The Protective Effects of Ganoderma lucidum Active Peptide GLP4 on Lung Injury Induced by Cadmium Poisoning in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
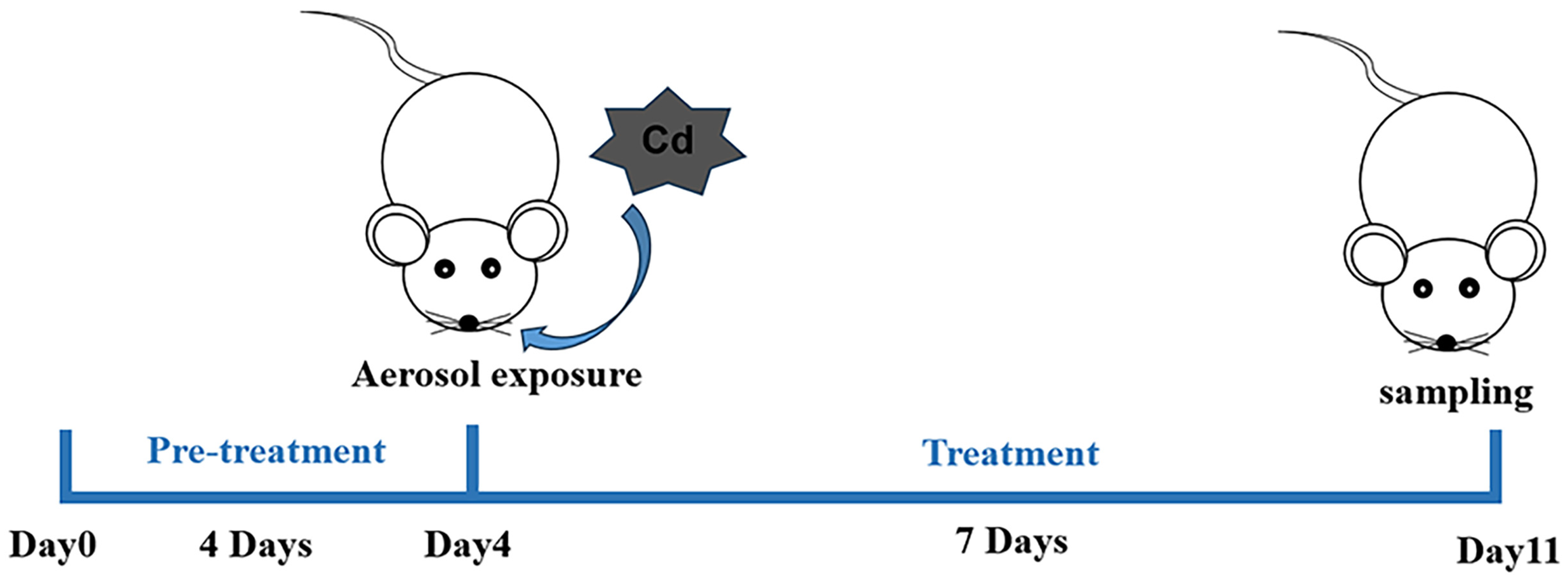
2.2. Animals and Treatments
2.3. Bronchoalveolar Lavage Fluid (BALF) Collection and Cell Counting
2.4. Histological and Morphological Analyses
2.5. Measurement of GOT and GPT Levels in Lung Tissues and BUN in Kidney Tissues, and Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Immunohistochemistry
2.7. Cell Culture and Treatment
2.8. Cell Viability Assay
2.9. Quantitative Real-Time PCR
2.10. Western Blot Assay
2.11. Statistical Analysis
3. Results
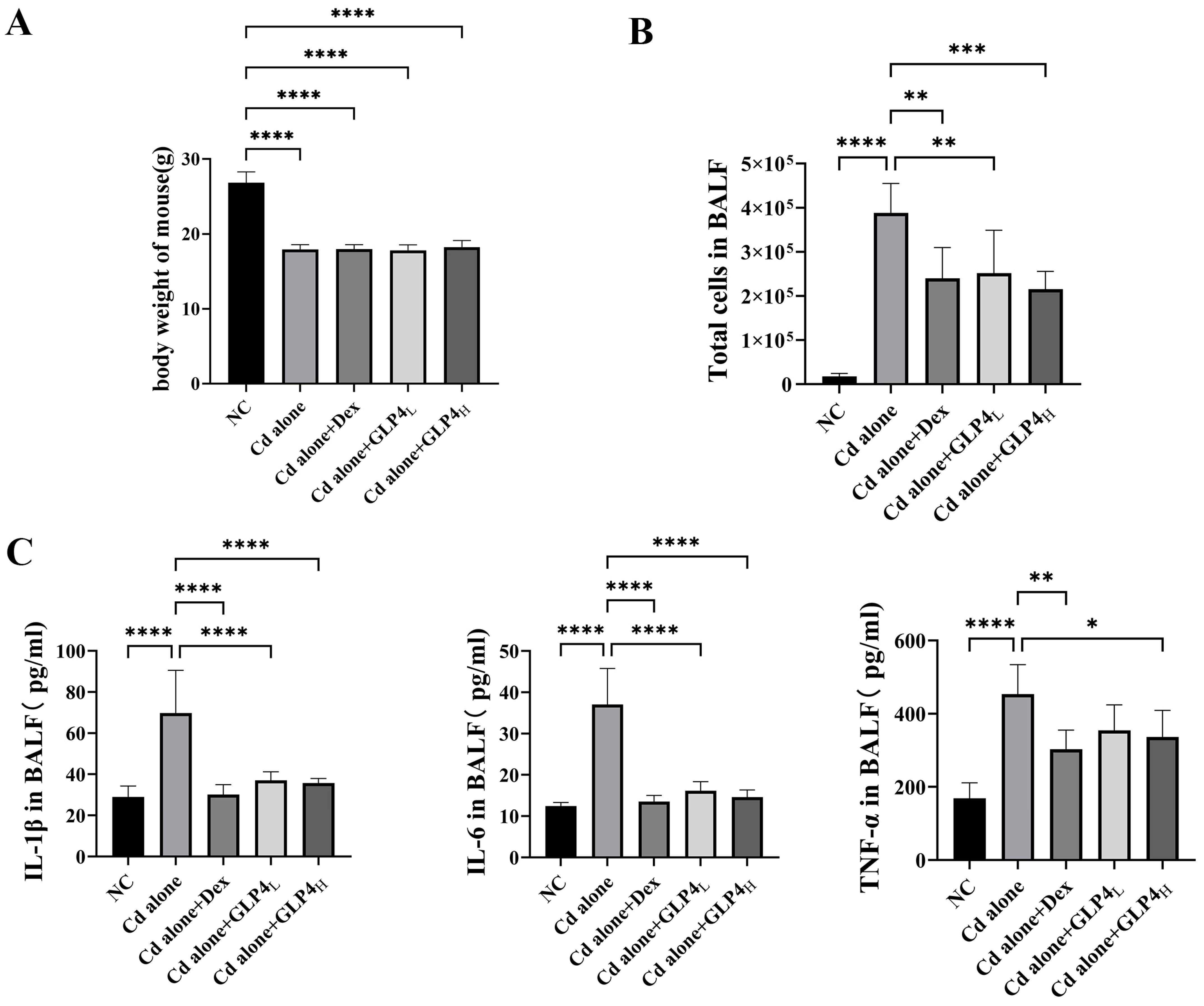
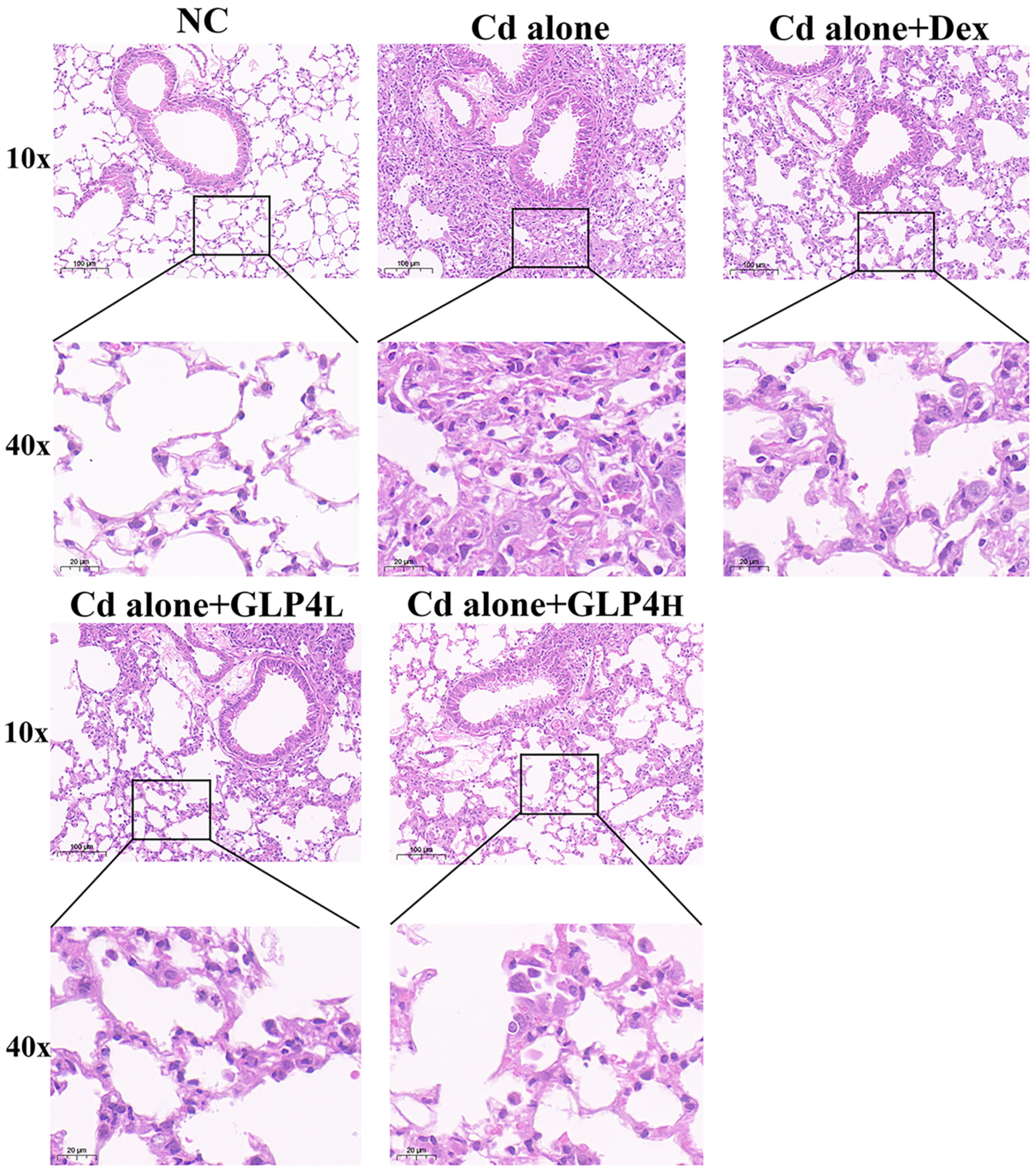
3.1. Protection by GLP4 against Cadmium Poisoning-Induced Lung Injury in Mice
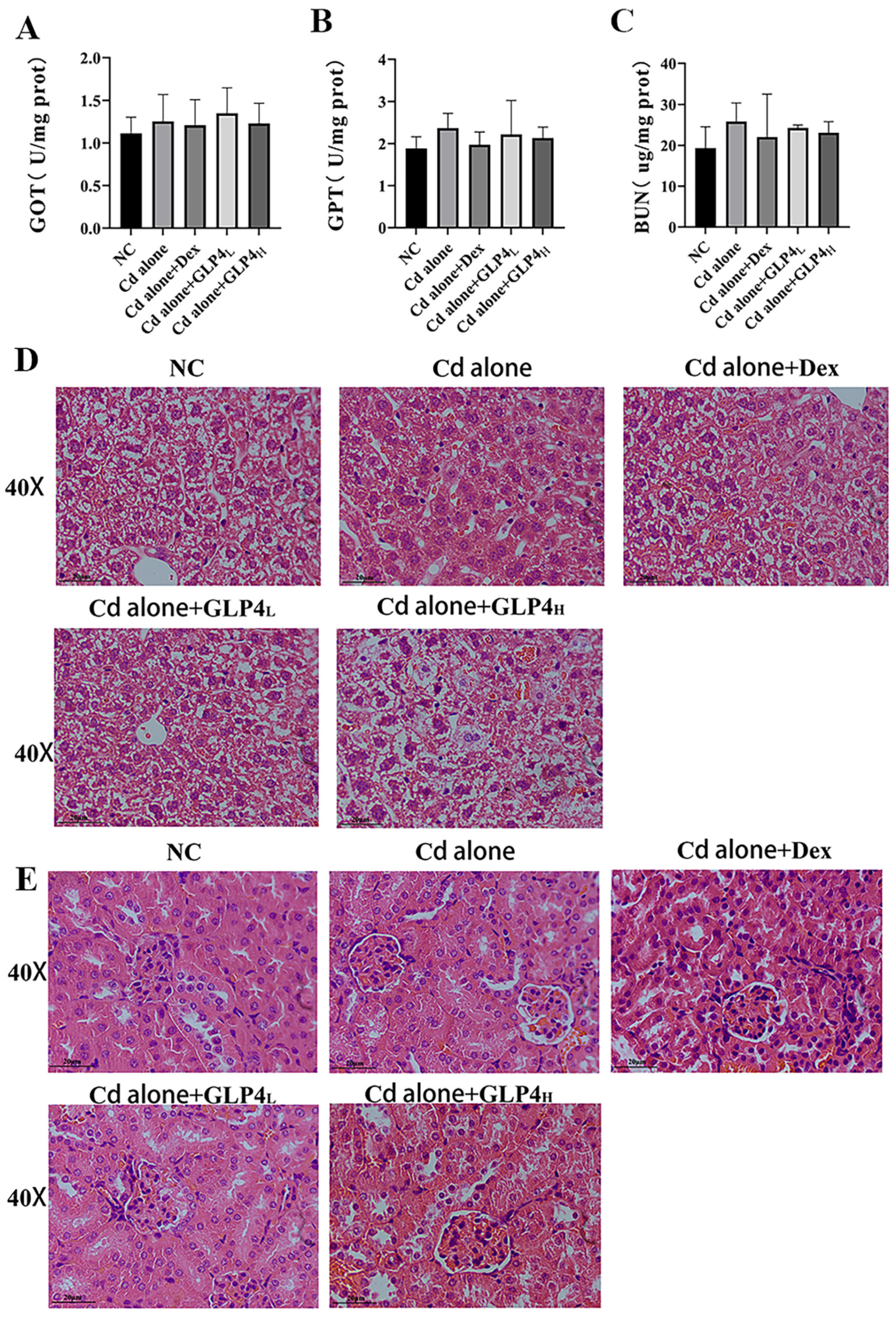
3.2. Respiratory Exposure to Acute Cadmium Does Not Affect Mouse Liver and Kidney Function
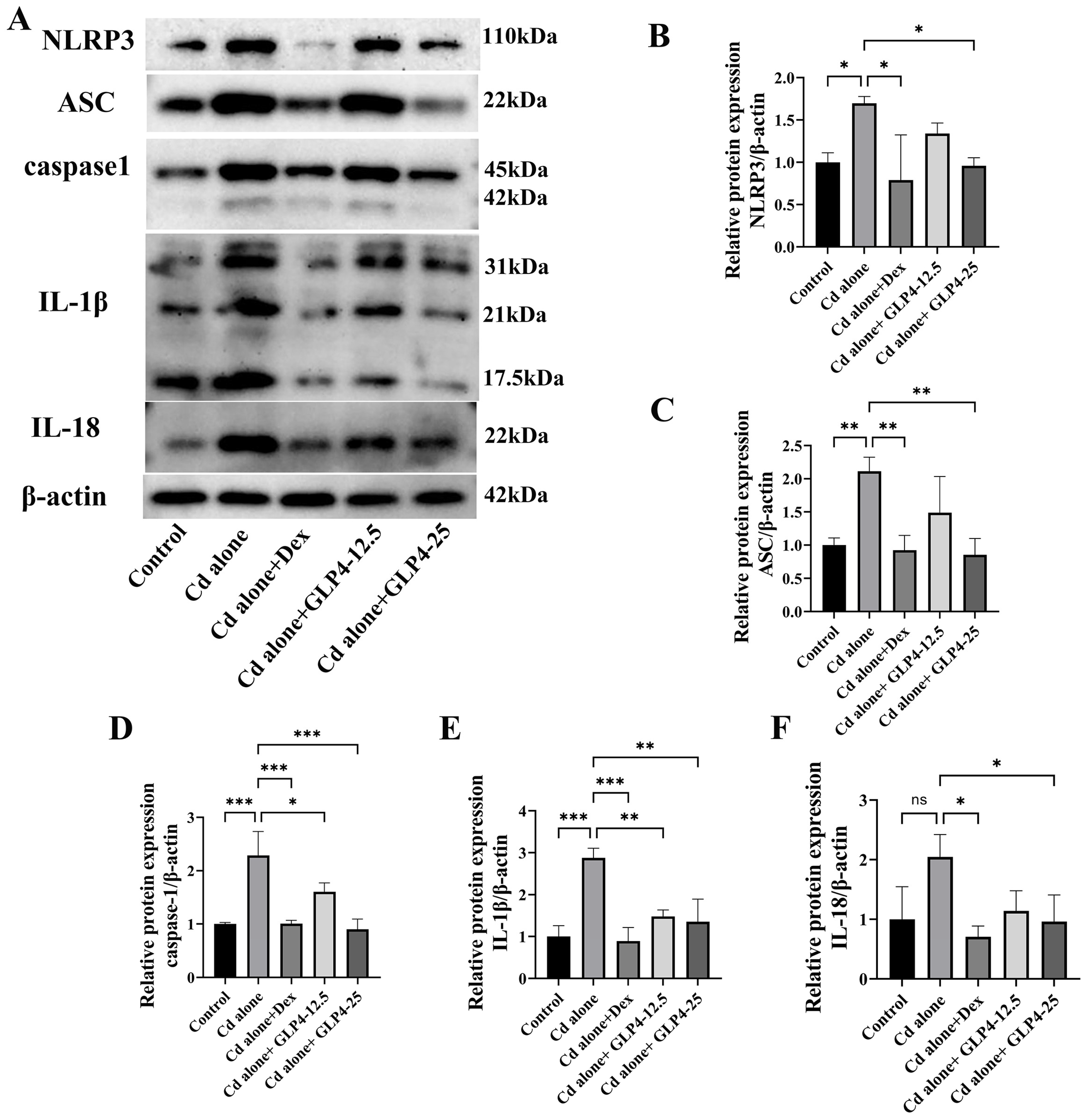
3.3. GLP4 Mitigates the Inflammatory Response in MHS Cells Induced by Cadmium by Suppressing NLRP3 Inflammasome Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, W.; Qi, Z.; Dong, Z.; Liu, W.; Xu, M.; Gao, M.; Liu, S. LncRNA MT1DP promotes cadmium-induced DNA replication stress by inhibiting chromatin recruitment of SMARCAL1. Sci. Total Environ. 2022, 807 Pt 3, 151078. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R.; Destefano, A.J.; Cecil, T.L.; Zaidi, K.; Williams, R.L. Metal impurities in food and drugs. Pharm. Res. 2010, 27, 750–755. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Mei, X.D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Z.; Lau, A.K.H.; Lin, C.Q.; Chuang, Y.C.; Chan, J.; Jiang, W.K.; Tam, T.; Yeoh, E.K.; Chan, T.C.; et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet. Health 2018, 2, e114–e125. [Google Scholar] [CrossRef]
- Han, C.; Oh, J.; Lim, Y.H.; Kim, S.; Hong, Y.C. Long-term exposure to fine particulate matter and development of chronic obstructive pulmonary disease in the elderly. Environ. Int. 2020, 143, 105895. [Google Scholar] [CrossRef]
- Li, X.; Yan, C.; Wang, C.; Ma, J.; Li, W.; Liu, J.; Liu, Y. PM (2.5)-bound elements in Hebei Province, China: Pollution levels, source apportionment and health risks. Sci. Total Environ. 2022, 806 Pt 1, 150440. [Google Scholar] [CrossRef]
- Xiong, Y.; Ning, Z.; Liu, Y.; Gomez, M.; Xiao, T. Emission and transformation behaviors of trace elements during combustion of Cd-rich coals from coal combustion related endemic fluorosis areas of Southwest, China. Ecotoxicol. Environ. Saf. 2022, 246, 114145. [Google Scholar] [CrossRef]
- Guan, W.J.; Zheng, X.Y.; Chung, K.F.; Zhong, N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Surolia, R.; Singh, P.; Dsouza, K.G.; Stephens, C.T.; Wang, Z.; Liu, R.M.; Bae, S.; Kim, Y.I.; Athar, M.; et al. Fibrinogen mediates cadmium-induced macrophage activation and serves as a predictor of cadmium exposure in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2022, 322, L593–L606. [Google Scholar] [CrossRef]
- Pearson, C.A.; Lamar, P.C.; Prozialeck, W.C. Effects of cadmium on E-cadherin and VE-cadherin in mouse lung. Life Sci. 2003, 72, 1303–1320. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Roede, J.R.; Orr, M.; Liang, Y.; Jones, D.P. Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of cd toxicity. Toxicol. Sci. 2014, 139, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Pei, Y.; Zhang, L.; Zhang, Y. Microbial communities respond to microenvironments in lungs of mice under simulated exposure to cadmium aerosols. Sci. Total Environ. 2020, 710, 136300. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Surolia, R.; Li, H.; Wang, Z.; Liu, G.; Liu, R.M.; Mirov, S.B.; Athar, M.; Thannickal, V.J.; Antony, V.B. Low-dose cadmium exposure induces peribronchiolar fibrosis through site-specific phosphorylation of vimentin. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L80–L91. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chandler, J.D.; Park, S.; Liu, K.; Fernandes, J.; Orr, M.; Smith, M.R.; Ma, C.; Kang, S.M.; Uppal, K.; et al. Low-dose cadmium disrupts mitochondrial citric acid cycle and lipid metabolism in mouse lung. Free Radic. Biol. Med. 2019, 131, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, D.Y.; Heo, H.R.; Choi, S.S.; Hong, S.H.; Kim, W.J. Role of miRNA-181a-2-3p in cadmium-induced inflammatory responses of human bronchial epithelial cells. J. Thorac. Dis. 2019, 11, 3055–3069. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, V.S.; Zhang, X.; Jagannathan, L.; Jose, C.C.; Cuddapah, S. Cadmium exposure upregulates SNAIL through miR-30 repression in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2019, 373, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Wang, H.; Jia, G.; Wang, T. Relationship between ambient PM (2.5) exposure and blood cadmium level in children under 14 years in Beijing, China. J. Hazard. Mater. 2021, 403, 123871. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liao, W.; Yin, X.; Liu, L.; Zhao, Z.; Lu, X.; Xu, F.; Lin, X.; Chen, Y.; Song, J.; et al. Hyperoside attenuates Cd-induced kidney injury via inhibiting NLRP3 inflammasome activation and ROS/MAPK/NF-κB signaling pathway in vivo and in vitro. Food Chem. Toxicol. 2023, 172, 113601. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Cao, Z.; Ma, Q.; Pi, H.; Fang, Y.; Yu, Z.; Hu, H.; Zhou, Z. Cadmium induces NLRP3 inflammasome-dependent pyroptosis in vascular endothelial cells. Toxicol. Lett. 2016, 246, 7–16. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef]
- Xu, S.; Xiaojing, L.; Xinyue, S.; Wei, C.; Honggui, L.; Shiwen, X. Pig lung fibrosis is active in the subacute CdCl2 exposure model and exerts cumulative toxicity through the M1/M2 imbalance. Ecotoxicol. Environ. Saf. 2021, 225, 112757. [Google Scholar] [CrossRef]
- Bhattacharya, S. The Role of Probiotics in the Amelioration of Cadmium Toxicity. Biol. Trace Elem. Res. 2020, 197, 440–444. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Borowska, S.; Tomczyk, M. Antioxidants as a Potential Preventive and Therapeutic Strategy for Cadmium. Curr. Drug Targets 2016, 17, 1350–1384. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; El-Gazzar, A.M.; El-Nahas, A.F.; Ashry, K.M. Vitamin C modulates cadmium-induced hepatic antioxidants’ gene transcripts and toxicopathic changes in Nile tilapia, Oreochromis niloticus. Environ. Sci. Pollut. Res. Int. 2016, 23, 1664–1670. [Google Scholar] [CrossRef]
- Kini, R.D.; Arunkumar, N.; Gokul, M. Potential Protective Role of Beta Carotene on Cadmium Induced Brain and Kidney Damage. Indian J. Public. Health Res. Dev. 2019, 10, 532. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, R.; Wang, H.; Yan, Y.; Ming, G. Protective Effect of Agaricus blazei Polysaccharide Against Cadmium-Induced Damage on the Testis of Chicken. Biol. Trace Elem. Res. 2018, 184, 491–500. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, L.; Ding, K. Structure elucidation and anti-tumor activity in vivo of a polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Int. J. Biol. Macromol. 2019, 141, 693–699. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.; Xu, H.; Yang, S.; Chen, Z.; Wang, H.; Zheng, B.; Mao, B.; Wu, X. Anti-inflammatory effects of Ganoderma lucidum sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2021, 150, 112073. [Google Scholar] [CrossRef]
- Fujita, R.; Liu, J.; Shimizu, K.; Konishi, F.; Noda, K.; Kumamoto, S.; Ueda, C.; Tajiri, H.; Kaneko, S.; Suimi, Y.; et al. Anti-androgenic activities of Ganoderma lucidum. J. Ethnopharmacol. 2005, 102, 107–112. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Peng, K.; Wang, X.L.; Ding, Z.; Liu, L.; Xu, P.; Liu, G.Q. Isolation and Characterization of Three Antihypertension Peptides from the Mycelia of Ganoderma Lucidum (Agaricomycetes). J. Agric. Food Chem. 2019, 67, 8149–8159. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmiański, J.; Szyjka, A.; Moreira, H.; Barg, E. Anticancer and Antioxidant Activities in Ganoderma lucidum Wild Mushrooms in Poland, as Well as Their Phenolic and Triterpenoid Compounds. Int. J. Mol. Sci. 2022, 23, 9359. [Google Scholar] [CrossRef]
- Wang, C.; Cao, B.; Liu, Q.Q.; Zou, Z.Q.; Liang, Z.A.; Gu, L.; Dong, J.P.; Liang, L.R.; Li, X.W.; Hu, K.; et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: A randomized trial. Ann. Intern. Med. 2011, 155, 217–225. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, F.; Wang, Y.; Liang, F.; Liang, J.; Lin, C.; Liang, J.; Gong, B.; Chan, S.; De Zhang, Z.; et al. A recombinant protein rLZ-8, originally extracted from Ganoderma lucidum, ameliorates OVA-induced lung inflammation by regulating Th17/Treg balance. J. Leukoc. Biol. 2020, 108, 531–545. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Tian, Y.; Chen, X.; Lan, J.; Wei, F.; Li, Y.; Luo, Y.; Sun, X. Ganoderma lucidum polysaccharides ameliorate lipopolysaccharide-induced acute pneumonia via inhibiting NRP1-mediated inflammation. Pharm. Biol. 2022, 60, 2201–2209. [Google Scholar] [CrossRef]
- Huang, P.; Luo, F.J.; Ma, Y.C.; Wang, S.X.; Huang, J.; Qin, D.D.; Xue, F.F.; Liu, B.Y.; Wu, Q.; Wang, X.L.; et al. Dual antioxidant activity and the related mechanisms of a novel pentapeptide GLP4 from the fermented mycelia of Ganoderma lingzhi. Food Funct. 2022, 13, 9032–9048. [Google Scholar] [CrossRef]
- Tao, C.; Tang, Y.; Zhang, L.; Tian, Y.; Zhang, Y. Atomization method for verifying size effects of inhalable particles on lung damage of mice. Sci. Total Environ. 2017, 579, 1476–1484. [Google Scholar] [CrossRef]
- Wang, W.J.; Peng, K.; Lu, X.; Zhu, Y.Y.; Li, Z.; Qian, Q.H.; Yao, Y.X.; Fu, L.; Wang, Y.; Huang, Y.C.; et al. Long-term cadmium exposure induces chronic obstructive pulmonary disease-like lung lesions in a mouse model. Sci. Total Environ. 2023, 879, 163073. [Google Scholar] [CrossRef]
- Lu, W.; Li, D.; Hu, J.; Mei, H.; Shu, J.; Long, Z.; Yuan, L.; Li, D.; Guan, R.; Li, Y.; et al. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J. Thorac. Dis. 2018, 10, 3232–3243. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Gu, L.; Fiehn, O.; Carter, A.B. Cadmium-mediated lung injury is exacerbated by the persistence of classically activated macrophages. J. Biol. Chem. 2020, 295, 15754–15766. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- El-Ebiary, A.A.; El-Ghaiesh, S.; Hantash, E.; Alomar, S. Mitigation of cadmium-induced lung injury by Nigella sativa oil. Environ. Sci. Pollut. Res. Int. 2016, 23, 25356–25363. [Google Scholar] [CrossRef]
- Jin, H.; Jin, F.; Jin, J.X.; Xu, J.; Tao, T.T.; Liu, J.; Huang, H.J. Protective effects of Ganoderma lucidum spore on cadmium hepatotoxicity in mice. Food Chem. Toxicol. 2013, 52, 171–175. [Google Scholar] [CrossRef]
- Dai, Z.; Li, G.; Wang, X.; Gao, B.; Gao, X.; Strappe, P.; Zhou, Z. Mapping the metabolic characteristics of probiotic-fermented Ganoderma lucidum and its protective mechanism against Cd-induced nephrotoxicity. Food Funct. 2023, 14, 8615–8630. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, H.; Song, Y.; Zhang, R.; Ge, M. Protective effects of Ganoderma triterpenoids on cadmium-induced oxidative stress and inflammatory injury in chicken livers. J. Trace Elem. Med. Biol. 2019, 52, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, K.; Kuang, W.; Huang, L. Exploration of the optimal strategy for dietary calcium intervention against the toxicity of liver and kidney induced by cadmium in mice: An in vivo diet intervention study. PLoS ONE 2021, 16, e0250885. [Google Scholar] [CrossRef]
- Madejczyk, M.S.; Baer, C.E.; Dennis, W.E.; Minarchick, V.C.; Leonard, S.S.; Jackson, D.A.; Stallings, J.D.; Lewis, J.A. Temporal changes in rat liver gene expression after acute cadmium and chromium exposure. PLoS ONE 2015, 10, e0127327. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, W.; Yan, Y.; Long, J.; Xie, X. Effects of waterborne cadmium exposure on Spinibarbus sinensis hepatopancreas and kidney: Mitochondrial cadmium accumulation and respiratory metabolism. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 248, 109115. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Bao, H.; Li, H.; Jia, Y.; Xiao, Y.; Luo, S.; Zhang, D.; Han, L.; Dai, L.; Xiao, C.; Feng, L.; et al. Ganoderic acid A exerted antidepressant-like action through FXR modulated NLRP3 inflammasome and synaptic activity. Biochem. Pharmacol. 2021, 188, 114561. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, Q.Z.; Wang, Z.J.; Hua, C. Anti-Inflammatory and Hepatoprotective Effects of Ganoderma lucidum Polysaccharides against Carbon Tetrachloride-Induced Liver Injury in Kunming Mice. Pharmacology 2019, 103, 143–150. [Google Scholar] [CrossRef]


| Genes | Primer Pairs |
|---|---|
| NLRP3 | Upper: AGGCTGCTATCTGGAGGAACT Lower: CCTTTCGGGCGGGTAATC |
| IL-1β | Upper: GAAATGCCACCTTTTGACAGTG Lower: TGGATGCTCTCATCAGGACAG |
| IL-6 | Upper: TAGTCCTTCCTACCCCAATTTCC Lower: TTGGTCCTTAGCCACTCCTTC |
| TNF-α | Upper: CAGGCGGTGCCTATGTCTC Lower: CGATCACCCCGAAGTTCAGTAG |
| GAPDH | Upper: CATCACTGCCACCCAGAAGACTG Lower: ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Wang, X.; Liu, G. The Protective Effects of Ganoderma lucidum Active Peptide GLP4 on Lung Injury Induced by Cadmium Poisoning in Mice. Toxics 2024, 12, 378. https://doi.org/10.3390/toxics12060378
Zhu S, Wang X, Liu G. The Protective Effects of Ganoderma lucidum Active Peptide GLP4 on Lung Injury Induced by Cadmium Poisoning in Mice. Toxics. 2024; 12(6):378. https://doi.org/10.3390/toxics12060378
Chicago/Turabian StyleZhu, Shirong, Xiaoling Wang, and Gaoqiang Liu. 2024. "The Protective Effects of Ganoderma lucidum Active Peptide GLP4 on Lung Injury Induced by Cadmium Poisoning in Mice" Toxics 12, no. 6: 378. https://doi.org/10.3390/toxics12060378
APA StyleZhu, S., Wang, X., & Liu, G. (2024). The Protective Effects of Ganoderma lucidum Active Peptide GLP4 on Lung Injury Induced by Cadmium Poisoning in Mice. Toxics, 12(6), 378. https://doi.org/10.3390/toxics12060378






