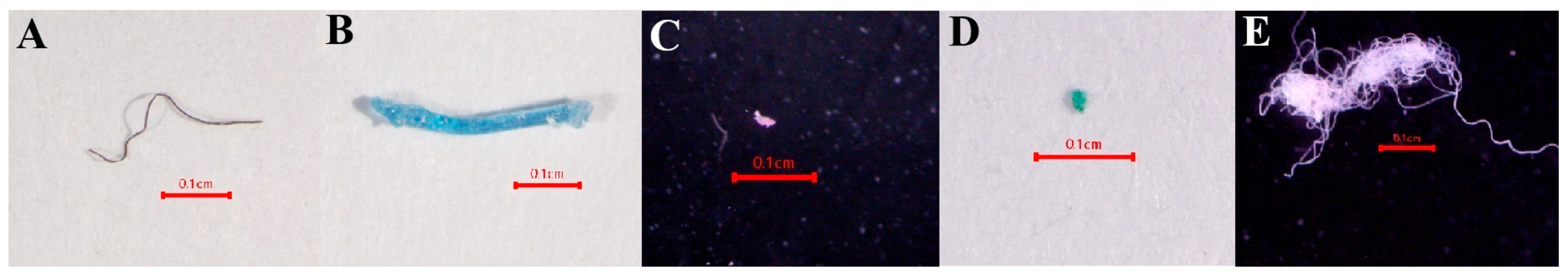
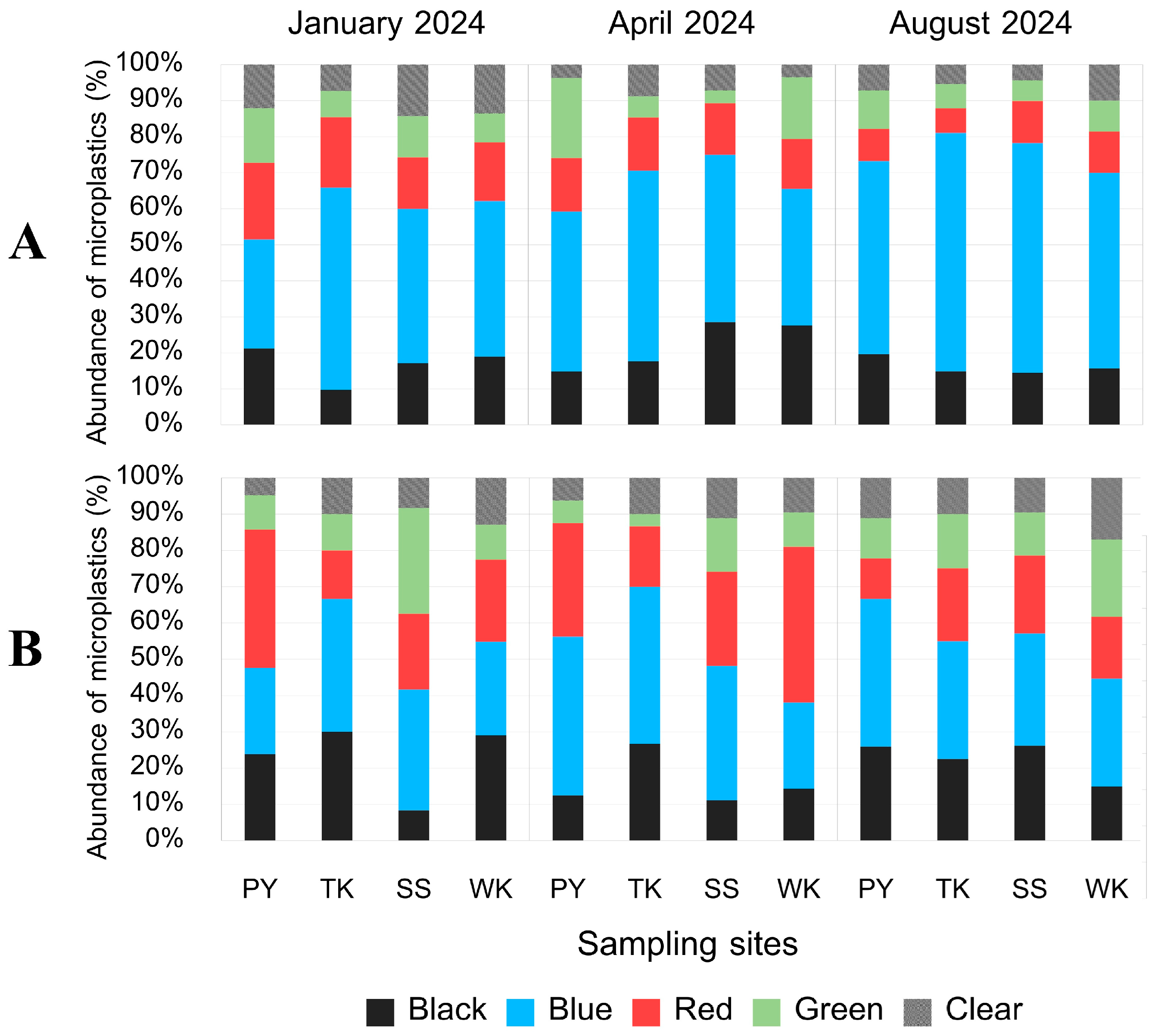

| SITE | PY | TK | SS | WK |
|---|---|---|---|---|
| Crab samples (individual) | 36 | 36 | 36 | 36 |
| Carapace width (cm) | 11.24 ± 0.97 | 11.02 ± 1.08 | 11.33 ± 0.98 | 11.15 ± 0.92 |
| Carapace length (cm) | 5.52 ± 0.54 | 5.38 ± 0.61 | 5.61 ± 0.60 | 5.53 ± 0.53 |
| Total weight (g) | 103.11 ± 25.28 | 97.71 ± 33.77 | 107.15 ± 27.15 | 98.89 ± 26.18 |
| Gut weight (g) | 1.01 ± 0.53 | 1.02 ± 0.51 | 1.01 ± 0.48 | 1.17 ± 0.56 |
| Gill weight (g) | 4.33 ± 1.19 | 4.15 ± 1.66 | 4.71 ± 1.30 | 4.54 ± 1.34 |
| Total external detection (individuals) | 22 | 23 | 23 | 22 |
| Total internal detection (individuals) | 25 | 29 | 24 | 26 |
| Total external MPs (items) | 64 | 100 | 93 | 99 |
| Total internal MPs (items) | 116 | 149 | 132 | 136 |
| MPs in gut (items) | 82 | 107 | 100 | 96 |
| MPs in gill (items) | 34 | 42 | 32 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jendanklang, P.; Ruengsorn, C.; Meksumpun, S.; Kasamesiri, P. The Contamination of Microplastic Debris in Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) from Artisanal Fisheries in the Eastern Gulf of Thailand. Toxics 2025, 13, 813. https://doi.org/10.3390/toxics13100813
Jendanklang P, Ruengsorn C, Meksumpun S, Kasamesiri P. The Contamination of Microplastic Debris in Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) from Artisanal Fisheries in the Eastern Gulf of Thailand. Toxics. 2025; 13(10):813. https://doi.org/10.3390/toxics13100813
Chicago/Turabian StyleJendanklang, Poratape, Chakhrit Ruengsorn, Shettapong Meksumpun, and Pattira Kasamesiri. 2025. "The Contamination of Microplastic Debris in Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) from Artisanal Fisheries in the Eastern Gulf of Thailand" Toxics 13, no. 10: 813. https://doi.org/10.3390/toxics13100813
APA StyleJendanklang, P., Ruengsorn, C., Meksumpun, S., & Kasamesiri, P. (2025). The Contamination of Microplastic Debris in Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) from Artisanal Fisheries in the Eastern Gulf of Thailand. Toxics, 13(10), 813. https://doi.org/10.3390/toxics13100813









