Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanomaterials and Chemicals
2.2. Characterization of the NPs
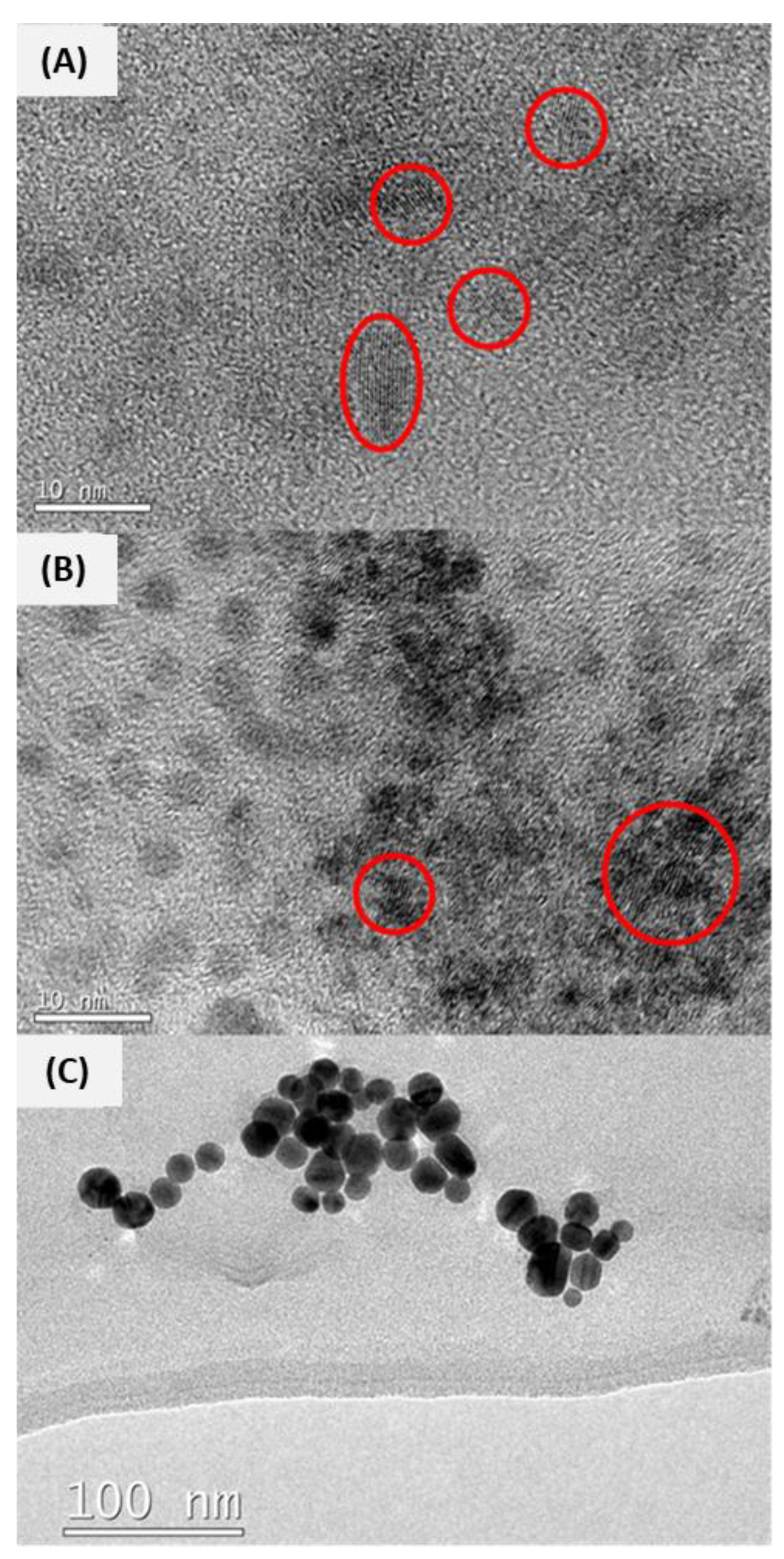
2.2.1. TEM Analyses
2.2.2. DLS Measurements
2.2.3. Metal Analyses via ICP-MS and ICP-OES
2.3. Cell Culture and Exposure
Cytotoxicity Assays
2.4. In Vivo Acute Toxicity Assays
2.4.1. Toxicity Assays in Daphnia Magna
2.4.2. Toxicity Assays in Fish
2.5. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles in the Stock Suspensions and Exposure Media
3.1.1. Dynamic Light Scattering (DLS)
3.1.2. TEM Analysis
3.1.3. Metal Analyses
3.2. Toxicity Assays
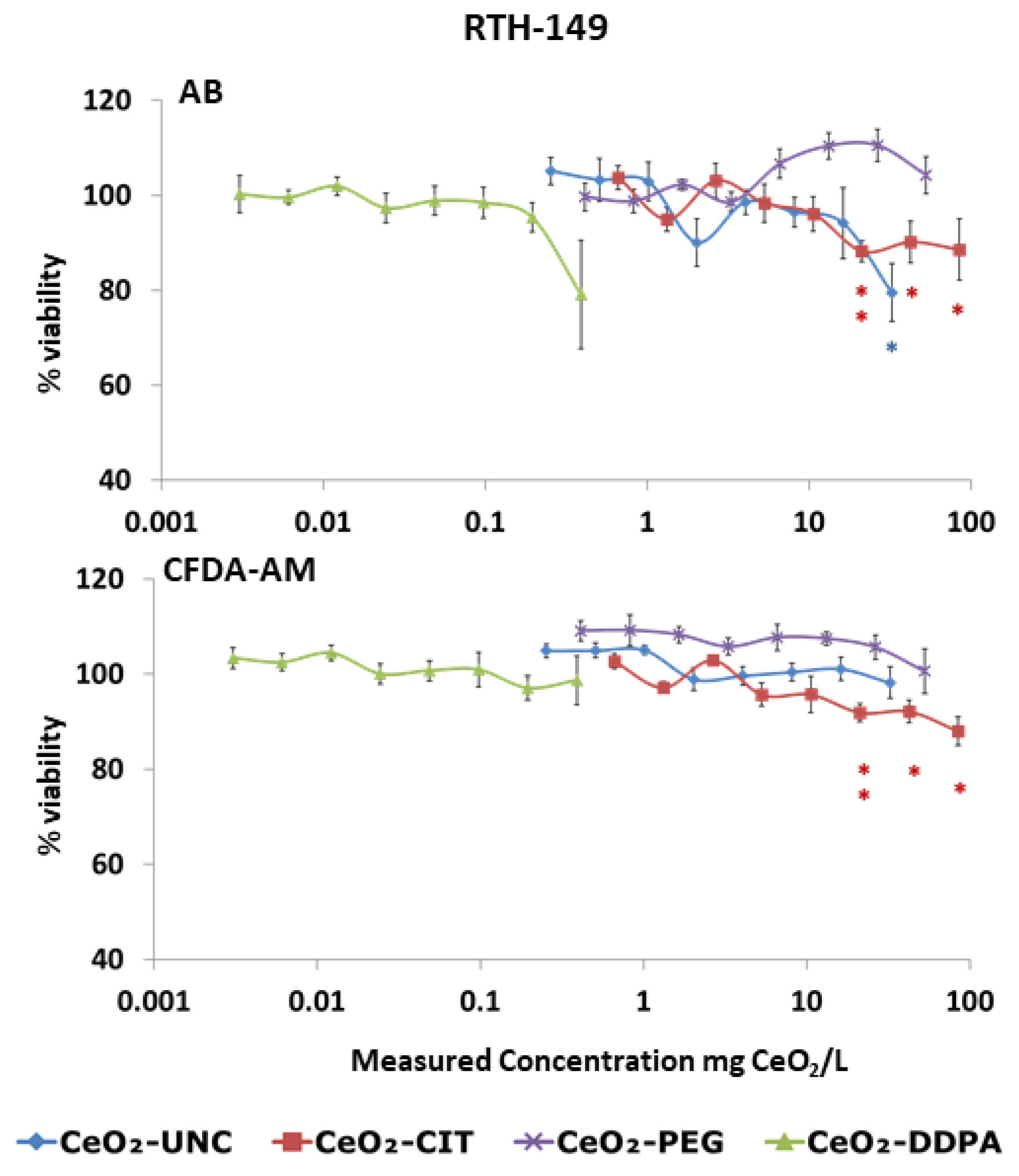
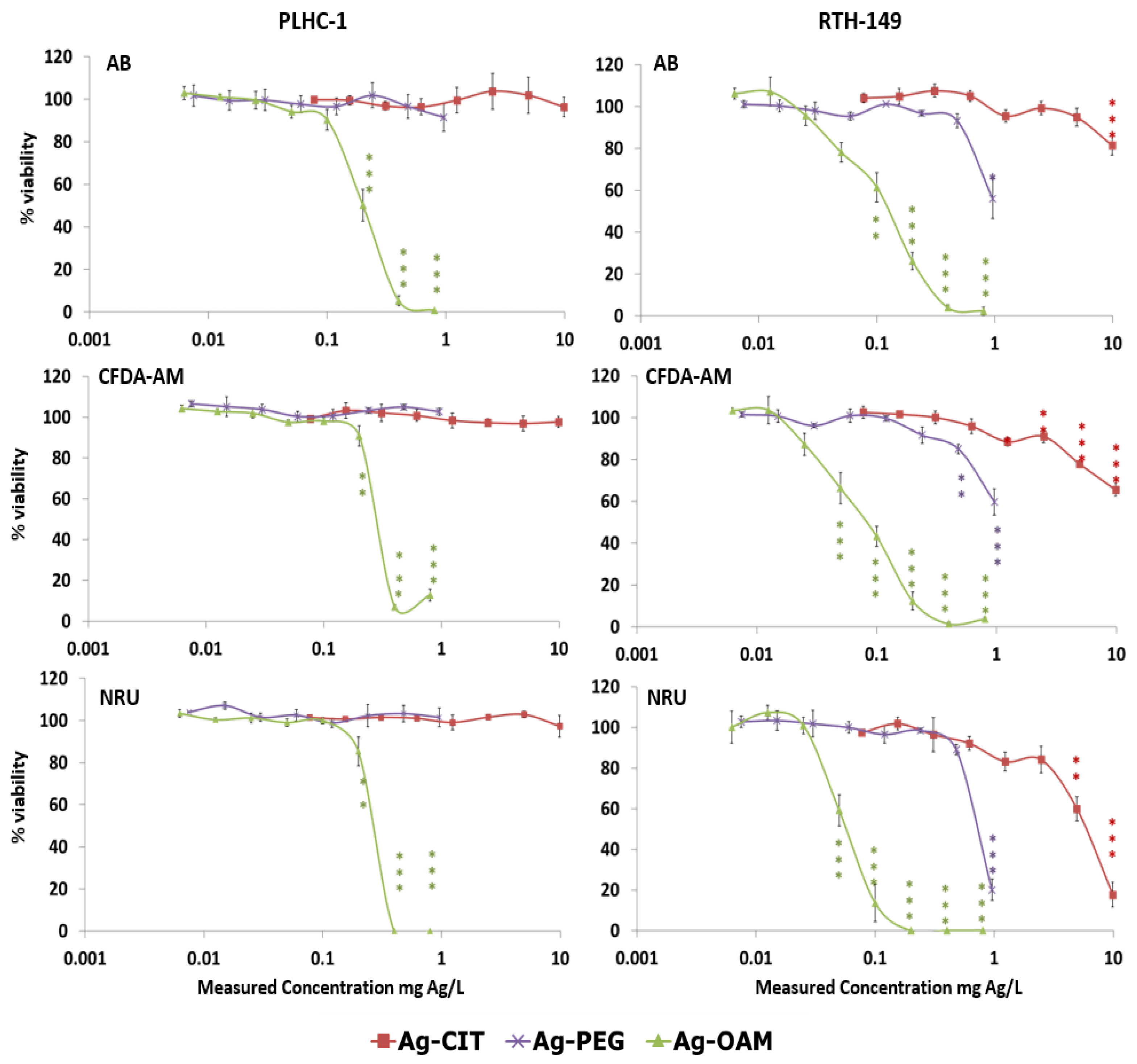
3.2.1. Cytotoxicity
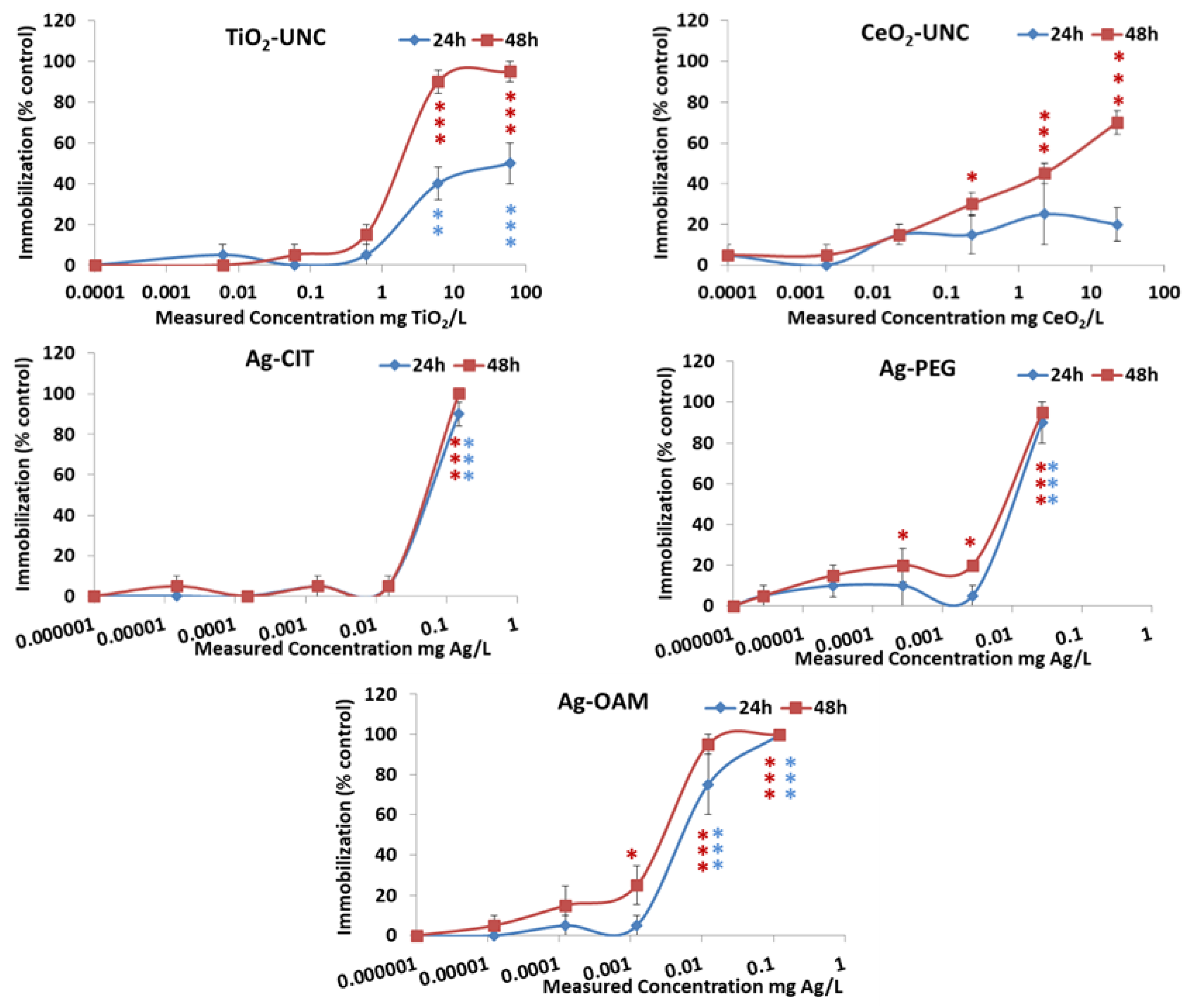
3.2.2. Acute Toxicity to Daphnia Magna
3.2.3. Acute Toxicity to Oncorhynchus Mykiss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreani, T.; Nogueira, V.; Pinto, V.V.; Ferreira, M.J.; Rasteiro, M.G.; Silva, A.M.; Pereira, R.; Pereira, C.M. Influence of the stabilizers on the toxicity of metallic nanomaterials in aquatic organisms and human cell lines. Sci. Total Environ. 2017, 607, 1264–1277. [Google Scholar] [CrossRef]
- Micheletti, C.; Roman, M.; Tedesco, E.; Olivato, I.; Benetti, F. Implementation of the NANoREG Safe-by-Design Approach for Different Nanomaterial Applications. In Proceedings of the 5th Nanosafe International Conference on Health and Safety Issues Related to Nanomaterials for a Socially Responsible Approach, Grenoble, France, 7–10 November 2016; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 838. [Google Scholar]
- Ates, M.; Demir, V.; Adiguzel, R.; Arslan, Z. Bioaccumulation, Subacute Toxicity, and Tissue Distribution of Engineered Titanium Dioxide Nanoparticles in Goldfish (Carassius auratus). J. Nanomater. 2013, 2013, 460518. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, W.; Gao, H.; Li, Y.; Tong, X.; Li, K.; Zhu, X.; Wang, Y.; Chen, Y. Behavior and Potential Impacts of Metal-Based Engineered Nanoparticles in Aquatic Environments. Nanomaterials 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Hernández-Moreno, D.; Conde, E.; Garnica, A.; Navas, J.M.; Torrent, F.; Rucandio, I.; Fernandez-Cruz, M.L. Influence of citrate and PEG coatings on the bioaccumulation of TiO2 and CeO2 nanoparticles following dietary exposure in rainbow trout. Environ. Sci. Eur. 2022, 34, 1. [Google Scholar] [CrossRef]
- Fernandez-Cruz, M.L.; Hernandez-Moreno, D.; Catalan, J.; Cross, R.K.; Stockmann-Juvala, H.; Cabellos, J.; Lopes, V.R.; Matzke, M.; Ferraz, N.; Izquierdo, J.J.; et al. Quality evaluation of human and environmental toxicity studies performed with nanomaterialssss—The GUIDEnano approach. Environ. Sci. Nano 2018, 5, 381–397. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Appendix R.6-1: Recommendations for Nanomaterials Applicable to the Guidance on QSARs and Grouping of Chemicals; ECHA: Helsinki, Finland, 2016. [Google Scholar]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. Approaches to Develop or Use Concepts of Grouping, Equivalence and Read-Across Based on Physical-Chemical Properties (GERA-PC) of Nanomaterials for Their Human Health and Ecosystem Hazard Assessment in Regulatory Regimes: Analysis of a Survey; Organisation for Economic Co-operation and Development: Paris, France, 2016. [Google Scholar]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Fernandez-Cruz, M.L.; Quesada-Garcia, A.; Alte, L.; Segner, H.; Navas, J.M. Comparative Cytotoxicity Study of Silver Nanoparticles (AgNPs) in a Variety of Rainbow Trout Cell Lines (RTL-W1, RTH-149, RTG-2) and Primary Hepatocytes. Int. J. Environ. Res. Public Health 2015, 12, 5386–5405. [Google Scholar] [CrossRef] [PubMed]
- Dayeh, V.R.; Schirmer, K.; Lee, L.E.J.; Bols, N.C. Rainbow Trout Gill Cell Line Microplate Cytotoxicity Test. In Small-Scale Freshwater Toxicity Investigations: Toxicity Test Methods; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 473–503. [Google Scholar]
- Lammel, T.; Boisseaux, P.; Fernandez-Cruz, M.L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004. [Google Scholar]
- OECD. Test No. 203: Fish, Acute Toxicity Test; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Bakshi, M.S. Impact of nanomaterials on ecosystems: Mechanistic aspects in vivo. Environ. Res. 2020, 182, 109099. [Google Scholar] [CrossRef]
- Baumann, J.; Köser, J.; Arndt, D.; Filser, J. The coating makes the difference: Acute effects of iron oxide nanoparticles on Daphnia magna. Sci. Total Environ. 2014, 484, 176–184. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological Considerations, Toxicity Assessment, and Risk Management of Inhaled Nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.; Hill, R.; Harper, S.; Rawle, A.; Hendren, C.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Seitz, F.; Rosenfeldt, R.R.; Storm, K.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Bundschuh, M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 111, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Truong, L.; Wehmas, L.; Tanguay, R.L. Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment. Nanotechnology 2013, 24, 115101. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.W.; Dong, W.; Marinakos, S.M.; Liu, J.; Chilkoti, A.; Wiesner, M.R.; Chernick, M.; Hinton, D.E. Silver nanoparticle toxicity is related to coating materials and disruption of sodium concentration regulation. Nanotoxicology 2016, 10, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Ribeiro, F.; Wojnarowicz, J.; Lojkowski, W.; Jurkschat, K.; Crossley, A.; Soares, A.M.; Loureiro, S. Zinc oxide nanoparticles toxicity to Daphnia magna: Size-dependent effects and dissolution. Environ. Toxicol. Chem. 2014, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cruz, M.L.; Lammel, T.; Connolly, M.; Conde, E.; Barrado, A.I.; Derick, S.; Perez, Y.; Fernandez, M.; Furger, C.; Navas, J.M. Comparative cytotoxicity induced by bulk and nanoparticulated ZnO in the fish and human hepatoma cell lines PLHC-1 and Hep G2. Nanotoxicology 2013, 7, 935–952. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Weiss, M.; Fan, J.; Claudel, M.; Sonntag, T.; Didier, P.; Ronzani, C.; Lebeau, L.; Pons, F. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential. J. Nanobiotechnol. 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, Z.; Xing, B. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J. Environ. Sci. 2009, 21, 1459–1466. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Diniz, M.S.; de Matos, A.P.; Lourenco, J.; Castro, L.; Peres, I.; Mendonca, E.; Picado, A. Liver alterations in two freshwater fish species (Carassius auratus and Danio rerio) following exposure to different TiO(2) nanoparticle concentrations. Microsc. Microanal. 2013, 19, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.M.; Choi, J. Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure. Environ. Toxicol. Pharmacol. 2009, 28, 86–91. [Google Scholar] [CrossRef]
- Van Hoecke, K.; Quik, J.T.; Mankiewicz-Boczek, J.; De Schamphelaere, K.A.; Elsaesser, A.; Van der Meeren, P.; Barnes, C.; McKerr, G.; Howard, C.V.; Van de Meent, D.; et al. Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ. Sci. Technol. 2009, 43, 4537–4546. [Google Scholar] [CrossRef]
- Johari, S.A.; Kalbassi, M.R.; Soltani, M.; Yu, I.J. Toxicity comparison of colloidal silver nanoparticles in various life stages of rainbow trout (Oncorhynchus mykiss). Iran. J. Fish. Sci. 2013, 12, 76–95. [Google Scholar]
- Gagné, F.; André, C.; Skirrow, R.; Gélinas, M.; Auclair, J.; van Aggelen, G.; Turcotte, P.; Gagnon, C. Toxicity of silver nanoparticles to rainbow trout: A toxicogenomic approach. Chemosphere 2012, 89, 615–622. [Google Scholar] [CrossRef]
- Sekine, R.; Khurana, K.; Vasilev, K.; Lombi, E.; Donner, E. Quantifying the adsorption of ionic silver and functionalized nanoparticles during ecotoxicity testing: Test container effects and recommendations. Nanotoxicology 2015, 9, 1005–1012. [Google Scholar] [CrossRef]
| PLHC-1 Medium | RTH-149 Medium | ||||||
|---|---|---|---|---|---|---|---|
| NM | Time (h) | PdI | Z-Average (nm; Mean ± SEM) | Main Peak Intensity (nm; Mean ± SEM) | PdI | Z-Average (nm; Mean ± SEM) | Main Peak Intensity (nm; Mean ± SEM) |
| TiO2-UNC | T0 | 0.25 ± 0.20 | 2409 ± 412 | 2159 ± 70 | 0.61 ± 0.03 | 603 ± 33 | 1047 ± 136 |
| T24 | 0.53 ± 0.10 | 2110 ± 400 | 1138 ± 70 | 0.68 ± 0.04 | 546 ± 49 | 958 ± 20 | |
| TiO2-CIT | T0 | 0.31 ± 0.01 | 281 ± 10 | 425 ± 22 | 0.25 ± 0.00 | 131 ± 7.6 | 171 ± 8 |
| T24 | 0.44 ± 0.02 | 719 ± 12 | 1324 ± 70 | 0.30 ± 0.05 | 178 ± 28 | 241 ± 48 | |
| TiO2-PEG | T0 | 0.16 ± 0.01 | 1190 ± 52 | 1227 ± 62 | 0.33 ± 0.04 | 941 ± 109 | 1215 ± 85 |
| T24 | 0.28 ± 0.01 | 1510 ± 16 | 1963 ± 50 | 0.34 ± 0.04 | 807 ± 47 | 1019 ± 93 | |
| TiO2-DDPA | T0 | * | - | - | * | - | - |
| T24 | * | - | - | * | - | - | |
| CeO2-UNC | T0 | 0.38 ± 0.03 | 2229 ± 234 | 1294 ± 219 | * | - | - |
| T24 | 0.34 ± 0.04 | 1658 ± 105 | 1117 ± 83 | * | - | - | |
| CeO2-CIT | T0 | 0.23 ± 0.02 | 596 ± 1 | 744 ± 21 | 0.47 ± 0.03 | 202 ± 23 | 354 ± 61 |
| T24 | 0.21 ± 0.02 | 590 ± 5 | 714 ± 12 | 0.43 ± 0.03 | 207 ± 25 | 343 ± 52 | |
| CeO2-PEG | T0 | 0.19 ± 0.05 | 821 ± 10 | 1015 ± 121 | 0.36 ± 0.02 | 431 ± 6 | 632 ± 22 |
| T24 | 0.11 ± 0.04 | 811 ± 23 | 870 ± 25 | 0.38 ± 0.05 | 452 ± 14 | 695 ± 59 | |
| CeO2-DDPA | T0 | * | - | - | 0.33 ± 0.08 | 242 ± 66 | 13 ± 3 |
| T24 | * | - | - | 0.67 ± 0.12 | 426 ± 11 | 11 ± 1 | |
| Ag-CIT | T0 | 0.32 ± 0.01 | 69 ± 1 | 99 ± 2 | 0.34 ± 0.01 | 61 ± 1 | 94 ± 2 |
| T24 | 0.31 ± 0.01 | 70 ± 1 | 104 ± 3 | 0.38 ± 0.05 | 64 ± 4 | 105 ± 16 | |
| Ag-PEG | T0 | 0.38 ± 0.01 | 325 ± 39 | 98 ± 13 | 0.51 ± 0.02 | 71 ± 5 | 118 ± 7 |
| T24 | 0.43 ± 0.01 | 421 ± 7 | 93 ± 30 | 0.29 ± 0.05 | 197 ± 55 | 108 ± 24 | |
| Ag-OAM | T0 | 0.33 ± 0.03 | 270 ± 21 | 187 ± 17 | 0.59 ± 0.10 | 413 ± 64 | 201 ± 87 |
| T24 | 0.38 ± 0.05 | 227 ± 45 | 589 ± 571 | 0.36 ± 0.01 | 198 ± 53 | 157 ± 5 | |
| Daphnia magna Medium | ||||
|---|---|---|---|---|
| NM | Time (h) | PdI | Z-Average (nm; Mean ± SEM) | Main Peak Intensity (nm; Mean ± SEM) |
| TiO2-UNC | T0 | 0.42 ± 0.10 | 7258 ± 640 | 3852 ± 591 |
| T48 | 0.46 ± 0.12 | 4681 ± 2711 | 1484 ± 871 | |
| TiO2-CIT | T0 | 0.16 ± 0.02 | 211 ± 101 | 255 ± 128 |
| T48 | 0.16 ± 0.01 | 322 ± 58 | 362 ± 67 | |
| TiO2-PEG | T0 | 0.29 ± 0.10 | 2914 ± 105 | 3115 ± 159 |
| T48 | 0.28 ± 0.06 | 4068 ± 512 | 3845 ± 427 | |
| TiO2-DDPA | T0 | 0.46 ± 0.18 | 441 ± 254 | 213 ± 47 |
| T48 | * | - | - | |
| CeO2-UNC | T0 | * | - | - |
| T48 | * | - | - | |
| CeO2-CIT | T0 | 0.20 ± 0.02 | 400 ± 32 | 462 ± 46 |
| T48 | 0.18 ± 0.02 | 482 ± 59 | 567 ± 84 | |
| CeO2-PEG | T0 | 0.53 ± 0.06 | 3939 ± 2467 | 1475 ± 448 |
| T48 | 0.21 ± 0.03 | 4451 ± 723 | 3915 ± 220 | |
| CeO2-DDPA | T0 | 0.60 ± 0.15 | 598 ± 252 | 256 ± 70 |
| T48 | * | - | - | |
| Ag-CIT | T0 | 0.47 ± 0.09 | 390 ± 128 | 222 ± 10 |
| T48 | 0.52 ± 0.02 | 512 ± 17 | 310 ± 7 | |
| Ag-PEG | T0 | 0.44 ± 0.01 | 345 ± 31 | 203 ± 19 |
| T48 | 0.62 ± 0.01 | 588 ± 24 | 224 ± 11 | |
| Ag-OAM | T0 | * | - | - |
| T48 | * | - | - | |
| Oncorhynchus mykiss Medium | ||||
|---|---|---|---|---|
| NM | Time (h) | PdI | Z-Average (nm; Mean ± SEM) | Main Peak Intensity (nm; Mean ± SEM) |
| TiO2-UNC | T0 | 0.68 ± 0.22 | 3095 ± 1650 | 1654 ± 1096 |
| T24 | 0.24 ± 0.08 | 4115 ± 281 | 3858 ± 36 | |
| T48 | * | - | - | |
| T72 | 0.46 ± 0.25 | 3286 ± 351 | 3246 ± 133 | |
| T96 | 0.52 ± 0.27 | 4117 ± 1475 | 2331 ± 1502 | |
| TiO2-CIT | T0 | 0.25 ± 0.02 | 143 ± 18 | 12 ± 0.64 |
| T24 | 0.28 ± 0.01 | 27 ± 0.32 | 27 ± 0.55 | |
| T48 | 0.26 ± 0.02 | 46 ± 9 | 59 ± 7 | |
| T72 | 0.27 ± 0.01 | 266 ± 6 | 346 ± 8 | |
| T96 | 0.49 ± 0.24 | 1942 ± 291 | 1969 ± 99 | |
| TiO2-PEG | T0 | 0.38 ± 0.23 | 2011 ± 877 | 1898 ± 1195 |
| T24 | 0.67 ± 0.10 | 933 ± 376 | 523 ± 245 | |
| T48 | 0.33 ± 0.20 | 3948 ± 864 | 3702 ± 476 | |
| T72 | 0.52 ± 0.20 | 2051 ± 691 | 1544 ± 1044 | |
| T96 | 0.68 ± 0.24 | 2095 ± 16 | 769 ± 167 | |
| CeO2-UNC | All Ts | * | - | - |
| CeO2-CIT | T0 | 0.34 ± 0.02 | 76 ± 3 | 97 ± 1 |
| T24 | 0.25 ± 0.01 | 71 ± 0.11 | 87 ± 2 | |
| T48 | 0.22 ± 0.01 | 141 ± 27 | 170 ± 32 | |
| T72 | 0.34 ± 0.01 | 240 ± 19 | 377 ± 38 | |
| T96 | 0.32 ± 0.05 | 1429 ± 295 | 1496 ± 575 | |
| CeO2-PEG | T0 | 0.41 ± 0.03 | 1501 ± 93 | 1084 ± 15 |
| T24 | 0.66 ± 0.03 | 1545 ± 21 | 886 ± 13 | |
| T48 | 0.38 ± 0.22 | 1515 ± 1 | 758 ± 27 | |
| T72/T96 | * | - | - | |
| Ag-CIT | T0 | 0.45 ± 0.01 | 360 ± 8 | 328 ± 29 |
| T24 | 0.37 ± 0.01 | 285 ± 2 | 260 ± 19 | |
| T48 | 0.38 ± 0.03 | 273 ± 13 | 267 ± 20 | |
| T72 | 0.37 ± 0.01 | 277 ± 3 | 286 ± 15 | |
| T96 | 0.39 ± 0.01 | 289 ± 11 | 259 ± 21 | |
| Ag-PEG | T0 | * | - | - |
| T24 | 0.54 ± 0.01 | 370 ± 40 | 318 ± 111 | |
| T48 | * | - | - | |
| T72 | 0.59 ± 0.02 | 442 ± 11 | 167 ± 17 | |
| T96 | 0.56 ± 0.02 | 389 ± 23 | 216 ± 21 | |
| Cell Medium (mV) | Daphnia magna Medium (mV) | Oncorhynchus mykiss Medium (mV) | ||||
|---|---|---|---|---|---|---|
| Time 0 | Time 24 h | Time 0 | Time 48 h | Time 0 | Time 96 h | |
| Medium | −8.32 | −10.35 | −6.02 | |||
| TiO2-UNC | −8.96 | −11.03 | 28.50 | 27.53 | 27.40 | 26.63 |
| TiO2-CIT | −11.40 | −14.87 | −33.80 | −35.13 | −36.63 | −28.2 |
| TiO2-PEG | −13.37 | −9.65 | −24.60 | −23.80 | −24.23 | −21.67 |
| CeO2-UNC | −9.72 | −10.13 | 41.30 | 40.93 | 34.70 | 37.43 |
| CeO2-CIT | −10.32 | −10.19 | −21.27 | −21.23 | −33.13 | −26.57 |
| CeO2-PEG | −9.36 | −9.455 | −12.33 | −12.1 | −15.60 | −20 |
| Type of Assay | Nominal (mg/L) | Measured (mg/L) | Nominal (mg/L) | Measured (mg/L) | Nominal (mg/L) | Measured (mg/L) | |||
|---|---|---|---|---|---|---|---|---|---|
| Fish cell lines | TiO2-UNC | 100 | 71.1 ± 18.9 | CeO2-UNC | 100 | 32.5 ± 2.9 | |||
| TiO2-CIT | 100 | 72.48 ± 9.3 | CeO2-CIT | 100 | 84.7 ± 5.2 | Ag-CIT | 10 | 9.9 ± 0.9 | |
| TiO2-PEG | 100 | 68.8 ± 0.03 | CeO2-PEG | 100 | 52.8 ± 2.7 | Ag-PEG | 1.62 | 0.96 ± 0.1 | |
| TiO2-DDPA | 100 | 0.28 ± 0.01 | CeO2-DDPA | 100 | 0.39 ± 0.16 | Ag-OAM | 8.6 | 0.80 ± 0.1 | |
| D. magna | TiO2-UNC | 100 | 60.4 ± 8.5 | CeO2-UNC | 100 | 22.8 ± 9.5 | |||
| TiO2-CIT | 100 | 170.4 ± 5.5 | CeO2-CIT | 100 | 49.4 ± 1.9 | Ag-CIT | 1 | 0.15 ± 0.007 | |
| TiO2-PEG | 100 | 82.6 ± 9.8 | CeO2-PEG | 100 | 29.6 ± 3.4 | Ag-PEG | 0.16 | 0.03 ± 0.001 | |
| TiO2-DDPA | 100 | 0.17 ± 0.01 | CeO2-DDPA | 100 | 1.1 ± 0.12 | Ag-OAM | 0.86 | 0.12 ± 0.001 | |
| O. mykiss | TiO2-UNC | 100 | 72.0 ± 6.1 | CeO2-UNC | 100 | 40.8 ± 3.4 | |||
| TiO2-CIT | 100 | 89.7 ± 6.6 | CeO2-CIT | 100 | 82.7 ± 8.8 | Ag-CIT | 1.5 | 0.035 ± 0.001 | |
| TiO2-PEG | 100 | 89.0 ± 11.8 | CeO2-PEG | 100 | 32.8 ± 3.3 | Ag-PEG | 0.24 | 0.08 ± 0.001 |
| PLHC-1 (mg/L) | RTH-149 (mg/L) | Daphnia magna (mg/L) | Oncorhynchus mykiss (mg/L) | |
|---|---|---|---|---|
| TiO2-UNC | NOEC > 71.1 | NOEC > 71.1 | NOEC = 0.60; EC20 = 1.7 ± 0.98; EC50 = 2.3 ± 1.09 | NOEC > 72.0 |
| TiO2-CIT | NOEC > 72.48 | NOEC > 72.4 | NOEC > 170.4 | NOEC > 89.7 |
| TiO2-PEG | NOEC > 68.8 | NOEC > 68.8 | NOEC > 82.6 | NOEC > 89.0 |
| TiO2-DDPA | NOEC > 0.28 | NOEC > 0.28 | NOEC > 0.17 | Not tested |
| CeO2-UNC | NOEC ≥ 32.5 | NOEC = 16.2; IC20 = 27.1 ± 4.8 | NOEC = 0.023; EC20 = 0.72 ± 0.40; EC50 = 5.4 ± 1.8 | NOEC > 40.8 |
| CeO2-CIT | NOEC > 84.7 | NOEC = 10.6 | NOEC > 49.4 | NOEC > 82.7 |
| CeO2-PEG | NOEC > 52.8 | NOEC > 52.8 | NOEC > 29.6 | NOEC > 32.8 |
| CeO2-DDPA | NOEC > 0.39 | NOEC > 0.39 | NOEC > 1.1 | Not tested |
| Ag-CIT | NOEC > 9.9 | NOEC = 0.62; IC20 = 3.6 ± 0.31; IC50 = 6.1 ± 0.63 | NOEC = 0.015; EC20 = 0.07 ± 0.02; EC50 = 0.08 ± 0.02 | NOEC > 0.035 |
| Ag-PEG | NOEC > 0.96 | NOEC = 0.24; IC20 = 0.56 ± 0.03; IC50 = 0.73 ± 0.04 | NOEC = 0.000027; EC20 = 0.006 ± 0.001; EC50 = 0.008 ± 0.002 | NOEC > 0.08 |
| Ag-OAM | NOEC = 0.10; IC20 = 0.16 ± 0.04; IC50 = 0.21 ± 0.01 | NOEC = 0.025; IC20 = 0.034 ± 0.007; IC50 = 0.056 ± 0.005 | NOEC = 0.00016; EC20 = 0.002 ± 0.0006; EC50 = 0.003 ± 0.0005 | Not tested |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Moreno, D.; Fernández-Díaz, M.; Rucandio, I.; Navas, J.M.; Fernández-Cruz, M.L. Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms. Toxics 2024, 12, 142. https://doi.org/10.3390/toxics12020142
Hernández-Moreno D, Fernández-Díaz M, Rucandio I, Navas JM, Fernández-Cruz ML. Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms. Toxics. 2024; 12(2):142. https://doi.org/10.3390/toxics12020142
Chicago/Turabian StyleHernández-Moreno, David, Marta Fernández-Díaz, Isabel Rucandio, José María Navas, and María Luisa Fernández-Cruz. 2024. "Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms" Toxics 12, no. 2: 142. https://doi.org/10.3390/toxics12020142
APA StyleHernández-Moreno, D., Fernández-Díaz, M., Rucandio, I., Navas, J. M., & Fernández-Cruz, M. L. (2024). Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms. Toxics, 12(2), 142. https://doi.org/10.3390/toxics12020142








