Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Epilepsy
2.3. Preparation of RO Aqueous Extract
2.4. Experimental Design
- Control group: Normal saline was administered.
- RO-treated group: RO extract (100 mg/kg) was administered.
- PTZ-treated group: Rats were treated with pentylenetetrazol (PTZ) (30 mg/kg).
- PTZ + RO-treated group: Rats were treated with RO (100 mg/kg) for 30 min before treatment with PTZ (30 mg/kg).
- PTZ + valproic acid (VA)-treated group (a positive control group): Rats were treated with VA (300 mg/kg) 30 min before treatment with PTZ (30 mg/kg).
2.5. Dose Selection
2.6. Scoring of Epileptic Seizures
2.7. Morris Water Maze (MWM) Test
2.8. Passive Avoidance Learning (PAL) Test
2.9. Tissue Collection and Processing
2.10. Biochemical Analysis in the Brain Homogenates
2.11. Histological Evaluations
2.12. Statistical Analysis
3. Results
3.1. Scoring of Epileptic Seizures
3.1.1. Assessment of Seizure Scores
3.1.2. Total Number of Myoclonic Jerks
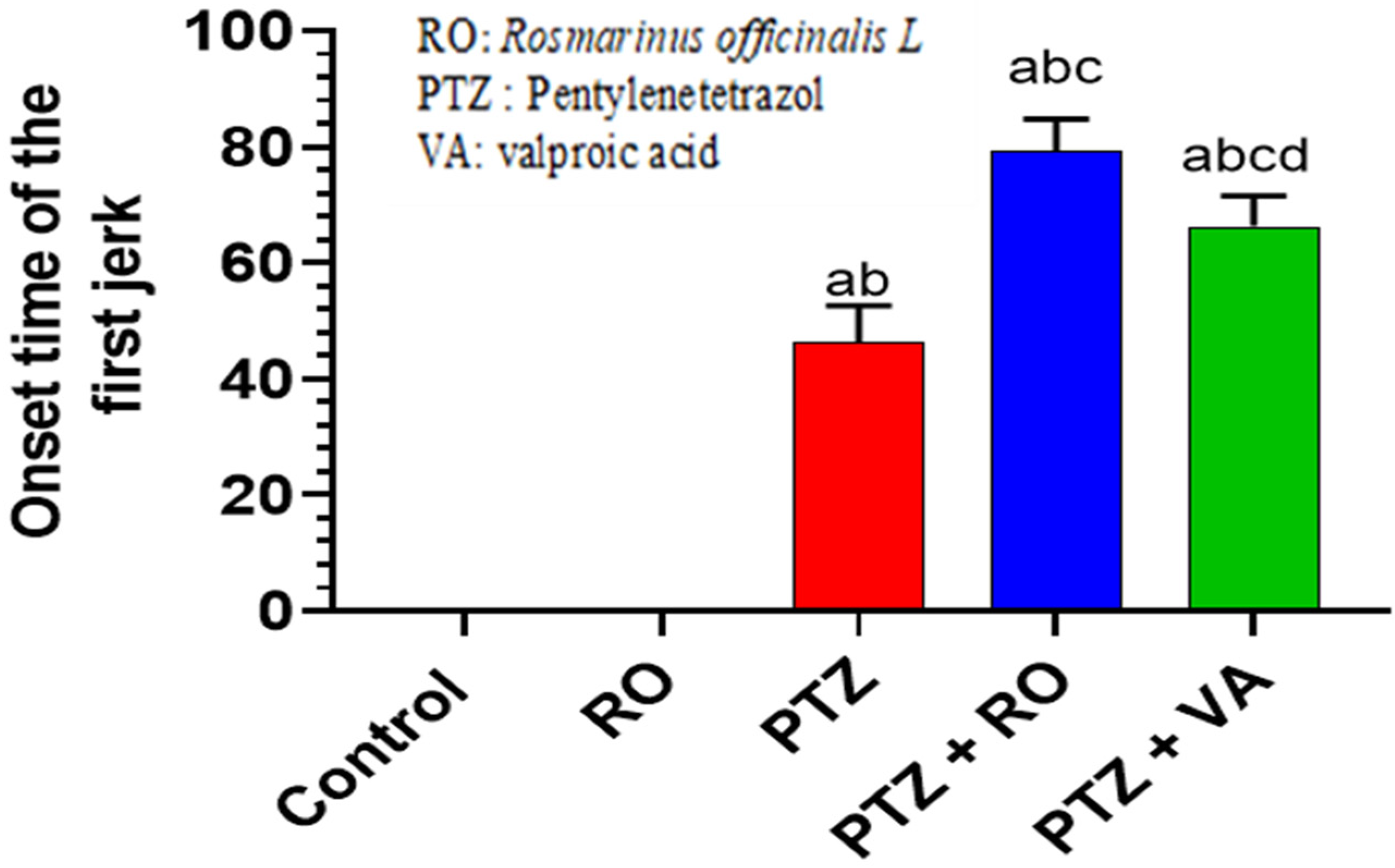
3.1.3. Onset of the First Myoclonic Jerk
3.1.4. Behavioral Tests
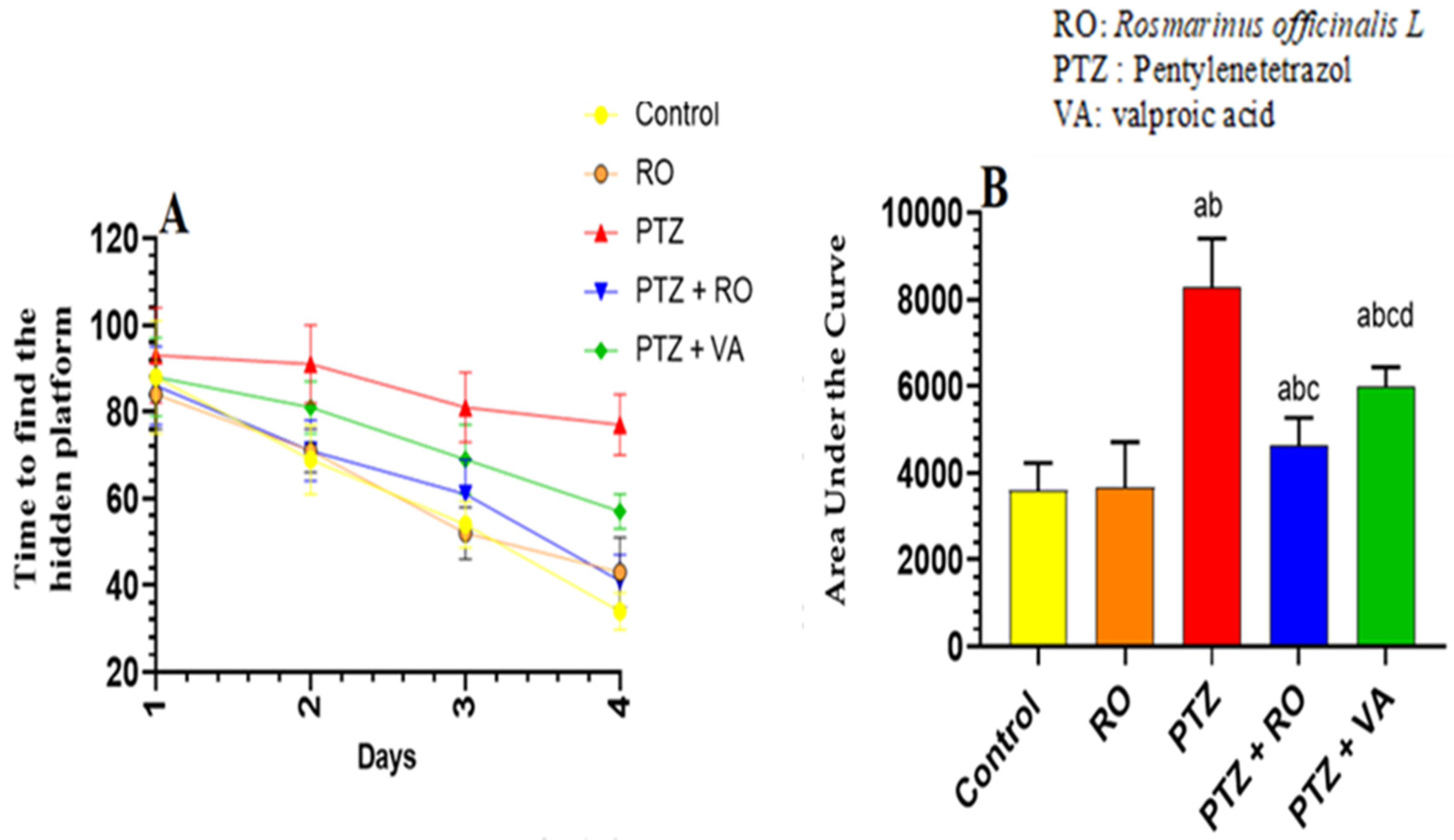
Time Required to Find the Hidden Platform during the MWM Test
Total Swimming Distance to Find the Hidden Platform during the MWM Test
Number of Crossing Times over the Removed Platform during the Probe Trial of the MWM Test
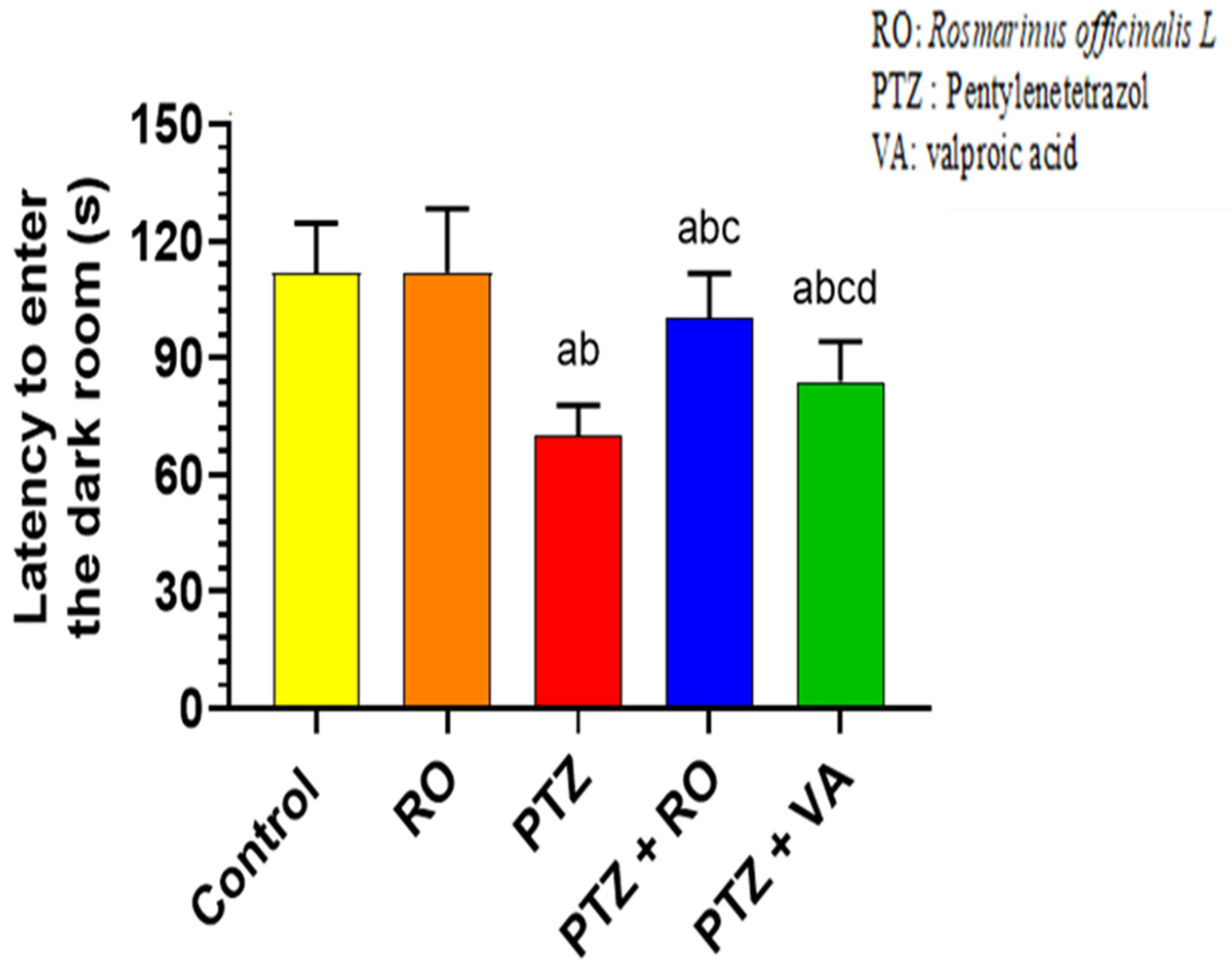
PAL Test
3.1.5. Biochemical Analysis
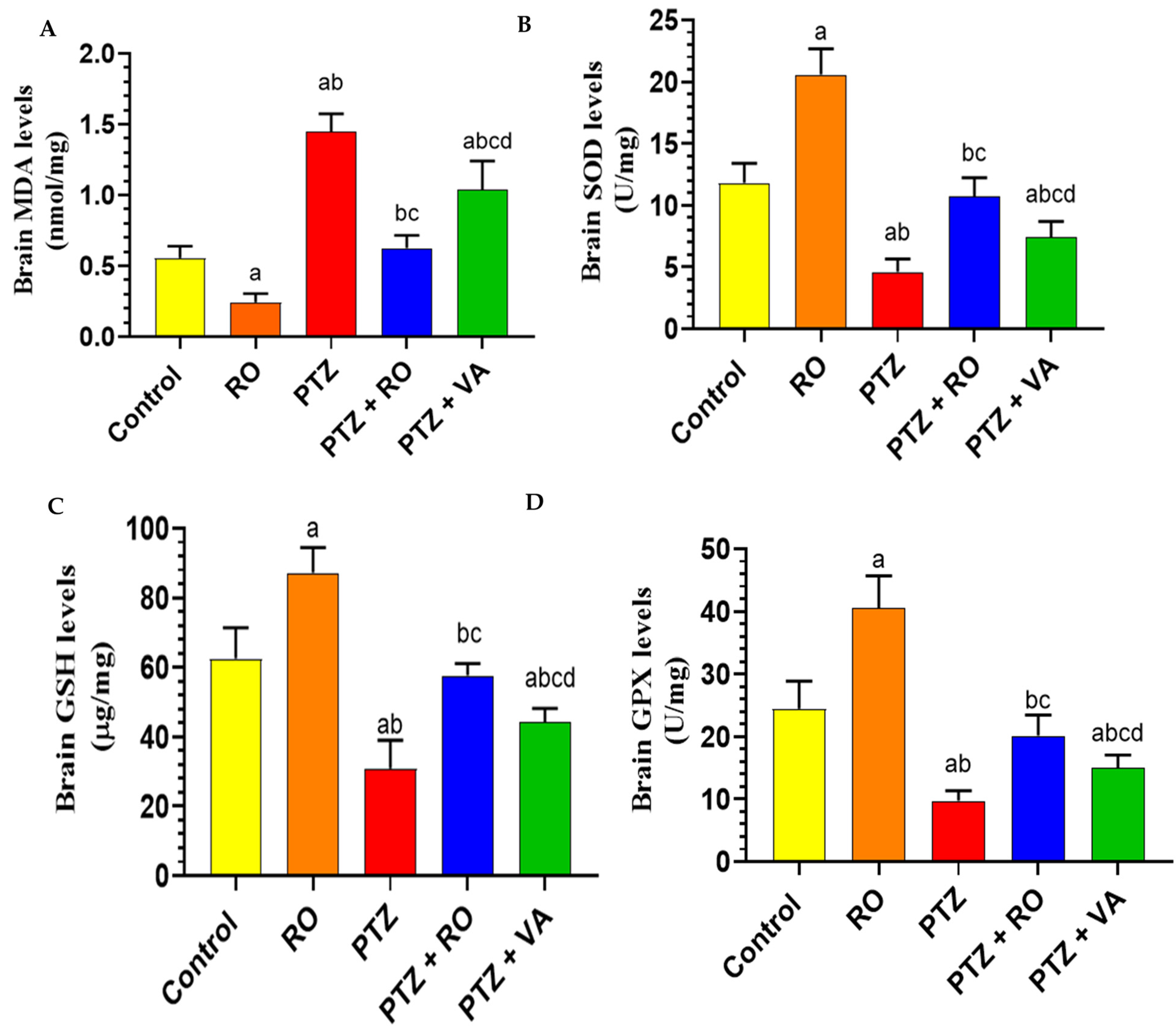
Levels of Antioxidant Markers
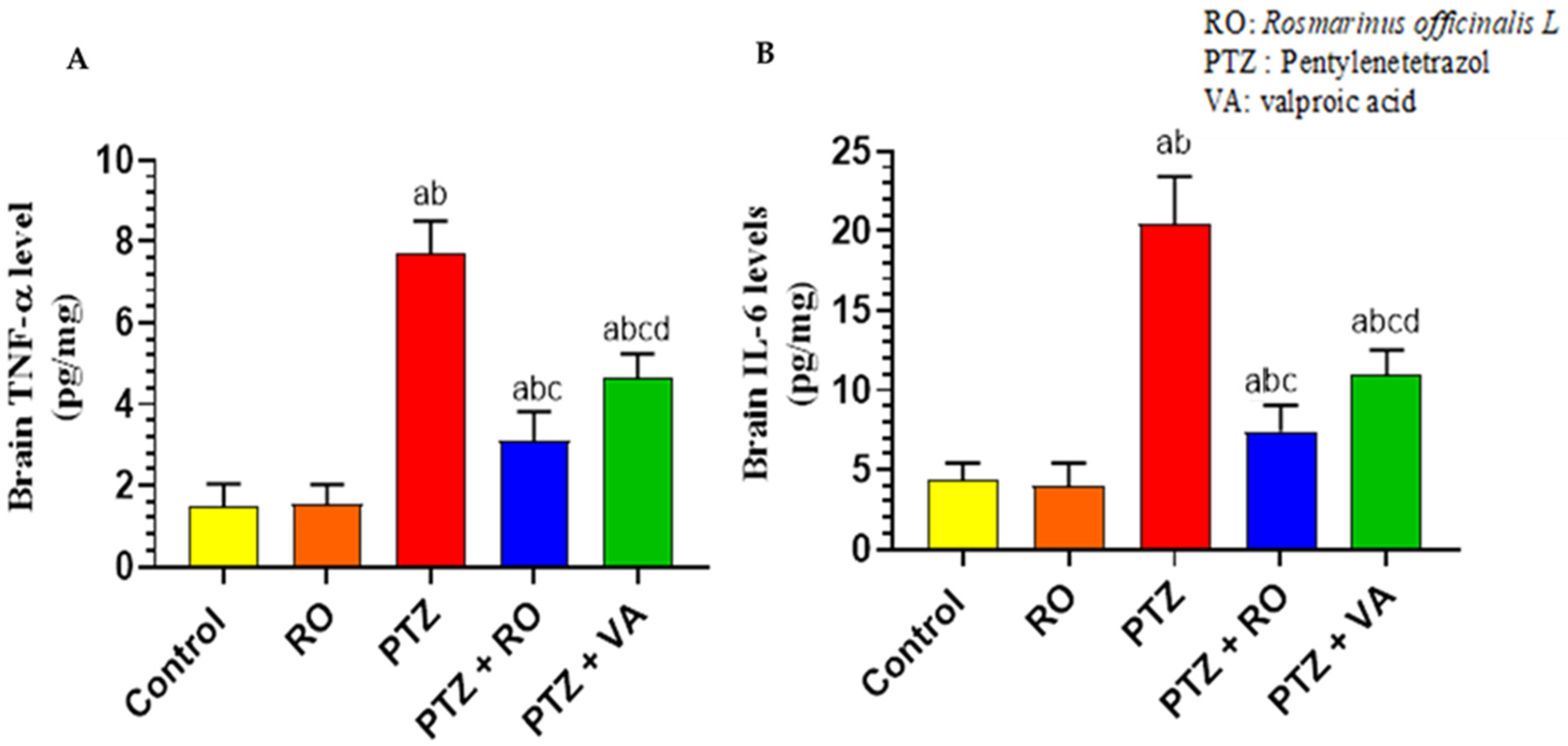
Levels of Inflammatory Markers
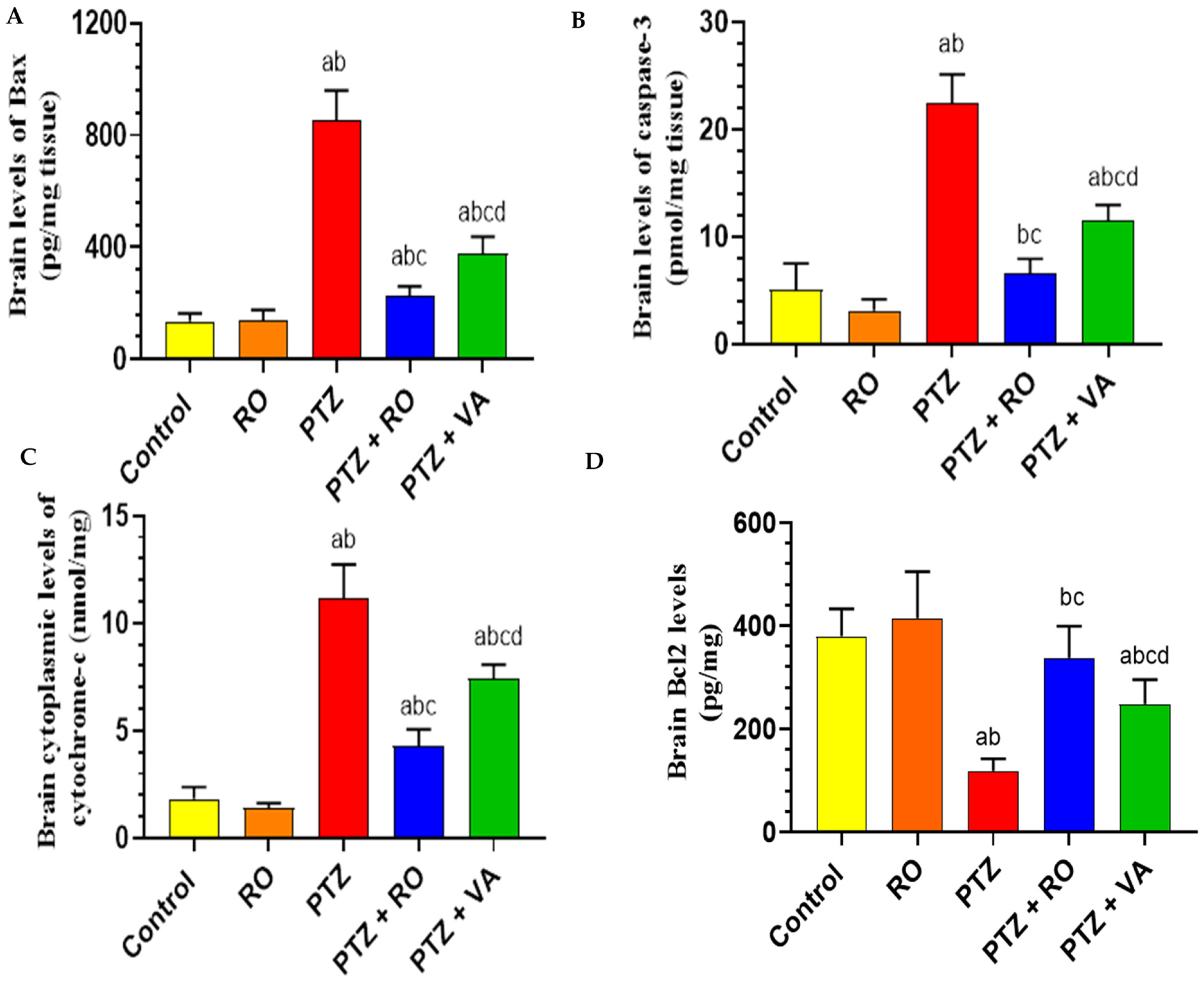
Levels of Apoptotic Proteins in the Brain
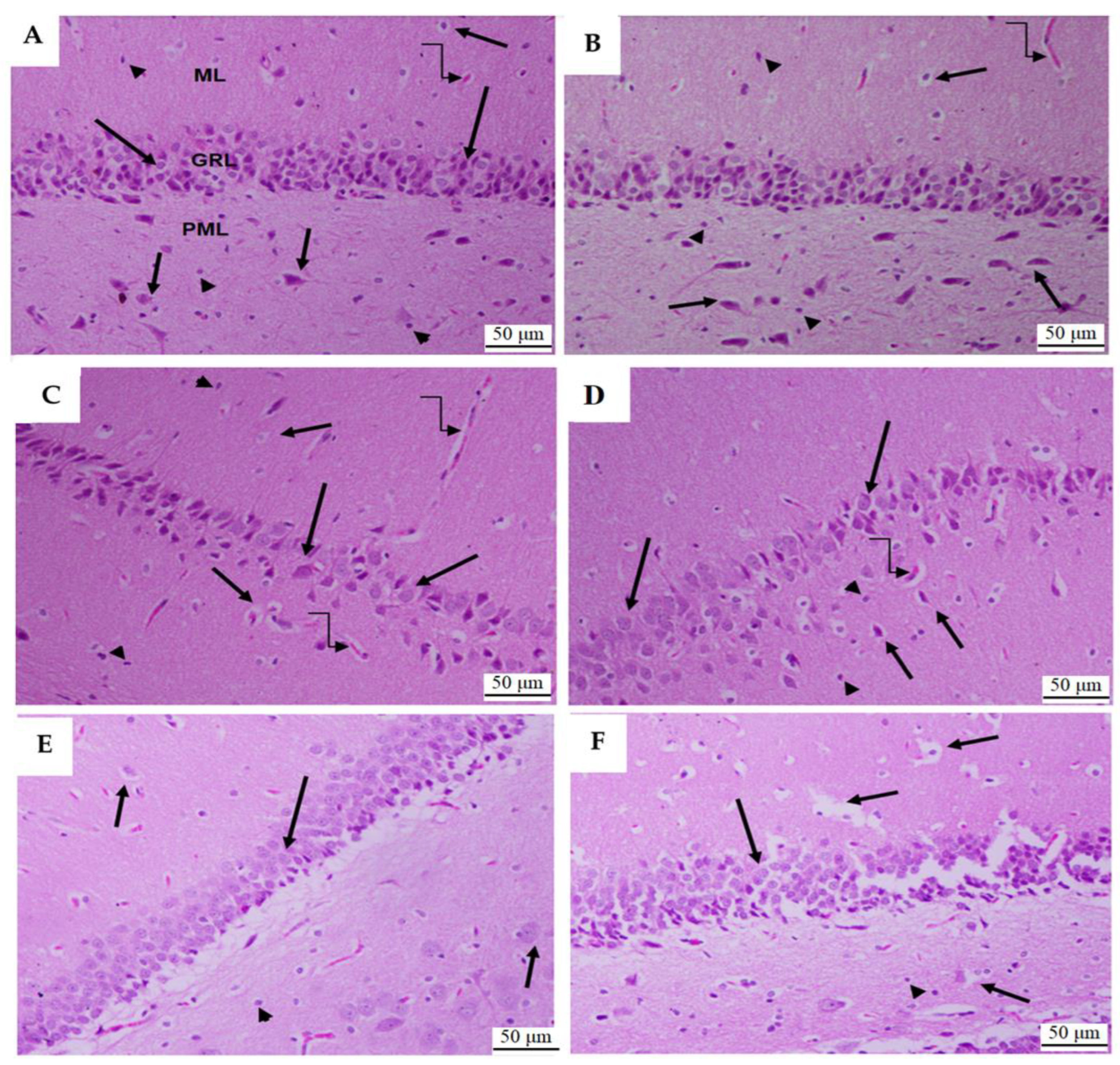
3.1.6. Histological Analysis of Dental Gyrus of the Hippocampi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.A.; Ahmad, I. Herbal medicine: Current trends and future prospects. In New Look to Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–13. [Google Scholar]
- Rotblatt, M. Herbal medicine: Expanded commission E monographs; American College of Physicians: Philadelphia, PA, USA, 2000. [Google Scholar]
- Zhang, Y.; Adelakun, T.A.; Qu, L.; Li, X.; Li, J.; Han, L.; Wang, T. New terpenoid glycosides obtained from Rosmarinus officinalis L. aerial parts. Fitoterapia 2014, 99, 78–85. [Google Scholar] [CrossRef]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Doolaege, E.H.; Vossen, E.; Raes, K.; De Meulenaer, B.; Verhe, R.; Paelinck, H.; De Smet, S. Effect of rosemary extract dose on lipid oxidation, colour stability and antioxidant concentrations, in reduced nitrite liver pates. Meat Sci. 2012, 90, 925–931. [Google Scholar] [CrossRef]
- Alvi, S.S.; Ahmad, P.; Ishrat, M.; Iqbal, D.; Khan, M.S. Secondary metabolites from rosemary (Rosmarinus officinalis L.): Structure, biochemistry and therapeutic implications against neurodegenerative diseases. In Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Springer: Singapore, 2019; pp. 1–24. [Google Scholar]
- Jennum, P.; Christensen, J.; Ibsen, R.; Kjellberg, J. Long-term socioeconomic consequences and health care costs of childhood and adolescent-onset epilepsy. Epilepsia 2016, 57, 1078–1085. [Google Scholar] [CrossRef]
- Kanner, A.M. Epilepsy, suicidal behaviour, and depression: Do they share common pathogenic mechanisms? Lancet Neurol. 2006, 5, 107–108. [Google Scholar] [CrossRef]
- Heidari, M.; Assadipour, A.; Rashid, F.P. Effect of Rosmarinus officinalis L. Extract on the seizure induced by picrotoxin in mice. Pak. J. Biol. Sci. 2005, 8, 1807–1811. [Google Scholar]
- Boroushaki, M.; Baharloo, A.; Malek, F. A comparative study on the anticonvulsive effects of the aqueous extract of the Rosmarinus officinalis plant with phenobarbital in pentylentetrazol-induced seizures in mice. Koomesh 2002, 3, 53–58. [Google Scholar]
- Li, M.; Cui, M.-M.; Kenechukwu, N.A.; Gu, Y.-W.; Chen, Y.-L.; Zhong, S.-J.; Gao, Y.-T.; Cao, X.-Y.; Wang, L.; Liu, F.-M. Rosmarinic acid ameliorates hypoxia/ischemia induced cognitive deficits and promotes remyelination. Neural Regen. Res. 2020, 15, 894. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Atef, R.M.; Fattah, I.O.A.; Mahmoud, O.M.; Abdel-Rahman, G.M.; Salem, N.A. Protective effects of Rosemary extract and/or Fluoxetine on Monosodium Glutamate-induced hippocampal neurotoxicity in rat. Rom. J. Morphol. Embryol. 2021, 62, 169. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sci. 2017, 179, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.N.; Ahmed, S.N.; Aalam, S.M.; Kumar, C.; Shafi, G. Evaluation of cichorium extract for the growth supporting property in rat hepatocyte primary culture. Asian J. Plant Sci. 2007, 6, 431–434. [Google Scholar] [CrossRef][Green Version]
- Naderali, E.; Nikbakht, F.; Ofogh, S.N.; Rasoolijazi, H. The role of rosemary extract in degeneration of hippocampal neurons induced by kainic acid in the rat: A behavioral and histochemical approach. J. Integr. Neurosci. 2018, 17, 31–43. [Google Scholar] [CrossRef]
- Uma Devi, P.; Kolappa Pillai, K.; Vohora, D. Modulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteine. Fundam. Clin. Pharmacol. 2006, 20, 247–253. [Google Scholar] [CrossRef]
- Kaboutari, J.; Zendehdel, M.; Habibian, S.; Azimi, M.; Shaker, M.; Karimi, B. The antiepileptic effect of sodium valproate during different phases of the estrous cycle in PTZ-induced seizures in rats. J. Physiol. Biochem. 2012, 68, 155–161. [Google Scholar] [CrossRef]
- Kilinc, E.; Ankarali, S.; Ayhan, D.; Ankarali, H.; Torun, I.E.; Cetinkaya, A. Protective effects of long-term probiotic mixture supplementation against pentylenetetrazole-induced seizures, inflammation and oxidative stress in rats. J. Nutr. Biochem. 2021, 98, 108830. [Google Scholar] [CrossRef]
- Kola, P.K.; Akula, A.; NissankaraRao, L.S.; Danduga, R.C.S.R. Protective effect of naringin on pentylenetetrazole (PTZ)-induced kindling; possible mechanisms of antikindling, memory improvement, and neuroprotection. Epilepsy Behav. 2017, 75, 114–126. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Al-Qahtani, W.H.; Alshuniaber, M.A.; Yagoub, A.E.A.; Al-Khalifah, A.S.; Al-Harbi, L.N.; Alhussain, M.H.; AlSedairy, S.A.; Yahya, M.A. Quercetin improves the impairment in memory function and attenuates hippocampal damage in cadmium chloride-intoxicated male rats by suppressing acetylcholinesterase and concomitant activation of SIRT1 signaling. J. Funct. Foods 2021, 86, 104675. [Google Scholar] [CrossRef]
- Attia, G.M.; Elmansy, R.A.; Elsaed, W.M. Neuroprotective effect of nilotinib on pentylenetetrazol-induced epilepsy in adult rat hippocampus: Involvement of oxidative stress, autophagy, inflammation, and apoptosis. Folia Neuropathol. 2019, 57, 146–160. [Google Scholar] [CrossRef]
- Koguchi, Y.; Kawakami, K.; Kon, S.; Segawa, T.; Maeda, M.; Uede, T.; Saito, A. Penicillium marneffei causes osteopontin-mediated production of interleukin-12 by peripheral blood mononuclear cells. Infect. Immun. 2002, 70, 1042–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshida, N.; Ikemoto, S.; Narita, K.; Sugimura, K.; Wada, S.; Yasumoto, R.; Kishimoto, T.; Nakatani, T. Interleukin-6, tumour necrosis factor α and interleukin-1β in patients with renal cell carcinoma. Br. J. Cancer 2002, 86, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, X.-Y.; Li, J.; Xu, Z.-G.; Chen, L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. J. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imada, T.; Yamaguchi, I.; Yoshitake, H.; Sanada, H.; Kashiwagi, T.; Takaba, K. Effects of prolonged water washing of tissue samples fixed in formalin on histological staining. Biotech. Histochem. 2012, 87, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K. Understanding one-way ANOVA using conceptual figures. Korean J. Anesthesiol. 2017, 70, 22–26. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. Recent progress of electrospun herbal medicine nanofibers. Biomolecules 2023, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Hixson, J.D. When and how to stop antiepileptic drugs. In Epilepsy; Wiley: Hoboken, NJ, USA, 2014; pp. 118–121. [Google Scholar]
- Wang, X.; Huang, S.; Liu, Y.; Li, D.; Dang, Y.; Yang, L. Effects of ketogenic diet on cognitive function in pentylenetetrazol-kindled rats. Epilepsy Res. 2021, 170, 106534. [Google Scholar] [CrossRef]
- Pappachan, F.; Suku, A.; Mohanan, S. Rosmarinus officinalis. In Herbs, Spices and Their Roles in Nutraceuticals and Functional Foods; Elsevier: Amsterdam, The Netherlands, 2023; pp. 149–170. [Google Scholar]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic. Med. Sci. 2020, 23, 1100. [Google Scholar]
- Grigoletto, J.; de Oliveira, C.V.; Grauncke, A.C.B.; de Souza, T.L.; Souto, N.S.; de Freitas, M.L.; Furian, A.F.; Santos, A.R.S.; Oliveira, M.S. Rosmarinic acid is anticonvulsant against seizures induced by pentylenetetrazol and pilocarpine in mice. Epilepsy Behav. 2016, 62, 27–34. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Nobakht, N. Effects of Rosmarinus officinalis L. aerial parts essential oil on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J. Med. Plants 2004, 3, 51–57. [Google Scholar]
- Rad, S.N.H.; Kashani, M.H.G.; Abrari, K. Pre-treatment by rosemary extract or cell transplantation improves memory deficits of parkinson’s disease: When tradition meets the future. Braz. Arch. Biol. Technol. 2021, 64, e21180392. [Google Scholar] [CrossRef]
- Al-Tawarah, N.M.; Al-Dmour, R.H.; Abu Hajleh, M.N.; Khleifat, K.M.; Alqaraleh, M.; Al-Saraireh, Y.M.; Jaradat, A.Q.; Al-Dujaili, E.A. Rosmarinus officinalis and Mentha piperita Oils Supplementation Enhances Memory in a Rat Model of Scopolamine-Induced Alzheimer’s Disease-like Condition. Nutrients 2023, 15, 1547. [Google Scholar] [CrossRef]
- Motlagh, M.K.; Sharafi, M.; Zhandi, M.; Mohammadi-Sangcheshmeh, A.; Shakeri, M.; Soleimani, M.; Zeinoaldini, S. Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze–thawing process of ram sperm. Cryobiology 2014, 69, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Asl, J.F.; Goudarzi, M.; Shoghi, H. The radio-protective effect of rosmarinic acid against mobile phone and Wi-Fi radiation-induced oxidative stress in the brains of rats. Pharmacol. Rep. 2020, 72, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Yesilbag, D.; SS, C.; Belenli, D. Effects of supplementation with rosemary (Rosmarinus officinalis L.) volatile oil on growth performance, meat MDA level and selected plasma antioxidant parameters in quail diets. Kafkas Üniversitesi Vet. Fakültesi Derg. 2017, 23, 283–288. [Google Scholar]
- Khalil, O.A.; Ramadan, K.S.; Danial, E.N.; Alnahdi, H.S.; Ayaz, N.O. Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. Afr. J. Pharm. Pharmacol. 2012, 6, 1031–1036. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Wu, Y.-n.; Huang, J.; Zuo, A.-l.; Yao, L. Research on the Effects of Rosemary (Rosmarinus officinalis L.) on the Blood Lipids and Anti-lipid Peroxidation in Rats. J. Essent. Oil Res. 2011, 23, 26–34. [Google Scholar] [CrossRef]
- Karataş, T.; Korkmaz, F.; Karataş, A.; Yildirim, S. Effects of Rosemary (Rosmarinus officinalis) extract on growth, blood biochemistry, immunity, antioxidant, digestive enzymes and liver histopathology of rainbow trout, Oncorhynchus mykiss. Aquac. Nutr. 2020, 26, 1533–1541. [Google Scholar] [CrossRef]
- Mengoni, E.S.; Vichera, G.; Rigano, L.A.; Rodriguez-Puebla, M.L.; Galliano, S.R.; Cafferata, E.E.; Pivetta, O.H.; Moreno, S.; Vojnov, A.A. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 2011, 82, 414–421. [Google Scholar] [CrossRef]
- Marklund, S. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J. Biol. Chem. 1992, 267, 6696–6701. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.P.; Nicks, M.; Tran, K.; Tanner, G.; Chang, L.-Y.; Young, S.K.; Worthen, G.S. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am. J. Respir. Cell Mol. Biol. 2004, 31, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cheon, I.-S.; Kim, B.-H.; Kwon, M.-J.; Lee, H.-W.; Kim, T.-Y. Loss of extracellular superoxide dismutase induces severe IL-23-mediated skin inflammation in mice. J. Investig. Dermatol. 2013, 133, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zachariae, E.D.; Larsen, U.G.; Vilhardt, F.; Petersen, S.V. The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response. Redox Biol. 2019, 26, 101268. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, B.; Mello, F.K.; Mallmann, M.P.; da Costa Sobral, K.G.; Fighera, M.R.; Royes, L.F.F.; Furian, A.F.; Sampaio, T.B.; Oliveira, M.S. Beneficial Effects of Rosmarinic Acid In Vitro and In Vivo Models of Epileptiform Activity Induced by Pilocarpine. Brain Sci. 2023, 13, 289. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef] [PubMed]
- Bedner, P.; Steinhäuser, C. Role of Impaired Astrocyte Gap Junction Coupling in Epileptogenesis. Cells 2023, 12, 1669. [Google Scholar] [CrossRef]
- Zappalà, A.; Vicario, N.; Calabrese, G.; Turnaturi, R.; Pasquinucci, L.; Montenegro, L.; Spadaro, A.; Parenti, R.; Parenti, C. Neuroprotective effects of Rosmarinus officinalis L. extract in oxygen glucose deprivation (OGD)-injured human neural-like cells. Nat. Prod. Res. 2021, 35, 669–675. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashdi, J.; Albasher, G.; Alanazi, M.M.; Al-Qahtani, W.S.; Alanezi, A.A.; Alasmari, F. Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy. Toxics 2023, 11, 826. https://doi.org/10.3390/toxics11100826
Alrashdi J, Albasher G, Alanazi MM, Al-Qahtani WS, Alanezi AA, Alasmari F. Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy. Toxics. 2023; 11(10):826. https://doi.org/10.3390/toxics11100826
Chicago/Turabian StyleAlrashdi, Jawaher, Gadah Albasher, Mohammed M. Alanazi, Wedad Saeed Al-Qahtani, Abdulkareem A. Alanezi, and Fawaz Alasmari. 2023. "Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy" Toxics 11, no. 10: 826. https://doi.org/10.3390/toxics11100826
APA StyleAlrashdi, J., Albasher, G., Alanazi, M. M., Al-Qahtani, W. S., Alanezi, A. A., & Alasmari, F. (2023). Effects of Rosmarinus officinalis L. Extract on Neurobehavioral and Neurobiological Changes in Male Rats with Pentylenetetrazol-Induced Epilepsy. Toxics, 11(10), 826. https://doi.org/10.3390/toxics11100826






