Biogenic Fabrication of Iron Oxide Nanoparticles from Leptolyngbya sp. L-2 and Multiple In Vitro Pharmacogenetic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterium
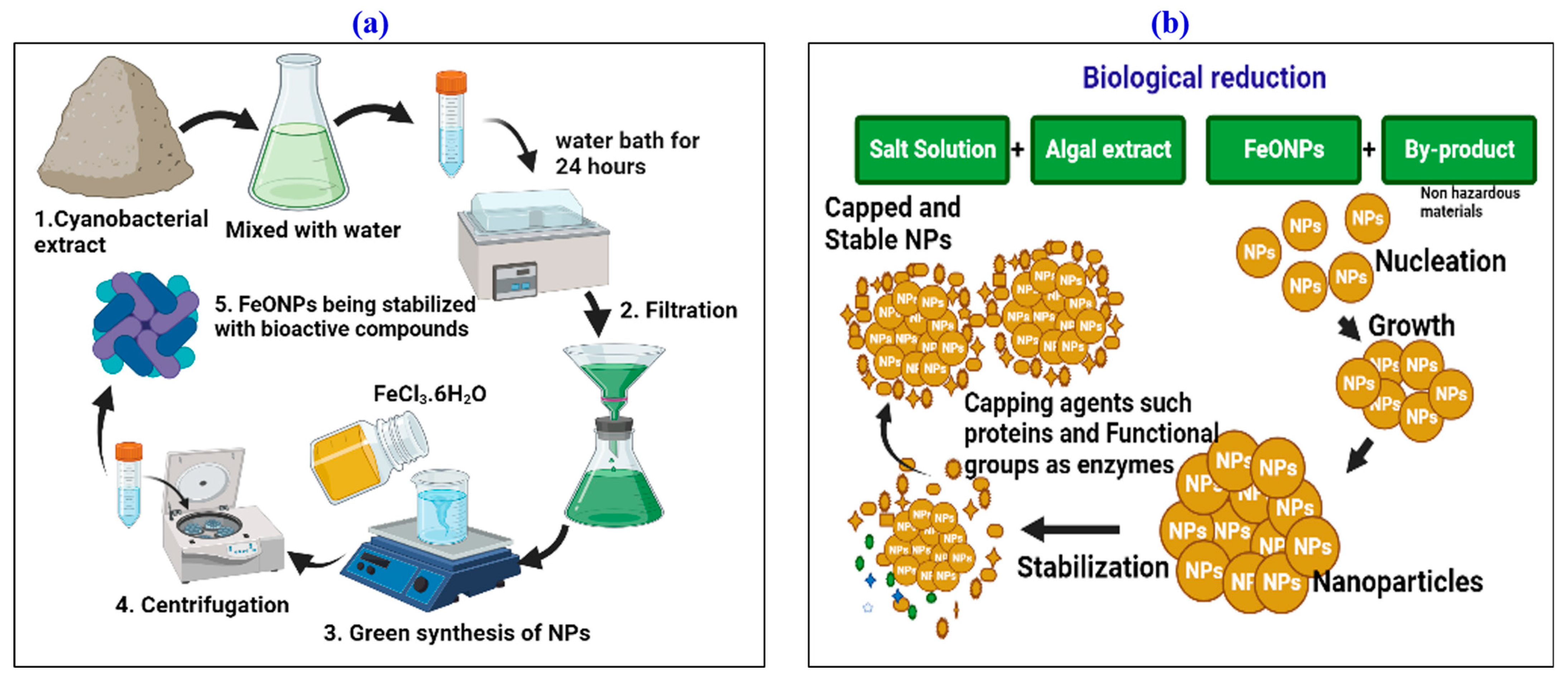
2.2. Biogenic Synthesis of Targeted FeONPs
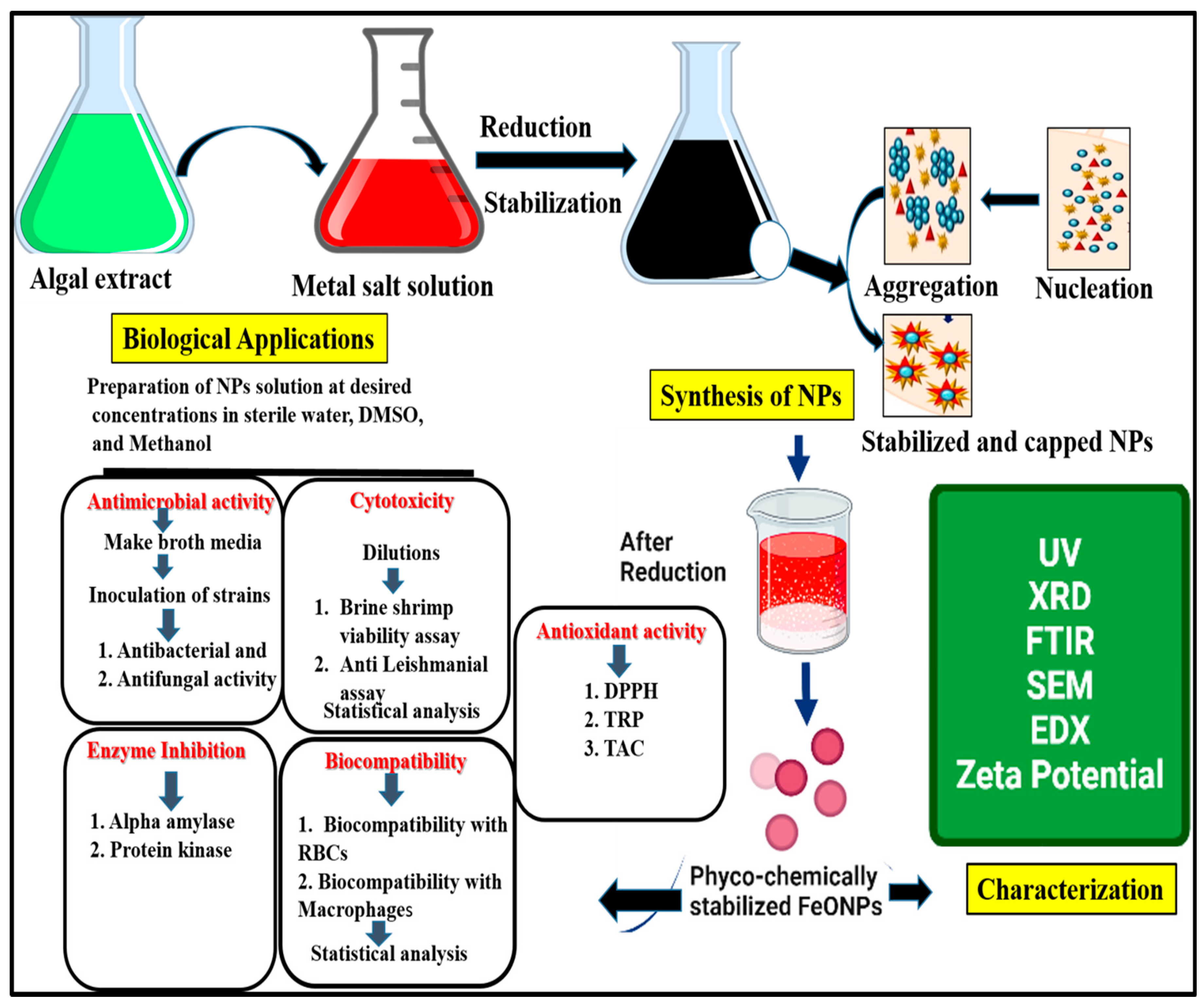
2.3. Physical and Chemical Characterization of FeONPs
2.4. Biomedical Properties of FeONPs
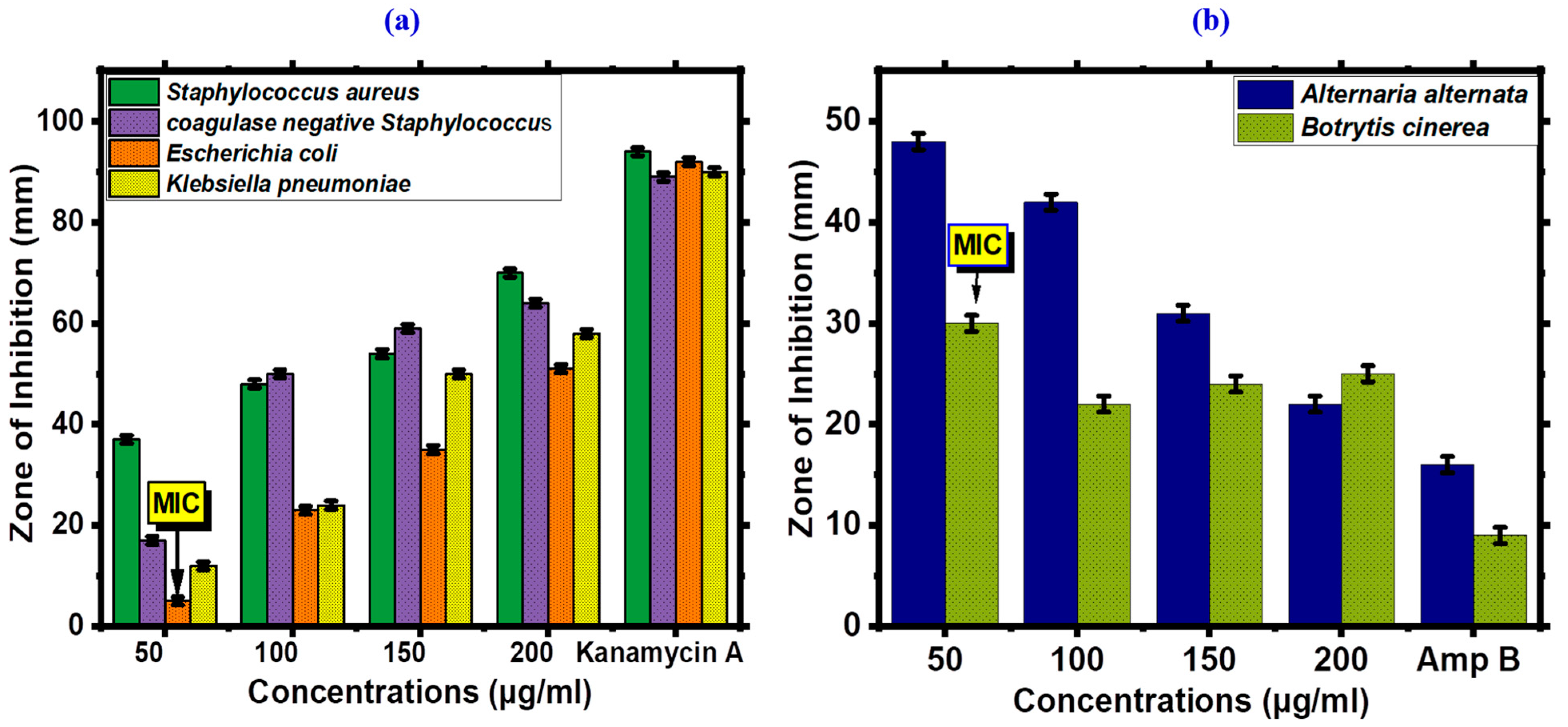
2.4.1. Antibacterial Activity of FeONPs
2.4.2. Antifungal Assay
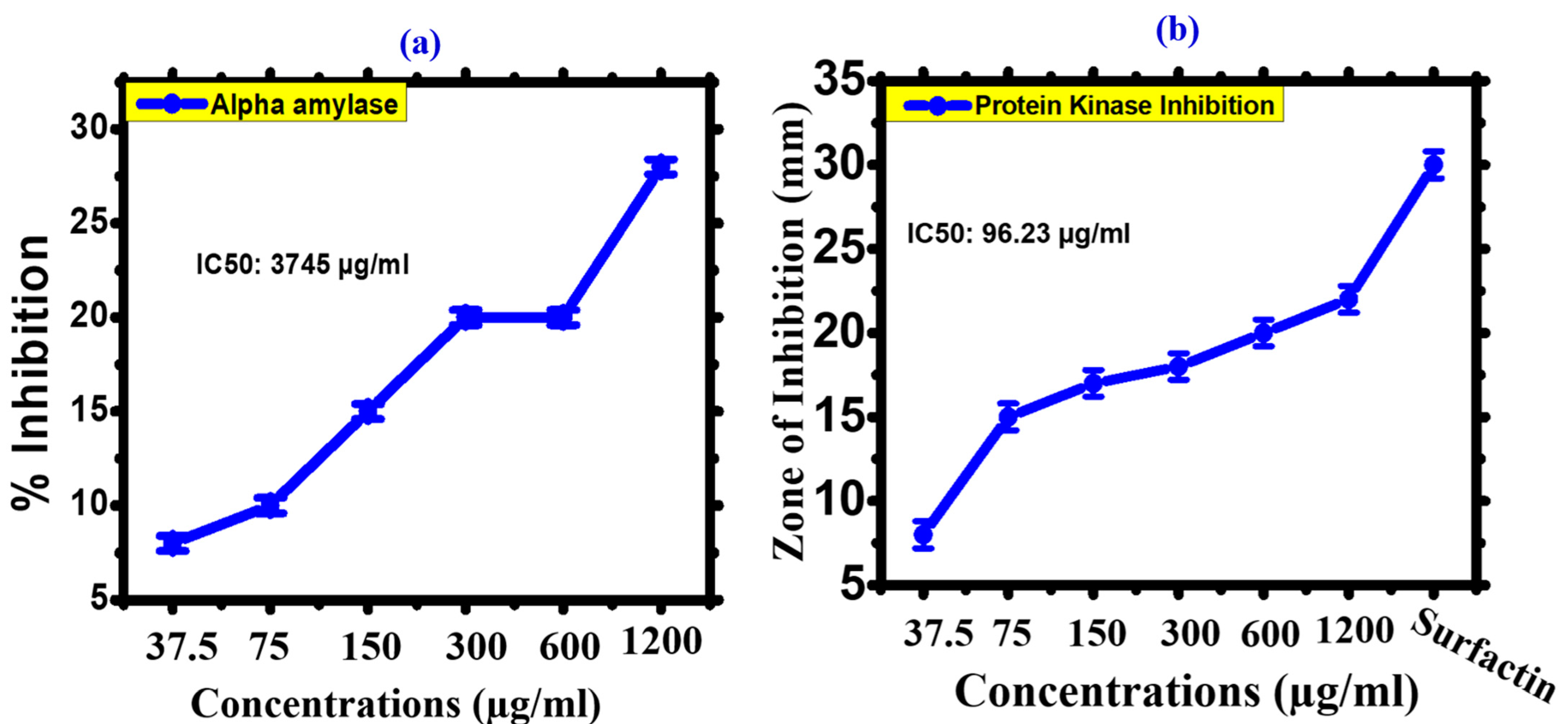
2.4.3. Alpha Amylase Inhibition (α-AI)
2.4.4. Protein Kinase Inhibition (PKI)
2.4.5. Brine Shrimps Cytotoxicity
2.4.6. Antileishmanial Assay
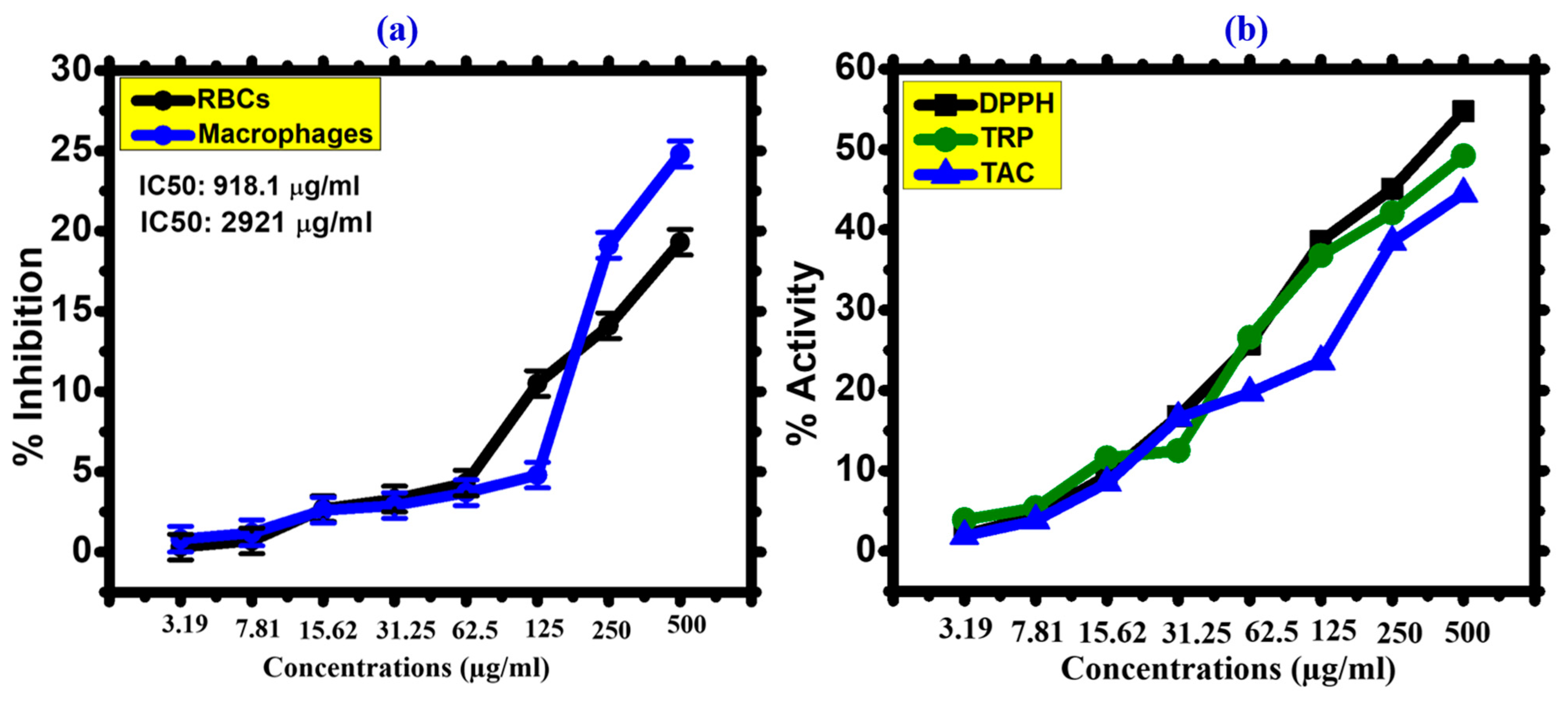
2.4.7. Biocompatibility against Human RBCs
2.4.8. Biocompatibility against Human Macrophages (HMs)
2.4.9. Antioxidant Assay
2.5. Statistical Analysis
3. Results and Discussion
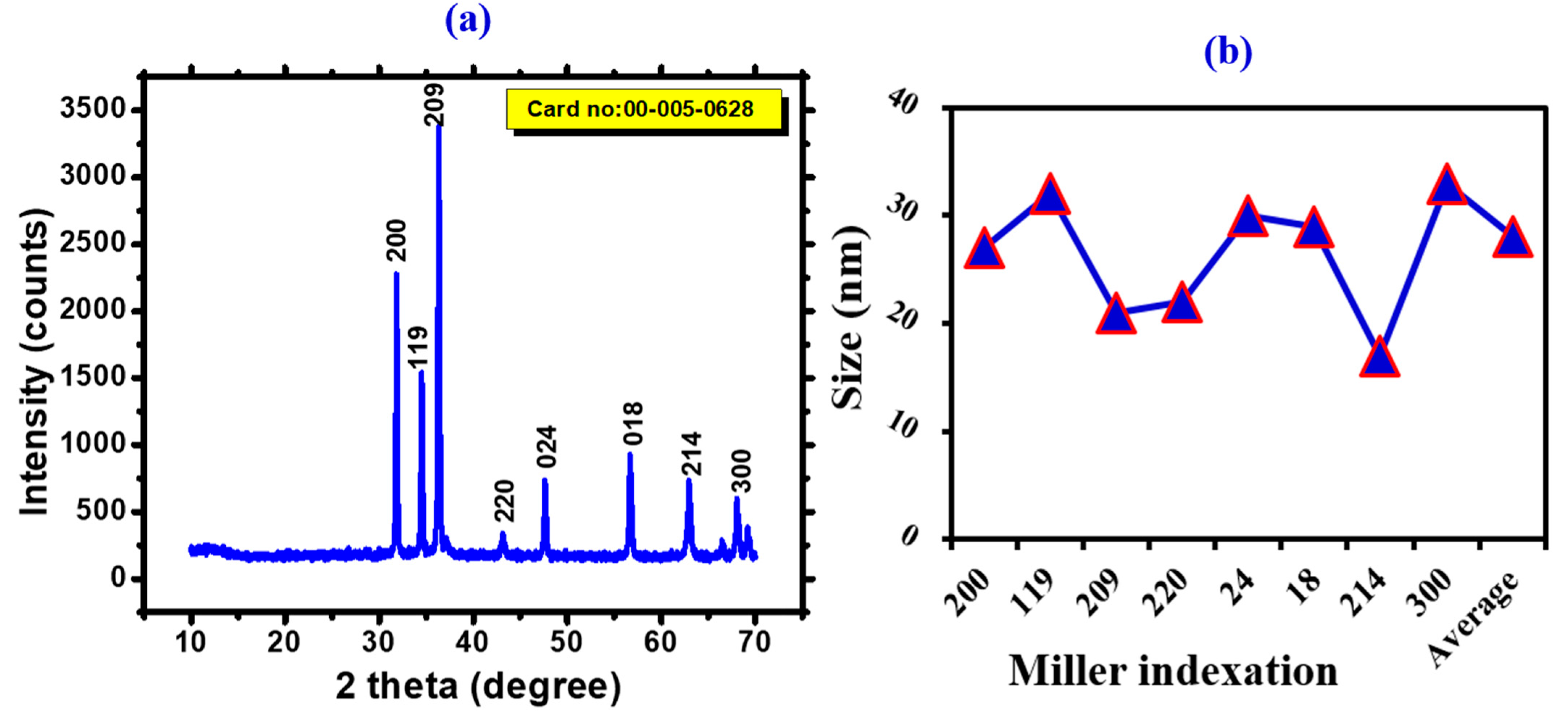
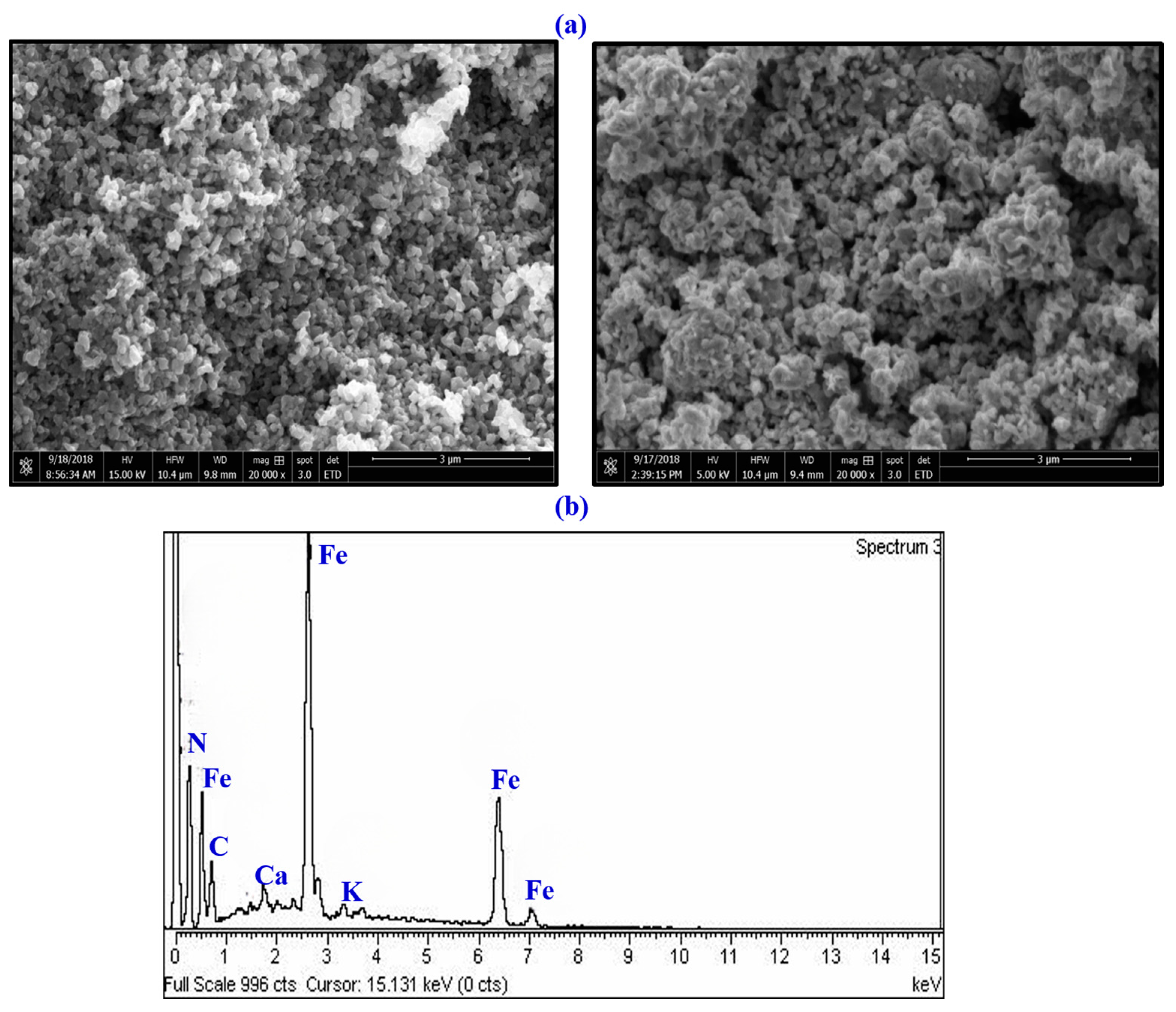
3.1. Physicochemical Properties of FeONPs
3.2. Pharmacogenetic Properties of FeONPs
3.2.1. Antimicrobial Assay
3.2.2. Enzyme Inhibition Potential
3.2.3. Cytotoxicity against Leishmania
3.2.4. Cytotoxicity against Brine Shrimp
3.2.5. Biocompatibility Assays
3.2.6. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, Z.; Taheri-Kafrani, A.; Bahrani, S.A.; Kajani, A.A. PEGAylated graphene oxide/superparamagnetic nanocomposite as a high-efficiency loading nanocarrier for controlled delivery of methotrexate. J. Biotechnol. 2019, 298, 88–97. [Google Scholar] [CrossRef]
- Batool, F.; Iqbal, M.S.; Khan, S.U.; Khan, J.; Ahmed, B.; Qadir, M.I. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci. Rep. 2021, 11, 22132. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Patra, P.; Pradhan, S.; Debnath, N.; Dey, K.K.; Sarkar, S.; Chattopadhyay, D.; Goswami, A. Microwave synthesis of ZnO@ mSiO2 for detailed antifungal mode of action study: Understanding the insights into oxidative stress. J. Colloid Interface Sci. 2015, 444, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yuvakkumar, R.; Suresh, J.; Saravanakumar, B.; Nathanael, A.J.; Hong, S.I.; Rajendran, V. Rambutan peels promoted biomimetic synthesis of bioinspired zinc oxide nanochains for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 250–258. [Google Scholar] [CrossRef]
- Devi, M.; Devi, S.; Sharma, V.; Rana, N.; Bhatia, R.K.; Bhatt, A.K. Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle marmelos and their antimicrobial potential against human bacterial pathogens. J. Tradit. Complement. Med. 2020, 10, 158–165. [Google Scholar] [CrossRef]
- Singh, A.K. A review on plant extract-based route for synthesis of cobalt nanoparticles: Photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100270. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendhi, A. Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Caf, F. Biogenic Synthesis of Iron Oxide Nanoparticle Using Padina Pavonica Extract: Application for Photocatalytic Degradation of Congo Red Dye, Neurotoxicity and Antioxidant Activity. Turk. J. Fish. Aquat. Sci. 2022, 23, TRJFAS21398. [Google Scholar] [CrossRef]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Namvar, F.; Mahdavi, M.; Ahmad, M.B.; Mohamad, R. Biosynthesis of silver nanoparticles using brown marine macroalga, Sargassum muticum aqueous extract. Materials 2013, 6, 5942–5950. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Yuvaraj, D.; Christudhas, L.; Ramesh, K.V. Bio synthesis of iron nanoparticles using the brown seaweed, Dictyota dicotoma. Biotechnol. Indian J. 2016, 12, 112. [Google Scholar]
- El-Kassas, H.Y.; Aly-Eldeen, M.A.; Gharib, S.M. Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: Characterization and application for lead bioremediation. Acta Oceanol. Sin. 2016, 35, 89–98. [Google Scholar] [CrossRef]
- Ercan, G.; Uzunoğlu, D.; Ergüt, M.; Ayla, Ö.Z. Biosynthesis and characterization of iron oxide nanoparticles from Enteromorpha spp. extract: Determination of adsorbent properties for copper (II) ions. Int. Adv. Res. Eng. J. 2019, 3, 65–74. [Google Scholar]
- Sahayaraj, K.; Rajesh, S.; Rathi, J. Silver Nanoparticles Biosynthesis using Marine algae Padina pavonica (linn) and its microbicidal activity. Dig. J. Nanomater. Biostructures 2012, 7, 1557–1567. [Google Scholar]
- Subhashini, G.; Ruban, P.; Daniel, T. Biosynthesis and characterization of magnetic (Fe3O4) iron oxide nanoparticles from a red seaweed gracilaria edulis and its antimicrobial activity. Int. J. Adv. Sci. Res. Manag 2018, 3, 184–189. [Google Scholar]
- Singh, Y.; Khattar, J.I.; Singh, D.P.; Rahi, P.; Gulati, A. Limnology and cyanobacterial diversity of high altitude lakes of Lahaul-Spiti in Himachal Pradesh, India. J. Biosci. 2014, 39, 643–657. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Komárek, J.; Johansen, J.R. Filamentous cyanobacteria, in Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 135–235. [Google Scholar]
- Ai, Y.; Lee, S.; Lee, J. Drinking water treatment residuals from cyanobacteria bloom-affected areas: Investigation of potential impact on agricultural land application. Sci. Total Environ. 2020, 706, 135756. [Google Scholar] [CrossRef]
- Rizzello, L.; Cingolani, R.; Pompa, P.P. Nanotechnology tools for antibacterial materials. Nanomedicine 2013, 8, 807–821. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.A.; Iqbal, J.; Zahra, S.A.; Shahbaz, A.; Kanwal, S.; Rabbani, A.; Mahmood, T. Bioinspired synthesis and activity characterization of iron oxide nanoparticles made using Rhamnus Triquetra leaf extract. Mater. Res. Express 2020, 6, 1250e7. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Singh, D.K.; Pandey, A.; Singh, S.P.; Sinha, R.P. Recent developments in green synthesis of metal nanoparticles utilizing cyanobacterial cell factories. Nanomater. Plants Algae Microorg. 2019, 2, 237–265. [Google Scholar] [CrossRef]
- Banerjee, S.; Banerjee, I.; Dutta, M.; Pal, R. Fabrication of iron nanoparticles using Leptolyngbya valderiana and investigation of its Cr (VI) removal potential in the free and biomass associated forms. Algal Res. 2021, 58, 102373. [Google Scholar] [CrossRef]
- Singh, Y.; Kaushal, S.; Sodhi, R.S. Biogenic synthesis of silver nanoparticles using cyanobacterium Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination. Nanoscale Adv. 2020, 2, 3972–3982. [Google Scholar] [CrossRef]
- Kumar, M.; Seth, K.; Choudhary, S.; Kumawat, G.; Nigam, S.; Joshi, G.; Saharan, V.; Meena, M.; Gupta, A.K.; Harish. Toxicity evaluation of iron oxide nanoparticles to freshwater cyanobacteria Nostoc ellipsosporum. Environ. Sci. Pollut. Res. 2023, 30, 55742–55755. [Google Scholar] [CrossRef]
- Brayner, R.; Coradin, T.; Beaunier, P.; Grenèche, J.M.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F. Intracellular biosynthesis of superparamagnetic 2-lines ferri-hydrite nanoparticles using Euglena gracilis microalgae. Colloids Surf. B Biointerfaces 2012, 93, 20–23. [Google Scholar] [CrossRef]
- Haris, M.; Fatima, N.; Iqbal, J.; Chalgham, W.; Mumtaz, A.S.; El-Sheikh, M.A.; Tavafoghi, M. Oscillatoria limnetica Mediated Green Synthesis of Iron Oxide (Fe2O3) Nanoparticles and Their Diverse In Vitro Bioactivities. Molecules 2023, 28, 2091. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Abdelmeguid, N.E.; Ali, M.A.; Bin-Meferij, M.M.; Khalil, M.I. Synthesis of silver nanoparticles using a novel cyanobacteria Desertifilum sp. extract: Their antibacterial and cytotoxicity effects. Int. J. Nanomed. 2020, 15, 49–63. [Google Scholar]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef] [PubMed]
- Boda, R.; Shareefuddin, M.D.; Chary, M.N.; Sayanna, R. FTIR and optical properties of europium doped lithium zinc bismuth borate glasses. Mater. Today Proc. 2016, 3, 1914–1922. [Google Scholar] [CrossRef]
- Minhas, L.A.; Mumtaz, A.S.; Kaleem, M.; Farraj, D.A.; Kamal, K.; Minhas, M.A.; Waqar, R.; Mahmoud, R.M. Green Synthesis of Zinc Oxide Nanoparticles Using Nostoc sp. and Their Multiple Biomedical Properties. Catalysts 2023, 13, 549. [Google Scholar]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Shahbaz, A.; Zahra, S.A.; Kanwal, S.; Munir, A.; Rabbani, A.; Mahmood, T. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct. 2020, 1199, 126979. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Ahmad, R.; Kanwal, S.; Afridi, S. Plant-mediated synthesis of nickel oxide nanoparticles (NiO) via Geranium wallichianum: Characterization and different biological applications. Mater. Res. Express 2019, 6, 0850a7. [Google Scholar] [CrossRef]
- De Faria, D.L.; Silva, S.V.; De Oliveira, M. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of zinc oxide nanoparticles using ixora coccinea leaf extract—A green approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Mahmood, T.; Qyyum, A.; Kanwal, S. Biofabrication of iron oxide nanoparticles by leaf extract of Rhamnus virgata: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Appl. Organomet. Chem. 2019, 33, e4947. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef]
- Lim, J.; Eggeman, A.; Lanni, F.; Tilton, R.D.; Majetich, S.A. Synthesis and single-particle optical detection of low-polydispersity plasmonic-superparamagnetic nanoparticles. Adv. Mater. 2008, 20, 1721–1726. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Ali, H.R.; Nassar, H.N.; El-Gendy, N.S. Green synthesis of α-Fe2O3 using Citrus reticulum peels extract and water decontamination from different organic pollutants. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1425–1434. [Google Scholar] [CrossRef]
- Ondiek, J.K. Synthesis and Characterization of Iron Nanoparticles Using Banana Peels Extracts and Their Application in Aptasensor. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2016. [Google Scholar]
- Cahyana, A.H.; Kam, N. Study on the stability of antioxidant and anti α-glucosidase activities using soaking treatment in Okra (Abelmoschus esculentus L.) mucilage extraction. Chem. Int. 2016, 3, 202–211. [Google Scholar]
- Iram, M.; Guo, C.; Guan, Y.; Ishfaq, A.; Liu, H. Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater. 2010, 181, 1039–1050. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Dubey, S.P.; Dwivedi, A.D.; Lahtinen, M.; Lee, C.; Kwon, Y.N.; Sillanpaa, M. Protocol for development of various plants leaves extract in single-pot synthesis of metal nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 103, 134–142. [Google Scholar] [CrossRef]
- De Jesus Oliveira, A.C.; de Araújo, A.R.; Quelemes, P.V.; Nadvorny, D.; Soares-Sobrinho, J.L.; de Almeida Leite, J.R.; da Silva-Filho, E.C.; da Silva, D.A. Solvent-free production of phthalated cashew gum for green synthesis of antimicrobial silver nanoparticles. Carbohydr. Polym. 2019, 213, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chopra, D.S.; Singh, D.; Singh, N. Optimization and ecofriendly synthesis of iron oxide nanoparticles as potential antioxidant. Arab. J. Chem. 2020, 13, 9034–9046. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Madkour, F.F.; El-Kassas, H.Y.; Mohamed, A.A.; Elgarahy, A.M. Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ. Sci. Pollut. Res. 2021, 28, 65549–65572. [Google Scholar] [CrossRef]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 21996. [Google Scholar] [CrossRef] [PubMed]
- Safawo, T.; Sandeep, B.V.; Pola, S.; Tadesse, A. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano 2018, 3, 56–63. [Google Scholar] [CrossRef]
- Salem, D.M.; Ismail, M.M.; Aly-Eldeen, M.A. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea, Egypt. Egypt. J. Aquat. Res. 2019, 45, 197–204. [Google Scholar] [CrossRef]
- Yao, G.; Sebisubi, F.M.; Voo, L.Y.; Ho, C.C.; Tan, G.T.; Chang, L.C. Citrinin derivatives from the soil filamentous fungus Penicillium sp. H9318. J. Braz. Chem. Soc. 2011, 22, 1125–1129. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in Southeastern Nigeria. Sci. World J. 2017, 2017, 3592491. [Google Scholar] [CrossRef]
- Waters, B.; Saxena, G.; Wanggui, Y.; Kau, D.; Wrigley, S.; Stokes, R.; Davies, J. Identifying protein kinase inhibitors using an assay based on inhibition of aerial hyphae formation in Streptomyces. J. Antibiot. 2002, 55, 407–416. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef]
- Hameed, S.; Khalil, A.T.; Ali, M.; Numan, M.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine 2019, 14, 655–673. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Baravalia, Y. Brine shrimp cytotoxicity of Caesalpinia pulcherrima aerial parts, antimicrobial activity and characterisation of isolated active fractions. Nat. Prod. Res. 2011, 25, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Method for analysis of nanoparticle hemolytic properties in vitro. Nano Lett. 2008, 8, 2180–2187. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Hameed, S.; Munir, A.; Kanwal, S. Green synthesis and characterizations of Nickel oxide nanoparticles using leaf extract of Rhamnus virgata and their potential biological applications. Appl. Organomet. Chem. 2019, 33, e4950. [Google Scholar] [CrossRef]
- Prach, M.; Stone, V.; Proudfoot, L. Zinc oxide nanoparticles and monocytes: Impact of size, charge and solubility on activation status. Toxicol. Appl. Pharmacol. 2013, 266, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Ahmad, M.S.; Mirza, B. Antioxidant and cytotoxic activities and phytochemical analysis of Euphorbia wallichii root extract and its fractions. Iran. J. Pharm. Res. 2012, 11, 241. [Google Scholar]




| Conditions for ZP Measurements | |
|---|---|
| Buffer Name | Phosphate-buffered saline |
| Dispersant RI | 1.330 |
| pH | 7.4 |
| Viscosity (cP) | 0.8872 |
| Dispersant Dielectric Constant | 78.5 |
| Temperature (°C) | 25.0 |
| Zeta Runs | 12 |
| Count Rate (kcps) | 70.1 |
| Measurement Position (mm) | 4.50 |
| Cell Description | Zeta dip cell |
| Attenuator | 10 |
| Zeta Size Distribution, Zeta Potential and PDI | Results |
|---|---|
| Zeta size | 266 |
| Zeta average size (d·nm) | 644.6 |
| PDI | 0.761 |
| Intercept | 0.929 |
| Zeta potential (mV) | −8.50 |
| Zeta Deviation (mV) | 16.1 |
| Conductivity (mS/cm) | 0.146 |
| Result Quality | Good |
| Source | Method | Chemical | Size | Shape | Surface Morphology | Nature | Reference |
|---|---|---|---|---|---|---|---|
| Oscillatoria limnetica | Green synthesis | FeCl3·6H2O | 23.33 nm | Trigonal rhombohedral | Agglomerated | Crystalline | [33] |
| Spirulina platensis | Green synthesis | FeCl3·6H2O | 10 nm | Non-regular | Agglomerated | Crystalline | [55] |
| Leptolyngbya valderiana | Green synthesis | FeCl3·6H2O | 47.42 nm | Spindle-like | Densely impregnated | Non-crystalline amorphous | [29] |
| Chlorella K01 | FeCl2·4H2O | 50 nm | Spherical | Monodispersed | Crystalline | [56] | |
| Leptolyngbya sp. L-2 | Green synthesis | FeCl3·6H2O | ~28 nm | Spherical | Polydisperse | Crystalline | Current Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minhas, L.A.; Kaleem, M.; Minhas, M.A.H.; Waqar, R.; Al Farraj, D.A.; Alsaigh, M.A.; Badshah, H.; Haris, M.; Mumtaz, A.S. Biogenic Fabrication of Iron Oxide Nanoparticles from Leptolyngbya sp. L-2 and Multiple In Vitro Pharmacogenetic Properties. Toxics 2023, 11, 561. https://doi.org/10.3390/toxics11070561
Minhas LA, Kaleem M, Minhas MAH, Waqar R, Al Farraj DA, Alsaigh MA, Badshah H, Haris M, Mumtaz AS. Biogenic Fabrication of Iron Oxide Nanoparticles from Leptolyngbya sp. L-2 and Multiple In Vitro Pharmacogenetic Properties. Toxics. 2023; 11(7):561. https://doi.org/10.3390/toxics11070561
Chicago/Turabian StyleMinhas, Lubna Anjum, Muhammad Kaleem, Malik Abrar Hassan Minhas, Rooma Waqar, Dunia A. Al Farraj, Mona Abdullah Alsaigh, Hussain Badshah, Muhammad Haris, and Abdul Samad Mumtaz. 2023. "Biogenic Fabrication of Iron Oxide Nanoparticles from Leptolyngbya sp. L-2 and Multiple In Vitro Pharmacogenetic Properties" Toxics 11, no. 7: 561. https://doi.org/10.3390/toxics11070561
APA StyleMinhas, L. A., Kaleem, M., Minhas, M. A. H., Waqar, R., Al Farraj, D. A., Alsaigh, M. A., Badshah, H., Haris, M., & Mumtaz, A. S. (2023). Biogenic Fabrication of Iron Oxide Nanoparticles from Leptolyngbya sp. L-2 and Multiple In Vitro Pharmacogenetic Properties. Toxics, 11(7), 561. https://doi.org/10.3390/toxics11070561






