The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Stock Culture of the Microalga
2.2. Seawater
2.3. Toluidine Blue Dye
2.4. Biosorption Assays
2.4.1. Effect of Light on the Biosorption Process by the Living Microalga
2.4.2. Determination of the Effect of pH on Biosorption Capacity
2.5. Analytical Methods
Analysis of Kinetics and Equilibrium Isotherms
2.6. Determination of the Zero Charge Point of the Biomass
2.7. Statistical Analysis
3. Results
3.1. Study of the Stability of the TB Dye in the Experimental Conditions
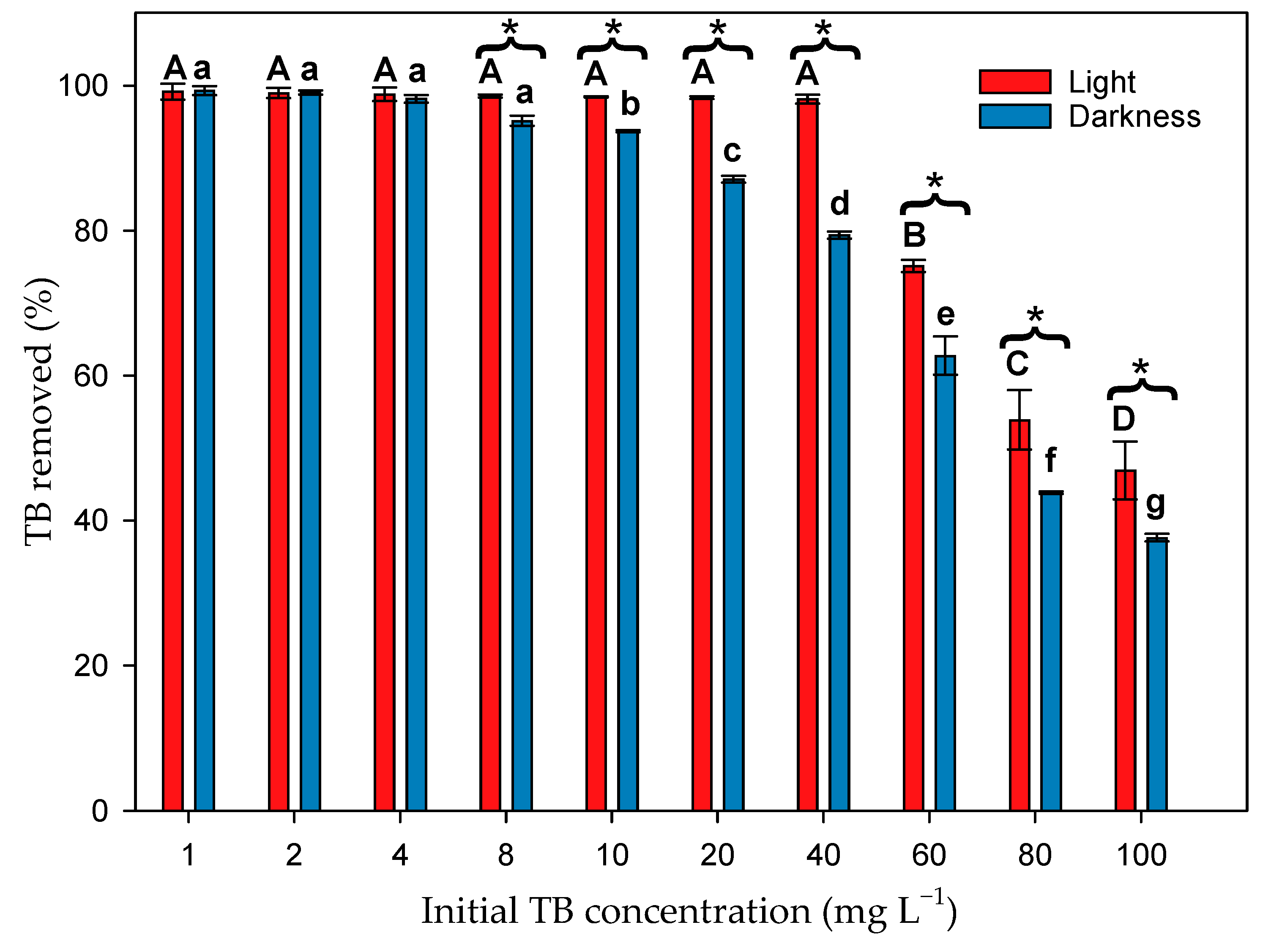
3.2. Effect of Microalgal Biomass on TB Dye Removal in the Presence of Light
3.3. Effect of Microalgal Biomass on Dye Removal in the Absence of Light
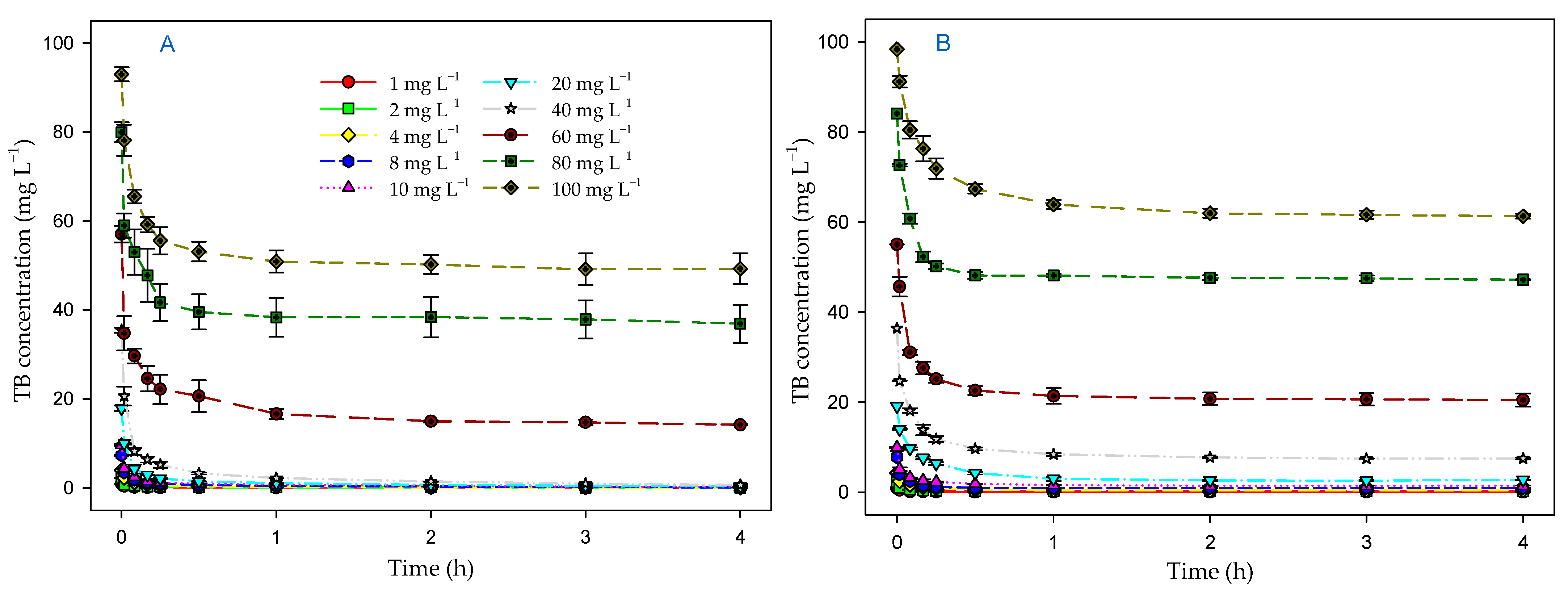
3.4. Effect of Contact Time and Initial Dye Concentration on Biosorption Process
3.5. Kinetic Models
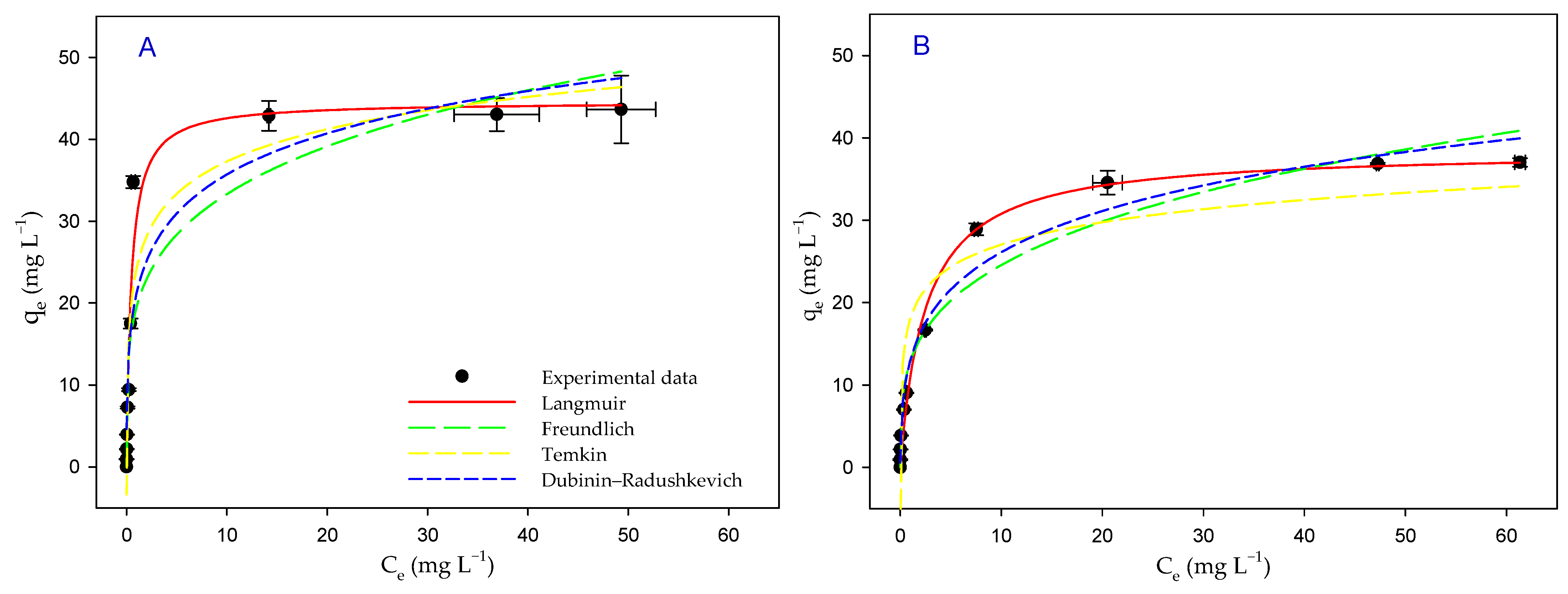
3.6. Biosorption Isotherms
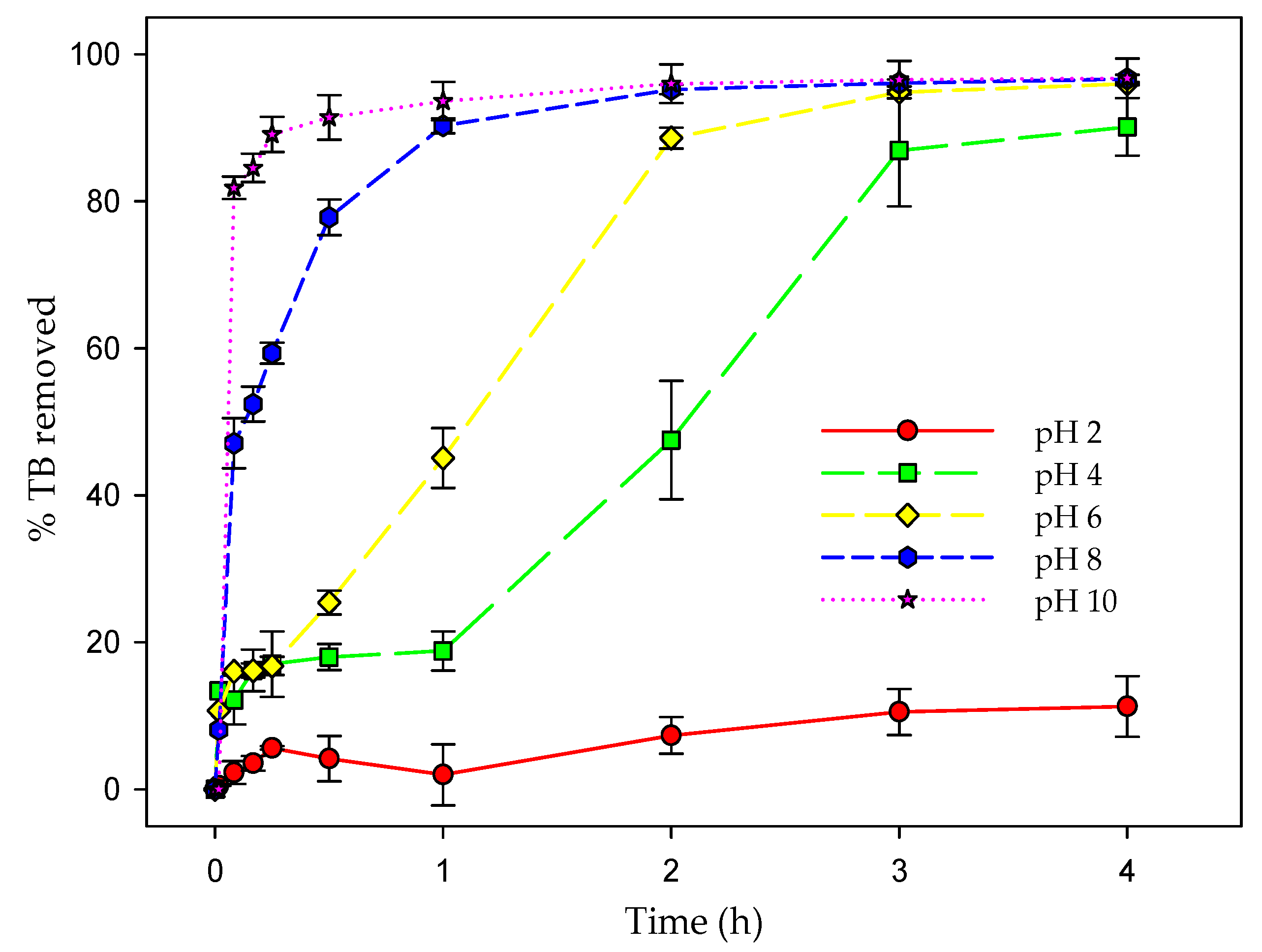
3.7. Effect of pH on Biosorption Efficiency
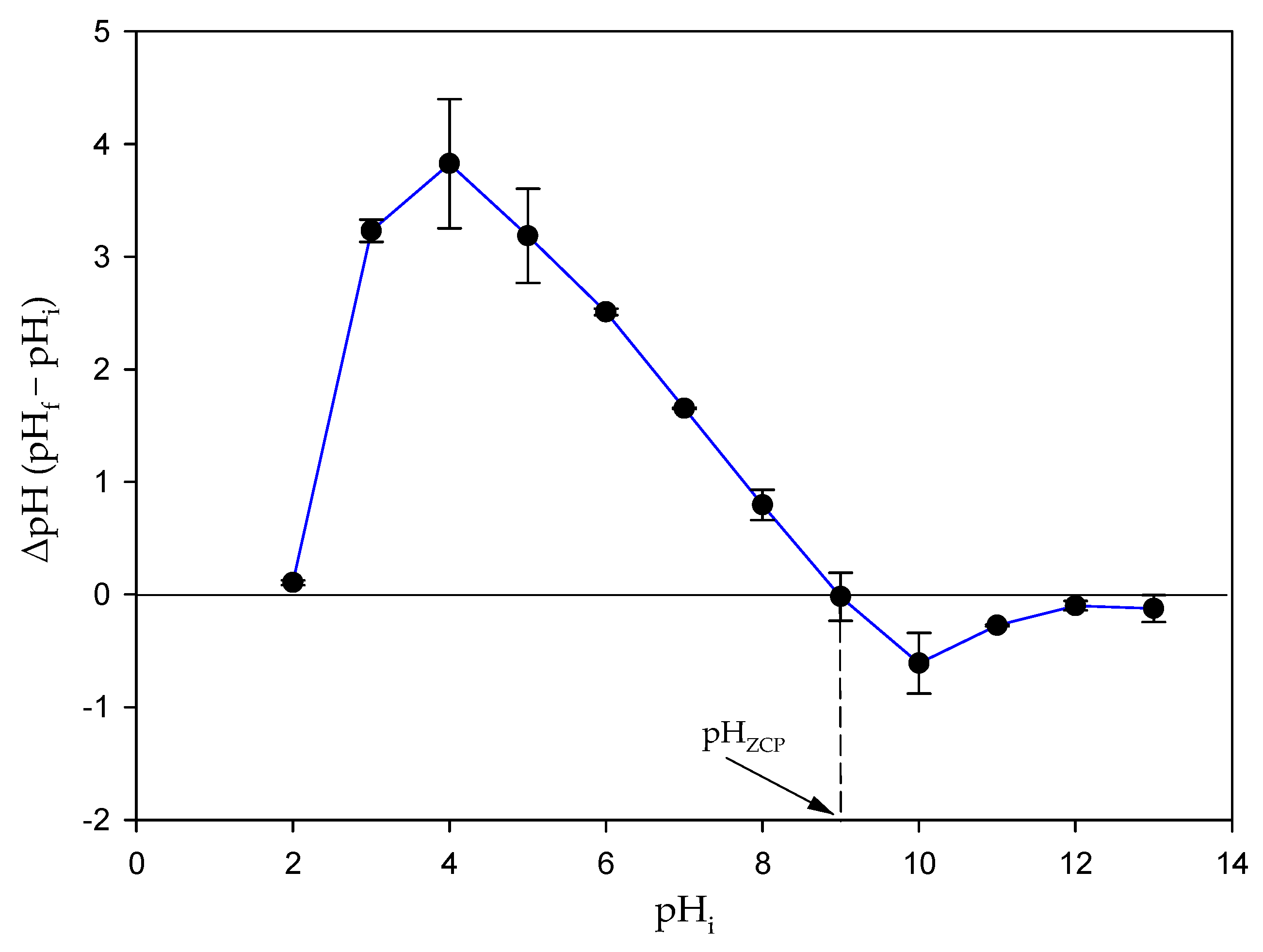
3.8. Determination of pHZCP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Textile dyeing industry an environmental hazard. J. Nat. Sci. 2011, 4, 22–26. [Google Scholar] [CrossRef]
- Rauf, M.A.; Qadri, S.M.; Ashraf, S.; Al-Mansoori, K.M. Adsorption studies of Toluidine Blue from aqueous solutions onto gypsum. J. Chem. Eng. 2009, 150, 90–95. [Google Scholar] [CrossRef]
- Khataee, A.R.; Movafeghi, A.; Torbati, S.; Salehi Lisar, S.Y.; Zarei, M. Phytoremediation potential of duckweed (Lemna minor L.) in degradation of C.I. Acid Blue 92: Artificial neural network modeling. Ecotoxicol. Environ. Saf. 2012, 80, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and micro algae in pollution control and biofuel production—A review. Chem. Bio Eng. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Poots, V.; Mckay, G.; Healy, J. The removal of acid dye from effluent using natural adsorbents—I peat. Water Res. 1976, 10, 1061–1066. [Google Scholar] [CrossRef]
- Nawar, S.S.; Doma, H.S. Removal of dyes from effluents using low-cost agricultural by-products. Sci. Total Environ. 1989, 79, 271–279. [Google Scholar] [CrossRef]
- Martínez-Jerónimo, F.; Cruz-Cisneros, J.L.; García-Hernández, L. A comparison of the response of Simocephalus mixtus (Cladocera) and Daphnia magna to contaminated freshwater sediments. Ecotoxicol. Environ. Saf. 2008, 71, 26–31. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Sánchez, E.; Baghour, M. The role of algae in bioremediation of organic pollutants. J. Pub. Environ. Heatlh 2014, 1, 19–32. [Google Scholar]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef]
- Salim, H.A.M.; Idrees, S.A.; Rashid, R.A.; Mohammed, A.A.; Simo, S.M.; Khalo, I.S. Photo-catalytic degradation of Toluidine Blue Dye in Aqueous Medium Under Fluorescent Light. In Proceedings of the International Conference on Advanced Science and Engineering, Duhok, Iraq, 9–11 October 2018. [Google Scholar]
- Venkatesan, D.; Umasankar, S.; Mangesh, V.L.; Krishnan, P.S.; Tamizhdurai, P.; Kumaran, R.; Baskaralingam, P. Removal of Toluidine blue in water using green synthesized nanomaterials. S. Afr. J. Chem. Eng. 2023, 45, 42–50. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Wu, L.; Yin, Y.; Jiang, B.; Lou, J. Enhanced performance of TiO2/reduced graphene oxide doped by rare-earth ions for degrading phenol in seawater excited by weak visible light. Adv. Powder Technol. 2019, 30, 1920–1931. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Z.; Feng, W.; Wang, T.; Li, Y. Mechanism of Photodegradation of Organic Pollutants in Seawater by TiO2-based Photocatalysts and Improvement in Their Performance. ACS Omega 2021, 6, 30698–30707. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Bhatti, H.N.; Maqbool, M.; Iqbal, M. Microalgae biosorption, bioaccumulation and biodegradation efficiency for the remediation of wastewater and carbon dioxide mitigation: Prospects, challenges and opportunities. J. Water Process. Eng. 2021, 41, 1–15. [Google Scholar] [CrossRef]
- Neag, E.; Malschi, D.; Măicăneanu, A. Isotherm and kinetic modelling of Toluidine Blue (TB) removal from aqueous solution using Lemna minor. Int. J. Phytoremediation 2018, 20, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Dukare, A. Microbe-mediated bioremediation: An eco-friendly sustainable approach for environmental clean-up. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer Nature: Singapore, 2018; pp. 145–163. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Abalde, J.; Torres, E. Eco-friendly rapid removal of triclosan from seawater using biomass of a microalgal species: Kinetic and equilibrium studies. J. Hazard. Mater. 2019, 369, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of dyes from seawater using as biosorbent the dead and living biomass of the microalga Phaeodactylum tricornutum: Equilibrium and kinetics studies. J. Appl. Phycol. 2021, 33, 3071–3090. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Abalde, J. Bioremediation of oxytetracycline in seawater by living and dead biomass of the microalga Phaeodactylum tricornutum. J. Hazard. Mater. 2016, 320, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Mohd Udaiyappan, A.F.; Abu Hasan, H.; Takriff, M.S.; Sheikh Abdullah, S.R. A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J. Water Process. Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Kurano, N.; Ikemoto, H.; Miyashita, H.; Hasegawa, T.; Hata, H.; Miyachi, S. Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Conv. Manag. 1995, 36, 689–692. [Google Scholar] [CrossRef]
- Kaithwas, A.; Prasad, M.; Kulshreshtha, A.; Verma, S. Industrial wastes derived solid adsorbents for CO2 capture: A mini review. Chem. Eng. Res. Des. 2012, 90, 1632–1641. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of carbon capture technology: Microalgal biorefinery concept and state-of-the-art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Torres, E.; Abalde, J. Biosorption of ibuprofen from aqueous solution using living and dead biomass of the microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2018, 30, 471–482. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta. Physiochim. URSS 1940, 12, 217–222. [Google Scholar]
- Chu, K.H. Revisiting the Temkin Isotherm: Dimensional Inconsistency and Approximate Forms. Ind. Eng. Chem. Res. 2021, 60, 13140–13147. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Dubinin, M.; Radushkevich, L. The equation of the characteristic curve of activated charcoal. Proc. Acad. Sci. Phys. Chem. Sect. USSR 1947, 55, 331–333. [Google Scholar]
- Quili, H.; Zhenya, Z. Application of Dubinin-Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Vacchi, F.I.; Vendemiatti, J.A.d.S.; da Silva, B.F.; Zanoni, M.V.B.; Umbuzeiro, G.d.A. Quantifying the contribution of dyes to the mutagenicity of waters under the influence of textile activities. Sci. Total Environ. 2017, 601–602, 230–236. [Google Scholar] [CrossRef]
- González, V.; Abalde, J.; Torres, E. Discoloration and biosorption of brilliant green dye in seawater using living biomass of the microalga Phaeodactylum tricornutum. J. Appl. Phycol. 2024. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Jeon, B.-H. Can microalgae remove pharmaceutical contaminants from water? Trends Biotechnol. 2018, 36, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Qingfeng, W.; Kristen, C.; Qi, C.; Xisen, W.; Zhaohui, L. Interactions between cationic dye toluidine blue and fibrous clay minerals. Crystals 2021, 11, 708. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Siaut, M.; Heijde, M.; Mangogna, M.; Montsant, A.; Coesel, S.; Allen, A.; Manfredonia, A.; Falciatore, A.; Bowler, C. Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene 2007, 406, 23–35. [Google Scholar] [CrossRef]
- Salim, H.A.M.; Salih, S.A.M. Photodegradation study of Toluidine Blue dye in aqueous solution using magnesium oxide as a photocatalyst. Int. J. Chem. 2015, 7, 143–149. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Zhang, W.; Li, B. Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv. Powder Technol. 2019, 30, 493–501. [Google Scholar] [CrossRef]
- Lafi, R.; Rezma, S.; Hafiane, A. Removal of toluidine blue from aqueous solution using orange peel waste (OPW). Desalin. Water Treat. 2015, 56, 2754–2765. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R. A study on removal of Toluidine blue dye from aqueous solution by adsorption onto Neem leaf powder. World Acad. Sci. Eng. Technol. 2010, 70, 831–836. [Google Scholar]

| Kinetics | Isotherms | ||
|---|---|---|---|
| |||
| (3) | ||
| (4) |
| ||
| (5) | |||
| (6) | |||
|
| ||
| (7) | (8) | ||
| (9) | |||
| q (mg g−1) is the mass of TB biosorbed per unit of biomass over the course of time t (h), k1 (h−1) is the constant of the first order kinetic model, qe (mg g−1) is the mass of TB absorbed at equilibrium, and k2 (g mg−1 h−1) is the constant of the second order kinetic model. | qe (mg g−1) is the mass of TB biosorbed per unit of biomass at equilibrium, qmax (mg g−1) is the maximum sorption capacity, KL (L mg−1) is the affinity constant of the material, Ce (mg L−1) is the TB concentration at equilibrium, KF (L mg−1) is the Freundlich constant, n is the intensity of the Freundlich constant, qT the surface capacity for pollutant sorption per unit binding energy (mg g−1), AT (L mg−1) is the binding energy constant, R is the ideal gas constant (8.314 J mol−1 K−1), T is temperature at 291 K, BD is the free energy of sorption per mole sorbate (mol2 J−2), sol is the solubility of the dye, and ED is the apparent energy (KJ mol−1). | ||
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (h−1) | radj2 | qe (mg g−1) | K2 (g mg−1 h−1) | radj2 | |
| 1 | 2.2 ± 0.0 | 56 ± 8 | 0.9733 | 2.2 ± 0.0 | 38 ± 4 | 0.9948 |
| 2 | 5.0 ± 0.1 | 72 ± 15 | 0.9503 | 5.2 ± 0.1 | 21 ± 4 | 0.9813 |
| 4 | 9.2 ± 0.3 | 26 ± 7 | 0.9320 | 9.6 ± 0.2 | 4.2 ± 0.6 | 0.9889 |
| 8 | 17 ± 1 | 46 ± 10 | 0.9445 | 18 ± 0 | 3.5 ± 0.5 | 0.9894 |
| 10 | 22 ± 0.6 | 50 ± 11 | 0.9451 | 23 ± 0 | 3.0 ± 0.4 | 0.9876 |
| 20 | 41 ± 1 | 32 ± 6 | 0.9651 | 43 ± 0 | 1.1 ± 0.1 | 0.9984 |
| 40 | 81 ± 2 | 29 ± 6 | 0.9624 | 85 ± 1 | 0.50 ± 0.04 | 0.9964 |
| 60 | 94 ± 5 | 43 ± 16 | 0.8530 | 101 ± 4 | 0.44 ± 0.13 | 0.9420 |
| 80 | 100 ± 5 | 18 ± 5 | 0.8800 | 104 ± 3 | 0.35 ± 0.09 | 0.9576 |
| 100 | 104 ± 3 | 13 ± 2 | 0.9564 | 109 ± 1 | 0.21 ± 0.02 | 0.9941 |
| Initial Concentration (mg L−1) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (h−1) | radj2 | qe (mg g−1) | K2 (g mg−1 h−1) | radj2 | |
| 1 | 2.1 ± 0.1 | 60 ± 18 | 0.9088 | 2.2 ± 0.1 | 32 ± 9 | 0.9616 |
| 2 | 4.9 ± 0.2 | 55 ± 156 | 0.9149 | 5.1 ± 0.1 | 13 ± 3 | 0.9673 |
| 4 | 9.0 ± 0.3 | 25 ± 7 | 0.9232 | 9.5 ± 0.2 | 4.1 ± 0.6 | 0.9853 |
| 8 | 17 ± 0 | 39 ± 7 | 0.9689 | 18 ± 0 | 3.3 ± 0.3 | 0.9962 |
| 10 | 22 ± 0 | 39 ± 6 | 0.9755 | 23 ± 0 | 2.5 ± 0.1 | 0.9990 |
| 20 | 40 ± 1 | 9.2 ± 1.6 | 0.9426 | 42 ± 1 | 0.39 ± 0.06 | 0.9856 |
| 40 | 69 ± 3 | 15 ± 3 | 0.9299 | 72 ± 1 | 0.39 ± 0.06 | 0.9841 |
| 60 | 83 ± 2 | 14 ± 2 | 0.9792 | 88 ± 1 | 0.27 ± 0.01 | 0.9989 |
| 80 | 90 ± 2 | 14 ± 1 | 0.9867 | 94 ± 1 | 0.26 ± 0.03 | 0.9921 |
| 100 | 88 ± 3 | 6.4 ± 0.8 | 0.9685 | 94 ± 1 | 0.10 ± 0.01 | 0.9952 |
| Isotherm | Parameters | Light | Darkness |
|---|---|---|---|
| Langmuir | qmax (mg g−1) | 45 ± 2 | 39 ± 1 |
| KL (L mg−1) | 2.1 ± 0.4 | 0.4 ± 0.1 | |
| radj2 | 0.9634 | 0.9880 | |
| Freundlich | KF (L mg−1) | 20 ± 3 | 13 ± 1 |
| 1/n | 4.3 ± 0.9 | 3.6 ± 0.4 | |
| radj2 | 0.8377 | 0.9581 | |
| Temkin | qT (mg g−1) | 5.7 ± 0.6 | 3.9 ± 0.6 |
| AT (L mg−1) | 70 ± 37 | 101 ± 82 | |
| radj2 | 0.8969 | 0.8429 | |
| Dubinin– Radushkevich | qmax (mg g−1) | 79 ± 13 | 75 ± 7 |
| BD (mol2 J−2) | 2.1 × 10−9 ± 4.1 × 10−10 | 2.9 × 10−9 ± 2.8 × 10−10 | |
| ED (KJ mol−1) | 15 ± 2 | 13 ± 1 | |
| radj2 | 0.8571 | 0.9714 |
| Sorbents | qmax † (mg g−1) | KF †† (L mg−1) | Contact Time (h) | pH | Sorbent Concentration (g L−1) | TB Concentration (mg L−1) | Reference |
|---|---|---|---|---|---|---|---|
| Graphene oxide/bentonite | 459 | 242 | 2 | 8 | 0.5 | 200–800 | [41] |
| Orange peel waste | 313 | 3.3 | 0.5 | 10 | 3 | 20–160 | [42] |
| Neem leaf powder | 187 | 4.0 | 8 | 7 | 3 | 0.0001–0.0009 | [43] |
| Phaeodactylum tricornutum | 45 | 20 | 4 | 8 | 0.4 | 1–100 | This work (seawater) |
| Gypsum | 28 | 6 | 1 | 6.5 | 1 | 0.03–0.3 | [3] |
| Lemna minor | 27 | 7.2 | 24 | --- | --- | 5–40 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, D.; Abalde, J.; Torres, E. The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater. Toxics 2024, 12, 277. https://doi.org/10.3390/toxics12040277
Fernández D, Abalde J, Torres E. The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater. Toxics. 2024; 12(4):277. https://doi.org/10.3390/toxics12040277
Chicago/Turabian StyleFernández, David, Julio Abalde, and Enrique Torres. 2024. "The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater" Toxics 12, no. 4: 277. https://doi.org/10.3390/toxics12040277
APA StyleFernández, D., Abalde, J., & Torres, E. (2024). The Biosorption Capacity of the Marine Microalga Phaeodactylum tricornutum for the Removal of Toluidine Blue from Seawater. Toxics, 12(4), 277. https://doi.org/10.3390/toxics12040277







