Melatonin Ameliorates Apoptosis of A549 Cells Exposed to Chicken House PM2.5: A Novel Insight in Poultry Production
Abstract
1. Introduction
2. Materials and Methods
2.1. PM2.5 Sampling and Extraction
2.2. Cell Culture and Treatment
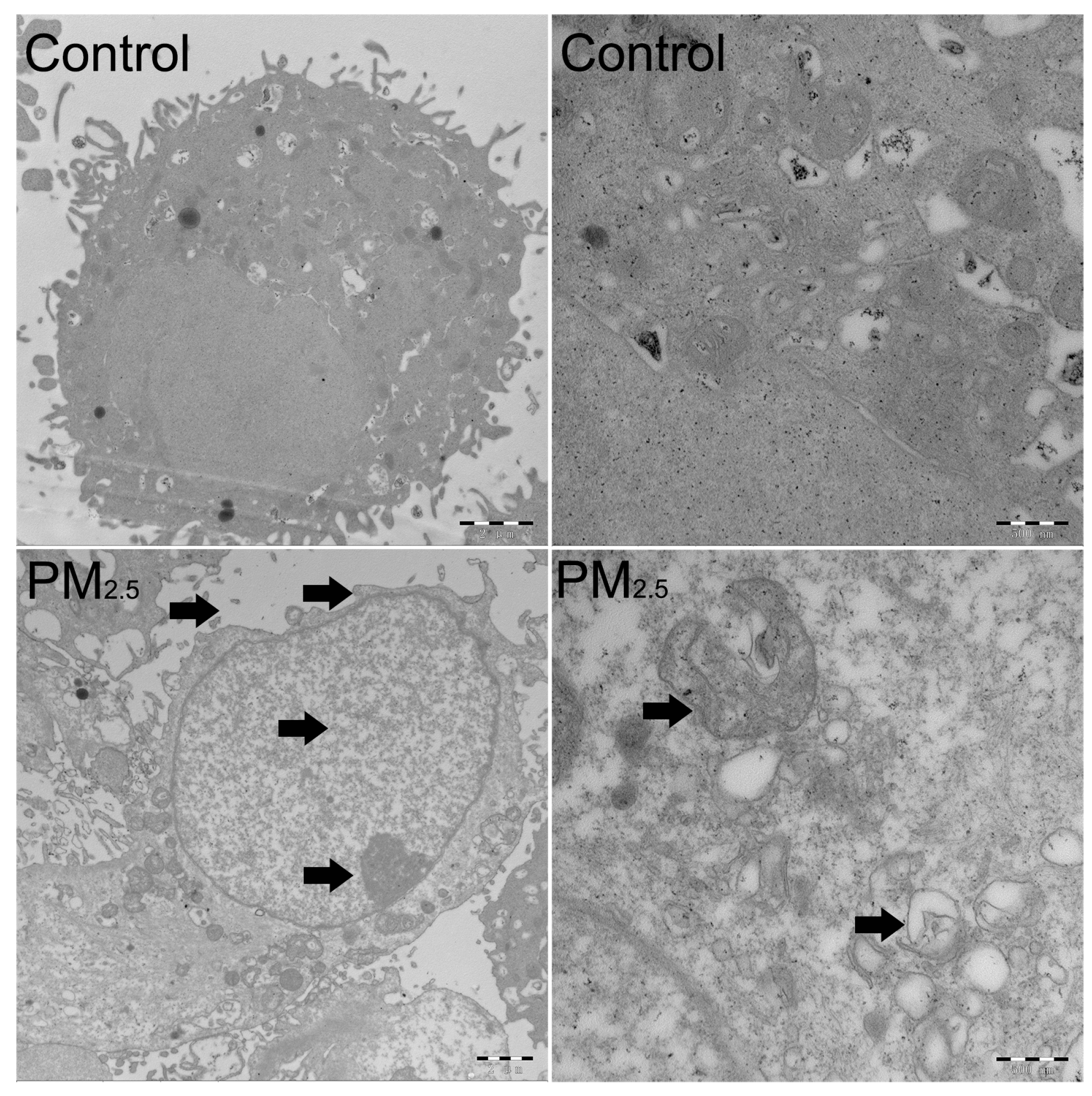
2.3. The Detection of the Cell Cycle, Cell Apoptosis, and Transmission Electron Microscopy (TEM)
2.4. Real-Time PCR and Western Blot Assays
2.5. The Detection of Cell Viability
2.6. The Detection of T-SOD, CAT, and MDA
2.7. Statistical Analysis
3. Results
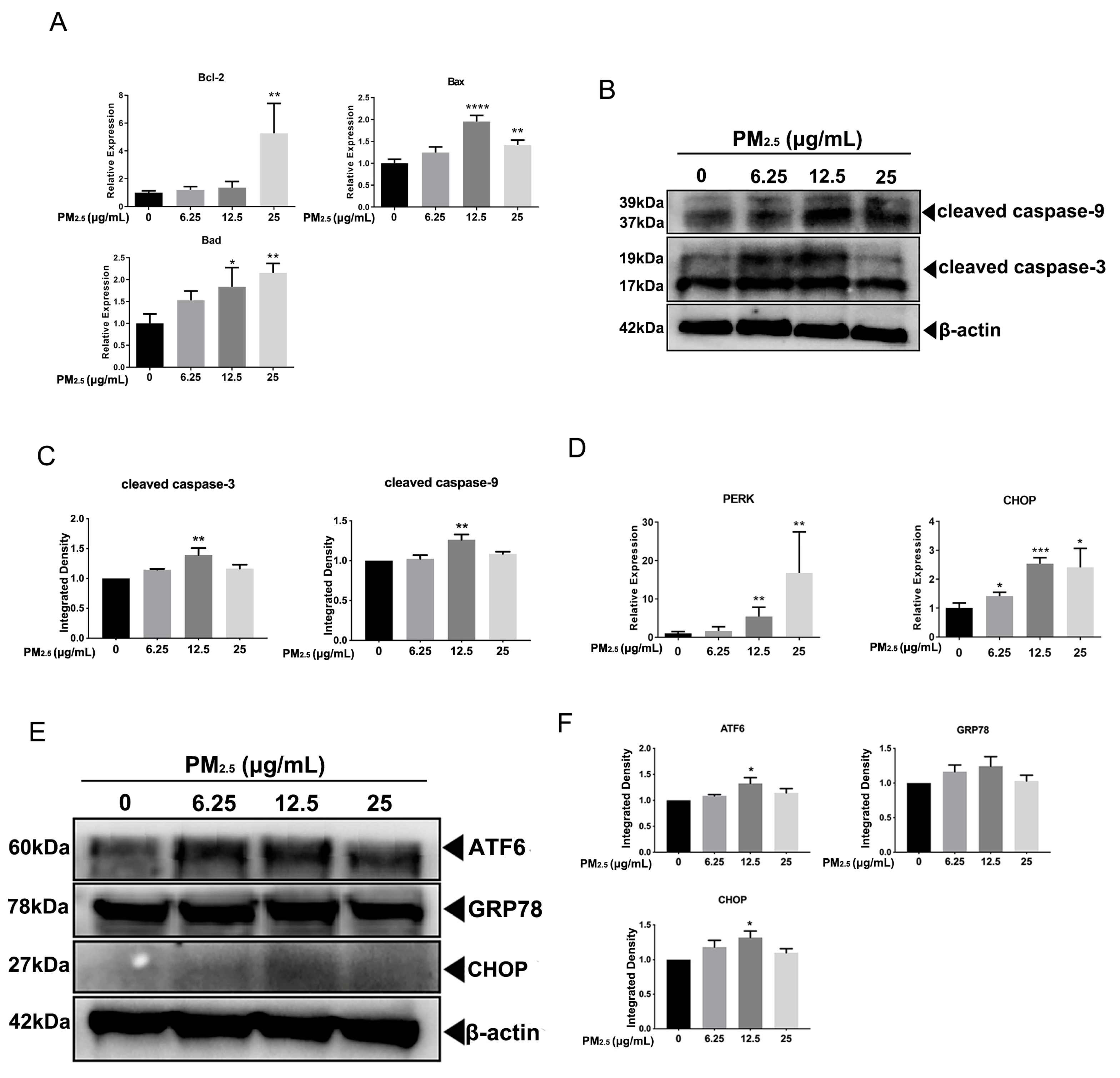
3.1. The Effects of PM2.5 on the Cell Cycle Distribution and Cell Apoptosis of A549 Cells
3.2. PM2.5 Caused ERS in A549 Cells
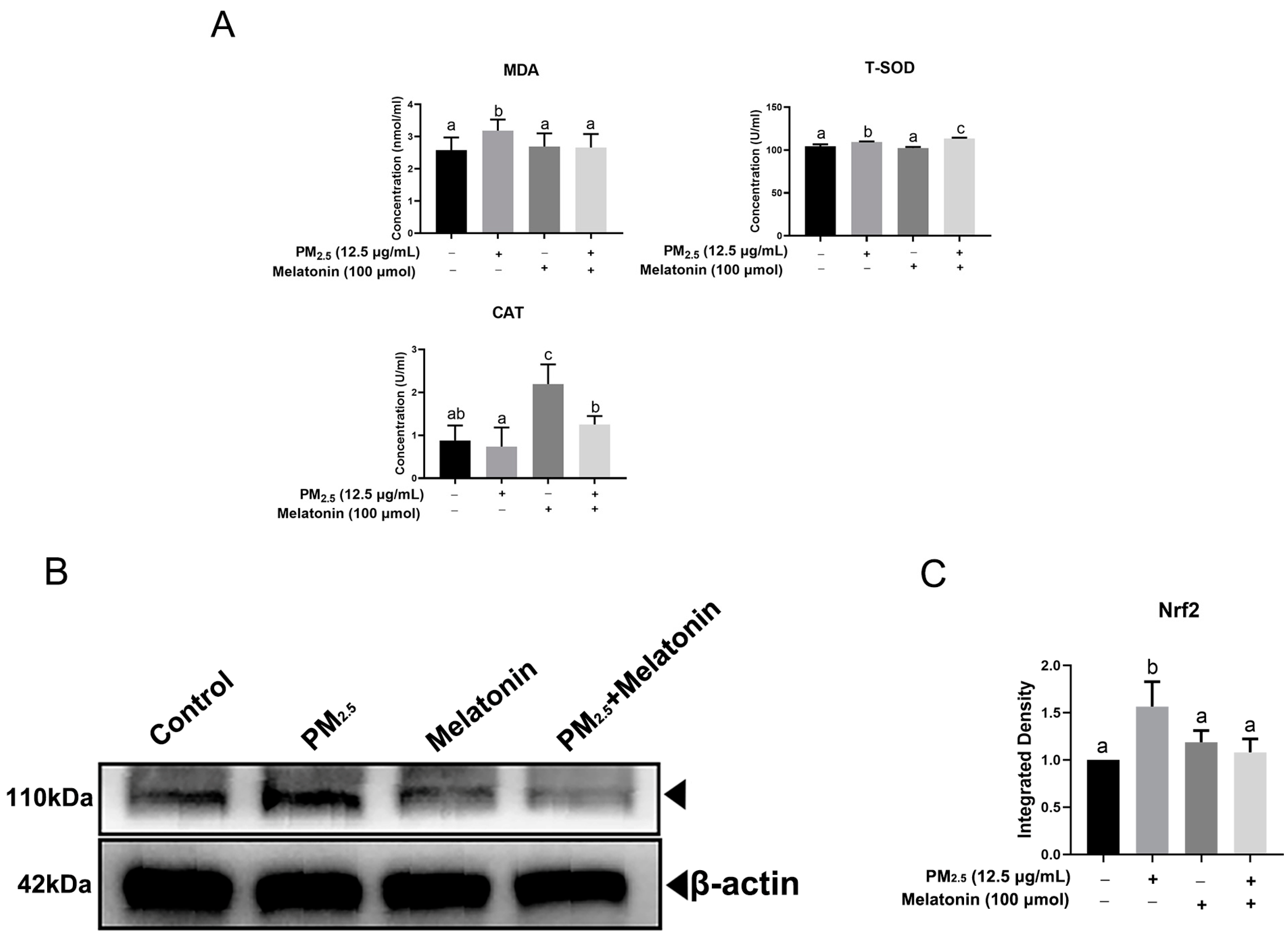
3.3. Melatonin Alleviated Apoptosis and Improved Cell Antioxidation after Exposure to PM2.5 from Chicken Houses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siah, K.T.; Wong, R.K.; Ho, K.Y. Melatonin for the treatment of irritable bowel syndrome. World J. Gastroentero. 2014, 20, 2492–2498. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Feng, Y.T.; Yan, J.J.; Fan, C.X.; Jiang, S.A.; Qu, Y. A review of melatonin in hepatic ischemia/reperfusion injury and clinical liver disease. Ann. Med. 2014, 46, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Chen, G.; Li, J.R.; Qian, C.; Mo, H.B.; Gu, C.; Yan, F.; Yan, W.; Wang, L. Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: A possible role for the regulation of pro-inflammatory cytokines. J. Pineal Res. 2015, 57, 340–347. [Google Scholar] [CrossRef]
- Ko, J.; Ryu, J.E.; Noh, S.W.; Choi, H.K. Melatonin Treatment Enhances the Growth and Productivity of Useful Metabolites in the In Vitro Culture of Spirodela polyrhiza. J. Agric. Food Chem. 2023, 71, 1748–1757. [Google Scholar] [CrossRef]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; Jiajia, L.; Yang, L. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial Effects of Melatonin on the In Vitro Maturation of Sheep Oocytes and Its Relation to Melatonin Receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Yang, M.H.; Zhu, K.F.; Wang, L.; Song, Y.K.; Wang, J.; Qin, W.; Xu, Z.; Chen, Y.; Liu, G. Melatonin implantation improved the egg-laying rate and quality in hens past their peak egg-laying age. Sci. Rep. 2016, 6, 39799. [Google Scholar] [CrossRef]
- Fagan, A.B.; Kennaway, D.J.; Oakley, A.P. Pinealectomy in the chicken: A good model of scoliosis? Eur. Spine J. 2009, 18, 1154–1159. [Google Scholar] [CrossRef]
- Taylor, A.C.; Horvat-Gordon, M.; Moore, A.; Bartell, P.A. The effects of melatonin on the physical properties of bones and egg shells in the laying hen. PLoS ONE 2013, 8, e55663. [Google Scholar] [CrossRef]
- Akbarian, A.; Kazerani, H.R.; Mohri, M.; Raji, A.R.; Jamshidi, A.; Golian, A. Exogenous melatonin improves growth performance, intestinal microbiota, and morphology in temporarily feed restricted broilers. Livest. Sci. 2014, 167, 400–407. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Liebana, R.; Herrera, T.; Rebollo-Hernanz, M.; Sanchez-Puelles, C.; Benitez, V.; Martín-Cabrejas, M.A. Effect of illumination on the content of melatonin, phenolic compounds, and antioxidant activity during germination of lentils (Lens culinaris L.) and kidney beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2014, 62, 10736–10743. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.Z.; Khatoon, R.; Talat, F.; Alam, M.M.; Tabassum, H.; Parvez, S. Melatonin Mitigates Rotenone-Induced Oxidative Stress and Mitochondrial Dysfunction in the Drosophila melanogaster Model of Parkinson’s Disease-like Symptoms. ACS Omega 2023, 8, 7279–7288. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yan, P.; Jing, Q.; Wei, X.; Liu, R.; Shi, T.; Wu, B. Effects of different padding on air quality in broiler house and growth physiological index of broilers. Agric. Sci. Technol. 2015, 16, 2764. [Google Scholar]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM2.5 from a broiler breeding production system: The characteristics and microbial community analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef]
- Cambra-Lopez, M.; Aarnink, A.J.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne particulate matter from livestock production systems: A review of an air pollution problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef]
- Lai, H.T.; Nieuwland, M.G.; Aarnink, A.J.; Kemp, B.; Parmentier, H.K. Effects of 2 size classes of intratracheally administered airborne dust particles on primary and secondary specific antibody responses and body weight gain of broilers: A pilot study on the effects of naturally occurring dust. Poult. Sci. 2012, 91, 604–615. [Google Scholar] [CrossRef]
- Almuhanna, E.; Ahmed, A.; Al-Yousif, Y. Effect of air contaminants on poultry immunological and production performance. Int. J. Poult. Sci. 2011, 10, 461–470. [Google Scholar] [CrossRef]
- Wood, D.J.; Van Heyst, B.J. A review of ammonia and particulate matter control strategies for poultry housing. J. Trans. ASABE 2016, 59, 329–344. [Google Scholar]
- Shen, D.; Guo, Z.; Huang, K.; Dai, P.; Jin, X.; Li, Y.; Li, C. Inflammation-associated pulmonary microbiome and metabolome changes in broilers exposed to particulate matter in broiler houses. J. Hazard. Mater. 2022, 421, 126710. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.; Liu, Y.; Du, X.; Zhang, W.; Zhang, J.; Shen, H. Comprehensive pulmonary metabolome responses to intratracheal instillation of airborne fine particulate matter in rats. Sci. Total Environ. 2017, 592, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Hennig, F.; Fuks, K.; Nonnemacher, M.; Jakobs, H.; Mohlenkamp, S.; Erbel, R.; Jöckel, K.-H.; Hoffmann, B. Long-term exposure to fine particulate matter and incidence of type 2 diabetes mellitus in a cohort study: Effects of total and traffic-specific air pollution. Env. Health 2015, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Mills, I.C.; Walton, H.A.; Anderson, H.R. Fine particle components and health—A systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.I.; Phalen, R.F.; Solomon, P.A. Airborne particulate matter and human health: A review. Aerosol. Sci. Technol. 2005, 39, 737–749. [Google Scholar] [CrossRef]
- Huang, X.; Yun, H.; Gong, Z.; Li, X.; He, L.; Zhang, Y.; Hu, M. Source apportionment and secondary organic aerosol estimation of PM2.5 in an urban atmosphere in China. Sci. China Earth Sci. 2014, 57, 1352–1362. [Google Scholar] [CrossRef]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H., Jr. Conventional transmission electron microscopy. Mol. Biol. Cell 2014, 25, 319–323. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Shen, J.; Tang, Q.; Xi, M.; Li, Y.; Li, C. The roles of Nrf2 and autophagy in modulating inflammation mediated by TLR4-NFκB in A549 cell exposed to layer house particulate matter 2.5 (PM2.5). Chemosphere 2019, 235, 1134–1145. [Google Scholar] [CrossRef]
- Li, M.X.; Dewson, G. Mitochondria and apoptosis: Emerging concepts. F1000Prime Rep. 2015, 7, 42. [Google Scholar] [CrossRef]
- Hector, S.; Prehn, J.H.M. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: A review. BBA-Rev. Cancer 2009, 1795, 117–129. [Google Scholar] [CrossRef]
- Han, M.; Li, Y.; Wen, D.; Liu, M.; Ma, Y.; Cong, B. NGAL protects against endotoxin-induced renal tubular cell damage by suppressing apoptosis. BMC Nephrol. 2018, 19, 168. [Google Scholar] [CrossRef]
- Xie, K.H.; Xie, H.M.; Su, G.Q.; Chen, D.W.; Yu, B.; Mao, X.B.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; et al. β-Defensin 129 Attenuates Bacterial Endotoxin-Induced Inflammation and Intestinal Epithelial Cell Apoptosis. Front. Immunol. 2019, 10, 2333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, R.; Dong, W.; Yang, H.; Zhang, L.; Chen, Y.; Wang, W.; Li, C.; Wu, Y.; Ye, Z.; et al. RIPK3 mediates renal tubular epithelial cell apoptosis in endotoxininduced acute kidney injury. Mol. Med. Rep. 2019, 20, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Luchetti, F.; Betti, M.; Canonico, B.; Arcangeletti, M.; Ferri, P.; Galli, F.; Papa, S. ERK MAPK activation mediates the antiapoptotic signaling of melatonin in UVB-stressed U937 cells. Free Radical. Bio. Med. 2009, 46, 339–351. [Google Scholar] [CrossRef]
- Culmsee, C.; Landshamer, S. Molecular insights into mechanisms of the cell death program: Role in the progression of neurodegenerative disorders. Curr. Alzheimer. Res. 2006, 3, 269–283. [Google Scholar] [CrossRef]
- Mattson, M.P.; Culmsee, C.; Yu, Z.F. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000, 301, 173–187. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Bazan, N.G. Survival signalling in Alzheimer’s disease. Biochem. Soc. Trans. 2006, 34 Pt 6, 1277–1282. [Google Scholar] [CrossRef]
- Seung-Yun, C.; Seol-Heui, H. Melatonin attenuates kainic acid-induced hippocampal neurodegeneration and oxidative stress through microglial inhibition. J. Pineal Res. 2010, 34, 95–102. [Google Scholar]
- Zhou, Q.; Gui, S.; Zhou, Q.; Wang, Y. Melatonin inhibits the migration of human lung adenocarcinoma A549 cell lines involving JNK/MAPK pathway. PLoS ONE 2014, 9, e101132. [Google Scholar] [CrossRef]
- Goradel, N.H.; Asghari, M.H.; Moloudizargari, M.; Negandari, B.; Haghi-Aminjan, H.; Abdollahi, M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol. Appl. Pharm. 2017, 335, 56–63. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L. Early metabolism evaluation making traditional Chinese medicine effective and safe therapeutics. J. Zhejiang Univ. Sci. B 2006, 7, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Ghanim, H.; Bandyopadhyay, A.; Korzeniewski, K.; Ling Sia, C.; Dhindsa, S.; Chaudhuri, A. Insulin suppresses endotoxin-induced oxidative, nitrosative, and inflammatory stress in humans. Diabetes Care 2010, 33, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Lin, Z.; Wang, Y.; He, H.; Liu, T.; Kamp, D.W. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 2016, 31, 923–936. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L.L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox. Sign. 2007, 9, 2277–2293. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, C.; Meng, C.J.; Zhu, G.Q.; Sun, X.B.; Huo, L.; Zhang, J.; Liu, H.-X.; He, W.-C.; Shen, X.-M.; et al. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 2012, 53, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.D.; Xu, J.G.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2–ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Di Bella, G.; Mascia, F.; Gualano, L.; Di Bella, L. Melatonin anticancer effects: Review. Int. J. Mol. Sci. 2013, 14, 2410–2430. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Sinclair, M.; Fryer, C.; Phillips, C.J.C. The Benefits of Improving Animal Welfare from the Perspective of Livestock Stakeholders across Asia. Animals 2019, 9, 123. [Google Scholar] [CrossRef]
- Jiang, H.C.; Yang, J.; Fan, Y.L.; Liu, Y.P. Synergistic effects of unripe raspberry extracts (Rubus chingii) and antibiotics against three bacteria. Food Sci. Technol. 2021, 41, 482–488. [Google Scholar] [CrossRef]
- da Silva, R.M.; da Silva, I.D.M.; Estevinho, M.M.; Estevinho, L.M. Anti-bacterial activity of Annona muricata Linnaeus extracts: A systematic review. Food Sci. Technol. 2022, 42, e13021. [Google Scholar] [CrossRef]
- Skwarlo-Sonta, K. Melatonin in immunity: Comparative aspects. J. Neuroendocrinol. Lett. 2002, 23, 61–66. [Google Scholar]
- Liu, Y.; Jia, Y.Q.; Yang, K.N.; Tong, Z.W.; Shi, J.R.; Li, R.C.; Xiao, X.; Ren, W.; Hardeland, R.; Reiter, R.J.; et al. Melatonin overcomes MCR-mediated colistin resistance in Gram-negative pathogens. Theranostics 2020, 10, 10697–10711. [Google Scholar] [CrossRef] [PubMed]
- Calislar, S.; Yeter, B.; Sahin, A. Importance of melatonin on poultry. KSU J. Agric. Nat. 2018, 21, 987–997. [Google Scholar] [CrossRef]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef]




| Gene | The Sequence of the Primers | Gene | The Sequence of the Primers |
|---|---|---|---|
| Bad | F: GATCGGGCTTGGGGTGAGAC R: TCATCTGTCTGCCGGGTCTG | CHOP | F: TTCACCACTCTTGACCCTGC R: TTCCTGCTTGAGCCGTTCAT |
| Bax | F: AGAAGCTGAGCGAGTGTCTC R: CGGAAAAAGACCTCTCGGGG | β-actin | F: GATCTTCATTGTGCTGGGTG R: GGGAAATCGTGCGTGACATT |
| Bcl-2 | F: CTTTGAGTTCGGTGGGGTCA R: GGGCCGTACAGTTCCACAAA | ||
| PERK | F: GCCAATGAGAGAGCAAACGC R: ATCTCGGACATCGCCCATTG | ||
| Component | Concentration (μg/m3) | Component | Concentration (μg/m3) |
|---|---|---|---|
| Organic carbon | 39.65 ± 6.91 | Cl− | 4.79 ± 2.93 |
| Elemental carbon | 11.58 ± 2.50 | NO3- | 30.25 ± 1.33 |
| Na+ | 0.7 ± 0.19 | SO42− | 14.43 ± 2.32 |
| NH4+ | 13.8 ± 0.97 | Mg | 0.12 ± 0.12 |
| K+ | 1.58 ± 0.77 | K | 1.39 ± 0.53 |
| Mg2+ | 0.34 ± 0.04 | Ca | 0.55 ± 0.29 |
| Ca2+ | 3.23 ± 0.18 | Fe | 1.01 ± 0.26 |
| F− | 0.29 ± 0.09 | Endotoxin | 0.3 EU/m3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Shen, J.; Shen, D.; Li, X.; Win-Shwe, T.-T.; Li, C. Melatonin Ameliorates Apoptosis of A549 Cells Exposed to Chicken House PM2.5: A Novel Insight in Poultry Production. Toxics 2023, 11, 562. https://doi.org/10.3390/toxics11070562
Dai P, Shen J, Shen D, Li X, Win-Shwe T-T, Li C. Melatonin Ameliorates Apoptosis of A549 Cells Exposed to Chicken House PM2.5: A Novel Insight in Poultry Production. Toxics. 2023; 11(7):562. https://doi.org/10.3390/toxics11070562
Chicago/Turabian StyleDai, Pengyuan, Jiakun Shen, Dan Shen, Xiaotong Li, Tin-Tin Win-Shwe, and Chunmei Li. 2023. "Melatonin Ameliorates Apoptosis of A549 Cells Exposed to Chicken House PM2.5: A Novel Insight in Poultry Production" Toxics 11, no. 7: 562. https://doi.org/10.3390/toxics11070562
APA StyleDai, P., Shen, J., Shen, D., Li, X., Win-Shwe, T.-T., & Li, C. (2023). Melatonin Ameliorates Apoptosis of A549 Cells Exposed to Chicken House PM2.5: A Novel Insight in Poultry Production. Toxics, 11(7), 562. https://doi.org/10.3390/toxics11070562








