Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure Conditions and Cell Models
2.2. Cellular Uptake of nPS/mPS
2.3. Viability Assays
2.4. Assessment of the Cellular Acidic Compartment
2.5. Evaluation of ROS Production
2.6. Mitochondrial Transmebrane Potential
2.7. Assessment of DNA Damage by the Comet Assay
2.8. Statistical Analyses
3. Results
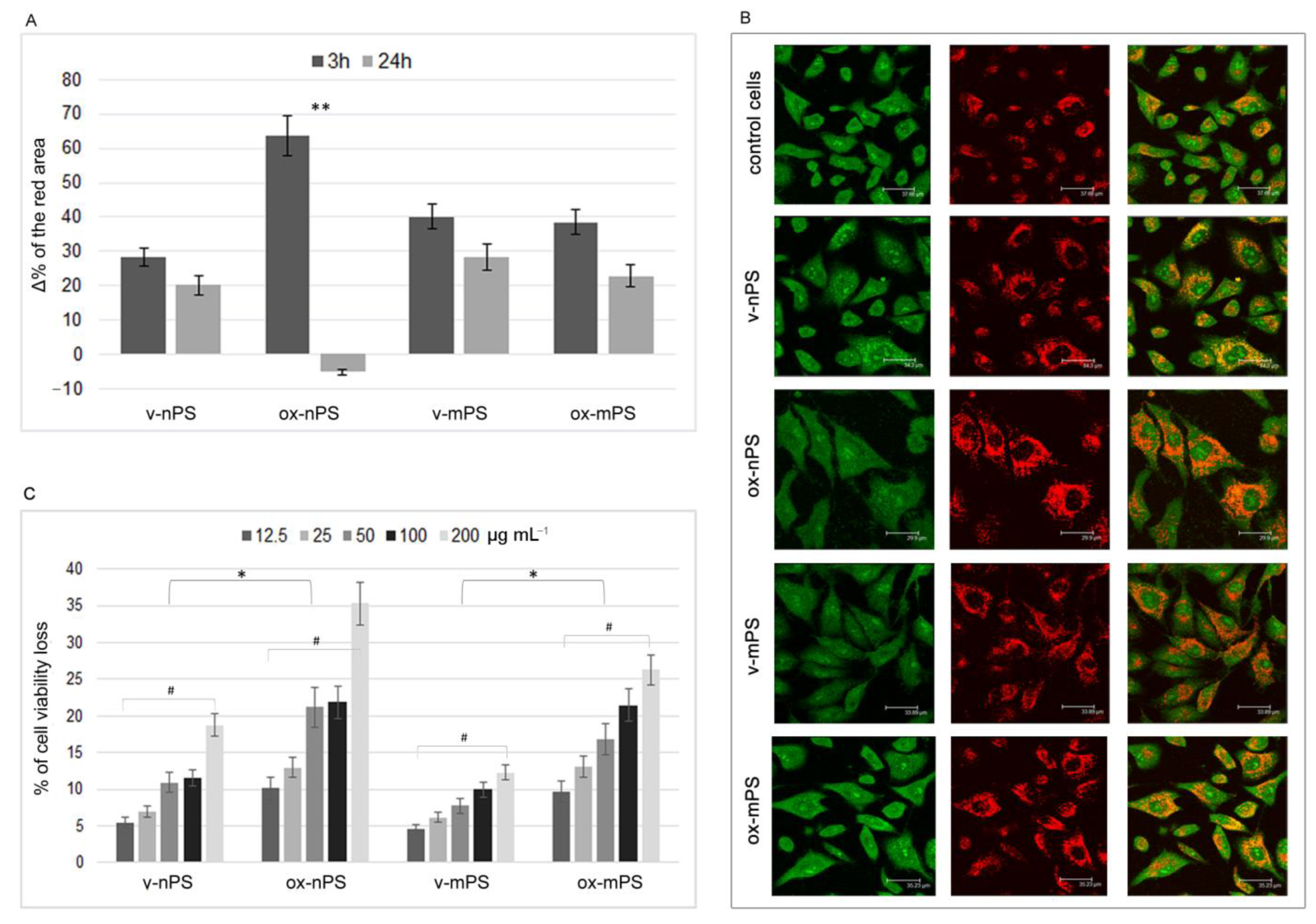
3.1. Cellular Uptakes of nPS/mPS
3.2. nPS/mPS-Induced Changes of the Acidic Compartment
3.3. Cytotoxicity nPS/mPS-Induced
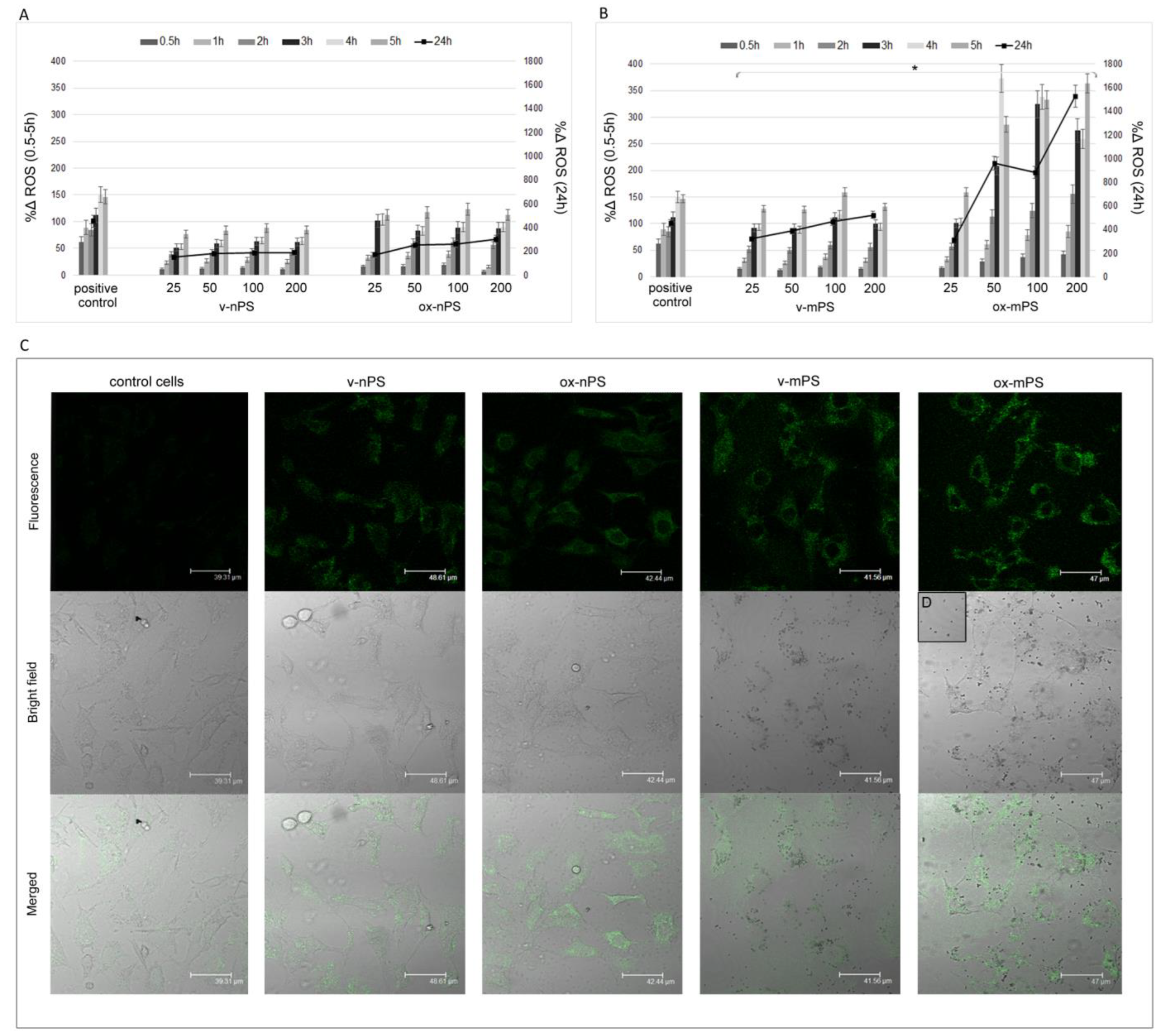
3.4. nPS/mPS Increased ROS Production
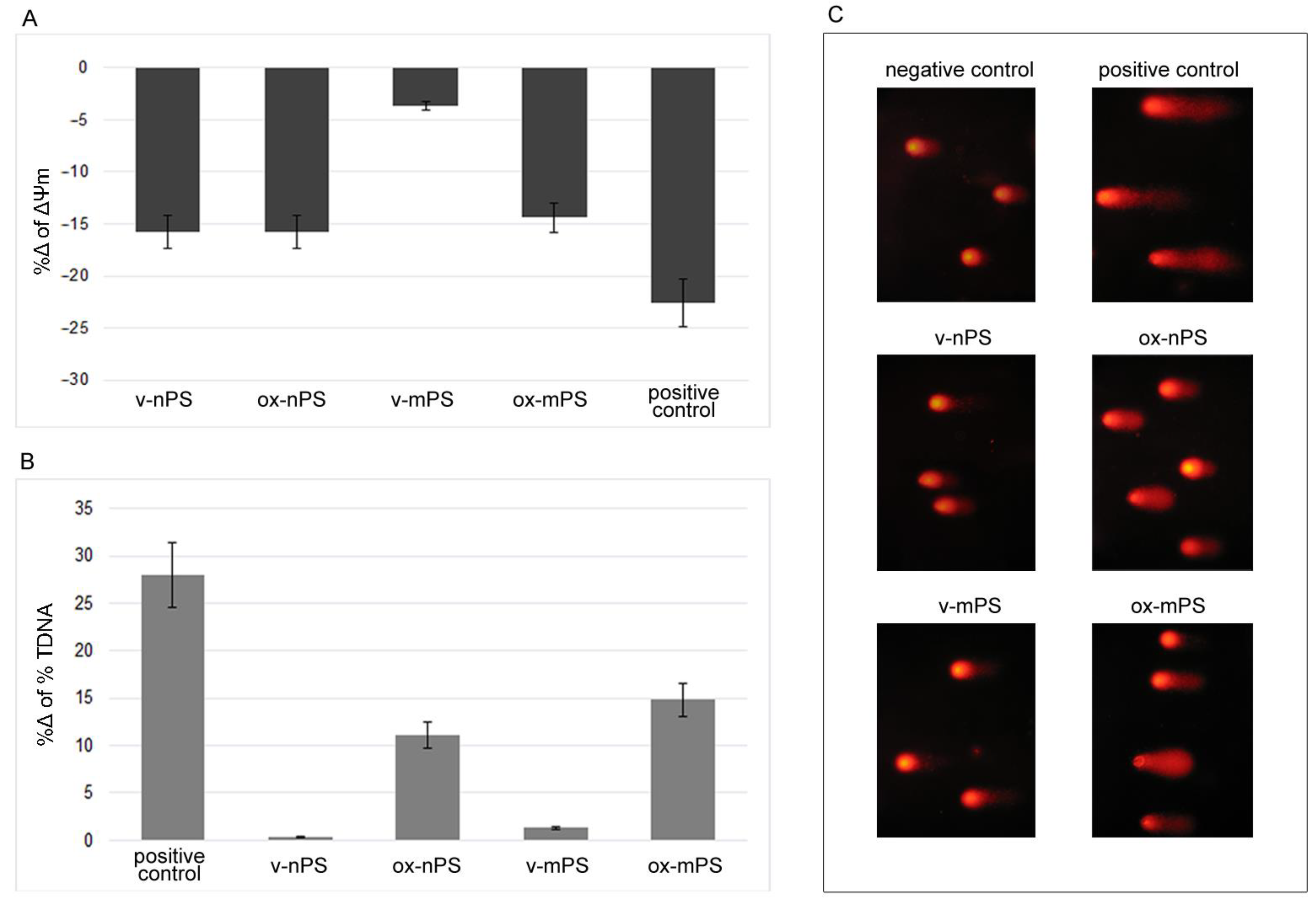
3.5. nPS/mPS Induced Mitochondrial Dysfunction
3.6. nPS/mPS-Induced DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar]
- Domínguez-Jaimes, L.P.; Cedillo-González, E.I.; Luévano-Hipólito, E.; Acuña-Bedoya, J.D.; Hernández-López, J.M. Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar]
- Prata, J.C.; Da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar]
- Najahi, H.; Alessio, N.; Squillaro, T.; Conti, G.O.; Ferrante, M.; Di Bernardo, G.; Galderisi, U.; Messaoudi, I.; Minucci, S.; Banni, M. Environmental microplastics (EMPs) exposure alter the differentiation potential of mesenchymal stromal cells. Environ. Res. 2022, 214, 114088. [Google Scholar]
- Li, Y.; Lu, Z.; Zheng, H.; Wang, J.; Chen, C. Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary, China. Environ. Sci. Eur. 2020, 32, 15. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Soto Villegas, C.; Macay, K.C.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2021, 11, 6424. [Google Scholar]
- Pastorino, P.; Prearo, M.; Pizzul, E.; Elia, A.C.; Renzi, M.; Ginebreda, A.; Barceló, D. High-mountain lakes as indicators of microplastic pollution: Current and future perspectives. Water Emerg. Contam. Nanoplast. 2022, 1, 3. [Google Scholar]
- Facciolà, A.; Visalli, G.; Pruiti Ciarello, M.; Di Pietro, A. Newly Emerging Airborne Pollutants: Current Knowledge of Health Impact of Micro and Nanoplastics. Int. J. Environ. Res. Public Health 2021, 18, 2997. [Google Scholar]
- Lim, X. Microplastics are everywhere—But are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef]
- Saley, A.M.; Smart, A.C.; Bezerra, M.F.; Burnham, T.L.U.; Capece, L.R.; Lima, L.F.O.; Carsh, A.C.; Williams, S.L.; Morgan, S.G. Microplastic accumulation and biomagnification in a coastal marine reserve situated in a sparsely populated area. Mar. Pollut. Bull. 2019, 146, 54–59. [Google Scholar]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Shafea, L.; Verla, A.W.; Verla, E.N.; Qingyue, W.; Chowdhury, T.; Paredes, M. Microplastics Exposure Routes and Toxicity Studies to Ecosystems: An Overview. Environ. Anal. Health Toxicol. 2020, 35, e2020004. [Google Scholar]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 μm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar]
- Zarus, G.M.; Muianga, C.; Hunter, C.M.; Pappas, R.S. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021, 756, 144010. [Google Scholar]
- Bredeck, G.; Halamoda-Kenzaoui, B.; Bogni, A.; Bogni, A.; Lipsa, D.; Bremer-Hoffmann, S. Tiered testing of micro- and nanoplastics using intestinal in vitro models to support hazard assessments. Environ. Int. 2022, 158, 106921. [Google Scholar]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar]
- Ibrahim, Y.S.; Tuan Anuar, S.; Azmi, A.A.; Wan Mohd Khalik, W.M.A.; Lehata, S.; Hamzah, S.R.; Ismail, D.; Ma, Z.F.; Dzulkarnaen, A.; Zakaria, Z.; et al. Detection of microplastics in human colectomy specimens. JGH Open 2020, 5, 116–121. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard Mater. 2021, 416, 126124. [Google Scholar]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar]
- Chen, G.; Feng, Q.; Wang, J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020, 703, 135504. [Google Scholar] [CrossRef]
- Liao, Z.; Ji, X.; Ma, Y.; Lv, B.; Huang, W.; Zhu, X.; Fang, M.; Wang, Q.; Wang, X.; Dahlgren, R.; et al. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007. [Google Scholar] [CrossRef]
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nat. Commun. 2020, 11, 3381. [Google Scholar] [CrossRef]
- Xumiao, L.; Prata, J.C.; Alves, J.R.; Duarte, A.C.; Rocha-Santos, T.; Cerqueira, M. Airborne microplastics and fibers in indoor residential environments in Aveiro, Portugal. Environ. Adv. 2021, 6, 100134. [Google Scholar] [CrossRef]
- Visalli, G.; Currò, M.; Iannazzo, D.; Pistone, A.; Pruiti Ciarello, M.; Acri, G.; Testagrossa, B.; Bertuccio, M.P.; Squeri, R.; Di Pietro, A. In vitro assessment of neurotoxicity and neuroinflammation of homemade MWCNTs. Environ. Toxicol. Pharmacol. 2017, 56, 121–128. [Google Scholar]
- Facciolà, A.; Visalli, G.; La Maestra, S.; Ceccarelli, M.; D’Aleo, F.; Nunnari, G.; Pellicanò, G.F.; Di Pietro, A. Carbon nanotubes and central nervous system: Environmental risks, toxicological aspects and future perspectives. Environ. Toxicol. Pharmacol. 2019, 65, 23–30. [Google Scholar]
- Visalli, G.; Facciolà, A.; Currò, M.; Laganà, P.; La Fauci, V.; Iannazzo, D.; Pistone, A.; Di Pietro, A. Mitochondrial Impairment Induced by Sub-Chronic Exposure to Multi-Walled Carbon Nanotubes. Int. J. Environ. Res. Public Health 2019, 16, 792. [Google Scholar]
- Puisney, C.; Baeza-Squiban, A.; Boland, S. Mechanisms of Uptake and Translocation of Nanomaterials in the Lung. Adv. Exp. Med. Biol. 2018, 1048, 21–36. [Google Scholar]
- Trovato, M.C.; Andronico, D.; Sciacchitano, S.; Ruggeri, R.M.; Picerno, I.; Di Pietro, A.; Visalli, G. Nanostructures: Between natural environment and medical practice. Rev. Environ. Health 2018, 33, 295–307. [Google Scholar]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Jeyachandran, Y.L.; Mielczarski, E.; Rai, B. Modification of polystyrene surface in aqueous solutions. J. Colloid Interface Sci. 2011, 362, 532–539. [Google Scholar]
- Visalli, G.; Laganà, A.; Facciolà, A.; Iaconis, A.; Curcio, J.; Pollino, S.; Celesti, C.; Scalese, S.; Libertino, S.; Iannazzo, D.; et al. Enhancement of biological effects of oxidised nano- and microplastics in human professional phagocytes. Environ. Toxicol. Pharmacol. 2023, 99, 104086. [Google Scholar] [CrossRef]
- Ter Liu, N.; Tang, M.; Ding, J. The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere 2020, 245, 125624. [Google Scholar]
- Visalli, G.; Bertuccio, M.P.; Iannazzo, D.; Piperno, A.; Pistone, A.; Di Pietro, A. Toxicological assessment of multi-walled carbon nanotubes on A549 human lung epithelial cells. Toxicol. In Vitro 2015, 29, 352–362. [Google Scholar] [CrossRef]
- Micale, R.T.; La Maestra, S.; Di Pietro, A.; Visalli, G.; Baluce, B.; Balansky, R.; Steele, V.E.; De Flora, S. Oxidative stress in the lung of mice exposed to cigarette smoke either early in life or in adulthood. Arch. Toxicol. 2013, 87, 915–918. [Google Scholar] [CrossRef]
- Di Pietro, A.; Baluce, B.; Visalli, G.; La Maestra, S.; Micale, R.; Izzotti, A. Ex vivo study for the assessment of behavioral factor and gene polymorphisms in individual susceptibility to oxidative DNA damage metals-induced. Int. J. Hygiene Environ. Health 2011, 214, 210–218. [Google Scholar]
- Visalli, G.; Baluce, B.; La Maestra, S.; Micale, R.T.; Cingano, L.; De Flora, S.; Di Pietro, A. Genotoxic damage in the oral mucosal cells of subjects carrying restorative dental fillings. Arch. Toxicol. 2013, 87, 2247–2248. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Zhao, J.; Cao, Z.; Zou, W.; Zhou, Q. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int. 2022, 158, 106922. [Google Scholar] [CrossRef]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Vinciguerra, V.; Castelvetro, V.; Modugno, F.A. Systematic Study on the Degradation Products Generated from Artificially Aged Microplastics. Polymers 2021, 13, 1997. [Google Scholar]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: Combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ. Int. 2020, 145, 106137. [Google Scholar]
- Shi, X.; Chen, Z.; Liu, X.; Wei, W.; Ni, B.J. The photochemical behaviors of microplastics through the lens of reactive oxygen species: Photolysis mechanisms and enhancing photo-transformation of pollutants. Sci. Total Environ. 2022, 846, 157498. [Google Scholar]
- Völkl, M.; Jérôme, V.; Weig, A.; Jasinski, J.; Meides, N.; Strohriegl, P.; Scheibel, T.; Freitag, R. Pristine and artificially-aged polystyrene microplastic particles differ in regard to cellular response. J. Hazard. Mater. 2022, 435, 128955. [Google Scholar] [CrossRef]
- Yu, X.; Lang, M.; Huang, D.; Yang, C.; Ouyang, Z.; Guo, X. Photo-transformation of microplastics and its toxicity to Caco-2 cells. Sci. Total Environ. 2022, 806, 150954. [Google Scholar]
- Visalli, G.; Facciolà, A.; Iannazzo, D.; Piperno, A.; Pistone, A.; Di Pietro, A. The role of the iron catalyst in the toxicity of multi-walled carbon nanotubes (MWCNTs). J. Trace Elem. Med. Biol. 2017, 43, 153–160. [Google Scholar]
- Katayama, K.; Nomura, H.; Ogataa, H.; Eitoku, T. Diffusion coefficients for nanoparticles under flow and stop-flow conditions. Phys. Chem. Chem. Phys. 2009, 11, 10494–10499. [Google Scholar]
- Varma, S.; Dey, S.; Palanisamy, D. Cellular Uptake Pathways of Nanoparticles: Process of Endocytosis and Factors Affecting their Fate. Curr. Pharm. Biotechnol. 2022, 23, 679–706. [Google Scholar] [PubMed]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene micro and nano-particles induce metabolic rewiring in normal human colon cells: A risk factor for human health. Chemosphere 2022, 303, 134947. [Google Scholar] [PubMed]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [PubMed]
- Visalli, G.; Baluce, B.; Bertuccio, M.; Picerno, I.; Di Pietro, A. Mitochondrial-mediated apoptosis pathway in alveolar epithelial cells exposed to the metals in combustion-generated particulate matter. J. Toxicol. Environ. Health A 2015, 78, 697–709. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laganà, A.; Visalli, G.; Facciolà, A.; Celesti, C.; Iannazzo, D.; Di Pietro, A. Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells. Toxics 2023, 11, 686. https://doi.org/10.3390/toxics11080686
Laganà A, Visalli G, Facciolà A, Celesti C, Iannazzo D, Di Pietro A. Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells. Toxics. 2023; 11(8):686. https://doi.org/10.3390/toxics11080686
Chicago/Turabian StyleLaganà, Antonio, Giuseppa Visalli, Alessio Facciolà, Consuelo Celesti, Daniela Iannazzo, and Angela Di Pietro. 2023. "Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells" Toxics 11, no. 8: 686. https://doi.org/10.3390/toxics11080686
APA StyleLaganà, A., Visalli, G., Facciolà, A., Celesti, C., Iannazzo, D., & Di Pietro, A. (2023). Uptake of Breathable Nano- and Micro-Sized Polystyrene Particles: Comparison of Virgin and Oxidised nPS/mPS in Human Alveolar Cells. Toxics, 11(8), 686. https://doi.org/10.3390/toxics11080686







