Treating Diphenhydramine Overdose: A Literature Review of Currently Available Treatment Methods
Abstract
1. Background
1.1. Diphenhydramine
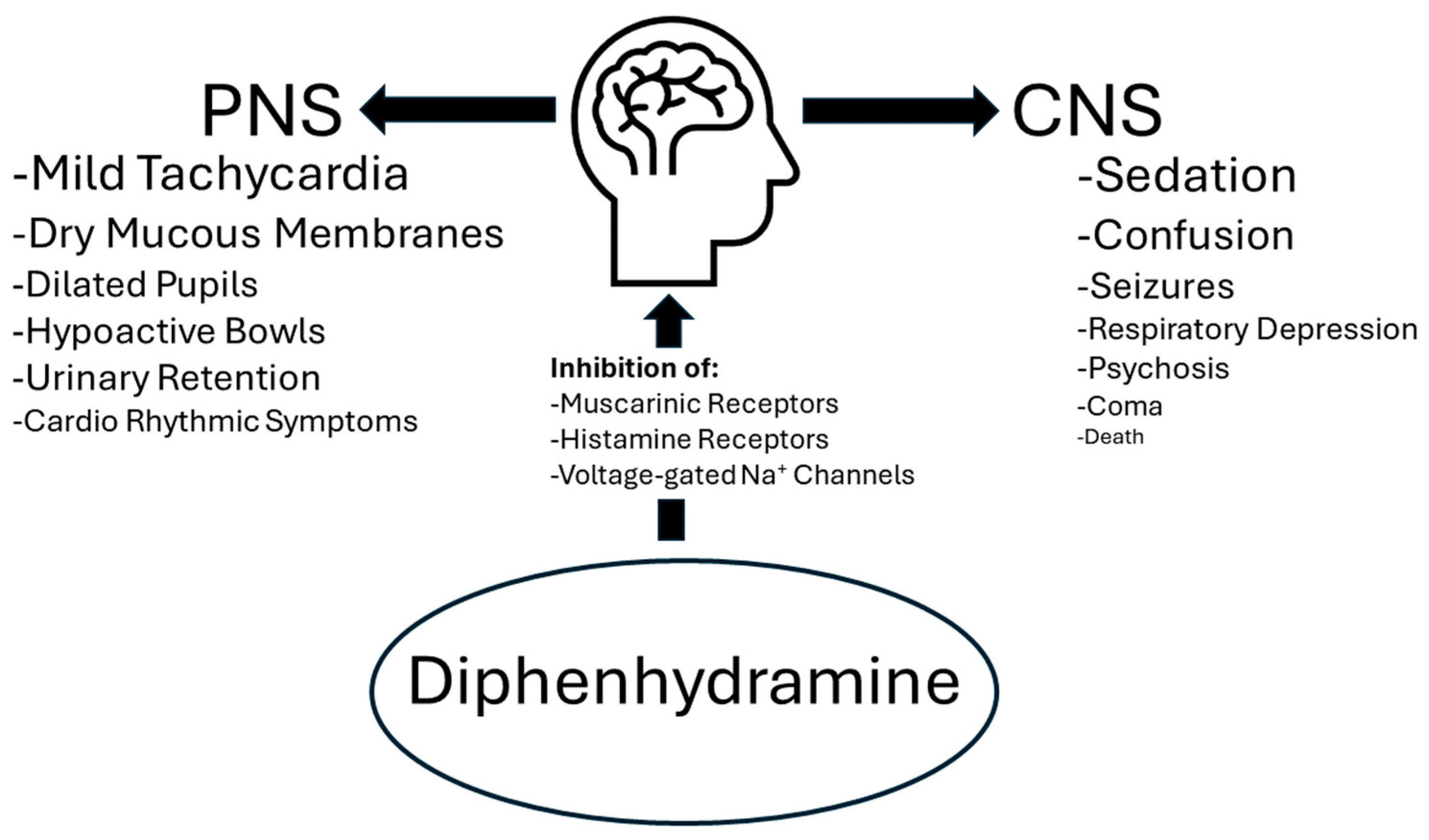
1.2. Diphenhydramine Risks
1.3. Diphenhydramine Overdoses
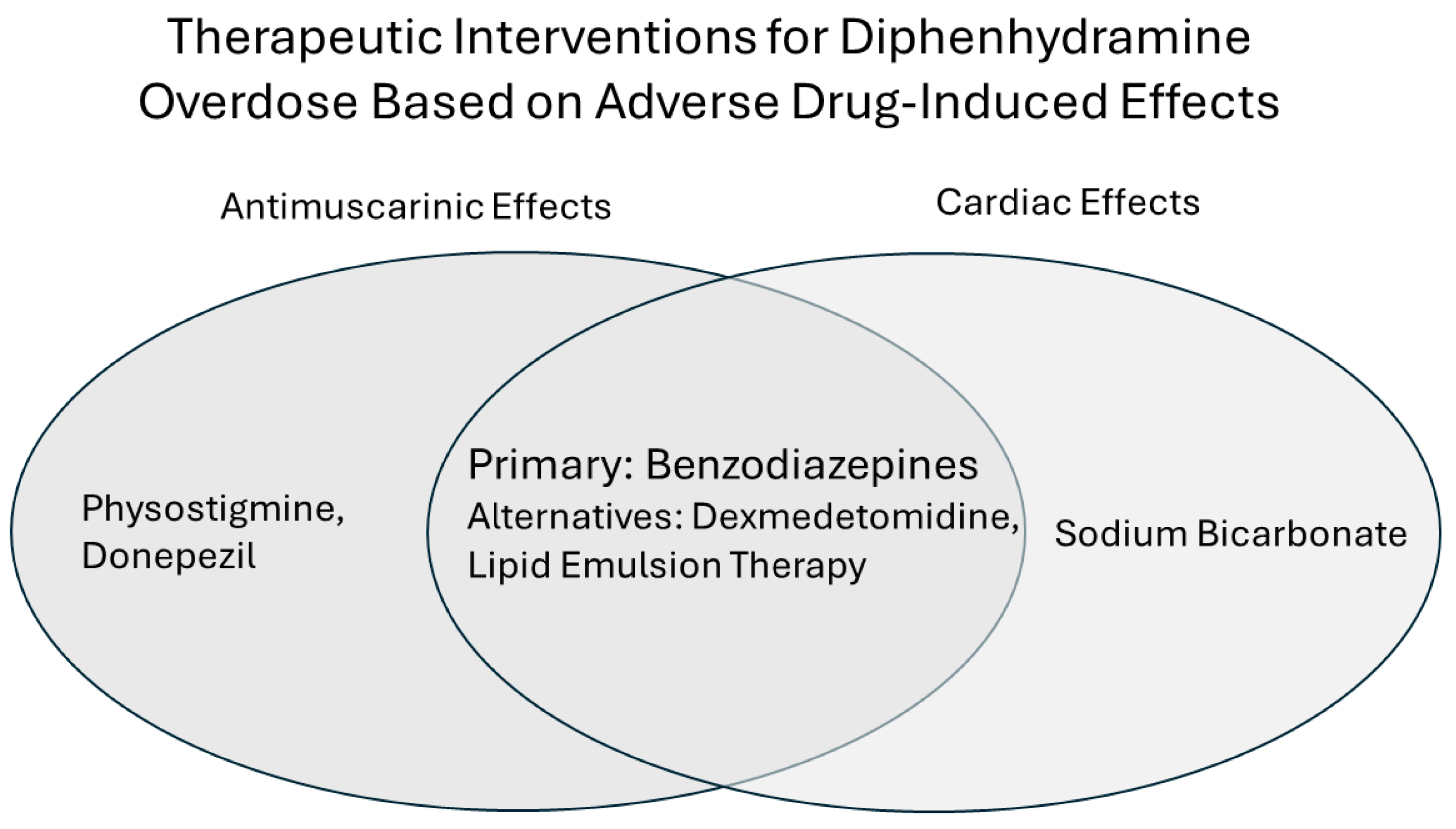
2. Treatments
2.1. Physostigmine
2.2. Sodium Bicarbonate
2.3. Donepezil
2.4. Dexmedetomidine
2.5. Lipid Emulsion Therapy
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolfson, A.R.; Wong, D.; Abrams, E.M.; Waserman, S.; Sussman, G.L. Diphenhydramine: Time to Move on? J. Allergy Clin. Immunol. Pract. 2022, 10, 3124–3130. [Google Scholar] [CrossRef]
- Merenstein, D.; Diener-West, M.; Halbower, A.C.; Krist, A.; Rubin, H.R. The trial of infant response to diphenhydramine: The TIRED study--a randomized, controlled, patient-oriented trial. Arch. Pediatr. Adolesc. Med. 2006, 160, 707–712. [Google Scholar] [CrossRef]
- Sharma, A.N.; Hexdall, A.H.; Chang, E.K.; Nelson, L.S.; Hoffman, R.S. Diphenhydramine-induced wide complex dysrhythmia responds to treatment with sodium bicarbonate. Am. J. Emerg. Med. 2003, 21, 212–215. [Google Scholar] [CrossRef]
- Okuno, T.; Morimoto, S.; Nishikawa, H.; Haraguchi, T.; Kojima, H.; Tsujino, H.; Arisawa, M.; Yamashita, T.; Nishikawa, J.; Yoshida, M.; et al. Bitterness-Suppressing Effect of Umami Dipeptides and Their Constituent Amino Acids on Diphenhydramine: Evaluation by Gustatory Sensation and Taste Sensor Testing. Chem. Pharm. Bull. 2020, 68, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Huang, R.C.; Lou, B.S. Inhibition of Na+ current by diphenhydramine and other diphenyl compounds: Molecular determinants of selective binding to the inactivated channels. Mol. Pharmacol. 2000, 57, 135–143. [Google Scholar] [PubMed]
- Simons, F.E.; Simons, K.J. Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011, 128, 1139–1150.e4. [Google Scholar] [CrossRef]
- FDA. Benadryl (Diphenhydramine): Drug Safety Communication—Serious Problems with High Doses of the Allergy Medicine. 2020. Available online: https://www.fda.gov/safety/medical-product-safety-information/benadryl-diphenhydramine-drug-safety-communication-serious-problems-high-doses-allergy-medicine (accessed on 7 January 2024).
- Spector, R.; Choudhury, A.K.; Chiang, C.K.; Goldberg, M.J.; Ghoneim, M.M. Diphenhydramine in Orientals and Caucasians. Clin. Pharmacol. Ther. 1980, 28, 229–234. [Google Scholar] [CrossRef]
- Ten Eick, A.P.; Blumer, J.L.; Reed, M.D. Safety of antihistamines in children. Drug Saf. 2001, 24, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Zekhtser, M.; Carroll, E.; Boyd, M.; Ambati, S. The Role of Dexmedetomidine in Pediatric Patients Presenting with an Anticholinergic Toxidrome. Case Rep. Crit. Care 2021, 2021, 7590960. [Google Scholar] [CrossRef]
- Walker, A.; Delle Donne, A.; Douglas, E.; Spicer, K.; Pluim, T. Novel use of dexmedetomidine for the treatment of anticholinergic toxidrome. J. Med. Toxicol. 2014, 10, 406–410. [Google Scholar] [CrossRef]
- Abdi, A.; Rose, E.; Levine, M. Diphenhydramine overdose with intraventricular conduction delay treated with hypertonic sodium bicarbonate and i.v. lipid emulsion. West. J. Emerg. Med. 2014, 15, 855–858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinwiddie, A.T.; Tanz, L.J.; Bitting, J. Notes from the Field: Antihistamine Positivity and Involvement in Drug Overdose Deaths—44 Jurisdictions, United States, 2019-2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1308–1310. [Google Scholar] [CrossRef]
- Padilla, R.B.; Pollack, M.L. The use of physostigmine in diphenhydramine overdose. Am. J. Emerg. Med. 2002, 20, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, S.V.; Purvis, A.W.; Tosto, S.T.; Velayati, A. IV Lipid Emulsion Infusion in the Treatment of Severe Diphenhydramine Overdose. Am. J. Case Rep. 2019, 20, 758–763. [Google Scholar] [CrossRef]
- Burns, M.J.; Linden, C.H.; Graudins, A.; Brown, R.M.; Fletcher, K.E. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann. Emerg. Med. 2000, 35, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Acquisto, N.M.; Gorodetsky, R.M.; Wiegand, T.J. Use of a physostigmine continuous infusion for the treatment of severe and recurrent antimuscarinic toxicity in a mixed drug overdose. J. Med. Toxicol. 2014, 10, 205–209. [Google Scholar] [CrossRef]
- Bruccoleri, R.E.; Burns, M.M. A Literature Review of the Use of Sodium Bicarbonate for the Treatment of QRS Widening. J. Med. Toxicol. 2016, 12, 121–129. [Google Scholar] [CrossRef]
- Cole, J.B.; Stellpflug, S.J.; Gross, E.A.; Smith, S.W. Wide complex tachycardia in a pediatric diphenhydramine overdose treated with sodium bicarbonate. Pediatr. Emerg. Care 2011, 27, 1175–1177. [Google Scholar] [CrossRef]
- Ahmad, J.; Hasan, M.J.; Anam, A.M.; Barua, D.K. Donepezil: An unusual therapy for acute diphenhydramine overdose. BMJ Case Rep. 2019, 12, e226836. [Google Scholar] [CrossRef]
- Rogers, S.L.; Friedhoff, L.T. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br. J. Clin. Pharmacol. 1998, 46 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Rogers, S.L.; Yamanishi, Y.; Yamatsu, K. E2020—The Pharmacology of a Piperidine Cholinesterase Inhibitor. In Cholinergic Basis for Alzheimer Therapy. Advances in Alzheimer Disease Therapy; Becker, R., Giacobini, E., Eds.; Springer: Boston, MA, USA, 1991. [Google Scholar] [CrossRef]
- Weinberg, G.L. Lipid emulsion infusion: Resuscitation for local anesthetic and other drug overdose. Anesthesiology 2012, 117, 180–187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.; Edwards, J. Treating Diphenhydramine Overdose: A Literature Review of Currently Available Treatment Methods. Toxics 2024, 12, 376. https://doi.org/10.3390/toxics12060376
Patel J, Edwards J. Treating Diphenhydramine Overdose: A Literature Review of Currently Available Treatment Methods. Toxics. 2024; 12(6):376. https://doi.org/10.3390/toxics12060376
Chicago/Turabian StylePatel, Jayna, and Joshua Edwards. 2024. "Treating Diphenhydramine Overdose: A Literature Review of Currently Available Treatment Methods" Toxics 12, no. 6: 376. https://doi.org/10.3390/toxics12060376
APA StylePatel, J., & Edwards, J. (2024). Treating Diphenhydramine Overdose: A Literature Review of Currently Available Treatment Methods. Toxics, 12(6), 376. https://doi.org/10.3390/toxics12060376





