Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Participants
2.2. Quantifying PFAS and HSV
2.3. Operationalizing Allostatic Load
2.4. Statistical Analysis
2.4.1. Descriptive Statistics
2.4.2. Multivariable Logistic Regression
2.4.3. Bayesian Kernel Machine Regression
3. Results
3.1. Characteristics of the Sample Participants
3.2. Correlations between PFAS Exposures
3.3. Results of Logistic Regression
3.3.1. Association between PFAS and AL Using Logistic Regression: Unadjusted for Covariates
3.3.2. Association between PFAS and AL by HSV Exposure Status, Adjusted for Study Covariates
3.3.3. Association between PFAS and AL, Accounting for Various PFAS Interactions
3.4. Results of Bayesian Kernel Machine Regression
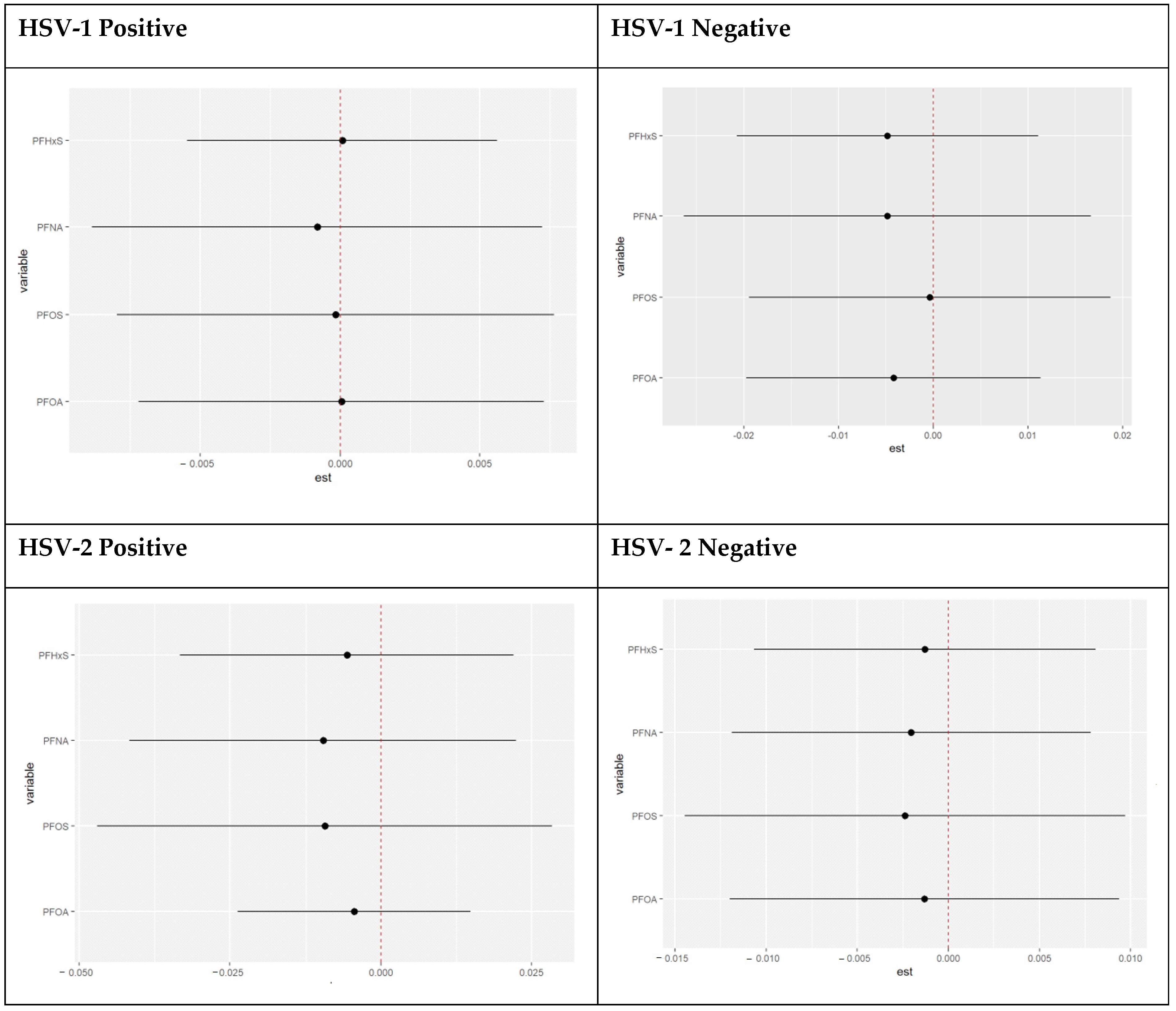
3.4.1. Variable Importance Results
3.4.2. Univariate Association of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
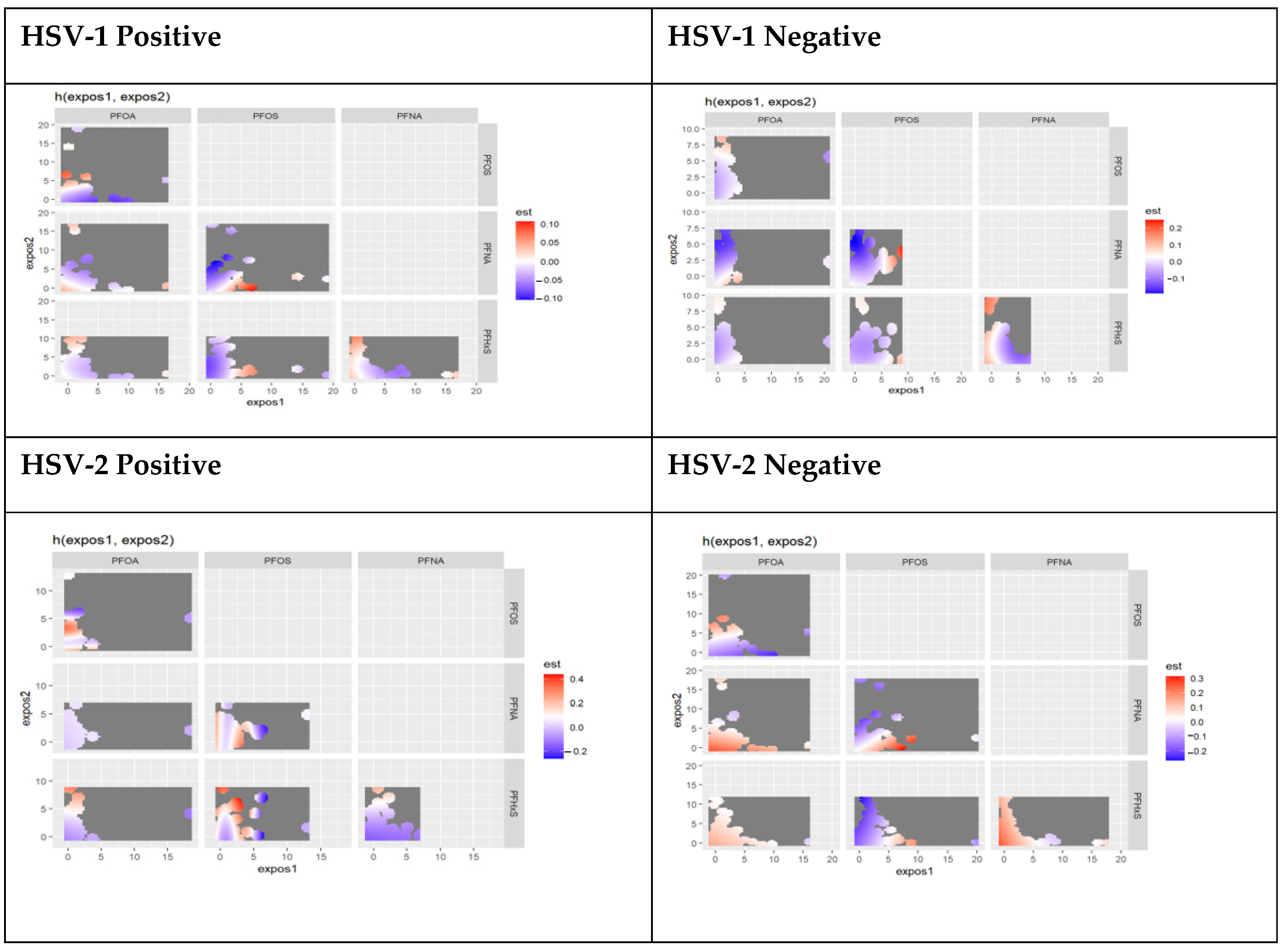
3.4.3. Bivariate Associations of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
3.4.4. Interactions of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
3.4.5. Three-Way Interactions of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
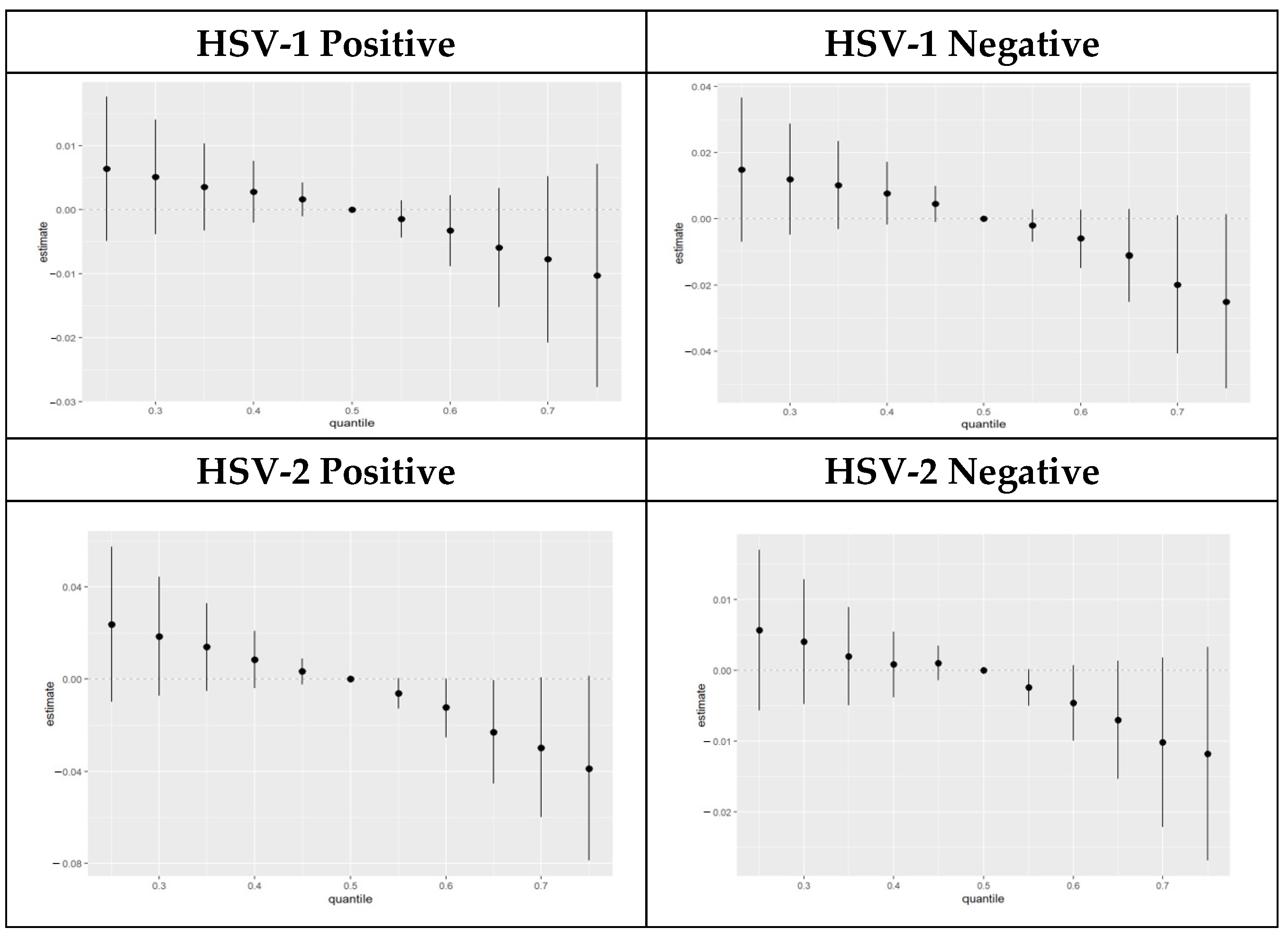
3.4.6. Overall Exposure Effect of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
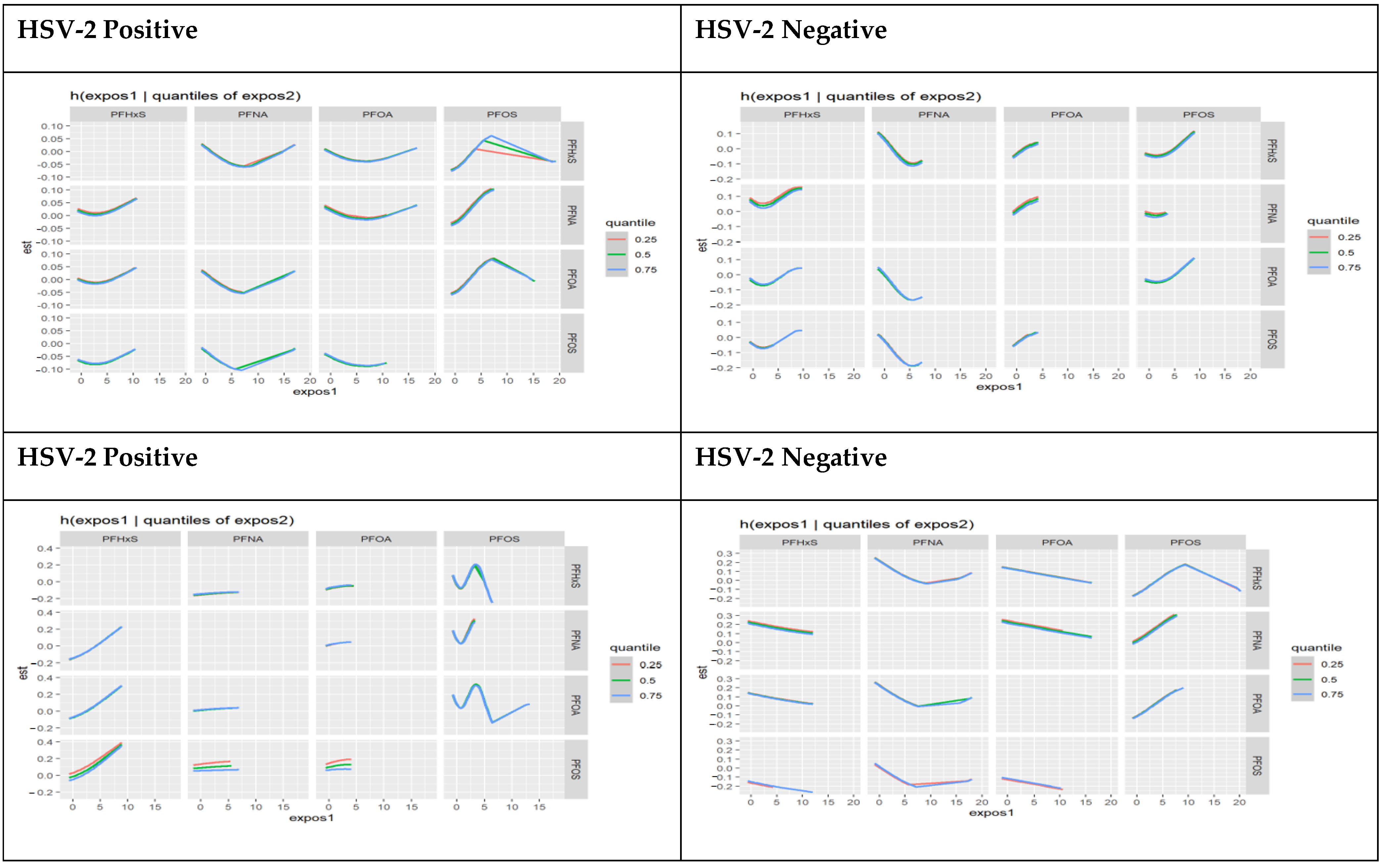
3.4.7. Single Variable Effects of PFAS Components Stratified by HSV-1 and HSV-2 Test Result
3.4.8. Single Variable Interaction Terms of PFAS Components Stratified by HSV-1 and HSV-2 Test Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2017–2018 Data Documentation, Codebook, and Frequencies Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS_J). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/PFAS_J.htm (accessed on 1 June 2023).
- Tool, L.E. Per-and Polyfluoroalkyl Substances (PFAS) Blood Level Estimation Tool. Available online: https://stacks.cdc.gov/view/cdc/129408 (accessed on 30 July 2023).
- Eick, S.M.; Enright, E.A.; Padula, A.M.; Aung, M.; Geiger, S.D.; Cushing, L.; Trowbridge, J.; Keil, A.P.; Baek, H.G.; Smith, S. Prenatal PFAS and psychosocial stress exposures in relation to fetal growth in two pregnancy cohorts: Applying environmental mixture methods to chemical and non-chemical stressors. Environ. Int. 2022, 163, 107238. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Jain, R.B. Time trends over 2003–2014 in the concentrations of selected perfluoroalkyl substances among US adults aged ≥20 years: Interpretational issues. Sci. Total Environ. 2018, 645, 946–957. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual: Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Adler, N.E.; Stewart, J. Preface to The Biology of Disadvantage: Socioeconomic Status and Health. Ann. N. Y. Acad. Sci. USA 2010, 1186, 1–4. [Google Scholar] [CrossRef]
- DeCaro, J.A.; Helfrecht, C. Applying minimally invasive biomarkers of chronic stress across complex ecological contexts. Am. J. Hum. Biol. 2022, 34, e23814. [Google Scholar] [CrossRef] [PubMed]
- Beese, S.; Postma, J.; Graves, J.M. Allostatic Load Measurement: A Systematic Review of Reviews, Database Inventory, and Considerations for Neighborhood Research. Int. J. Environ. Res. Public Health 2022, 19, 17006. [Google Scholar] [CrossRef] [PubMed]
- Geronimus, A.T.; Hicken, M.; Keene, D.; Bound, J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 2006, 96, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Maranhao Neto, G.A.; Polcrova, A.B.; Pospisilova, A.; Blaha, L.; Klanova, J.; Bobak, M.; Gonzalez-Rivas, J.P. Associations between Per-and Polyfluoroalkyl Substances (PFAS) and Cardiometabolic Biomarkers in Adults of Czechia: The Kardiovize Study. Int. J. Environ. Res. Public Health 2022, 19, 13898. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Obeng-Gyasi, E. The Association of Combined Per-and Polyfluoroalkyl Substances and Metals with Allostatic Load Using Bayesian Kernel Machine Regression. Diseases 2023, 11, 52. [Google Scholar] [CrossRef]
- Bashir, T.; Obeng-Gyasi, E. The Association between Multiple Per-and Polyfluoroalkyl Substances’ Serum Levels and Allostatic Load. Int. J. Environ. Res. Public Health 2022, 19, 5455. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Bingham, B.A.; Aldana, P.C.; Chung, S.T.; Sumner, A.E. Variation in the calculation of allostatic load score: 21 examples from NHANES. J. Racial Ethn. Health Disparities 2017, 4, 455–461. [Google Scholar] [CrossRef]
- Yang, D.; Wheeler, M.; Karanth, S.D.; Aduse-Poku, L.; Leeuwenburgh, C.; Anton, S.; Guo, Y.; Bian, J.; Liang, M.; Yoon, H.S. Allostatic load and risk of all-cause, cancer-specific, and cardiovascular mortality in older cancer survivors: An analysis of the National Health and Nutrition Examination Survey 1999–2010. Aging Cancer 2023, 4, 74–84. [Google Scholar] [CrossRef]
- Boldogh, I.; Albrecht, T.; Porter, D.D. Persistent viral infections. In Medical Microbiology, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2015-2016 Data Documentation, Codebook, and Frequencies Herpes Simplex Virus Type-1 & Type-2 (HSV_I). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/HSV_I.htm (accessed on 20 July 2023).
- McQuillan, G.M.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016. 2018. Available online: https://www.cdc.gov/nchs/data/databriefs/db304.pdf (accessed on 20 July 2023).
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Guo, X.; Li, N.; Wang, H.; Su, W.; Song, Q.; Liang, Q.; Liang, M.; Sun, C.; Li, Y.; Lowe, S. Combined exposure to multiple metals on cardiovascular disease in NHANES under five statistical models. Environ. Res. 2022, 215, 114435. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: 1999–2022 Survey Content Brochur. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 20 July 2023).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: What to Expect and Infomation Collected. Available online: https://www.cdc.gov/nchs/nhanes/participant/information-collected.htm (accessed on 20 July 2023).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Informed Consent. Available online: https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm (accessed on 20 July 2023).
- Centers-For-Disease-Control-and-Prevention. Laboratory Procedure Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PFAS_I_MET.pdf (accessed on 20 July 2023).
- Centers-For-Disease-Control-and-Prevention. Herpes Simplex Virus Type 1 & 2. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/HSV_I_MET.pdf (accessed on 20 July 2023).
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Bobb, J.F. Example Using the BKMR R Package with Simulated Data from the NIEHS Mixtures Workshop. Available online: https://jenfb.github.io/bkmr/SimData1 (accessed on 20 July 2023).
- Hill, M.; Mostafa, S.; Muganda, P.M.; Jeffers-Francis, L.K.; Obeng-Gyasi, E. The Association of Cytomegalovirus and Allostatic Load by Country of Birth and Length of Time in the United States. Diseases 2023, 11, 101. [Google Scholar] [CrossRef]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. USA 2004, 1032, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aldwin, C.M. The elders life stress. In Stress and Coping in Later-Life Families; Taylor & Francis: Abingdon, UK, 2018; pp. 49–69. [Google Scholar]
- Omoike, O.E.; Pack, R.P.; Mamudu, H.M.; Liu, Y.; Strasser, S.; Zheng, S.; Okoro, J.; Wang, L. Association between per and polyfluoroalkyl substances and markers of inflammation and oxidative stress. Environ. Res. 2021, 196, 110361. [Google Scholar] [CrossRef]
- Ehrlich, V.; Bil, W.; Vandebriel, R.; Granum, B.; Luijten, M.; Lindeman, B.; Grandjean, P.; Kaiser, A.-M.; Hauzenberger, I.; Hartmann, C. Consideration of pathways for immunotoxicity of per-and polyfluoroalkyl substances (PFAS). Environ. Health 2023, 22, 19. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Germano, M.L.; dos Santos Gomes, C.; de Souza Barbosa, J.F.; Neto, N.J.; Pereira, D.S.; Ahmed, T.; Borrero, C.L.C.; Guerra, R.O. Allostatic load and physical performance in older adults: Findings from the International Mobility in Aging Study (IMIAS). Arch. Gerontol. Geriatr. 2023, 109, 104961. [Google Scholar] [CrossRef] [PubMed]
- Lueth, A.J.; Allshouse, A.A.; Blue, N.M.; Grobman, W.A.; Levine, L.D.; Catov, J.; Saade, G.; Yee, L.M.; Wilson, F.A.; Murtaugh, M. Can allostatic load in pregnancy explain the association between race and subsequent cardiovascular disease risk: A cohort study. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Guo, X.; Ding, X.; Song, Q.; Wang, H.; Li, N.; Su, W.; Liang, Q.; Sun, Y. Combined effects of multiple metals on hearing loss: A Bayesian kernel machine regression approach. Ecotoxicol. Environ. Saf. 2022, 247, 114279. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, H.; Song, Q.; Li, N.; Liang, Q.; Su, W.; Liang, M.; Ding, X.; Sun, C.; Lowe, S. Association between exposure to organophosphorus pesticides and the risk of diabetes among US Adults: Cross-sectional findings from the National Health and Nutrition Examination Survey. Chemosphere 2022, 301, 134471. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J. Introduction to Bayesian Kernel Machine Regression and the BKMR R Package. 2017. Available online: https://jenfb.github.io/bkmr/overview.html (accessed on 30 July 2023).




| Characteristics | Total Sample Population |
|---|---|
| N (%) | 1784 |
| Age: mean (SD) | 33.62 (8.95) |
| PFOA: Mean (SD) | 3.47 (3.32) |
| PFOS: Mean (SD) | 11.08 (11.77) |
| PFNA: Mean (SD) | 1.32 (1.36) |
| PFHxS: Mean (SD) | 2.11 (2.73) |
| HSV-1 | |
| Yes | 60.59% |
| No | 39.41% |
| HSV-2 | |
| Yes | 19.99% |
| No | 80.01% |
| Gender | |
| Female | 52.28% |
| Male | 47.72% |
| Education | |
| Less than high school | 22.86% |
| High school or equivalent | 23.21% |
| Some college/associate degree | 33.30% |
| Bachelor’s degree or higher | 20.63% |
| Race/ethnicity | |
| Black | 14.84% |
| White | 57.08% |
| Hispanic | 20.80% |
| Other | 7.29% |
| Household Income | |
| <25,000 | 30.18% |
| 25,000–<55,000 | 44.59% |
| 55,000–<75,000 | 19.95% |
| 75,000+ | 5.29% |
| Marital Status | |
| Married or Living with a partner | 54.07% |
| Single | 45.93% |
| Non-Elevated AL | Elevated AL | p-Value * | |
|---|---|---|---|
| N (%) | 1476 (82.7%) | 307 (16.3%) | |
| Age: mean (SD) | 32.95 (8.89) | 37 (8.40) | |
| PFOA: Mean (SD) | 3.50 (3.50) | 3.28 (2.13) | 0.7698 |
| PFOS: Mean (SD) | 10.98 (11.71) | 11.56 (12.14) | 0.5881 |
| PFNA: Mean (SD) | 1.34 (1.43) | 1.22 (0.90) | 0.5278 |
| PFHxS: Mean (SD) | 2.11 (2.64) | 2.07 (3.14) | 0.4129 |
| HSV-1 | |||
| Yes | 82.77% | 17.23% | 0.1507 |
| No | 85.65% | 14.35% | |
| HSV-2 | |||
| Yes | 82.83% | 17.17% | 0.6009 |
| No | 84.18% | 15.82% | |
| Gender | |||
| Female | 81.02% | 13.36% | 0.0050 |
| Male | 86.72% | 19.08% | |
| Education | |||
| Less than high school | 81.02% | 18.98% | 0.2121 |
| High school or equivalent | 84.03% | 15.97% | |
| Some college/associate degree | 83.60% | 16.40% | |
| Bachelor’s degree or higher | 87.48% | 12.52% | |
| Race/ethnicity | |||
| Black | 84.12% | 15.88% | 0.9370 |
| White | 84.32% | 15.68% | |
| Hispanic | 82.77% | 17.23% | |
| Other | 83.50% | 16.50% | |
| Household Income | |||
| <25,000 | 82.67% | 17.33% | 0.3544 |
| 25,000–<55,000 | 83.06% | 16.94% | |
| 55,000–<75,000 | 86.05% | 13.95% | |
| 75,000+ | 90.10% | 9.90% | |
| Marital Status | |||
| Married or Living with a partner | 81.82% | 18.18% | 0.0206 |
| Single | 86.37% | 13.63% |
| Stratum | HSV-1 Negative | HSV-1 Positive | HSV-2 Negative | HSV-2 Positive | ||||
|---|---|---|---|---|---|---|---|---|
| Term | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Intercept | 0.23 (0.15, 0.37) | <0.0001 | 0.21 (0.18, 0.24) | <0.0001 | 0.21 (0.15, 0.28) | 0 | 0.23 (0.14, 0.39) | 0.0000 |
| PFOS | 1.02 (1.00, 1.05) | 0.0349 | – | – | 1.02 (1.00, 1.04) | 0.0900 | 0.96 (0.91, 1.02) | 0.1630 |
| PFNA | 0.60 (0.42, 0.86) | 0.0063 | – | – | 0.85 (0.73, 1.00) | 0.0556 | – | – |
| PFOA | – | – | – | – | – | – | – | – |
| PFHxS | – | – | – | – | 0.95 (0.89, 1.01) | 0.1087 | 1.15 (0.98, 1.35) | 0.0870 |
| Stratum | HSV-1 Negative | HSV-1 Positive | HSV-2 Negative | HSV-2 Positive | ||||
|---|---|---|---|---|---|---|---|---|
| Term | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Intercept | 0.23 (0.15, 0.37) | <0.0001 | 0.03 (0.01, 0.08) | <0.0001 | 0.21 (0.15, 0.28) | <0.0001 | 0.23 (0.14, 0.39) | <0.0001 |
| Gender: Male | – | – | 1.51 (1.07, 2.14) | 0.0206 | – | – | – | – |
| Age | – | – | 1.06 (1.03, 1.08) | <0.0001 | – | – | – | – |
| HH Income: 25,000–<55,000 | – | – | 0.81 (0.52, 1.27) | 0.3432 | – | – | – | – |
| HH Income: 55,000–<75,000 | – | – | 0.59 (0.33, 1.05) | 0.0724 | – | – | – | – |
| HH Income: 75,000+ | – | – | 0.40 (0.18, 0.91) | 0.0287 | – | – | – | – |
| Marital Status: Single | – | – | 0.54 (0.34, 0.85) | 0.0085 | – | – | – | – |
| PFOS | 1.02 (1.00, 1.05) | 0.0350 | – | – | 1.02 (1.00, 1.04) | 0.0900 | 0.96 (0.91, 1.02) | 0.1630 |
| PFNA | 0.60 (0.42, 0.86) | 0.0063 | – | – | 0.85 (0.73, 1.00) | 0.0556 | 1.15 (0.98, 1.35) | 0.0870 |
| PFHxS | – | – | – | – | 0.95 (0.89, 1.01) | 0.1087 | – | – |
| Stratum | HSV-1 Negative | HSV-1 Positive | HSV-2 Negative | HSV-2 Positive | ||||
|---|---|---|---|---|---|---|---|---|
| Term | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Intercept | 0.23 (0.12, 0.37) | <0.0001 | 0.03 (0.01, 0.08) | <0.0001 | 0.21 (0.15, 0.28) | <0.00001 | 0.23 (0.14, 0.39) | <0.0001 |
| PFOS | 1.02 (1.00, 1.05) | 0.03495 | 1.04 (0.96, 1.14) | 0.0206 | 1.02 (1.00, 1.04) | 0.0900 | 0.96 (0.91, 1.02) | 0.163 |
| PFOA | – | – | 1.01 (0.78, 1.32) | <0.0001 | – | – | – | |
| PFNA | 0.60 (0.42, 0.86) | 0.00634 | 1.22 (0.71, 2.10) | 0.3432 | 0.85 (0.73, 1.00) | 0.0556 | – | – |
| PFHxS | – | – | 0.56 (0.32, 0.99) | 0.0724 | 0.95 (0.89, 1.01) | 0.1087 | 1.15 (0.98, 1.35) | 0.0870 |
| PFOS: PFOA | – | – | 1.00 (0.98, 1.01) | 0.7401 | – | – | – | – |
| PFOS: PFNA | – | – | 0.97 (0.94, 1.00) | 0.0389 | – | – | – | – |
| PFOA: PFNA | 0.91 (0.77, 1.07) | 0.2251 | – | – | ||||
| PFOS: PFHxS | – | – | 1.02 (1.00, 1.03) | 0.0643 | – | – | – | – |
| PFOA: PFHxS | – | – | 1.07 (0.97, 1.18) | 0.1713 | – | – | – | – |
| PFNA: PFHxS | – | – | 1.17 (0.84, 1.64) | 0.3408 | – | – | – | – |
| PFOS:PFOA: PFNA | – | – | 1.00 (1.00, 1.01) | 0.0344 | – | – | – | – |
| PFOS:PFOA: PFHxS | – | – | 1.00 (0.99, 1.00) | 0.1469 | – | – | – | – |
| PFOS:PFNA: PFHxS | – | – | 1.00 (0.99, 1.00) | 0.3540 | – | – | – | – |
| PFOA:PFNA: PFHxS | – | – | 1.00 (0.94, 1.06) | 0.9149 | – | – | – | – |
| Gender: Male | – | – | 1.61 (1.02, 2.54) | 0.0411 | – | – | – | – |
| Age | – | – | 1.06 (1.04, 1.09) | <0.00001 | – | – | – | – |
| HH Income: 25,000–<55,000 | – | – | 0.80 (0.51, 1.26) | 0.3270 | – | – | – | – |
| HH Income: 55,000–<75,000 | – | – | 0.58 (0.32, 1.04) | 0.0685 | – | – | – | – |
| HH Income: 75,000+ | – | – | 0.40 (0.17, 0.92) | 0.0334 | – | – | – | – |
| Marital Status: Single | – | – | 0.55 (0.35, 0.88) | 0.0138 | – | – | – | – |
| HSV-1 | HSV-2 | |||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| PFOA | 0.4659 | 0.3305 | 0.4408 | 0.2970 |
| PFOS | 0.4700 | 0.4060 | 0.4526 | 0.4732 |
| PFNA | 0.4604 | 0.4853 | 0.3435 | 0.4553 |
| PFHxS | 0.4934 | 0.3486 | 0.4473 | 0.36072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boafo, Y.S.; Mostafa, S.; Obeng-Gyasi, E. Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure. Toxics 2023, 11, 745. https://doi.org/10.3390/toxics11090745
Boafo YS, Mostafa S, Obeng-Gyasi E. Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure. Toxics. 2023; 11(9):745. https://doi.org/10.3390/toxics11090745
Chicago/Turabian StyleBoafo, Yvonne S., Sayed Mostafa, and Emmanuel Obeng-Gyasi. 2023. "Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure" Toxics 11, no. 9: 745. https://doi.org/10.3390/toxics11090745
APA StyleBoafo, Y. S., Mostafa, S., & Obeng-Gyasi, E. (2023). Association of Per- and Polyfluoroalkyl Substances with Allostatic Load Stratified by Herpes Simplex Virus 1 and 2 Exposure. Toxics, 11(9), 745. https://doi.org/10.3390/toxics11090745








