Mechanistic Assessment of Anise Seeds and Clove Buds against the Neurotoxicity Caused by Metronidazole in Rats: Possible Role of Antioxidants, Neurotransmitters, and Cytokines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Animals
2.2. Chemicals and Kits
2.3. Extract Preparation
2.4. Phenolic Compounds Analysis
2.5. Study Model
2.6. Sampling and Biochemical Investigation
2.7. Preparation of Brain Homogenates and Biochemical Analysis
2.8. Histopathological Examination
2.9. qPCR
2.10. Statistical Analysis
3. Results
3.1. The Polyphenolic Compounds of the Extract
3.2. Body and Brain Weights
3.3. Protective Effect of Anise and Clove Seeds Extract on GABA, ACHE, DA, and ST Quantity in the Brain Rats Administered MET
3.4. Anise and Clove Extract to Improve the Altered Antioxidant Status Due to MET
3.5. Histopathological Results
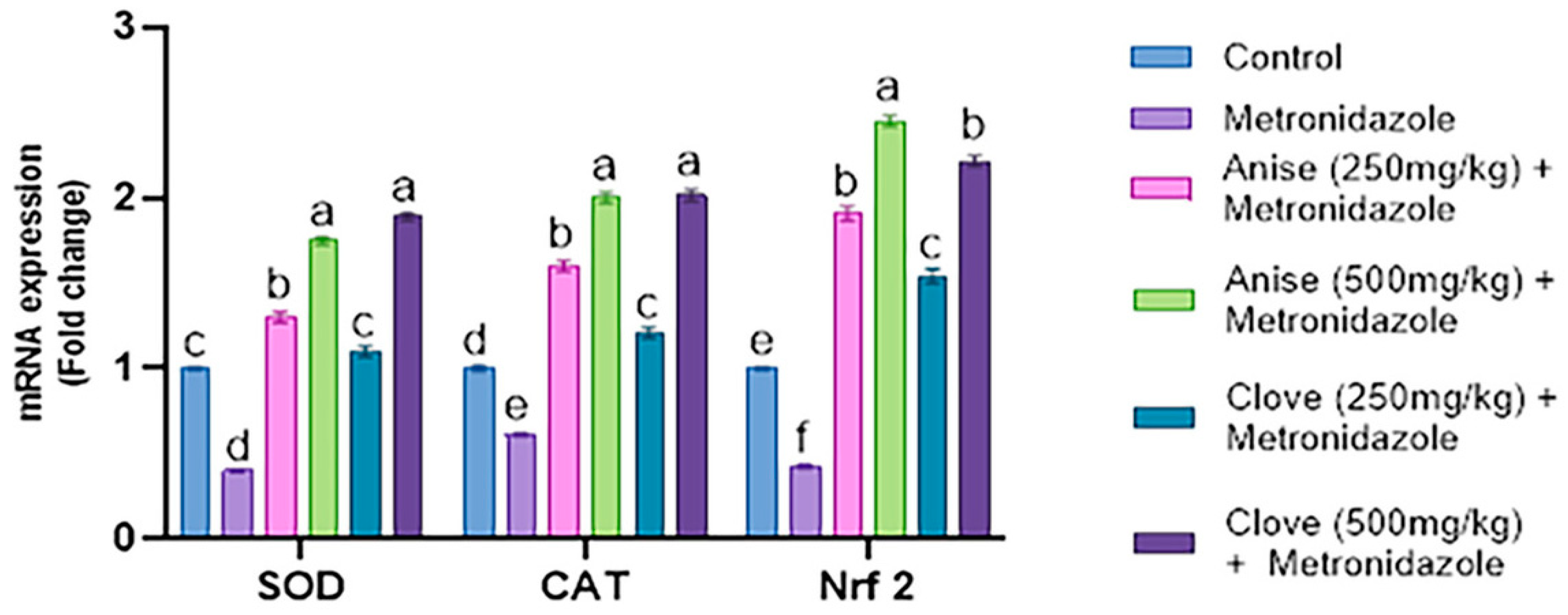
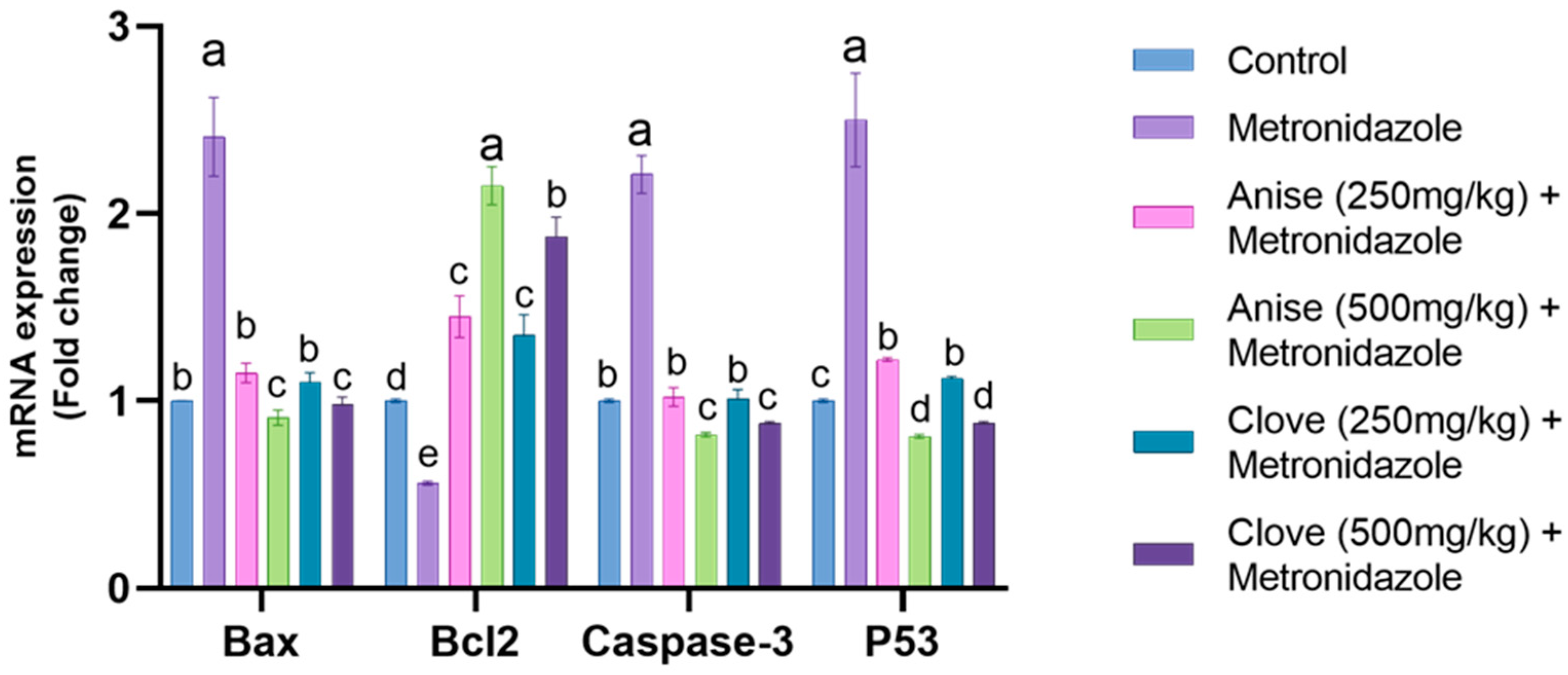
3.6. Effect of the Anise and Clove Extract on the Antioxidants and Apoptotic Gene Expression of the Brain Subjected to MET
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AbdRabou, M.A.; Alrashdi, B.M.; Alruwaili, H.K.; Elmazoudy, R.H.; Alwaili, M.A.; Othman, S.I.; Alghamdi, F.A.; Fahmy, G.H. Exploration of Maternal and Fetal Toxicity Risks for Metronidazole-Related Teratogenicity and Hepatotoxicity through an Assessment in Albino Rats. Toxics 2023, 11, 303. [Google Scholar] [CrossRef]
- Stoian, I.-A.; Iacob, B.-C.; Dudaș, C.-L.; Barbu-Tudoran, L.; Bogdan, D.; Marian, I.O.; Bodoki, E.; Oprean, R. Biomimetic electrochemical sensor for the highly selective detection of azithromycin in biological samples. Biosens. Bioelectron. 2020, 155, 112098. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Malik, M.Y.; Rashid, M.; Singh, S.; Tiwari, V.; Gupta, P.; Shukla, S.; Singh, S.; Wahajuddin, M. Mechanistic exploration of quercetin against metronidazole induced neurotoxicity in rats: Possible role of nitric oxide isoforms and inflammatory cytokines. Neurotoxicology 2020, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vilian, A.E.; Ranjith, K.S.; Lee, S.J.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hierarchical dense Ni−Co layered double hydroxide supported carbon nanofibers for the electrochemical determination of metronidazole in biological samples. Electrochim. Acta 2020, 354, 136723. [Google Scholar] [CrossRef]
- Sørensen, C.G.; Karlsson, W.K.; Amin, F.M.; Lindelof, M. Metronidazole-induced encephalopathy: A systematic review. J. Neurol. 2020, 267, 1–13. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 546–556. [Google Scholar] [CrossRef]
- Bekara, A.; Hamadouche, N.A.; Kahloula, K.; Harouat, S.; Tabbas, D.; Aoues, A. Effect of Pimpinella anisum L (Aniseed) Aqueous Extract against Lead (Pb) Neurotoxicity: Neurobehavioral Study. Int. J. Neurosci. Behav. Sci. 2015, 3, 32–40. [Google Scholar] [CrossRef]
- Bekara, A.; Aithamadouche, N.; Kahloula, K.; Sadi, N.; Aoues, A. Effect of Pimpinella anisum L. on Histological and Biochemical Damage in Cerebrum and Cerebellum of Young Rats Intoxicated by Lead Acetate. Group 2016, 11, 12. [Google Scholar]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Methanolic extract of Pimpinella anisum L. prevents dementia by reducing oxidative stress in neuronal pathways of hypermnesic mice. Pak. J. Zool. 2020, 52, 1779–1786. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Tayebwa, D.S.; Shaheen, H.M.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick-Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Shaaban, H.A. Essential oil as antimicrobial agents: Efficacy, stability, and safety issues for food application. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, A.; Pozos-Guillén, A.; Ruiz-Rodríguez, S.; Garrocho-Rangel, A.; Vértiz-Hernández, A.; Escobar-García, D.M. Effect of 4-allyl-1-hydroxy-2-methoxybenzene (eugenol) on inflammatory and apoptosis processes in dental pulp fibroblasts. Mediat. Inflamm. 2016, 2016, 9371403. [Google Scholar] [CrossRef] [PubMed]
- Amber, S.; Shah, S.A.A.; Ahmed, T.; Zahid, S. Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression. Asian Pac. J. Trop. Med. 2018, 11, 123. [Google Scholar]
- Kadri, Y.; Nciri, R.; Bardaa, S.; Brahmi, N.; Saber, S.; Harrath, A.H.; Aldahmash, W.; Alwasel, S.; Mohany, M.; El Feki, A.; et al. Syzygium Aromaticum Alleviates Cerium Chloride-Induced Neurotoxic Effect In The Adult Mice. Toxicol. Mech. Methods 2019, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, A.M.; El-Gerby, M.; Azim, M.H.A.E.; Awad, A. Antioxidant, antimicrobial activities and volatile constituents of clove flower buds oil. J. Essent. Oil Bear. Plants 2012, 15, 900–907. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]. Food Chem. 2001, 74, 443–448. [Google Scholar] [CrossRef]
- Nassar, M.I.; Gaara, A.H.; El-Ghorab, A.H.; Farrag, A.; Shen, H.; Huq, E.; Mabry, T.J. Chemical constituents of clove (Syzygium aromaticum, Fam. Myrtaceae) and their antioxidant activity. Rev. Latinoam. Química 2007, 35, 47. [Google Scholar]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Moon, S.-E.; Kim, H.-Y.; Cha, J.-D. Synergistic effect between clove oil and its major compounds and antibiotics against oral bacteria. Arch. Oral Biol. 2011, 56, 907–916. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ogbonye, E.; Ejimofor, O.; Ogbodo, E.; Ezeugwunne, I.; Madukwe, D.; Odumodu, I.; Agada, U.; Okezie, A.; Amah, A. The effect of metronidazole on the histology of the cerebellum and pituitary gland in female wistar rats. IP Indian J. Neurosci. 2020, 6, 67–72. [Google Scholar]
- Oda, S.S. Histopathological and biochemical alterations of metronidazole-induced toxicity in male rats. GV 2012, 9, 303–310. [Google Scholar]
- Tarola, A.M.; Van de Velde, F.; Salvagni, L.; Preti, R. Determination of phenolic compounds in strawberries (Fragaria ananassa Duch) by high performance liquid chromatography with diode array detection. Food Anal. Methods 2013, 6, 227–237. [Google Scholar] [CrossRef]
- Schermer, S. The Blood Morphology of Laboratory Animals, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 1967; pp. 42–48. [Google Scholar]
- Lasley, S.M.; Greenland, R.D.; Michaelson, I.A. Determination of gamma-aminobutyric and glutamic acids in rat brain by liquid chromatography with electrochemical detection. Life Sci. 1984, 35, 1921–1930. [Google Scholar] [CrossRef]
- Carageorgiou, H.; Tzotzes, V.; Sideris, A.; Zarros, A.; Tsakiris, S. Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: Modulation by zinc, calcium and L-cysteine co-administration. Basic Clin. Pharmacol. Toxicol. 2005, 97, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Sasa, S.; Blank, C.L. Determination of serotonin and dopamine in mouse brain tissue by high performance liquid chromatography with electrochemical detection. Anal. Chem. 1977, 49, 354–359. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Methods Enzymol. 2008, 440, 361–380. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.; Spitz, D.; Greenwald, R. CRC handbook of methods for oxygen radical research. In CRC Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985; Volume 2. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sarna, J.R.; Brownell, A.K.W.; Furtado, S. Reversible cerebellar syndrome caused by metronidazole. Can. Med Assoc. J. 2009, 181, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Sarna, J.R.; Furtado, S.; Brownell, A.K.W. Neurologic complications of metronidazole. Can. J. Neurol. Sci. 2013, 40, 768–776. [Google Scholar] [CrossRef]
- Evans, J.; Levesque, D.; Knowles, K.; Longshore, R.; Plummer, S. Diazepam as a treatment for metronidazole toxicosis in dogs: A retrospective study of 21 cases. J. Vet. Intern. Med. 2003, 17, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-Safi, I.; Mohamed Al Kamaly, O.; Bousta, D. Insight into gentisic acid antidiabetic potential using in vitro and in silico approaches. Molecules 2021, 26, 1932. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Calarco, A.; Boukhira, S.; Noman, O.M.; Mothana, R.A.; Nasr, F.A.; Bekkari, H.; Bousta, D. Defatted Hydroethanolic Extract of Ammodaucus leucotrichus Cosson and Durieu Seeds: Antidiabetic and Anti-Inflammatory Activities. Appl. Sci. 2020, 10, 9147. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Jaberi, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef]
- Shojaii, A.; Abdollahi Fard, M. Review of pharmacological properties and chemical constituents of Pimpinella anisum. Int. Sch. Res. Not. 2012, 2012, 510795. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef]
- Johannah, N.; Renny, R.; Gopakumar, G.; Maliakel, B.; Sureshkumar, D.; Krishnakumar, I. Beyond the flavour: A de-flavoured polyphenol rich extract of clove buds (Syzygium aromaticum L.) as a novel dietary antioxidant ingredient. Food Funct. 2015, 6, 3373–3382. [Google Scholar]
- Tahoun, E.A.E.-A.M. Protective Effect of Moringa Oleifera against Metronidazole-induced toxicity in male albino rats. J. Biosci. Appl. Res. 2017, 3, 137–149. [Google Scholar]
- Sohrabi, D.; Alipour, M.; Melati, A. Effect of metronidazole on spermatogenesis, plasma gonadotrophins and testosterone in rats. Iran. J. Pharm. Res. 2007, 6, 279–283. [Google Scholar]
- Chukwu, V.; Akudike, C.; Ezejindu, D.; Ofoego, U.; Chukwuocha, C. The protective effects of turmeric on testicular tissues, after treatment with metronidazole in adult male Wistar rats. CIBTech. J. Pharm. Sci. 2015, 4, 62–68. [Google Scholar]
- Dusengeyezu, E.; Kadima, J.N. How do metronidazole drawbacks impact patient compliance and therapeutic outcomes in treating amoebiasis in Rwanda. Int. J. Trop. Dis. Health 2016, 17, 1–7. [Google Scholar] [CrossRef]
- Bradley, W.G.; Karlsson, I.; Rassol, C. Metronidazole neuropathy. Br. Med. J. 1977, 2, 610. [Google Scholar] [CrossRef]
- Hassan, M.; Awadalla, E.; Ali, R.; Fouad, S.; Abdel-Kahaar, E. Thiamine deficiency and oxidative stress induced by prolonged metronidazole therapy can explain its side effects of neurotoxicity and infertility in experimental animals: Effect of grapefruit co-therapy. Hum. Exp. Toxicol. 2020, 39, 834–847. [Google Scholar] [CrossRef]
- Agarwal, A.; Kanekar, S.; Sabat, S.; Thamburaj, K. Metronidazole-induced cerebellar toxicity. Neurol. Int. 2016, 8, 6365. [Google Scholar] [CrossRef]
- Kalia, V.; Saggar, K. Case report: MRI of the brain in metronidazole toxicity. Indian J. Radiol. Imaging 2010, 20, 195–197. [Google Scholar] [CrossRef]
- Ligha, A.; Paul, C. Oxidative effect of metronidazole on the testis of Wistar rats. Aus. J. Bas. Appl. Sci. 2011, 5, 1339–1344. [Google Scholar]
- Cabuk, M.; Alcicek, A.; Bozkurt, M.; Imre, N. Antimicrobial properties of the essential oils isolated from aromatic plants and using possibility as alternative feed additives. In Proceedings of the National Animal Nutrition Congress, Konya, Turkey, 18–20 September 2003; pp. 184–187. [Google Scholar]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [PubMed]
- Samojlik, I.; Mijatović, V.; Petković, S.; Škrbić, B.; Božin, B. The influence of essential oil of aniseed (Pimpinella anisum, L.) on drug effects on the central nervous system. Fitoterapia 2012, 83, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Andallu, B.; Rajeshwari, C.U. Chapter 20—Aniseeds (Pimpinella anisum L.) in Health and Disease. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 175–181. [Google Scholar] [CrossRef]
- Kahloula, K.; Slimani, M.; Adli, D.E.H.; Rachdi, S.; Boumediene, D. Neuro beneficial effects of Pimpinella anisum against lead exposure. Int. J. Green Pharm. (IJGP) 2013, 7, 18. [Google Scholar] [CrossRef]
- Gorji, A.; Ghadiri, M.K. History of epilepsy in Medieval Iranian medicine. Neurosci. Biobehav. Rev. 2001, 25, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, F.; Hosseini, M.; Mangeng, D.; Alavi, H.; Hassanzadeh, G.R.; Bayat, M.; Jafarian, M.; Kazemi, H.; Gorji, A. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement. Altern. Med. 2012, 12, 76. [Google Scholar] [CrossRef]
- Kassab, R.B.; Bauomy, A.A. The neuroprotective efficency of the aqueous extract of clove (Syzygium aromaticum) in aluniniuminduced neurotoxicity. Int. J. Pharm. Pharm. Sci. 2014, 6, 503–508. [Google Scholar]
- Prasad, S.N. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 2013, 38, 330–345. [Google Scholar] [CrossRef]
- Gülçin, Ì.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Wang, E.K.; Lee, H.-C.; Tsay, H.-J.; Wei, Y.-H. Increased expression of manganese-superoxide dismutase in fibroblasts of patients with CPEO syndrome. Mol. Genet. Metab. 2003, 80, 321–329. [Google Scholar] [CrossRef]
- Adegbola, M.V.; Anyim, G.; Ntwasa, M.; Ayeleso, A.O.; Oyedepo, T.A. Potential Effect of Syzygium aromaticum (Cloves) Extract on Serum Antioxidant Status and Lipid Profiles in Wistar Rats with Artesunate Toxicity. Appl. Sci. 2022, 12, 8216. [Google Scholar] [CrossRef]
- Dashti-R, M.H.; Morshedi, A. The effects of Syzygium aromaticum (clove) on learning and memory in mice. Asian J. Tradit. Med. 2009, 4, 128–133. [Google Scholar]
- Faried, M.A.; Issa, N.M. Comparative study on the effect of the pimpinella anisum and estradiol on the hippocampus and dentate gyrus of ovariectomized rats. Anat. Physiol. Biochem. Int. J. 2017, 2. [Google Scholar] [CrossRef]
- Moghimian, M.; Abtahi-Evari, S.-H.; Shokoohi, M.; Amiri, M.; Soltani, M. Effect of Syzygium aromaticum (clove) extract on seminiferous tubules and oxidative stress after testicular torsion in adult rats. Physiol. Pharmacol. 2017, 21, 343–350. [Google Scholar]
- Soltani, M.; Moghimian, M.; Abtahi-Evari, S.-H.; Esmaeili, S.-A.; Mahdipour, R.; Shokoohi, M. The Effects of Clove Oil on The Biochemical and Histological Parameters, and Autophagy Markers in Polycystic Ovary Syndrome-Model Rats. Int. J. Fertil. Steril. 2023, 17, 187–194. [Google Scholar] [PubMed]

| Anise (1 g/15 mL) | Clove (1 g/15 mL) | |||
|---|---|---|---|---|
| Compounds | ||||
| Area | Conc. (µg/mL) | Area | Conc. (µg/mL) | |
| Gallic acid | 721.26 | 63.38 | 14,131.40 | 1241.87 |
| Chlorogenic acid | 2050.14 | 153.15 | 3252.87 | 243.00 |
| Catechin | 28.44 | 3.41 | 2029.36 | 243.16 |
| Methyl gallate | 0.00 | 0.00 | 1014.85 | 13.78 |
| Coffeic acid | 1085.13 | 46.38 | 0.00 | 0.00 |
| Syringic acid | 475.21 | 20.80 | 4503.74 | 197.08 |
| Pyro catechol | 202.00 | 14.33 | 473.29 | 33.58 |
| Rutin | 89.69 | 12.21 | 10.48 | 1.43 |
| Ellagic acid | 645.97 | 43.22 | 3844.39 | 257.22 |
| Coumaric acid | 48.63 | 0.83 | 1967.15 | 33.44 |
| Vanillin | 259.17 | 5.83 | 103.12 | 2.32 |
| Ferulic acid | 66.09 | 2.31 | 0.00 | 0.00 |
| Naringenin | 2417.75 | 133.64 | 2310.65 | 127.72 |
| Taxifolin | 800.27 | 58.17 | 313.15 | 22.76 |
| Cinnamic acid | 570.17 | 5.76 | 220.99 | 2.23 |
| Kaempferol | 9.14 | 0.36 | 20.42 | 0.80 |
| Groups | FI (g/d) | BWG % | FER | Brain % |
|---|---|---|---|---|
| Control | 23.35 ± 0.28 a | 29.90 ± 2.12 a | 0.19 ± 0.01 a | 1.58 ± 0.13 a |
| Metronidazole | 17.87 ± 0.12 e | 8.85 ± 0.23 e | 0.02 ± 0.005 d | 0.76 ± 0.04 c |
| Anise (250 mg/kg) + Metronidazole | 22.49 ± 0.12 ab | 26.17 ± 0.64 b | 0.04 ± 0.002 c | 0.97 ± 0.01 b |
| Anise (500 mg/kg) + Metronidazole | 20.95 ± 0.64 c | 23.94 ± 0.68 c | 0.05 ± 0.004 c | 1.56 ± 0.98 a |
| Clove (250 mg/kg) + Metronidazole | 19.87 ± 0.94 d | 22.47 ± 1.02 c | 0.07 ± 0.003 b | 1.02 ± 0.003 b |
| Clove (500 mg/kg) + Metronidazole | 21.79 ± 0.19 bc | 19.49 ± 1.55 d | 0.06 ± 0.005 b | 0.95 ± 0.133 b |
| Groups | GABA (Pg/mL) | ACHE (Pg/mL) | DA (ng/mL) | ST (ng/mL) |
|---|---|---|---|---|
| control | 404.02 ± 22 a | 11.21 ± 21 e | 2.51 ± 0.12 a | 67.5 ± 6.5 a |
| Metronidazole | 46.86 ± 4.26 f | 87.12 ± 6.1 a | 0.15 ± 0.005 f | 5.21 ± 0.9 e |
| Anise (250 mg/kg) + Metronidazole | 97.41 ± 12.9 e | 67.35 ± 2 b | 0.37 ± 0.01 e | 25.31 ± 6.8 d |
| Anise (500 mg/kg) + Metronidazole | 274.75 ± 26.55 c | 20.51 ± 3.5 d | 0.94 ± 0.03 c | 50.41 ± 20 c |
| Clove (250 mg/kg) + Metronidazole | 157.5 2 ± 9.5 d | 42.51 ± 5.5 c | 0.76 ± 0.04 d | 32.21 ± 5.7 d |
| Clove (500 mg/kg) + Metronidazole | 335.51 ± 10.5 b | 19.11 ± 1.1 d | 1.12 ± 0.03 b | 59.14 ± 1.2 b |
| Groups | MDA (nmol/gm) | NO (umol/L) | SOD (U/mg) | CAT (U/mg) | GSH (mg/gm) |
|---|---|---|---|---|---|
| Control | 1.24 ± 0.51 f | 5 ± 1 e | 222 ± 16 a | 9.91 ± 1.09 a | 192.5 ± 11.5 a |
| Metronidazole | 25.75 ± 2 a | 86 ± 7 a | 30.5 ± 2.5 e | 0.59 ± 0.03 d | 24.5 ± 2.5 d |
| Anise (250 mg/kg) + Metronidazole | 18.6 ± 1.12 b | 45 ± 5 c | 48.5 ± 9.5 d | 2.22 ± 0.26 c | 70 ± 3 c |
| Anise (500 mg/kg) + Metronidazole | 9.92 ± 0.92 d | 19.5 ± 1.5 d | 130 ± 9 b | 5.52 ± 0.72 b | 95 ± 5 b |
| Clove (250 mg/kg) + Metronidazole | 12.65 ± 0.15 c | 56.5 ± 6.5 b | 80.5 ± 0.13 c | 1.86 ± 0.5 c | 73.5 ± 5.74 c |
| Clove (500 mg/kg) + Metronidazole | 6.45 ± 1.39 e | 27.5 ± 3.5 d | 126 ± 7 b | 5.08 ± 0.82 b | 93 ± 3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Moslemany, A.M.; Abd-Elfatah, M.H.; Tahoon, N.A.; Bahnasy, R.M.; Alotaibi, B.S.; Ghamry, H.I.; Shukry, M. Mechanistic Assessment of Anise Seeds and Clove Buds against the Neurotoxicity Caused by Metronidazole in Rats: Possible Role of Antioxidants, Neurotransmitters, and Cytokines. Toxics 2023, 11, 724. https://doi.org/10.3390/toxics11090724
El-Moslemany AM, Abd-Elfatah MH, Tahoon NA, Bahnasy RM, Alotaibi BS, Ghamry HI, Shukry M. Mechanistic Assessment of Anise Seeds and Clove Buds against the Neurotoxicity Caused by Metronidazole in Rats: Possible Role of Antioxidants, Neurotransmitters, and Cytokines. Toxics. 2023; 11(9):724. https://doi.org/10.3390/toxics11090724
Chicago/Turabian StyleEl-Moslemany, Amira M., Mai Hussein Abd-Elfatah, Nawal A. Tahoon, Rasha M. Bahnasy, Badriyah S. Alotaibi, Heba I. Ghamry, and Mustafa Shukry. 2023. "Mechanistic Assessment of Anise Seeds and Clove Buds against the Neurotoxicity Caused by Metronidazole in Rats: Possible Role of Antioxidants, Neurotransmitters, and Cytokines" Toxics 11, no. 9: 724. https://doi.org/10.3390/toxics11090724
APA StyleEl-Moslemany, A. M., Abd-Elfatah, M. H., Tahoon, N. A., Bahnasy, R. M., Alotaibi, B. S., Ghamry, H. I., & Shukry, M. (2023). Mechanistic Assessment of Anise Seeds and Clove Buds against the Neurotoxicity Caused by Metronidazole in Rats: Possible Role of Antioxidants, Neurotransmitters, and Cytokines. Toxics, 11(9), 724. https://doi.org/10.3390/toxics11090724








