The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
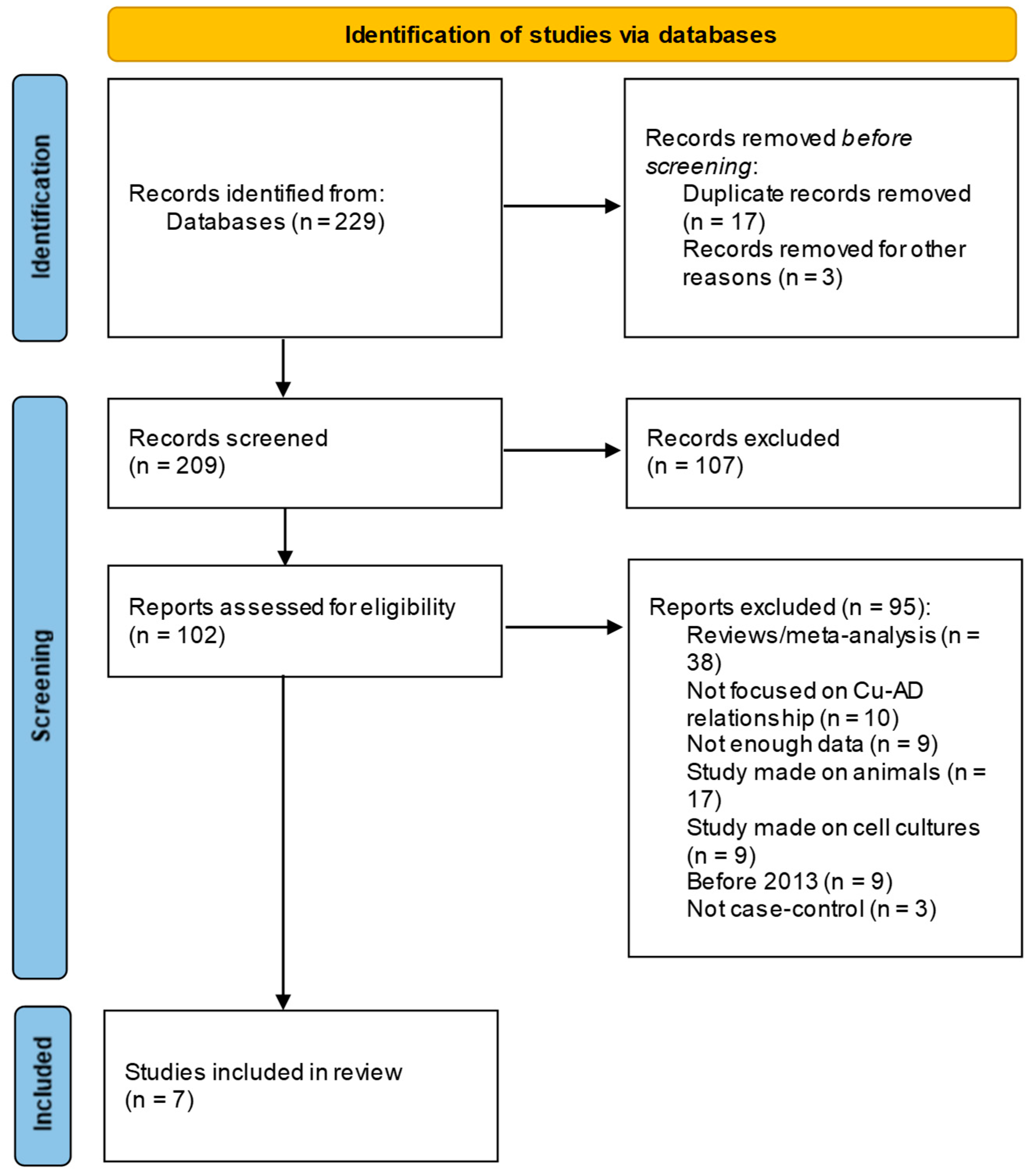
2.2. Eligibility Criteria, Search Method, Information Sources, and Study Selection
3. Results
3.1. Ceruloplasmin-Bound Copper Results
3.2. Non-Ceruloplasmin-Bound Copper (Non-Cp-Cu) Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial Dysfunction: The Missing Link between Aging and Sporadic Alzheimer’s Disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Hebert, L.E.; Beckett, L.A.; Evans, D.A.; Scherr, P.A.; Albert, M.S.; Pilgrim, D.M.; Chown, M.J.; Harris Funkenstein, H. Age-Specific Incidence of Alzheimer’s Disease in a Community Population. JAMA 1995, 273, 1354–1359. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- 2022 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef]
- Majeed, A.; Marwick, B.; Yu, H.; Fadavi, H.; Tavakoli, M. Ophthalmic Biomarkers for Alzheimer’s Disease: A Review. Front. Aging Neurosci. 2021, 13, 720167. [Google Scholar] [CrossRef]
- Hart, N.J.; Koronyo, Y.; Black, K.L.; Koronyo-Hamaoui, M. Ocular Indicators of Alzheimer’s: Exploring Disease in the Retina. Acta Neuropathol. 2016, 132, 767–787. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease: How Far Have We Come in the Clinic? J. Alzheimers Dis. 2018, 62, 1369–1379. [Google Scholar] [CrossRef]
- Davies, K.M.; Hare, D.J.; Cottam, V.; Chen, N.; Hilgers, L.; Halliday, G.; Mercer, J.F.B.; Double, K.L. Localization of Copper and Copper Transporters in the Human Brain. Metallomics 2013, 5, 43–51. [Google Scholar] [CrossRef]
- Grubman, A.; White, A.R. Copper as a Key Regulator of Cell Signalling Pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Zhang, C.; Zhou, S.; Ji, G. Molecular Functions of Ceruloplasmin in Metabolic Disease Pathology. Diabetes Metab. Syndr. Obes. 2022, 15, 695–711. [Google Scholar] [CrossRef]
- Squitti, R.; Ventriglia, M.; Gennarelli, M.; Colabufo, N.A.; El Idrissi, I.G.; Bucossi, S.; Mariani, S.; Rongioletti, M.; Zanetti, O.; Congiu, C.; et al. Non-Ceruloplasmin Copper Distincts Subtypes in Alzheimer’s Disease: A Genetic Study of ATP7B Frequency. Mol. Neurobiol. 2017, 54, 671–681. [Google Scholar] [CrossRef]
- Al-Khateeb, E.; Al-Zayadneh, E.; Al-Dalahmah, O.; Alawadi, Z.; Khatib, F.; Naffa, R.; Shafagoj, Y. Relation between Copper, Lipid Profile, and Cognition in Elderly Jordanians. J. Alzheimers Dis. 2014, 41, 203–211. [Google Scholar] [CrossRef]
- Squitti, R.; Polimanti, R.; Siotto, M.; Bucossi, S.; Ventriglia, M.; Mariani, S.; Vernieri, F.; Scrascia, F.; Trotta, L.; Rossini, P.M. ATP7B Variants as Modulators of Copper Dyshomeostasis in Alzheimer’s Disease. Neuromolecular Med. 2013, 15, 515–522. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Negahdar, H.; Hosseini, S.R.; Parsian, H.; Kheirkhah, F.; Mosapour, A.; Khafri, S.; Haghighi, A.H. agh Homocysteine, Trace Elements and Oxidant/Antioxidant Status in Mild Cognitively Impaired Elderly Persons: A Cross-Sectional Study. Rom. J. Intern. Med. 2015, 53, 336–342. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Squitti, R.; Mendez, A.J.; Simonelli, I.; Ricordi, C. Diabetes and Alzheimer’s Disease: Can Elevated Free Copper Predict the Risk of the Disease? J. Alzheimers Dis. 2017, 56, 1055–1064. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals Toxicity and Its Correlation with the Gene Expression in Alzheimer’s Disease. Mol. Biol. Rep. 2021, 48, 3245–3252. [Google Scholar] [CrossRef]
- Bush, A.I.; Tanzi, R.E. Therapeutics for Alzheimer’s Disease Based on the Metal Hypothesis. Neurotherapeutics 2008, 5, 421–432. [Google Scholar] [CrossRef]
- Gupta, A.; Chattopadhyay, I.; Mukherjee, S.; Sengupta, M.; Das, S.K.; Ray, K. A Novel COMMD1 Mutation Thr174Met Associated with Elevated Urinary Copper and Signs of Enhanced Apoptotic Cell Death in a Wilson Disease Patient. Behav. Brain Funct. 2010, 6, 33. [Google Scholar] [CrossRef]
- Simon, I.; Schaefer, M.; Reichert, J.; Stremmel, W. Analysis of the Human Atox 1 Homologue in Wilson Patients. World J. Gastroenterol. 2008, 14, 2383–2387. [Google Scholar] [CrossRef]
- Liu, H.P.; Lin, W.Y.; Wang, W.F.; Tsai, C.H.; Wu, W.C.; Chiou, M.T.; Shen, C.P.; Wu, B.T.; Tsai, F.J. Genetic Variability in Copper-Transporting P-Type Adenosine Triphosphatase (ATP7B) Is Associated with Alzheimer’s Disease in a Chinese Population. J. Biol. Regul. Homeost. Agents 2013, 27, 319–327. [Google Scholar]
- Hozumi, I.; Hasegawa, T.; Honda, A.; Ozawa, K.; Hayashi, Y.; Hashimoto, K.; Yamada, M.; Koumura, A.; Sakurai, T.; Kimura, A.; et al. Patterns of Levels of Biological Metals in CSF Differ among Neurodegenerative Diseases. J. Neurol. Sci. 2011, 303, 95–99. [Google Scholar] [CrossRef]
- Brewer, G.J.; Kanzer, S.H.; Zimmerman, E.A.; Molho, E.S.; Celmins, D.F.; Heckman, S.M.; Dick, R. Subclinical Zinc Deficiency in Alzheimer’s Disease and Parkinson’s Disease. Am. J. Alzheimers Dis. Other Dement. 2010, 25, 572. [Google Scholar] [CrossRef]
- Damante, C.A.; Ösz, K.; Nagy, Z.; Grasso, G.; Pappalardo, G.; Rizzarelli, E.; Sóvágó, I. Zn2+’s Ability to Alter the Distribution of Cu2+ among the Available Binding Sites of Aβ(1-16)-Polyethylenglycol-Ylated Peptide: Implications in Alzheimer’s Disease. Inorg. Chem. 2011, 50, 5342–5350. [Google Scholar] [CrossRef]
- Tiiman, A.; Palumaa, P.; Tõugu, V. The Missing Link in the Amyloid Cascade of Alzheimer’s Disease—Metal Ions. Neurochem. Int. 2013, 62, 367–378. [Google Scholar] [CrossRef]
- Zhu, D.; Su, Y.; Fu, B.; Xu, H. Magnesium Reduces Blood-Brain Barrier Permeability and Regulates Amyloid-β Transcytosis. Mol. Neurobiol. 2018, 55, 7118–7131. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Ma, Z.T.; Lv, J.Y.; Jiang, W.J.; Liu, M.Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease from 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13, 799824. [Google Scholar] [CrossRef]
- Maynard, C.J.; Bush, A.I.; Masters, C.L.; Cappai, R.; Li, Q.X. Metals and Amyloid-Beta in Alzheimer’s Disease. Int. J. Exp. Pathol. 2005, 86, 147–159. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Wang, Z.X.; Tan, L.; Wang, H.F.; Ma, J.; Liu, J.; Tan, M.S.; Sun, J.H.; Zhu, X.C.; Jiang, T.; Yu, J.T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimers Dis. 2015, 47, 565–581. [Google Scholar] [CrossRef]
- Wenstrup, D.; Ehman, W.D.; Markesbery, W.R. Trace Element Imbalances in Isolated Subcellular Fractions of Alzheimer’s Disease Brains. Brain Res. 1990, 533, 125–131. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum in Hippocampal Neurons from Humans with Alzheimer’s Disease. Neurotoxicology 2006, 27, 385–394. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front. Cell. Infect. Microbiol. 2017, 7, 396. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Dutta, K.; Prasad, P.; Sinha, D. Chronic Low Level Arsenic Exposure Evokes Inflammatory Responses and DNA Damage. Int. J. Hyg. Environ. Health 2015, 218, 564–574. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Mumford, S.L.; Sjaarda, L.; Perkins, N.J.; Malik, F.; Wactawski-Wende, J.; Schisterman, E.F. Blood Lead, Cadmium and Mercury in Relation to Homocysteine and C-Reactive Protein in Women of Reproductive Age: A Panel Study. Environ. Health 2017, 16, 84. [Google Scholar] [CrossRef]
- Wang, T.C.; Song, Y.S.; Yu, S.F.; Zhang, J.; Wang, H.; Gu, Y.E.; Chen, T.; Jia, G. Association of Folate Deficiency and Selected Tumor Marker Concentrations in Long-Term Hexavalent Chromium Exposed Population. Int. J. Hyg. Environ. Health 2014, 217, 88–94. [Google Scholar] [CrossRef]
- Haan, M.N.; Miller, J.W.; Aiello, A.E.; Whitmer, R.A.; Jagust, W.J.; Mungas, D.M.; Allen, L.H.; Green, R. Homocysteine, B Vitamins, and the Incidence of Dementia and Cognitive Impairment: Results from the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2007, 85, 511–517. [Google Scholar] [CrossRef]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, Á.M.; Lasheras, C. Homocysteine Increases the Risk of Mortality in Elderly Individuals. Br. J. Nutr. 2007, 97, 1138–1143. [Google Scholar] [CrossRef]
- Pal, A.; Siotto, M.; Prasad, R.; Squitti, R. Towards a Unified Vision of Copper Involvement in Alzheimer’s Disease: A Review Connecting Basic, Experimental, and Clinical Research. J. Alzheimers Dis. 2015, 44, 343–354. [Google Scholar] [CrossRef]
- Perrone, L.; Grant, W.B. Observational and Ecological Studies of Dietary Advanced Glycation End Products in National Diets and Alzheimer’s Disease Incidence and Prevalence. J. Alzheimers Dis. 2015, 45, 965–979. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common Neurodegenerative Pathways in Obesity, Diabetes, and Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Sun, D.-X.; Hu, B.; Shan, Q.; Zhang, Z.-F.; Fan, S.-H. Trace Amounts of Copper Exacerbate Beta Amyloid-Induced Neurotoxicity in the Cholesterol-Fed Mice through TNF-Mediated Inflammatory Pathway. Brain Behav. Immun. 2009, 23, 193–203. [Google Scholar] [CrossRef]
- Hofman, A.; Ott, A.; Breteler, M.M.B.; Bots, M.L.; Slooter, A.J.C.; Van Harskamp, F.; Van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, Apolipoprotein E, and Prevalence of Dementia and Alzheimer’s Disease in the Rotterdam Study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.L.; Hänninen, T.; Laakso, M.P.; Hallikainen, M.; Alhainen, K.; Soininen, H.; Tuomilehto, J.; Nissinen, A. Midlife Vascular Risk Factors and Late-Life Mild Cognitive Impairment: A Population-Based Study. Neurology 2001, 56, 1683–1689. [Google Scholar] [CrossRef]
- Singh, I.; Sagare, A.P.; Coma, M.; Perlmutter, D.; Gelein, R.; Bell, R.D.; Deane, R.J.; Zhong, E.; Parisi, M.; Ciszewski, J.; et al. Low Levels of Copper Disrupt Brain Amyloid-β Homeostasis by Altering Its Production and Clearance. Proc. Natl. Acad. Sci. USA 2013, 110, 14771–14776. [Google Scholar] [CrossRef]
- Stern, Y.; Alexander, G.E.; Prohovnik, I.; Mayeux, R. Inverse Relationship between Education and Parietotemporal Perfusion Deficit in Alzheimer’s Disease. Ann. Neurol. 1992, 32, 371–375. [Google Scholar] [CrossRef]
| Authors (Publication Year) | Country | Cases | Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu Type | Gender Number | Age (Mean ± Sd) | Cu (Mean ± Sd) | Subject Gender | Age (Mean ± Sd) | Cu (Mean ± Sd) | p-Value (Cu) | ||
| Al-khateeb E. et al. (2014) [12] | Jordan | Cu | 23F/29M | 70.7 ± 7.63 | 126.12 ± 71.78 (μg/dL) | 17F/33M | 68.9 ± 7.11 | 114.55 ± 57.6 (μg/dL) | <0.377 |
| Negahdar H. et al. (2015) [16] | Iran | Cu | 60F/60M | 74.2 ± 6.9 | 0.99 ± 0.4 (ppm) | 60M/60F | 67.7 ± 7.3 | 0.88 ± 0.3 (ppm) | <0.068 |
| Paglia G. et al. (2016) [17] | Italy | Cu | 25F/9M | 72.4 ± 7.48 | 815.75 ± 206 (μg/L) | 25F/15M | 65.53 ± 6.37 | 703.88 ± 244.03 (μg/L) | <0.033 |
| Squitti R. et al. (2013) [13] | Italy | Non-cp Cu | 294F/140M | 74.9 ± 8.1 | 2.24 ± 3.14 (μmol/L) | 207F/96M | 66.5 ± 10.5 | 0.28 ± 2.98 (μmol/L) | <0.001 |
| Squitti R. et al. (2017) [11] | Italy | Non-cp Cu | 52F/37M | 73 ± 8.5 | 2.3 ± 1.5 (μmol/L) | 77F/70M | 49 ± 12.7 | 1.07 ± 0.6 (μmol/L) | <0.001 |
| Squitti R. et al. (2017) [18] | Italy | Non-cp Cu | 118F/58M | 80.7 ± 6.9 | 2.5 ± 0.5 (μmol/L) | 76F/35M | 81.5 ± 6.8 | 1.6 ± 0.3 (μmol/L) | <0.0001 |
| Yadav J. et al. (2021) [19] | India | Cu | 32F/28M | 74.1 ± 1.68 | 0.127 ± 0.024 (mg/L) | - | 74.13 ± 1.68 | 0.069 ± 0.0068 (mg/L) | <0.0254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabalic, A.; Mei, V.; Solinas, G.; Madeddu, R. The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review. Toxics 2024, 12, 755. https://doi.org/10.3390/toxics12100755
Sabalic A, Mei V, Solinas G, Madeddu R. The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review. Toxics. 2024; 12(10):755. https://doi.org/10.3390/toxics12100755
Chicago/Turabian StyleSabalic, Angela, Veronica Mei, Giuliana Solinas, and Roberto Madeddu. 2024. "The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review" Toxics 12, no. 10: 755. https://doi.org/10.3390/toxics12100755
APA StyleSabalic, A., Mei, V., Solinas, G., & Madeddu, R. (2024). The Role of Copper in Alzheimer’s Disease Etiopathogenesis: An Updated Systematic Review. Toxics, 12(10), 755. https://doi.org/10.3390/toxics12100755









