Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Rearing
2.2. Herbicide and Fertilizer
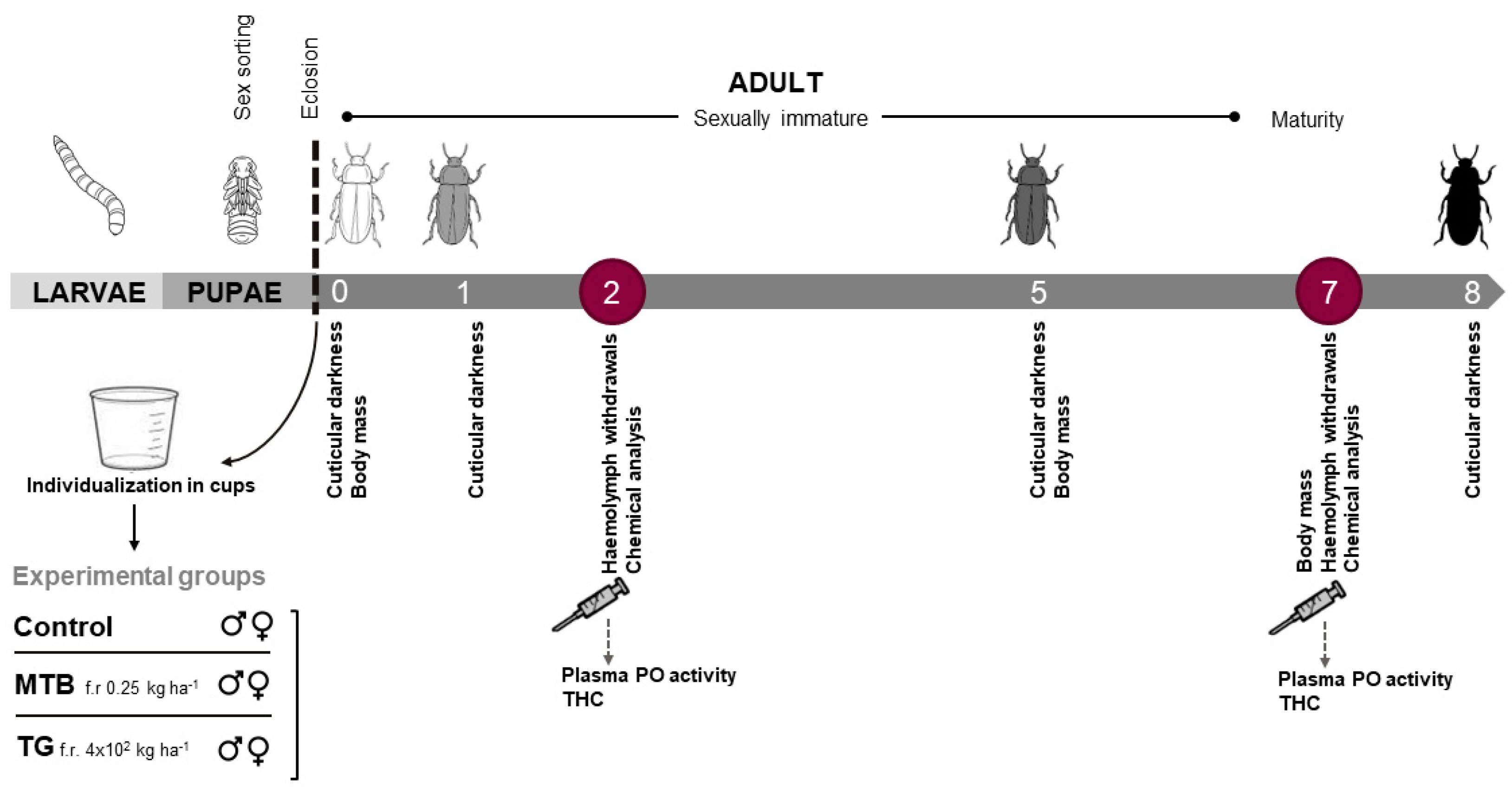
2.3. Experimental Setup and Treatments
2.4. Chemical Analyses
2.5. Body Conditions and Cuticular Darkness
2.6. Hemolymph Sampling, Total Hemocyte Counts, and Phenoloxidase Enzymatic Assay
2.7. Statistical Analyses
3. Results
3.1. Element Concentration in NPK Fertilizer, Metribuzin-Based Herbicide, and Wheat Bran
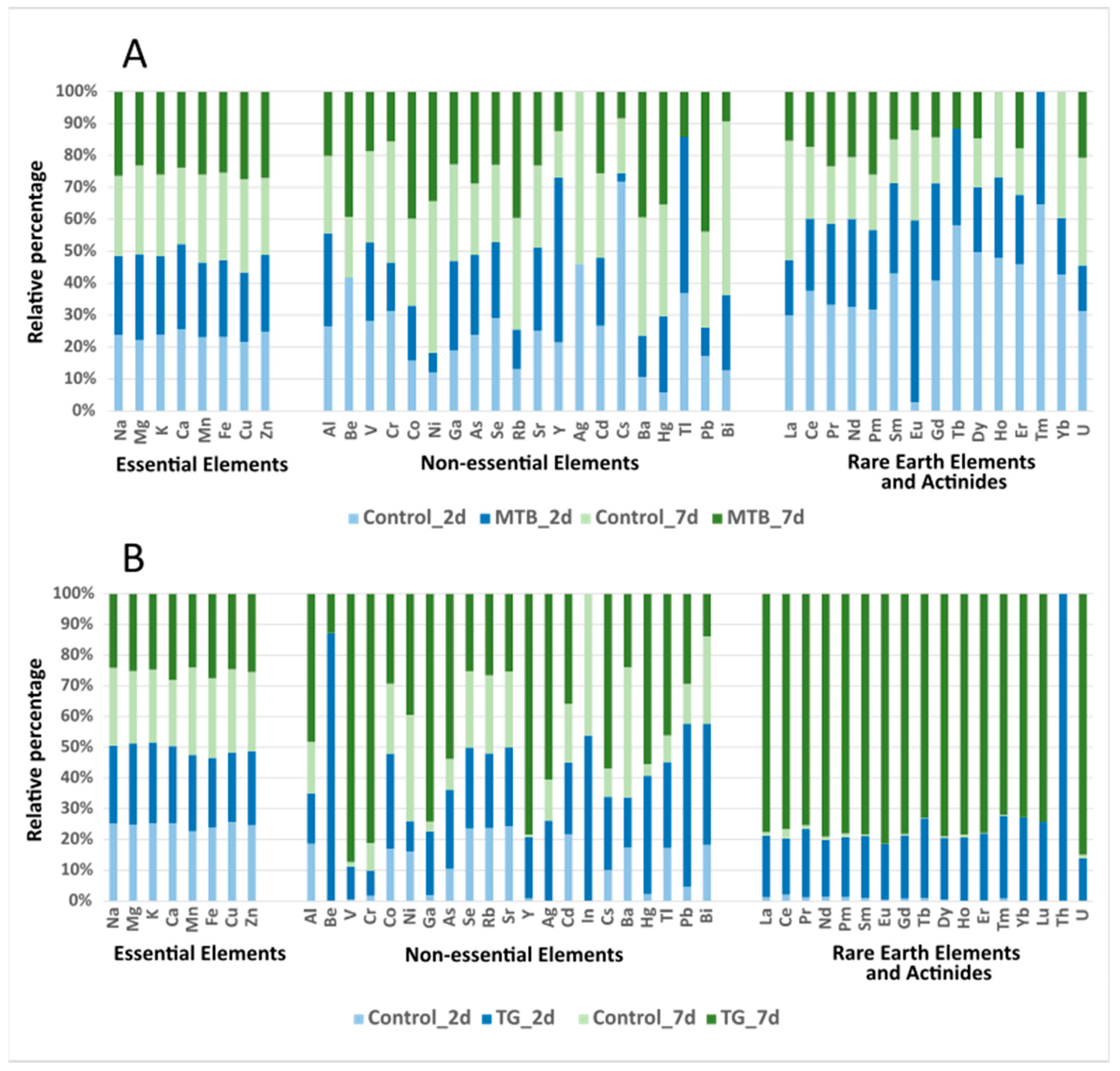
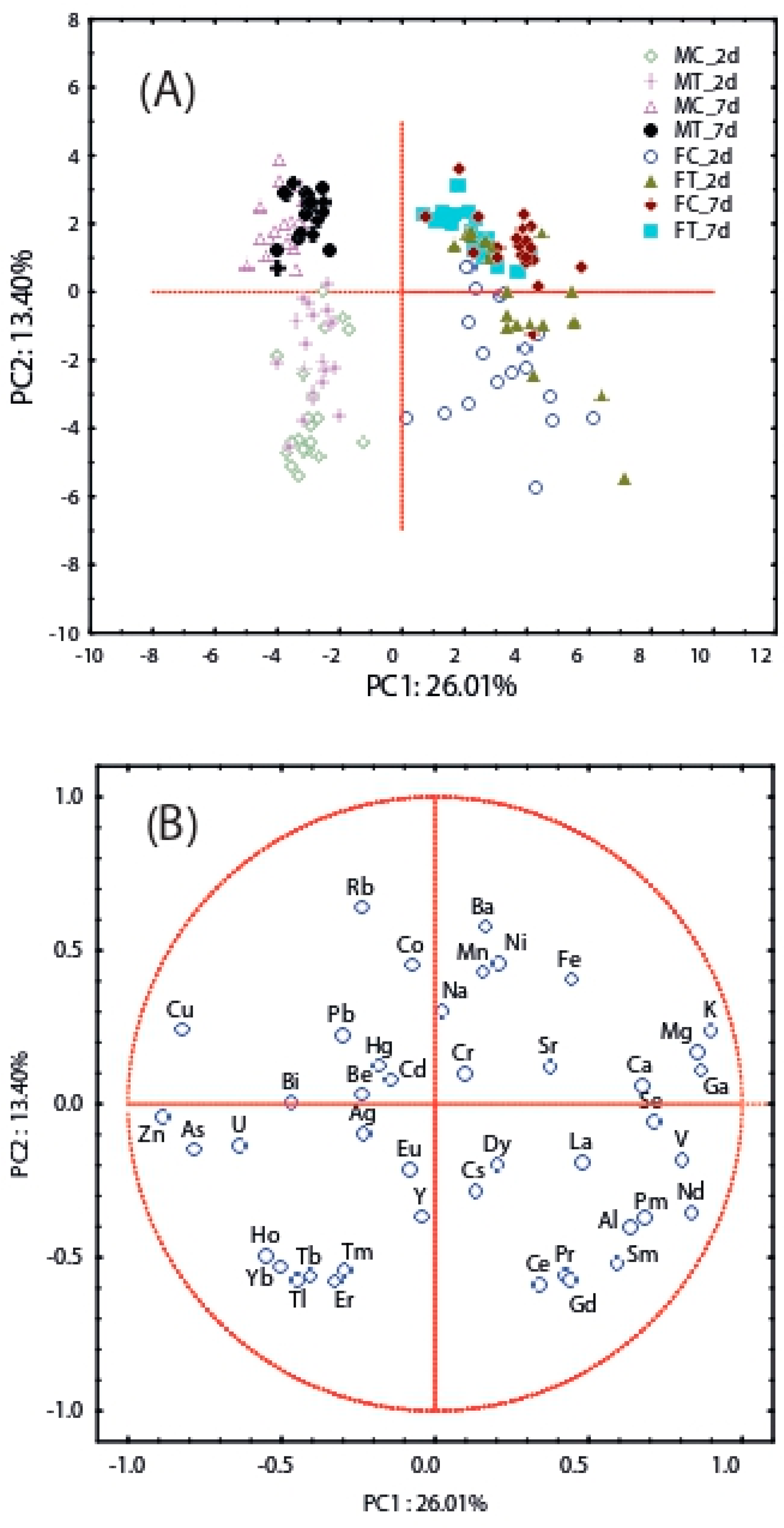
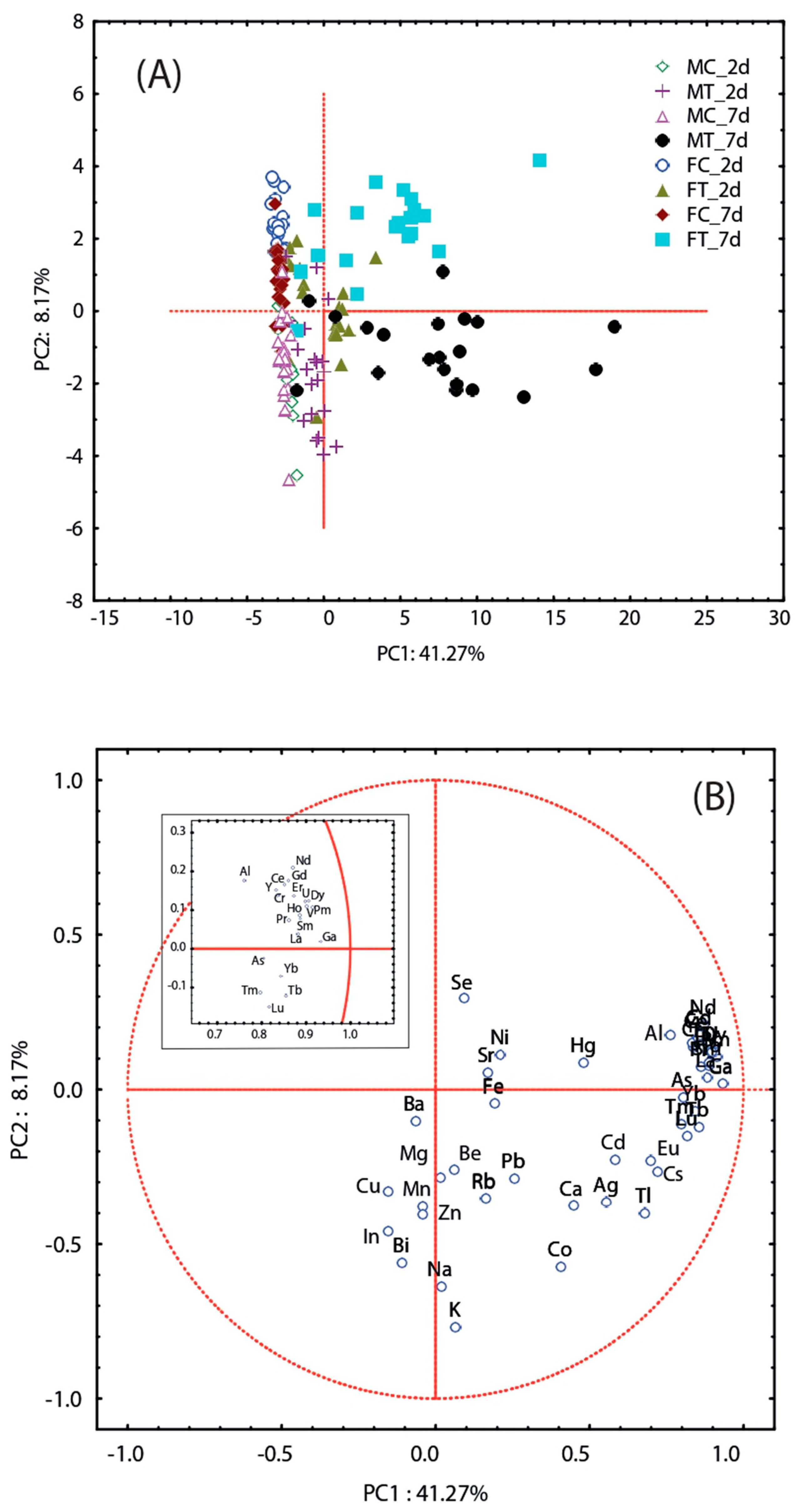
3.2. Element Accumulation in T. molitor
3.3. Biomagnification Factor
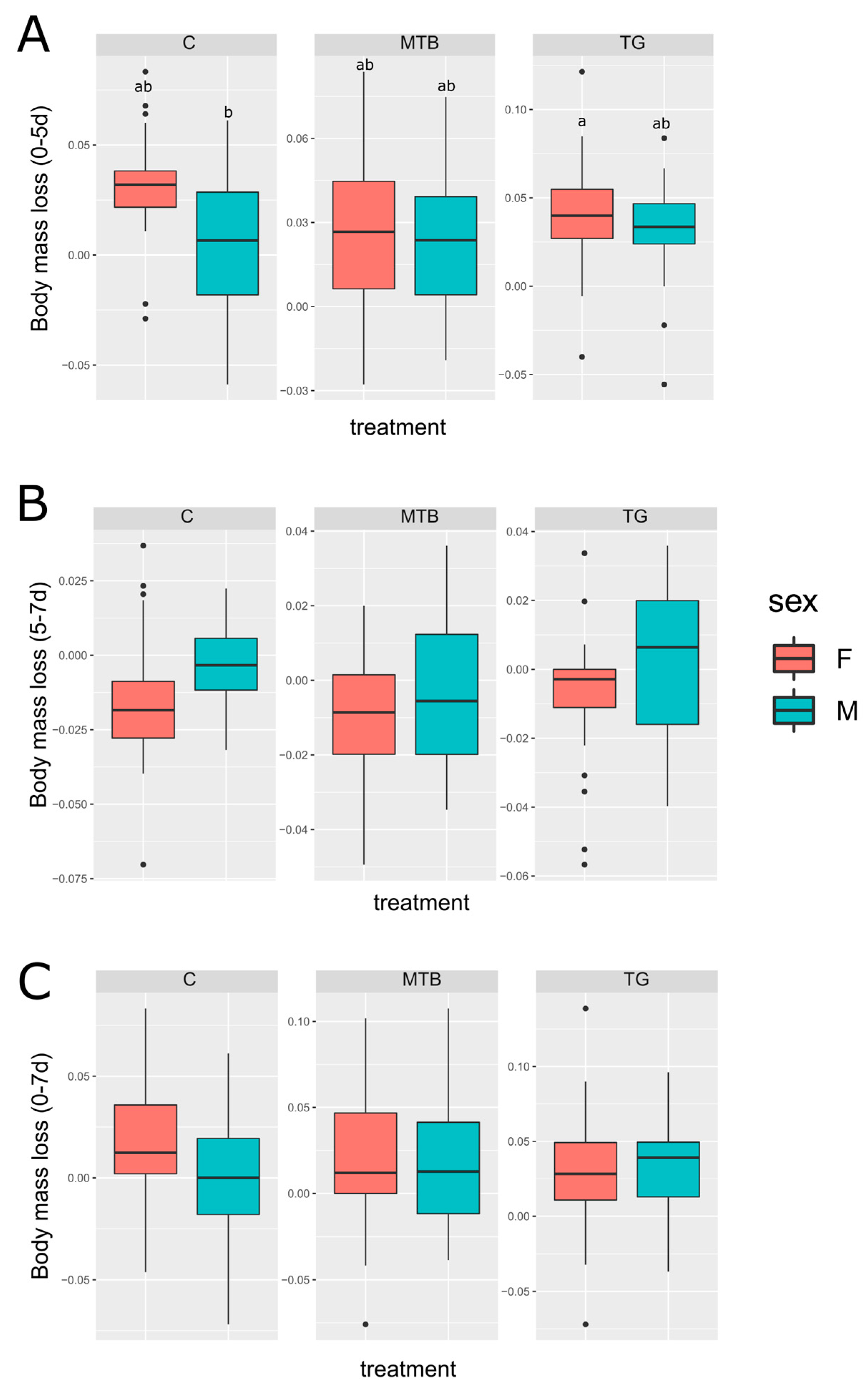
3.4. Body Conditions
3.5. Total Hemocyte Counts
3.6. Plasmatic Phenoloxidase Enzyme Activity
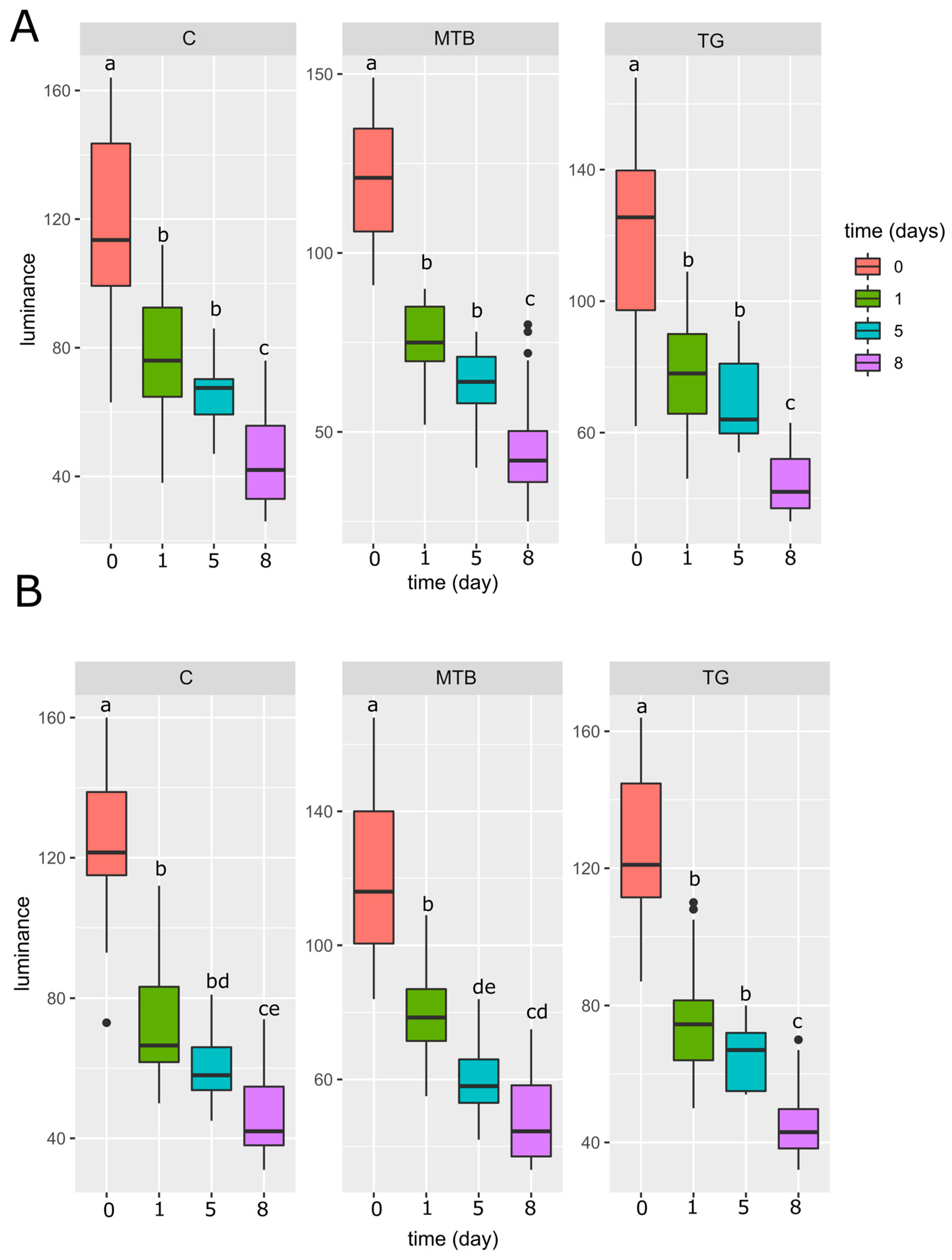
3.7. Cuticular Darkness
4. Discussion
4.1. Trace Elements in Metribuzin-Based Herbicide and NPK Fertilizer
4.2. Accumulation and Biomagnification of Trace Elements in Males and Females of T. molitor
4.3. Exposure Effects on Physiological Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malingreau, J.-P.; Eva, H.; Maggio, A. NPK: Will There Be Enough Plant Nutrients to Feed a World of 9 Billion in 2050; JRC: Tokyo, Japan, 2012; ISBN 9789279249105. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations. FAO World Fertilizer Trends and Outlook to 2020: Summary Report; Food and Agriculture Organization of United Nations: Rome, Italy, 2017; p. 38. [Google Scholar]
- Randive, K.; Raut, T.; Jawadand, S. An overview of the global fertilizer trends and India’s position in 2020. Miner. Econ. 2021, 34, 371–384. [Google Scholar] [CrossRef]
- EFMA European Fertilizer Manufacturers Association, EFMA. Available online: https://www.fertilizerseurope.com (accessed on 21 March 2023).
- Nziguheba, G.; Smolders, E. Inputs of trace elements in agricultural soils via phosphate fertilizers in european countries. Sci. Total Environ. 2008, 390, 53–57. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef]
- Mortvedt, J.J. Heavy metal contaminants in inorganic and organic fertilizers. In Fertilizer Research; Springer: Berlin/Heidelberg, Germany, 1995; Volume 43, pp. 55–61. [Google Scholar]
- Ashitha, A.; Rakhimol, K.R.; Jyothis, M. Fate of the conventional fertilizers in environment. In Controlled Release Fertilizers for Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–39. [Google Scholar]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- European Commission Regulation (EU). 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. J. Eur. Union 2019, 170, 1–114. [Google Scholar]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 0471370517. [Google Scholar]
- De, A.; Bose, R.; Kumar, A.; Mozumdar, S. Worldwide pesticide use. In Targeted Delivery of Pesticides Using Biodegradable Polymeric Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2014; pp. 5–6. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Korenko, S.; Niedobová, J.; Kolářová, M.; Hamouzová, K.; Kysilková, K.; Michalko, R. The Effect of Eight Common herbicides on the predatory activity of the agrobiont spider Pardosa agrestis. BioControl 2016, 61, 507–517. [Google Scholar] [CrossRef]
- Sharma, A.; Jha, P.; Reddy, G.V.P. Multidimensional relationships of herbicides with insect-crop food webs. Sci. Total Environ. 2018, 643, 1522–1532. [Google Scholar] [CrossRef]
- Giglio, A.; Vommaro, M.L. Dinitroaniline Herbicides: A comprehensive review of toxicity and side effects on animal non-target organisms. Environ. Sci. Pollut. Res. 2022, 29, 76687–76711. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticides: Environmental impacts and management strategies. In Pesticides—Toxic Aspects; Larramendy, M.L., Soloneski, S., Eds.; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Giglio, A.; Cavaliere, F.; Giulianini, P.G.; Mazzei, A.; Talarico, F.; Vommaro, M.L.; Brandmayr, P. Impact of agrochemicals on non-target species: Calathus fuscipes Goeze 1777 (Coleoptera: Carabidae) as model. Ecotoxicol. Environ. Saf. 2017, 142, 522–529. [Google Scholar] [CrossRef]
- Rose, M.T.; Cavagnaro, T.R.; Scanlan, C.A.; Rose, T.J.; Vancov, T.; Kimber, S.; Kennedy, I.R.; Kookana, R.S.; Van Zwieten, L. Impact of herbicides on soil biology and function. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 136, pp. 133–220. ISBN 0065-2113. [Google Scholar]
- Prosser, R.S.; Anderson, J.C.; Hanson, M.L.; Solomon, K.R.; Sibley, P.K. Indirect effects of herbicides on biota in terrestrial edge-of-field habitats: A critical review of the literature. Agri. Ecosyst. Environ. 2016, 232, 59–72. [Google Scholar] [CrossRef]
- Vonk, J.A.; Kraak, M.H.S. Herbicide exposure and toxicity to aquatic primary producers. Rev. Environ. Contam. Toxicol. 2020, 250, 119–171. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F. Indirect effect of pesticides on insects and other arthropods. Toxics 2021, 9, 177. [Google Scholar] [CrossRef]
- Giglio, A.; Cavaliere, F.; Giulianini, P.G.; Kurtz, J.; Vommaro, M.L.; Brandmayr, P. Continuous agrochemical treatments in agroecosystems can modify the effects of pendimethalin-based herbicide exposure on immunocompetence of a beneficial ground beetle. Diversity 2019, 11, 241. [Google Scholar] [CrossRef]
- Bohnenblust, E.W.; Vaudo, A.D.; Egan, J.F.; Mortensen, D.A.; Tooker, J.F. Effects of the herbicide dicamba on nontarget plants and pollinator visitation. Environ. Toxicol. Chem. 2016, 35, 144–151. [Google Scholar] [CrossRef]
- Egan, J.F.; Bohnenblust, E.; Goslee, S.; Mortensen, D.; Tooker, J. Herbicide drift can affect plant and arthropod communities. Agric. Ecosyst. Environ. 2014, 185, 77–87. [Google Scholar] [CrossRef]
- Abuzeid, M.A. Effects of two herbicides on healthy and Nosema infected honey bee workers. Arthropods 2018, 7, 31–41. [Google Scholar]
- Giglio, A.; Vommaro, M.L.; Gionechetti, F.; Pallavicini, A. Gut microbial community response to herbicide exposure in a ground beetle. J. Appl. Entomol. 2021, 145, 986–1000. [Google Scholar] [CrossRef]
- Motta, E.V.S.; Raymann, K.; Moran, N.A. Glyphosate perturbs the gut microbiota of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 10305–10310. [Google Scholar] [CrossRef]
- Mesnage, R.; Antoniou, M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health 2018, 5, 361. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Seralini, G.E.; Jungers, G. Toxic compounds in herbicides without glyphosate. Food Chem. Toxicol. 2020, 146, 111770. [Google Scholar] [CrossRef]
- LeBaron, H.M.; McFarland, J.E.; Burnside, O.C. The Triazine Herbicides; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780444511676. [Google Scholar]
- Travlos, I.S.; Gkotsi, T.; Roussis, I.; Kontopoulou, C.K.; Kakabouki, I.; Bilalis, D.J. Effects of the herbicides benfluralin, metribuzin and propyzamide on the survival and weight of earthworms (Octodrilus complanatus). Plant Soil Environ. 2017, 63, 117–124. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Rao, H.; Liu, X. Potential neurotoxicity, immunotoxicity, and carcinogenicity induced by metribuzin and tebuconazole exposure in earthworms (Eisenia fetida) revealed by transcriptome analysis. Sci. Total Environ. 2022, 807, 150760. [Google Scholar] [CrossRef]
- Alhewairini, S.S. Toxicity effects of glyphosate and metribuzin on five species of soil-dwelling predatory mites. Pak. J. Agric. Sci. 2020, 57, 1429–1435. [Google Scholar]
- Wolfson, J.L.; Yeargan, K.V. The effects of metribuzin on larval populations of alfalfa weevil, Hypera postica (Coleoptera: Curculionidae). J. Kans Entomol. Soc. 1983, 56, 40–46. [Google Scholar]
- Kaya, B.; Yanikoǧlu, A.; Creus, A.; Marcos, R. Genotoxicity testing of five herbicides in the Drosophila wing spot test. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2000, 465, 77–84. [Google Scholar] [CrossRef]
- Cavaliere, F.; Brandmayr, P.; Giglio, A. DNA damage in haemocytes of Harpalus (Pseudophonus) rufipes (De Geer, 1774) (Coleoptera, Carabidae) as an indicator of sublethal effects of exposure to herbicides. Ecol. Indic. 2019, 98, 88–91. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Gureev, A.P.; Starkova, N.N.; Savinkova, O.V.; Starkov, A.A.; Lopatin, A.V.; Popov, V.N. Method for detection of mtDNA damages for evaluating of pesticides toxicity for bumblebees (Bombus terrestris L.). Pestic. Biochem. Physiol. 2020, 169, 104675. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Novotny, L.; Blahova, J.; Sudova, E.; Maly, V. Effects of metribuzin on rainbow trout (Oncorhynchus mykiss). Vet. Med. 2008, 53, 324–332. [Google Scholar] [CrossRef]
- Velisek, J.; Stastna, K.; Sudova, E.; Turek, J.; Svobodova, Z.; Velíšek, J. Effects of subchronic simazine exposure on some biometric, biochemical, hematological and histopathological parameters of common carp (Cyprinus carpio L.). Neuroendocrinol. Lett. 2009, 30, 300709–300749. [Google Scholar]
- Koutnik, D.; Stara, A.; Zuskova, E.; Kouba, A.; Velisek, J. The effect of subchronic metribuzin exposure to signal crayfish (Pacifastacus leniuscutus Dana 1852). Neuroendocrinol. Lett. 2014, 35, 51–56. [Google Scholar] [PubMed]
- Samir, D.; Asma, S. Triazinone herbicide metribuzin induced acute liver injury: A study of animal model. J. Acute Dis. 2018, 7, 152. [Google Scholar] [CrossRef]
- Medjdoub, A.; Merzouk, S.A.; Merzouk, H.; Chiali, F.Z.; Narce, M. Effects of mancozeb and metribuzin on in vitro proliferative responses and oxidative stress of human and rat spleen lymphocytes stimulated by mitogens. Pestic. Biochem. Physiol. 2011, 101, 27–33. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Chang, A.C.; Page, A.L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environ. Pollut. 2012, 168, 44–53. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface and groundwater. Encycl. Anthr. 2017, 1–5, 225–240. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida Basin, Peloponnese, Greece. Geoderma 2014, 221, 82–90. [Google Scholar] [CrossRef]
- WHO Chemicals. Compendium of WHO and Other UN Guidance on Health and Environment; WHO: Geneva, Switzerland, 2021.
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Cell Biol. Toxicol. 2012, 28, 31–46. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Belon, E.; Boisson, M.; Deportes, I.Z.; Eglin, T.K.; Feix, I.; Bispo, A.O.; Galsomies, L.; Leblond, S.; Guellier, C.R. An inventory of trace elements inputs to french agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ennaji, W.; Barakat, A.; El Baghdadi, M.; Rais, J. Heavy metal contamination in agricultural soil and ecological risk assessment in the northeast area of Tadla Plain, Morocco. J. Sediment. Environ. 2020, 5, 307–320. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in china: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic Transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess 2015, 187, 201. [Google Scholar] [CrossRef]
- Dar, M.I.; Green, I.D.; Khan, F.A. Trace metal contamination: Transfer and fate in food chains of terrestrial invertebrates. Food Webs 2019, 20, e00116. [Google Scholar] [CrossRef]
- Monchanin, C.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Current permissible levels of metal pollutants harm terrestrial invertebrates. Sci. Total Environ. 2021, 779, 146398. [Google Scholar] [CrossRef]
- United Nations Sustainable Development Goals (SDGs). Available online: https://sdgs.un.org/goals (accessed on 23 April 2023).
- EFSA European Food Safety Authority, EFSA. Available online: https://www.efsa.europa.eu/it/applications/pesticides/regulationsandguidance (accessed on 21 March 2023).
- EPA EPA—Regulatory and Guidance Information by Topic: Pesticides. Available online: https://www.epa.gov/regulatory-information-topic/regulatory-and-guidance-information-topic-pesticides (accessed on 21 March 2023).
- Tao, Y.; Shen, L.; Feng, C.; Yang, R.; Qu, J.; Ju, H.; Zhang, Y. Distribution of Rare Earth Elements (REEs) and their roles in plant growth: A review. Environ. Pollut. 2022, 298, 118540. [Google Scholar] [CrossRef] [PubMed]
- Balaram, V. Rare Earth Elements: A Review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Tommasi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of Rare Earth Elements as fertilizers and feed additives: A Knowledge Gap Analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef]
- Pagano, G.; Thomas, P.J.; Di Nunzio, A.; Trifuoggi, M. Human Exposures to Rare Earth Elements: Present knowledge and research prospects. Environ. Res 2019, 171, 493–500. [Google Scholar] [CrossRef]
- Tedesco, R.; Hidalgo, M.D.C.V.; Varde, M.; Kehrwald, N.M.; Barbante, C.; Cozzi, G. Trace and rare earth elements determination in milk whey from the Veneto region, Italy. Food Control 2021, 121, 107595. [Google Scholar] [CrossRef]
- Li, J.; Verweij, R.A.; van Gestel, C.A.M. Lanthanum toxicity to five different species of soil invertebrates in relation to availability in soil. Chemosphere 2018, 193, 412–420. [Google Scholar] [CrossRef]
- Li, J.; Hong, M.; Yin, X.; Liu, J. Effects of the accumulation of the rare earth elements on soil macrofauna community. J. Rare Earths 2010, 28, 957–964. [Google Scholar] [CrossRef]
- Rucki, M.; Kejlova, K.; Vlkova, A.; Jirova, D.; Dvorakova, M.; Svobodova, L.; Kandarova, H.; Letasiova, S.; Kolarova, H.; Mannerstrom, M. Evaluation of toxicity profiles of rare earth elements salts (lanthanides). J. Rare Earths 2021, 39, 225–232. [Google Scholar] [CrossRef]
- Swift, M.J.; Izac, A.M.N.; Van Noordwijk, M. Biodiversity and ecosystem services in agricultural landscapes—Are we asking the right questions? Agric. Ecosyst. Environ. 2004, 104, 113–134. [Google Scholar] [CrossRef]
- Köhler, H.R.; Triebskorn, R. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M.; et al. Beetles as model organisms in physiological, biomedical and environmental studies—A review. Front. Physiol. 2019, 10, 319. [Google Scholar] [CrossRef]
- Howard, R.S. The Biology of the Grain Beetle Tenebrio molitor with particular reference to its behavior. Ecology 1955, 36, 262–269. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune defenses of a beneficial pest: The mealworm beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Moreno-García, M.; Córdoba-Aguilar, A.; Condé, R.; Lanz-Mendoza, H. Current immunity markers in insect ecological immunology: Assumed trade-offs and methodological issues. Bull Entomol. Res. 2013, 103, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, D.; Du, X.; Guo, X.; Zhang, F.; Desneux, N.; Zang, L.; Wang, S. The role of the dopamine melanin pathway in the ontogeny of elytral melanization in Harmonia axyridis. Front. Physiol. 2019, 10, 1066. [Google Scholar] [CrossRef]
- Mun, S.; Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. Gene functions in adult cuticle pigmentation of the yellow mealworm, Tenebrio molitor. Insect Biochem. Mol. Biol. 2020, 117, 103291. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The Insect Cuticle. Biol. Rev. 1948, 23, 408–451. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.W.S.; Araujo, L.S.; Azevedo, D.O.; Serrão, J.E.; Elliot, S.L. Physical and chemical properties of primary defences in Tenebrio molitor. Physiol. Entomol. 2016, 41, 121–126. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle formation and pigmentation in beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Evison, S.E.F.; Gallagher, J.D.; Thompson, J.J.W.; Siva-Jothy, M.T.; Armitage, S.A.O. Cuticular colour reflects underlying architecture and is affected by a limiting resource. J. Insect Physiol. 2017, 98, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Armitage, S.A.O.; Siva-Jothy, M.T. Immune function responds to selection for cuticular colour in Tenebrio molit. Heredity 2005, 94, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.I.; Siva-Jothy, M.T. Density–dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 177–182. [Google Scholar] [CrossRef]
- Giglio, A.; Ammendola, A.; Battistella, S.; Naccarato, A.; Pallavicini, A.; Simeon, E.; Tagarelli, A.; Giulianini, P.G. Apis mellifera ligustica, Spinola 1806 as bioindicator for detecting environmental contamination: A preliminary study of heavy metal pollution in Trieste, Italy. Environ. Sci. Pollut. Res. 2017, 24, 659–665. [Google Scholar] [CrossRef]
- Talarico, F.; Brandmayr, P.; Giulianini, P.G.; Ietto, F.; Naccarato, A.; Perrotta, E.; Tagarelli, A.; Giglio, A. Effects of metal pollution on survival and physiological responses in Carabus (Chaetocarabus) lefebvrei (Coleoptera, Carabidae). Eur. J. Soil. Biol. 2014, 61, 80–89. [Google Scholar] [CrossRef]
- Naccarato, A.; Tagarelli, A. Recent applications and newly developed strategies of solid-phase microextraction in contaminant analysis: Through the environment to humans. Separations 2019, 6, 54. [Google Scholar] [CrossRef]
- Chianese, E.; Tirimberio, G.; Dinoi, A.; Cesari, D.; Contini, D.; Bonasoni, P.; Marinoni, A.; Andreoli, V.; Mannarino, V.; Moretti, S.; et al. Particulate matter ionic and elemental composition during the winter season: A comparative study among rural, urban and remote sites in southern Italy. Atmosphere 2022, 13, 356. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Martino, M.; Elliani, R.; Sprovieri, F.; Pirrone, N.; Tagarelli, A. An innovative green protocol for the quantification of benzothiazoles, benzotriazoles and benzosulfonamides in PM10 using microwave-assisted extraction coupled with solid-phase microextraction Gas Chromatography Tandem-Mass Spectrometry. Environ. Pollut. 2021, 285, 117487. [Google Scholar] [CrossRef]
- Knapp, M.; Řeřicha, M. Effects of the winter temperature regime on survival, body mass loss and post-winter starvation resistance in laboratory-reared and field-collected ladybirds. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.J.W.; Armitage, S.A.O.; Siva-Jothy, M.T. Cuticular colour change after imaginal eclosion is time-constrained: Blacker beetles darken faster. Physiol. Entomol. 2002, 27, 136–141. [Google Scholar] [CrossRef]
- Cavaliere, F.; Brandmayr, P.; Giulianini, P.G.; Vommaro, M.L.; Giglio, A. Harpalus (Pseudoophonus) rufipes as a model to study cellular and humoral immune defence strategies in coleopteran species. Invertebr. Surviv. J. 2019, 16, 92–104. [Google Scholar] [CrossRef]
- Naccarato, A.; Gionfriddo, E.; Elliani, R.; Pawliszyn, J.; Sindona, G.; Tagarelli, A. Investigating the robustness and extraction performance of a matrix-compatible solid-phase microextraction coating in human urine and its application to assess 2-6-ring polycyclic aromatic hydrocarbons using GC-MS/MS. J. Sep. Sci. 2018, 41, 929–939. [Google Scholar] [CrossRef]
- Moretti, S.; Salmatonidis, A.; Querol, X.; Tassone, A.; Andreoli, V.; Bencardino, M.; Pirrone, N.; Sprovieri, F.; Naccarato, A. Contribution of volcanic and fumarolic emission to the aerosol in marine atmosphere in the central mediterranean sea: Results from Med-Oceanor 2017 cruise campaign. Atmosphere 2020, 11, 149. [Google Scholar] [CrossRef]
- Elliani, R.; Tagarelli, A.; Naccarato, A. Assessment of benzothiazoles, benzotriazoles and benzenesulfonamides in environmental waters using an optimized combination of microextraction by packed sorbent with programmed temperature vaporization-Gas Chromatography Tandem-Mass Spectrometry. Talanta 2023, 258, 124410. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and Environment for Statistical Computing. r Foundation for Statistical Computing, Vienna, Austria. 2013. Available online: https://www.r-project.org/ (accessed on 21 March 2023).
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of Aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Campos, V. Trace Elements in Pesticides. Commun. Soil. Sci. Plant Anal. 2003, 34, 1261–1268. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Merrikhpour, H. Investigation of the effects of phosphate fertilizer application on the heavy metal content in agricultural soils with different cultivation patterns. Biol. Trace. Elem. Res. 2012, 145, 87–92. [Google Scholar] [CrossRef]
- Shuaib, M.; Azam, N.; Bahadur, S.; Romman, M.; Yu, Q.; Xuexiu, C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb. Pathog. 2021, 150, 104713. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Afonne, O.J.; Ifediba, E.C. Heavy metals risks in plant foods—Need to step up precautionary measures. Curr. Opin. Toxicol. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Lindqvist, L. Accumulation of Cadmium, Copper, and Zinc in five species of phytophagous insects. Environ. Entomol. 1992, 21, 160–163. [Google Scholar] [CrossRef]
- Gintenreiter, S.; Ortel, J.; Nopp, H.J. Bioaccumulation of Cadmium, Lead, Copper, and Zinc in successive developmental stages of Lymantria dispar L. (Lymantriidae, Lepid)—A Life Cycle Study. Arch. Environ. Contam. Toxicol. 1993, 25, 55–61. [Google Scholar] [CrossRef]
- Butt, A.; Qurat-ul-Ain; Rehman, K.; Khan, M.X.; Hesselberg, T. Bioaccumulation of Cadmium, Lead, and Zinc in agriculture-based insect food chains. Environ. Monit. Assess. 2018, 190, 698. [Google Scholar] [CrossRef]
- Green, I.D.; Jeffries, C.; Diaz, A.; Tibbett, M. Contrasting behaviour of Cadmium and Zinc in a soil-plant-arthropod system. Chemosphere 2006, 64, 1115–1121. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Gao, Z.; Wen, Y.; Wang, W.; Liu, W.; Wang, X.; Zhu, F. Identification of three metallothioneins in the black soldier fly and their functions in Cd accumulation and detoxification. Environ. Pollut. 2021, 286, 117146. [Google Scholar] [CrossRef]
- Ballan-Dufrançais, C. Localization of metals in cells of pterygote insects. Microsc. Res. Tech. 2002, 56, 403–420. [Google Scholar] [CrossRef]
- Stewart, A.D.; Anand, R.R.; Laird, J.S.; Verrall, M.; Ryan, C.G.; de Jonge, M.D.; Paterson, D.; Howard, D.L. Distribution of metals in the termite Tumulitermes tumuli (Froggatt): Two types of Malpighian tubule concretion host Zn and Ca mutually exclusively. PLoS ONE 2011, 6, e27578. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.M.; Pierce, L.M.; Gillis, P.L.; Wood, C.M.; O’Donnell, M.J. Cadmium transport by the gut and malpighian tubules of Chironomus Riparius. Aquat. Toxicol. 2009, 92, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, A.J.; Świątek, Z.M.; Labecka, A.M. Effects of cadmium bioavailability in food on its distribution in different tissues in the ground beetle Pterostichus oblongopunctatus. Bull Environ. Contam. Toxicol. 2019, 103, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.; Brandmayr, P. Structural and functional alterations in Malpighian tubules as biomarkers of environmental pollution: Synopsis and prospective. J. Appl. Toxicol. 2017, 37, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Qin, Q.; Zhou, B. Functional studies of drosophila zinc transporters reveal the mechanism for zinc excretion in Malpighian tubules. BMC Biol. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Belowitz, R.; O’Donnell, M.J. Ion-selective microelectrode measurements of Tl+ and K+ transport by the gut and associated epithelia in Chironomus Riparius. Aquat. Toxicol. 2013, 138–139, 70–80. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Świątek, Z.M.; Paciorek, K.; Kubińska, N. Effect of cadmium bioavailability in food on its compartmentalisation in Carabids. Ecotoxicology 2017, 26, 1259–1270. [Google Scholar] [CrossRef]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The effects of essential and non-essential metal toxicity in the Drosophila Melanogaster Insect Model: A Review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef]
- Bednarska, A.J.; Świątek, Z. Subcellular partitioning of cadmium and zinc in mealworm beetle (Tenebrio molitor) larvae exposed to metal-contaminated flour. Ecotoxicol. Environ. Saf. 2016, 133, 82–89. [Google Scholar] [CrossRef]
- Pedersen, S.A.; Kristiansen, E.; Andersen, R.A.; Zachariassen, K.E. Cadmium is deposited in the gut content of larvae of the beetle Tenebrio molitor and involves a Cd-binding protein of the low cysteine type. Comp. Biochem. Physiol. Part—C Toxicol. Pharm. 2008, 148, 217–222. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Dong, X.; Meng, Z.; Yan, S. Accumulation of Cd and Pb in various body parts, organs and tissues of Lymantria dispar asiatica (Lepidoptera: Erebidae). J. Asia Pac. Entomol. 2020, 23, 963–969. [Google Scholar] [CrossRef]
- Lindqvist, L.; Block, M. Losses of Cd, Hg, and Zn during metamorphosis in the beetle Tenebrio Molitor (Coleoptera: Tenebrionidae). Bull. Environ. Contam. Toxicol. 1997, 58, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Kafel, A.; Rozpedek, K.; Szulińska, E.; Zawisza-Raszka, A.; Migula, P. The effects of Cadmium or Zinc multigenerational exposure on metal tolerance of Spodoptera Exigua (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2014, 21, 4705–4715. [Google Scholar] [CrossRef]
- Filipiak, M.; Woyciechowski, M.; Czarnoleski, M. Stoichiometric niche, nutrient partitioning and resource allocation in a solitary bee are sex-specific and Phosphorous is allocated mainly to the cocoon. Sci. Rep. 2021, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, M.; Migula, P. Chapter 16 Body burden with metals and detoxifying abilities of the grasshopper—Chorthippus brunneus (Thunberg) from industrially polluted areas. In Trace Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2000; Volume 4, pp. 423–454. ISBN 0927-5215. [Google Scholar]
- Balinski, M.A.; Woodruff, R.C. Differential sexual survival of Drosophila Melanogaster on Copper sulfate. Genetica 2017, 145, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R. Are the top carnivores endangered by heavy metal biomagnification? Oikos 1991, 60, 387. [Google Scholar] [CrossRef]
- Su, H.; Wu, J.; Zhang, Z.; Ye, Z.; Chen, Y.; Yang, Y. Effects of Cadmium stress at different concentrations on the reproductive behaviors of beet armyworm Spodoptera Exigua (Hübner). Ecotoxicology 2021, 30, 402–410. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nogara, P.A.; Lima, L.S.; Galiciolli, M.E.; Souza, J.V.; Aschner, M.; Rocha, J.B. Toxic Metals That Interact with thiol groups and alteration in insect behavior. Curr. Opin. Insect Sci. 2022, 52, 100923. [Google Scholar] [CrossRef]
- Gkinali, A.A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor larva-based ingredients for the food industry: A review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Fur. Nat.—Sect. C J. Biosci. 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Selaledi, L.; Mbajiorgu, C.A.; Mabelebele, M. The use of yellow mealworm (T. molitor) as alternative source of protein in poultry diets: A review. Trop. Anim. Health Prod. 2020, 52, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of frozen and dried formulations from whole yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to regulation (EU) 2015/2283. EFSA J. 2021, 19, e06778. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods; European Commission: Brussels, Belgium, 2017.
- Van Der Fels-Klerx, H.J.; Camenzuli, L.; Van Der Lee, M.K.; Oonincx, D.G.A.B. Uptake of Cadmium, Lead and Arsenic by Tenebrio molitor and Hermetia illucens from contaminated substrates. PLoS ONE 2016, 11, e0166186. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio molitor larvae as an alternative food source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Truzzi, C.; Illuminati, S.; Girolametti, F.; Antonucci, M.; Scarponi, G.; Ruschioni, S.; Riolo, P.; Annibaldi, A. Influence of feeding substrates on the presence of toxic metals (Cd, Pb, Ni, as, Hg) in larvae of Tenebrio molitor: Risk assessment for human consumption. Int. J. Environ. Res. Public Health 2019, 16, 4815. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.; Vignati, D.A.L.; Leyval, C.; Giamberini, L. Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int 2014, 71, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sharma, A.; Talukder, G. Effects of Lanthanum in cellular systems—A review. Biol. Trace Elem. Res. 1988, 18, 201–228. [Google Scholar] [CrossRef]
- Huang, P.; Li, J.; Zhang, S.; Chen, C.; Han, Y.; Liu, N.; Xiao, Y.; Wang, H.; Zhang, M.; Yu, Q. Effects of Lanthanum, Cerium, and Neodymium on the nuclei and mitochondria of hepatocytes: Accumulation and oxidative damage. Environ. Toxicol. Pharm. 2011, 31, 25–32. [Google Scholar] [CrossRef]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 2015, 142, 215–220. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Arakane, Y.; Noh, M.Y.; Asano, T.; Kramer, K.J. Tyrosine metabolism for insect cuticle pigmentation and sclerotization. In Extracellular Composite Matrices in Arthropods; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–220. [Google Scholar]
- Gibert, P.; Debat, V.; Ghalambor, C.K. Phenotypic plasticity, global change, and the speed of adaptive evolution. Curr. Opin. Insect Sci. 2019, 35, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Simpson, S.J.; Wilson, K. Dietary protein-quality influences melanization and immune function in an insect. Funct. Ecol. 2008, 22, 1052–1061. [Google Scholar] [CrossRef]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction–immunity trade-offs in insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Vommaro, M.L.; Giulianini, P.G.; Giglio, A. Pendimethalin-based herbicide impairs cellular immune response and haemocyte morphology in a beneficial ground beetle. J. Insect Physiol. 2021, 131, 104236. [Google Scholar] [CrossRef]
- Cestonaro, L.V.; Macedo, S.M.D.; Piton, Y.V.; Garcia, S.C.; Arbo, M.D. Toxic effects of pesticides on cellular and humoral immunity: An overview. Immunopharmacol. Immunotoxicol. 2022, 44, 816–831. [Google Scholar] [CrossRef]
- Babcock, D.T.; Brock, A.R.; Fish, G.S.; Wang, Y.; Perrin, L.; Krasnow, M.A.; Galko, M.J. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 10017–10022. [Google Scholar] [CrossRef]
- Chaitanya, R.K.; Shashank, K.; Sridevi, P. Oxidative Stress in Invertebrate systems. Free Radic. Dis. 2016, 19, 51–68. [Google Scholar]
- Rantala, M.J.; Jokinen, I.; Kortet, R.; Vainikka, A.; Suhonen, J. Do pheromones reveal male immunocompetence? Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1681–1685. [Google Scholar] [CrossRef]
- Ruiz-Guzmán, G.; Cordero-Molina, S.; Krams, I.; Contreras-Garduño, J. Interactions between oxidative stress and attractiveness to mates and individual mate choice in the beetle Tenebrio molitor. Ethology 2021, 127, 109–116. [Google Scholar] [CrossRef]
- McCallum, M.L.; Matlock, M.; Treas, J.; Safi, B.; Sanson, W.; McCallum, J.L. Endocrine disruption of sexual selection by an estrogenic herbicide in the mealworm beetle (Tenebrio molitor). Ecotoxicology 2013, 22, 1461–1466. [Google Scholar] [CrossRef]
- Vommaro, M.L.; Kurtz, J.; Giglio, A. morphological characterisation of haemocytes in the mealworm beetle Tenebrio molitor (Coleoptera, Tenebrionidae). Insects 2021, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yi, Y. Effects of dietary heavy metals on the immune and antioxidant systems of Galleria Mellonella larvae. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2015, 167, 131–139. [Google Scholar] [CrossRef]
- Cardoso-Jaime, V.; Broderick, N.A.; Maya-Maldonado, K. Metal ions in insect reproduction: A crosstalk between reproductive physiology and immunity. Curr. Opin. Insect Sci. 2022, 52, 100924. [Google Scholar] [CrossRef]
- Coskun, M.; Kayis, T.; Yilmaz, M.; Dursun, O.; Emre, I. Copper and Zinc Impact on stress biomarkers and growth parameters in a model organism, Galleria mellonella Larvae. BioMetals 2021, 34, 1263–1273. [Google Scholar] [CrossRef]
- Augustyniak, M.; Babczyńska, A.; Kozłowski, M.; Sawczyn, T.; Augustyniak, M. Effects of Zinc and female aging on nymphal life history in a grasshopper from polluted sites. J. Insect Physiol. 2008, 54, 41–50. [Google Scholar] [CrossRef]
- Filipiak, M.; Bilska, E.; Tylko, G.; Pyza, E. Effects of Zinc on programmed cell death of Musca domestica and Drosophila Melanogaster Blood Cells. J. Insect Physiol. 2010, 56, 383–390. [Google Scholar] [CrossRef]
- Borowska, J.; Pyza, E. Effects of heavy metals on insect immunocompetent cells. J. Insect Physiol. 2011, 57, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Pipe, R.K.; Coles, J.A.; Carissan, F.M.M.; Ramanathan, K. Copper induced immunomodulation in the marine mussel, Mytilus Edulis. Aquat. Toxicol. 1999, 46, 43–54. [Google Scholar] [CrossRef]
- Lorenzon, S.; Francese, M.; Smith, V.J.; Ferrero, E.A. Heavy metals affect the circulating haemocyte number in the shrimp Palaemon Elegans. Fish Shellfish Immunol. 2001, 11, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.; Iatsenko, I. The roles of metals in insect–microbe interactions and immunity. Curr. Opin. Insect Sci. 2022, 49, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.Q.; Camacho, E.; Thakur, R.; Barron, A.J.; Dong, Y.; Dimopoulos, G.; Broderick, N.A.; Casadevall, A. Glyphosate inhibits melanization and increases susceptibility to infection in insects. PLoS Biol. 2021, 19, e3001182. [Google Scholar] [CrossRef] [PubMed]


| Treatments | ||||||
|---|---|---|---|---|---|---|
| CTRL a | MTB b | TG b | ||||
| Females | Males | Females | Males | Females | Males | |
| Na | 16.25–17.13 | 20.48–19.85 | 0.09–0.09 | 0.07–0.09 | 0.68–0.59 | 0.62–0.65 |
| K | 0.57–0.61 | 0.81–0.68 | 28.89–28.36 | 12.90–15.67 | 0.02–0.02 | 0.02–0.02 |
| Mn | 0.08–0.10 | 0.10–0.13 | 4.04–3.97 | 3.15–4.01 | 0.07–0.06 | 0.05–0.06 |
| Cu | 1.43–1.52 | 1.63–1.68 | 7.49–8.14 | 12.18–16.61 | 5.05–5.63 | 5.58–5.91 |
| Zn | 1.49–1.59 | 1.64–1.68 | 31.74–32.86 | 57.58–67.53 | 2.73–3.10 | 3.17–3.15 |
| Se | 3.05–2.53 | 1.70–2.47 | - | - | 0.68–0.59 | 0.62–0.65 |
| Cr | 0.97–5.28 | 0.20–0.84 | - | - | - | - |
| Cd | 1.15–1.53 | 2.49–1.67 | - | - | - | - |
| Bi | 0–0.45 | 1.41–3.95 | - | - | - | - |
| U | 0–0.08 | 0.04–2.44 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naccarato, A.; Vommaro, M.L.; Amico, D.; Sprovieri, F.; Pirrone, N.; Tagarelli, A.; Giglio, A. Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor. Toxics 2023, 11, 499. https://doi.org/10.3390/toxics11060499
Naccarato A, Vommaro ML, Amico D, Sprovieri F, Pirrone N, Tagarelli A, Giglio A. Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor. Toxics. 2023; 11(6):499. https://doi.org/10.3390/toxics11060499
Chicago/Turabian StyleNaccarato, Attilio, Maria Luigia Vommaro, Domenico Amico, Francesca Sprovieri, Nicola Pirrone, Antonio Tagarelli, and Anita Giglio. 2023. "Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor" Toxics 11, no. 6: 499. https://doi.org/10.3390/toxics11060499
APA StyleNaccarato, A., Vommaro, M. L., Amico, D., Sprovieri, F., Pirrone, N., Tagarelli, A., & Giglio, A. (2023). Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor. Toxics, 11(6), 499. https://doi.org/10.3390/toxics11060499










