Methamphetamine Shows Different Joint Toxicity for Different Types of Microplastics on Zebrafish Larvae by Mediating Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. High-Performance Liquid Chromatography Tandem Mass Spectrometry (HPLC-MS/MS) Detection Method
2.3. Behavioral Function Measurement
2.4. Histological Examination
2.5. Quantitative Real-Time RT-PCR Assay
2.6. Statistical Analysis
3. Results
3.1. Characterization Map, Zeta Potential, and Analysis of MPs
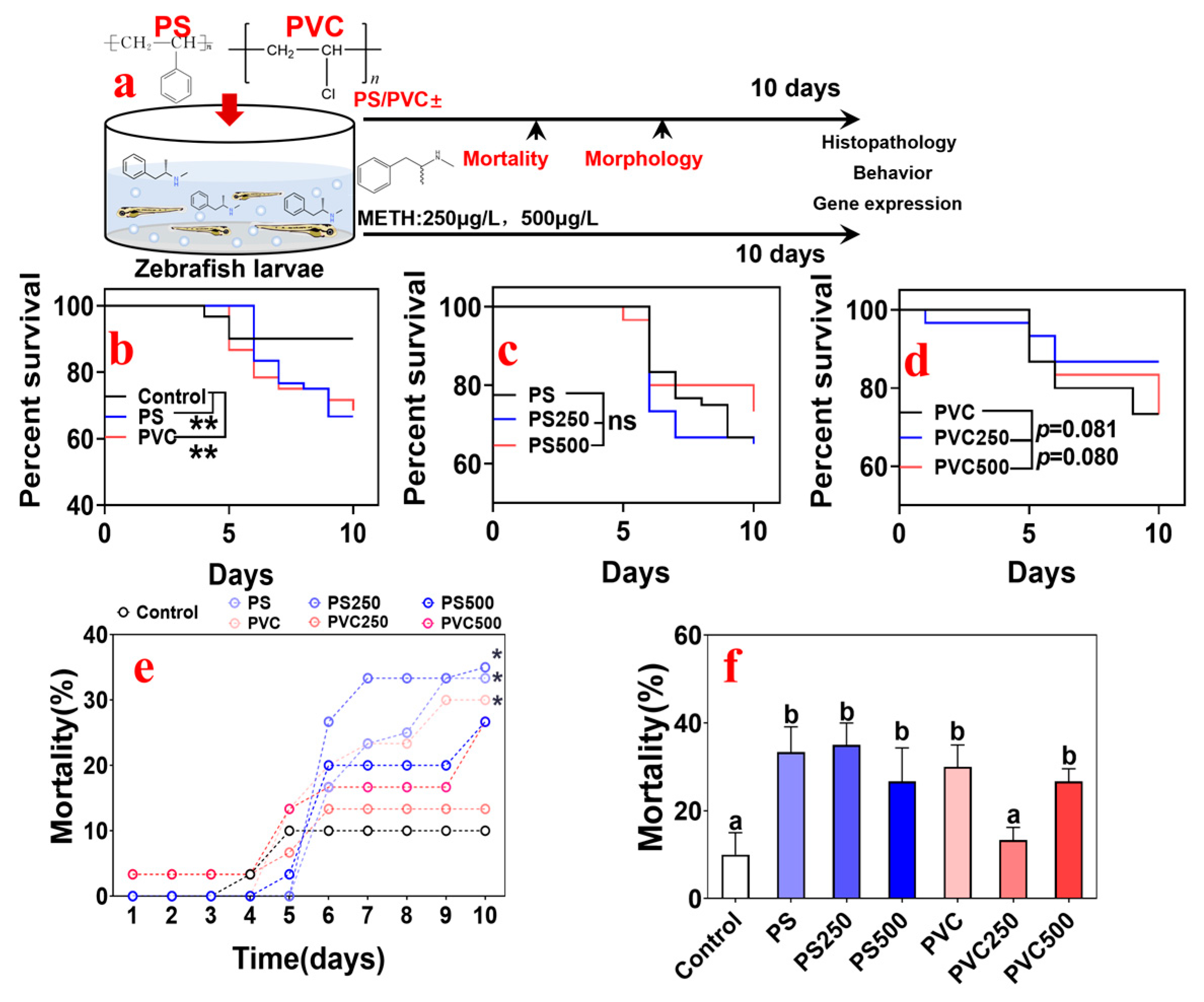
3.2. Effects of Combined MPs and METH Exposure on the Mortality of Zebrafish Larvae
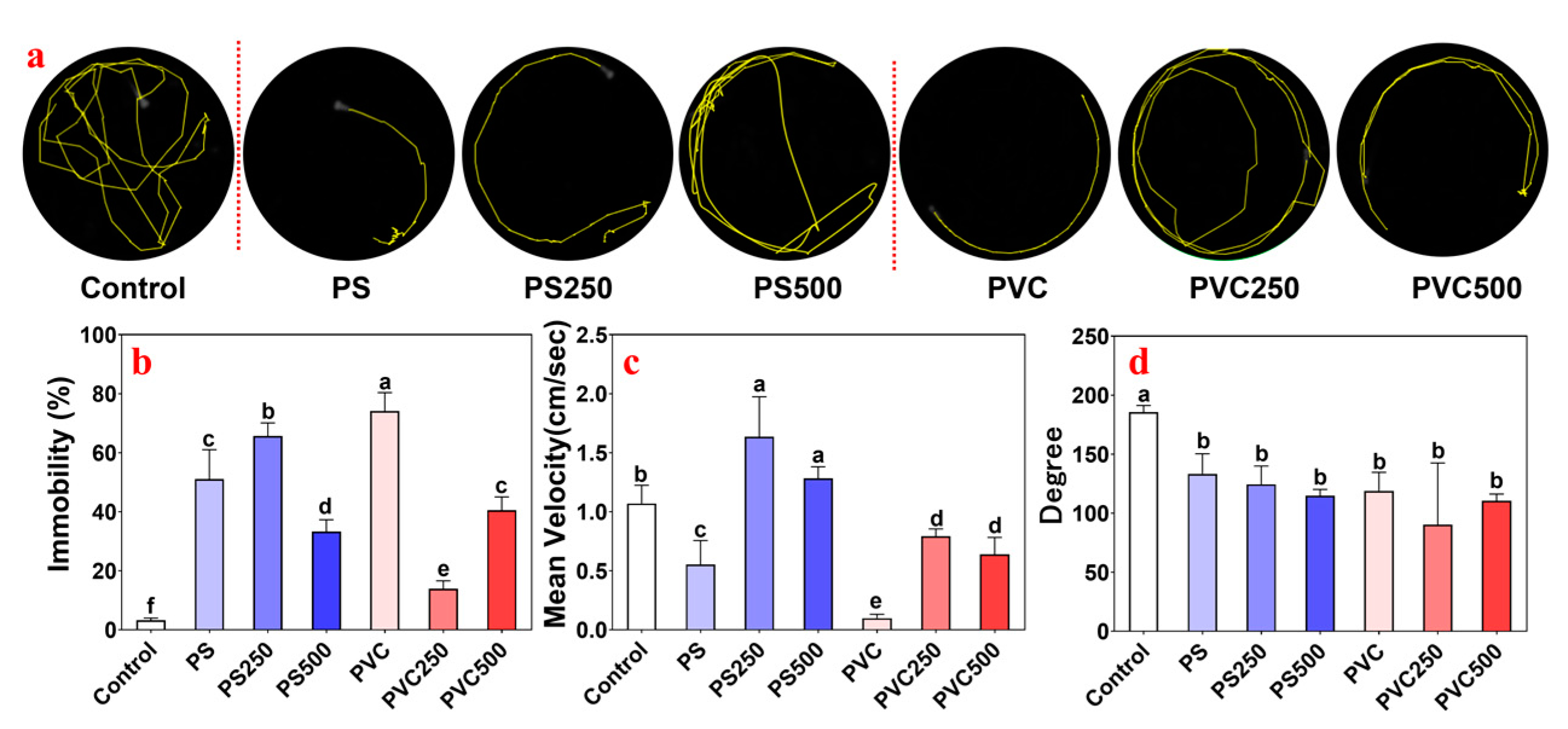
3.3. Effects of Combined MPs and METH Exposure on the Locomotor Behavior of Zebrafish Larvae
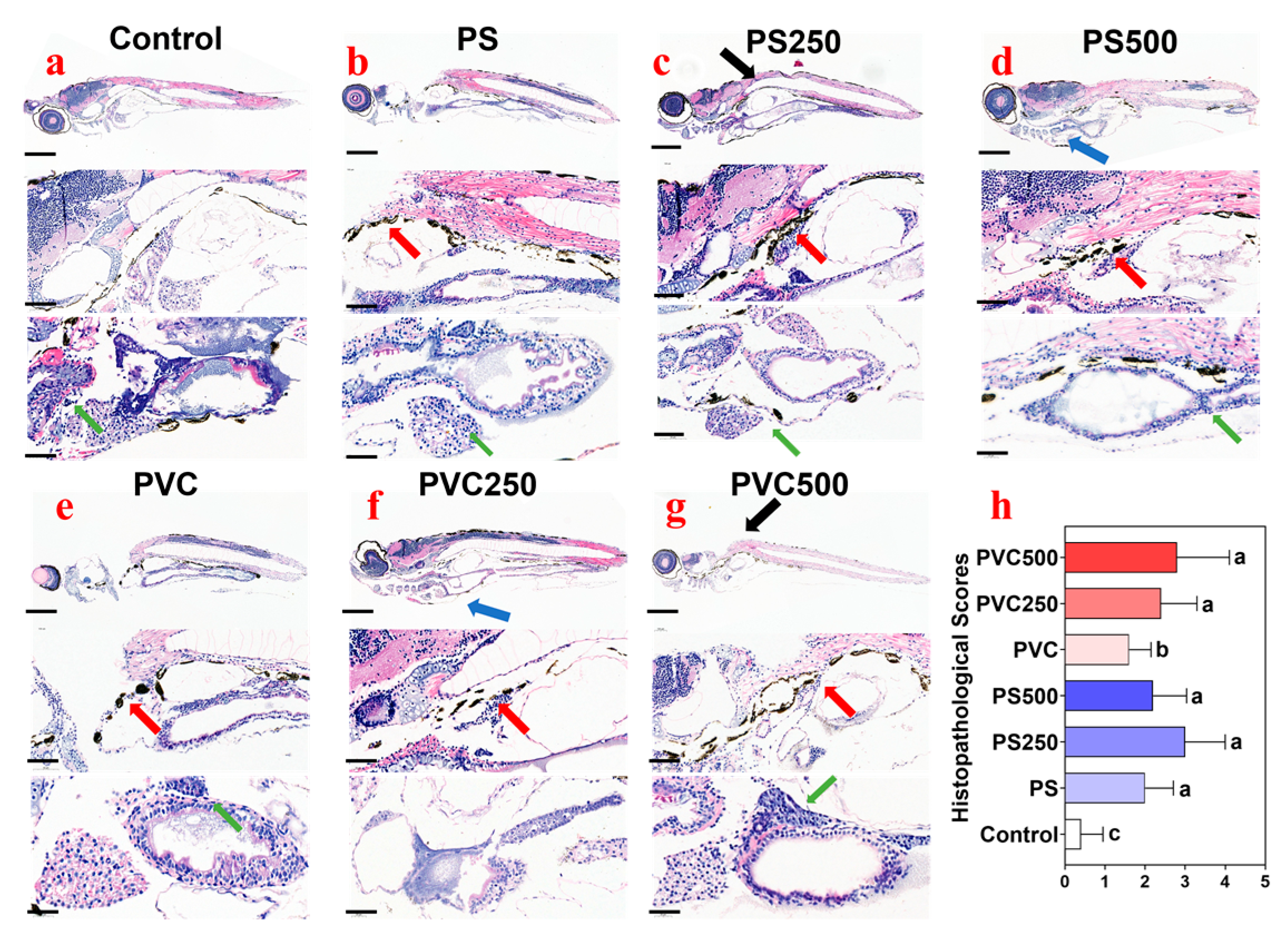
3.4. Histopathological Effects of Combined MPs and METH Exposure on Zebrafish Larvae
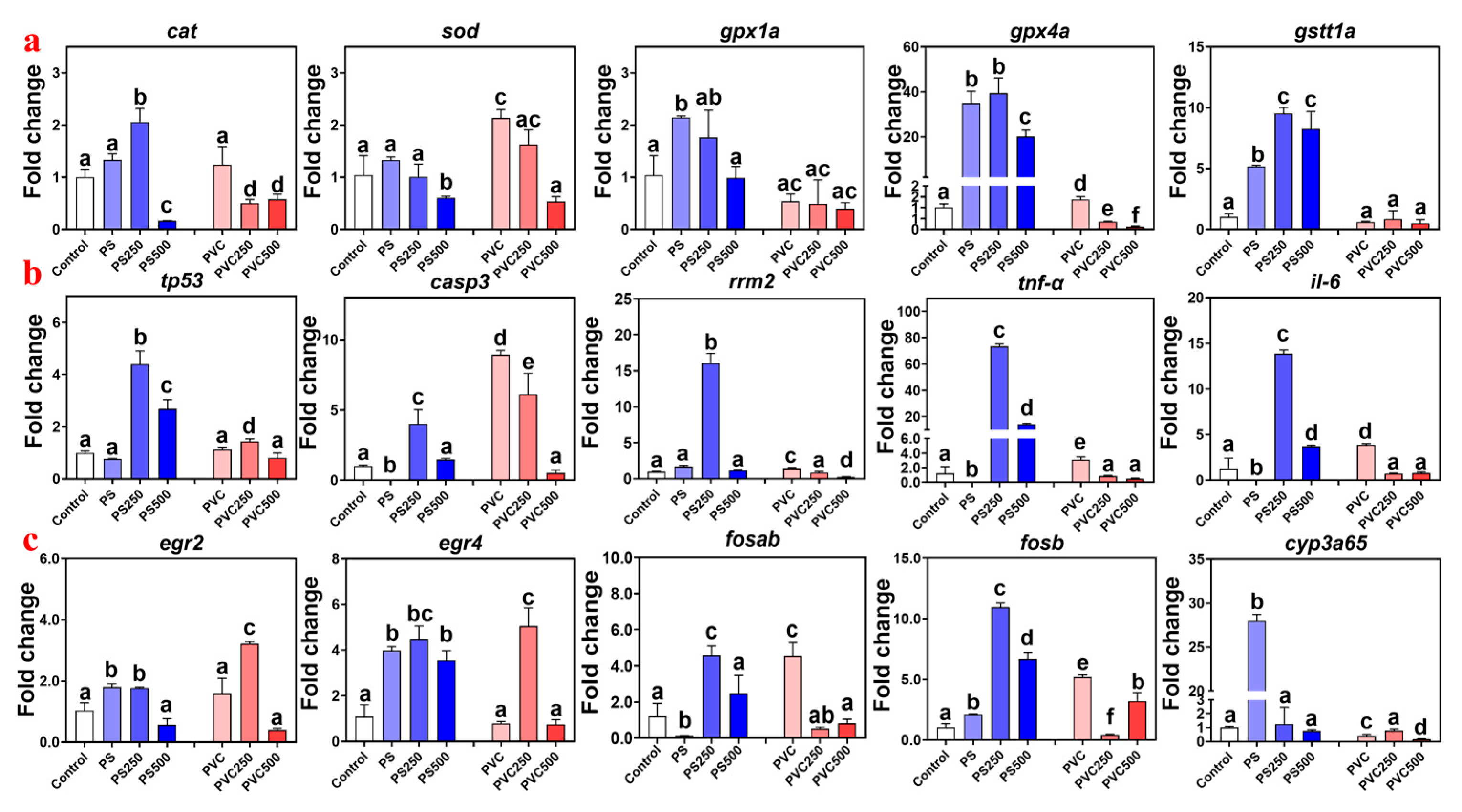
3.5. Effect of Combined MPs and METH Exposure on Gene Expression in Zebrafish Larvae
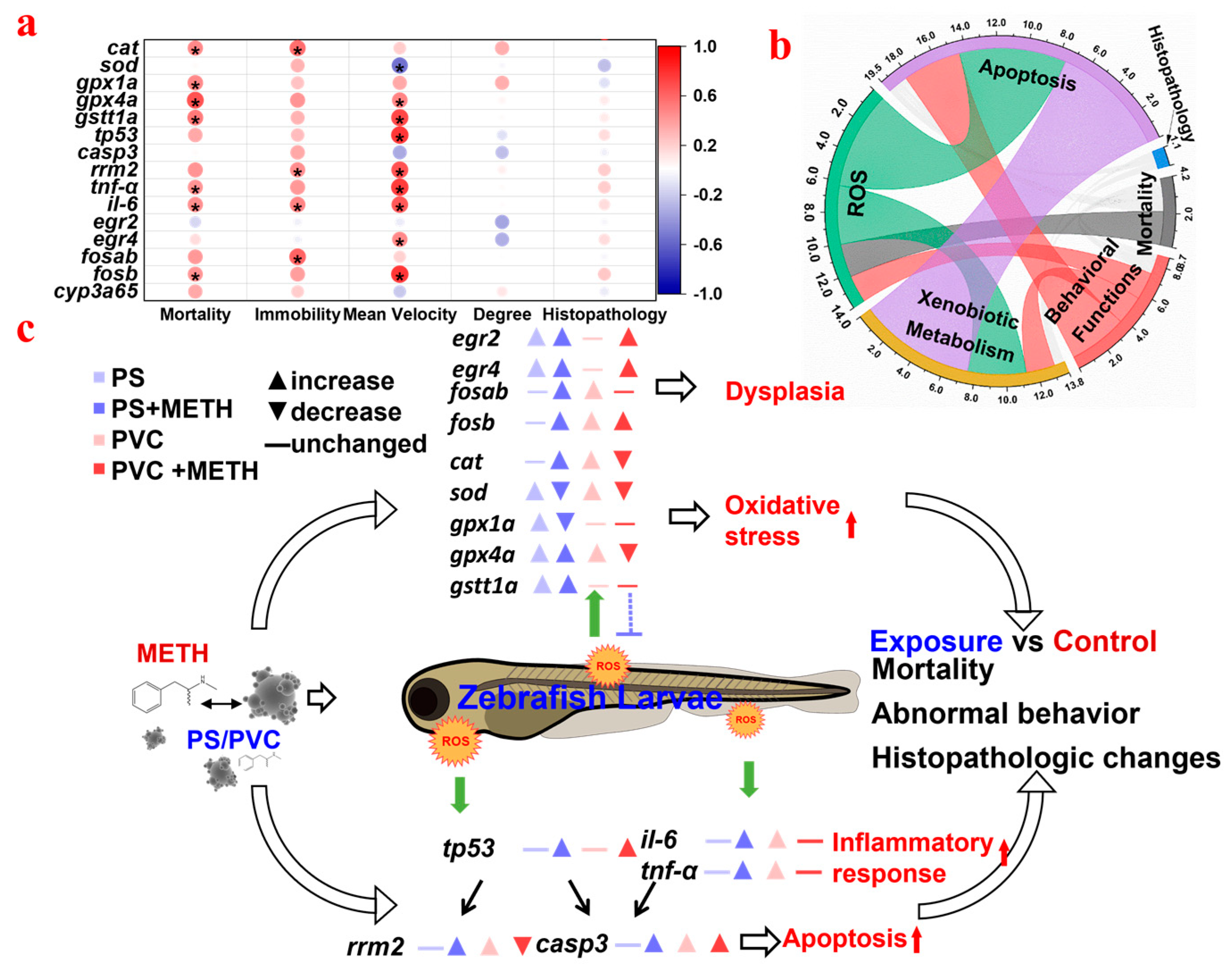
3.6. Correlation Analysis between the Different Biomarkers of Fish in Different Groups
4. Discussion
4.1. The Effects on the Ecological Function Associated Indicators of Zebrafish Larvae by MPs
4.2. Oxidative Stress
4.3. Ecological Implications
4.4. Perspectives for Joint Toxicity of METH and PS/PVC MPs for Aquatic Organisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yang, C.; Zhang, T.; Tang, C.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Europe, P. An analysis of European Plastics Production, Demand and Waste Data, Plastics-The Facts 2019. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2019/ (accessed on 10 March 2023).
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, University of Washington Tacoma, Tacoma, WA, USA, 9–11 September 2008.
- Su, L.; Xue, Y.; Li, L.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in Taihu Lake, China. Environ. Pollut. 2016, 216, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Welden, N.; Sobral, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. In Analysis of Nanoplastics and Microplastics in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–148. [Google Scholar]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef]
- De Marco, G.; Conti, G.O.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Pulvirenti, E.; Capparucci, F.; Mauceri, A.; Ferrante, M.; et al. Embryotoxicity of polystyrene microplastics in zebrafish Danio rerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef]
- Corami, F.; Rosso, B.; Morabito, E.; Rensi, V.; Gambaro, A.; Barbante, C. Small microplastics (<100 μm), plasticizers and additives in seawater and sediments: Oleo-extraction, purification, quantification, and polymer characterization using Micro-FTIR. Sci. Total Environ. 2021, 797, 148937. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Liu, C.; Huang, W.; Gu, Y.; Rao, Z. Recent advances in high value added reuse of waste polystyrene in environment and energy. CIESC J. 2020, 71, 2956–2972. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.-H.; Luo, G.; Kang, Y.; Zhang, L.; Pan, Y.; Zhou, X.; Fan, L.; Liang, B.; Wang, A. The contamination of microplastics in China’s aquatic environment: Occurrence, detection and implications for ecological risk. Environ. Pollut. 2022, 296, 118737. [Google Scholar] [CrossRef] [PubMed]
- Strom, S.L.; Fredrickson, K.A.; Bright, K.J. Microzooplankton in the coastal Gulf of Alaska: Regional, seasonal and interannual variations. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 192–202. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.; Wang, S.; Fang, H.; Ye, N.; Wang, D. Ecotoxicological effects on Scenedesmus obliquus and Danio rerio Co-exposed to polystyrene nano-plastic particles and natural acidic organic polymer. Environ. Toxicol. Pharmacol. 2019, 67, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Thanh Truc, N.T.; Lee, C.-H.; Lee, B.-K.; Mallampati, S.R. Development of hydrophobicity and selective separation of hazardous chlorinated plastics by mild heat treatment after PAC coating and froth flotation. J. Hazard. Mater. 2017, 321, 193–202. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, K.; Du, W.; Cai, M.; Zhang, Z.; Li, X. Diluted concentrations of methamphetamine in surface water induce behavior disorder, transgenerational toxicity, and ecosystem-level consequences of fish. Water Res. 2020, 184, 116164. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a Vector for Chemicals in the Aquatic Environment: Critical Review and Model-Supported Reinterpretation of Empirical Studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Granby, K.; Rainieri, S.; Rasmussen, R.R.; Kotterman, M.J.J.; Sloth, J.J.; Cederberg, T.L.; Barranco, A.; Marques, A.; Larsen, B.K. The influence of microplastics and halogenated contaminants in feed on toxicokinetics and gene expression in European seabass (Dicentrarchus labrax). Environ. Res. 2018, 164, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.; Wang, B.; Yang, J.; Duan, L.; Yu, G. Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. J. Hazard. Mater. 2019, 370, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Ding, G.; Shi, H.; Cong, Y.; Li, Z.; Wang, J. Polystyrene microplastics alleviate adverse effects of benzo[a]pyrene on tissues and cells of the marine mussel, Mytilus galloprovincialis. Aquat. Toxicol. 2023, 256, 106430. [Google Scholar] [CrossRef] [PubMed]
- Syberg, K.; Nielsen, A.; Khan, F.R.; Banta, G.T.; Palmqvist, A.; Jepsen, P.M. Microplastic potentiates triclosan toxicity to the marine copepod Acartia tonsa (Dana). J. Toxicol. Environ. Health Part A 2017, 80, 1369–1371. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Wang, Q.; Lv, M.; Zhao, X.; Ji, Y.; Han, X.; Wang, X.; Chen, L. The combined toxic effects of polyvinyl chloride microplastics and di(2-ethylhexyl) phthalate on the juvenile zebrafish (Danio rerio). J. Hazard. Mater. 2022, 440, 129711. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Wang, S.-C.; Zhao, F.-F.; Wang, S.-G.; Liu, F.-F.; Liu, G.-Z. Joint toxicity of microplastics with triclosan to marine microalgae Skeletonema costatum. Environ. Pollut. 2019, 246, 509–517. [Google Scholar] [CrossRef]
- Rubin, A.E.; Zucker, I. Interactions of microplastics and organic compounds in aquatic environments: A case study of augmented joint toxicity. Chemosphere 2022, 289, 133212. [Google Scholar] [CrossRef]
- Chen, Y.; Wisner, A.S.; Schiefer, I.T.; Williams, F.E.; Hall, F.S. Methamphetamine-induced lethal toxicity in zebrafish larvae. Psychopharmacology 2022, 239, 3833–3846. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Wang, X.-H.; Lin, C.-F. Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere 2010, 81, 562–570. [Google Scholar] [CrossRef]
- Paciuszkiewicz, K.; Ryan, M.; Wright, I.A.; Reynolds, J.K. Variations in Illicit Compound Discharged from Treated Wastewater. Water 2019, 11, 1071. [Google Scholar] [CrossRef]
- Mastroianni, N.; Bleda, M.J.; López de Alda, M.; Barceló, D. Occurrence of drugs of abuse in surface water from four Spanish river basins: Spatial and temporal variations and environmental risk assessment. J. Hazard. Mater. 2016, 316, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-H.; Hwang, C.-C.; Chen, T.-H.; Chen, P.-J. Developmental exposures to waterborne abused drugs alter physiological function and larval locomotion in early life stages of medaka fish. Aquat. Toxicol. 2015, 165, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Horký, P.; Grabic, R.; Grabicová, K.; Brooks, B.W.; Douda, K.; Slavík, O.; Hubená, P.; Sancho Santos, E.M.; Randák, T. Methamphetamine pollution elicits addiction in wild fish. J. Exp. Biol. 2021, 224, jeb242145. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Ma, R.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Chen, P.; Wang, W.; Peng, G.; Yu, G. How microplastics affect chiral illicit drug methamphetamine in aquatic food chain? From green alga (Chlorella pyrenoidosa) to freshwater snail (Cipangopaludian cathayensis). Environ. Int. 2020, 136, 105480. [Google Scholar] [CrossRef]
- Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef]
- Xue, Y.-H.; Feng, L.-S.; Xu, Z.-Y.; Zhao, F.-Y.; Wen, X.-L.; Jin, T.; Sun, Z.-X. The time-dependent variations of zebrafish intestine and gill after polyethylene microplastics exposure. Ecotoxicology 2021, 30, 1997–2010. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.; Wang, J.; Du, W.; Zhu, L. Assessment on chronic and transgenerational toxicity of methamphetamine to Caenorhabditis elegans and associated aquatic risk through toxicity indicator sensitivity distribution (TISD) analysis. Environ. Pollut. 2021, 288, 117696. [Google Scholar] [CrossRef]
- Gulyás, M.; Bencsik, N.; Pusztai, S.; Liliom, H.; Schlett, K. AnimalTracker: An ImageJ-Based Tracking API to Create a Customized Behaviour Analyser Program. Neuroinformatics 2016, 14, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, Y.; Nel, A.E.; Lin, S. Zebrafish: An in vivo model for nano EHS studies. Small 2013, 9, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Qi, S.; Liu, J.; Yuan, L.; Huang, Y.; Xue, J.; Qian, L.; Wang, C.; Li, Y. Toxicity and behavioral response of zebrafish exposed to combined microplastic and bisphenol analogues. Environ. Chem. Lett. 2022, 20, 41–48. [Google Scholar] [CrossRef]
- Félix, L.M.; Vidal, A.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Campos, S.; Matos, M.; Monteiro, S.M.; Coimbra, A.M. Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos. RSC Adv. 2016, 6, 61254–61266. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Perfettini, J.-L.; Roumier, T.; Castedo, M.; Larochette, N.; Boya, P.; Raynal, B.; Lazar, V.; Ciccosanti, F.; Nardacci, R.; Penninger, J.; et al. NF-κB and p53 Are the Dominant Apoptosis-inducing Transcription Factors Elicited by the HIV-1 Envelope. J. Exp. Med. 2004, 199, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Yabu, T.; Kishi, S.; Okazaki, T.; Yamashita, M. Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001, 360, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Niida, H.; Katsuno, Y.; Sengoku, M.; Shimada, M.; Yukawa, M.; Ikura, M.; Ikura, T.; Kohno, K.; Shima, H.; Suzuki, H. Essential role of Tip60-dependent recruitment of ribonucleotide reductase at DNA damage sites in DNA repair during G1 phase. Genes Dev. 2010, 24, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, D.; Fan, H.; Li, X.; Gu, Z.; Zhang, X. Preparation of nano-HA/PLA composite by modified-PLA for controlling the growth of HA crystals. Mater. Lett. 2007, 61, 59–62. [Google Scholar] [CrossRef]
- Johansson, F.; Brodin, T. Effects of Fish Predators and Abiotic Factors on Dragonfly Community Structure. J. Freshw. Ecol. 2003, 18, 415–423. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Magurran, A.E. Development of predator defences in fishes. Rev. Fish Biol. Fish. 1994, 4, 145–183. [Google Scholar] [CrossRef]
- Campos-Sánchez, J.C.; Esteban, M.Á. Review of inflammation in fish and value of the zebrafish model. J. Fish Dis. 2021, 44, 123–139. [Google Scholar] [CrossRef]
- Kirsten, K.; Soares, S.M.; Koakoski, G.; Carlos Kreutz, L.; Barcellos, L.J.G. Characterization of sickness behavior in zebrafish. Brain Behav. Immun. 2018, 73, 596–602. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Amran, N.H.; Zaid, S.S.M.; Mokhtar, M.H.; Manaf, L.A.; Othman, S. Exposure to Microplastics during Early Developmental Stage: Review of Current Evidence. Toxics 2022, 10, 597. [Google Scholar] [CrossRef]
- Jeong, S.; Jang, S.; Kim, S.S.; Bae, M.A.; Shin, J.; Lee, K.-B.; Kim, K.-T. Size-dependent seizurogenic effect of polystyrene microplastics in zebrafish embryos. J. Hazard. Mater. 2022, 439, 129616. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Chen, D.; Guo, J.-L.; Feng, D.-F.; Sun, M.-Z.; Lu, Y.; Chen, D.-Y.; Zhao, X.; Feng, X.-Z. Evaluating the biological impact of polyhydroxyalkanoates (PHAs) on developmental and exploratory profile of zebrafish larvae. RSC Adv. 2016, 6, 37018–37030. [Google Scholar] [CrossRef]
- Lee, C.-W.; Hsu, L.-F.; Wu, I.L.; Wang, Y.-L.; Chen, W.-C.; Liu, Y.-J.; Yang, L.-T.; Tan, C.-L.; Luo, Y.-H.; Wang, C.-C.; et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice. J. Hazard. Mater. 2022, 430, 128431. [Google Scholar] [CrossRef] [PubMed]
- Laue, K.; Jänicke, M.; Plaster, N.; Sonntag, C.; Hammerschmidt, M. Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 2008, 135, 3775–3787. [Google Scholar] [CrossRef]
- Addison, M.; Xu, Q.; Cayuso, J.; Wilkinson, D.G. Cell Identity Switching Regulated by Retinoic Acid Signaling Maintains Homogeneous Segments in the Hindbrain. Dev. Cell 2018, 45, 606–620.e603. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yang, W.; Wang, D.; Wang, Z.; Liu, C.; Li, J. Methamphetamine Shows Different Joint Toxicity for Different Types of Microplastics on Zebrafish Larvae by Mediating Oxidative Stress. Toxics 2024, 12, 9. https://doi.org/10.3390/toxics12010009
Xu J, Yang W, Wang D, Wang Z, Liu C, Li J. Methamphetamine Shows Different Joint Toxicity for Different Types of Microplastics on Zebrafish Larvae by Mediating Oxidative Stress. Toxics. 2024; 12(1):9. https://doi.org/10.3390/toxics12010009
Chicago/Turabian StyleXu, Jindong, Wenqi Yang, Dongyi Wang, Zhenglu Wang, Chuang Liu, and Jiana Li. 2024. "Methamphetamine Shows Different Joint Toxicity for Different Types of Microplastics on Zebrafish Larvae by Mediating Oxidative Stress" Toxics 12, no. 1: 9. https://doi.org/10.3390/toxics12010009
APA StyleXu, J., Yang, W., Wang, D., Wang, Z., Liu, C., & Li, J. (2024). Methamphetamine Shows Different Joint Toxicity for Different Types of Microplastics on Zebrafish Larvae by Mediating Oxidative Stress. Toxics, 12(1), 9. https://doi.org/10.3390/toxics12010009








