Validation of a Method Scope Extension for Simple Biomonitoring of 353 Pollutants in Serum Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Instrumental Analysis
2.2.1. UHPLC-MS/MS
2.2.2. GC-MS/MS
2.3. Sample Preparation and Extraction Procedure
2.4. Validation Experiments/Procedures
2.5. Applicability of the Method
3. Results and Discussion
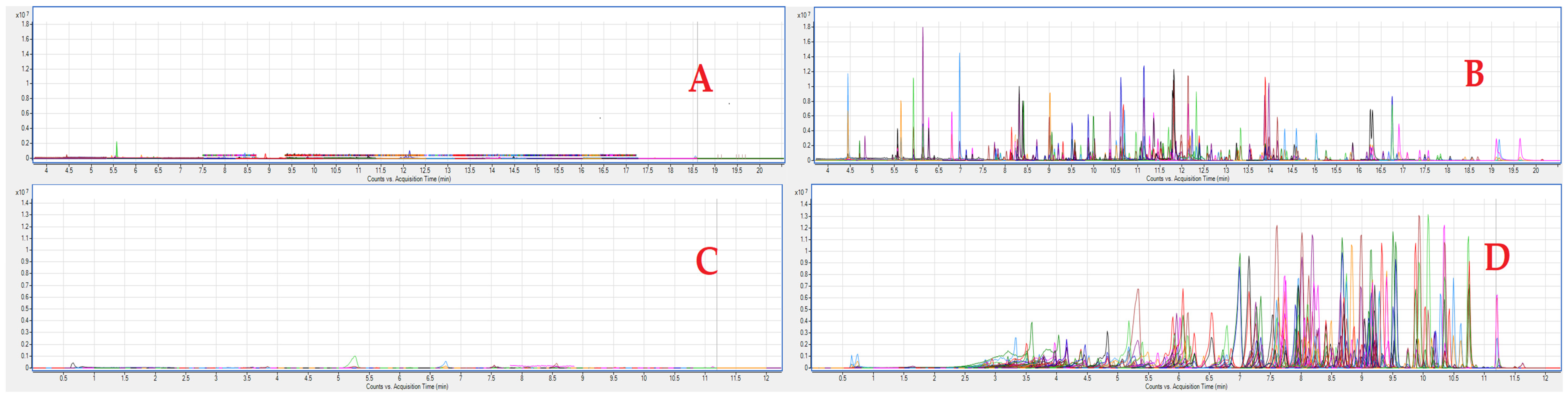
3.1. Optimization of Extraction, Separation and Detection
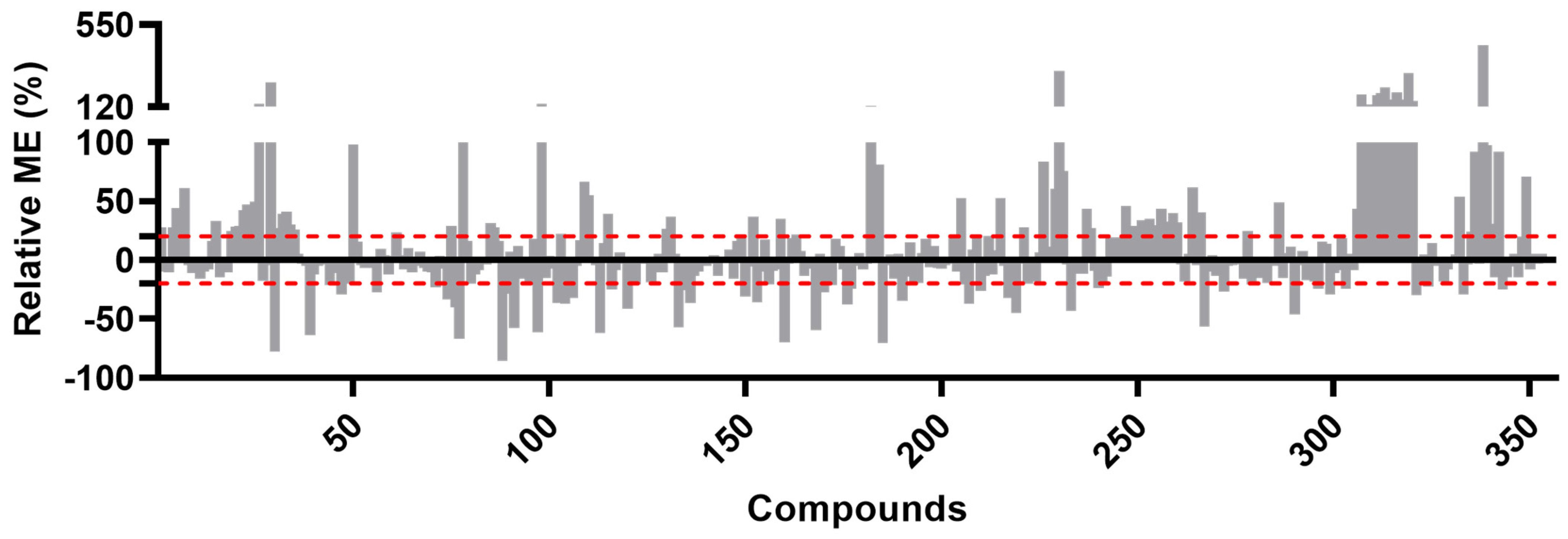
3.2. Validation Experiments
3.3. Application to Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Summary of Method Validation Results (Measured Uncertainty (MU), Recoveries and Inter- and Intraday Precisions (n = 5))
| No. | Compound | MU | 0.3 ng/mL | 1.25 ng/mL | 5 ng/mL | 20 ng/mL | 40 ng/mL | ||||||||||
| Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | Precision (RSD %) | |||||||||||||
| Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | Rec. (%) | Intraday | Interday | |||
| 1 | 2-Phenylphenol | 22.71 | 111.20 | 11.92 | 11.29 | 93.26 | 17.78 | 20.06 | 100.24 | 17.65 | 18.54 | 98.44 | 7.68 | 8.21 | |||
| 2 | 4,4′-Dichlorobenzophenone (metabolite of dicofol) | 24.86 | 93.72 | 17.18 | 19.30 | 113.48 | 15.09 | 14.00 | 89.56 | 13.23 | 15.55 | 99.40 | 14.69 | 15.56 | 103.47 | 8.37 | 8.51 |
| 3 | Abamectine | 31.79 | 97.94 | 13.71 | 14.74 | 98.51 | 11.04 | 11.80 | 101.43 | 10.98 | 11.40 | ||||||
| 4 | Acenaphthene | 25.01 | 94.41 | 15.26 | 17.02 | 97.82 | 4.08 | 4.40 | 94.69 | 11.06 | 12.29 | 96.61 | 18.23 | 19.86 | 101.84 | 8.60 | 8.89 |
| 5 | Acenaphtylene | 33.02 | 114.97 | 21.82 | 19.98 | 100.11 | 14.39 | 15.13 | 98.15 | 18.65 | 20.01 | 98.35 | 11.22 | 12.00 | |||
| 6 | Acephate | 16.55 | 97.41 | 9.85 | 10.65 | 98.76 | 3.85 | 4.10 | 101.74 | 5.62 | 5.82 | ||||||
| 7 | Acetaminophen (Paracetemol) | 16.63 | 118.40 | 15.13 | 13.45 | 87.58 | 11.04 | 13.27 | 99.23 | 7.33 | 7.77 | 102.05 | 5.61 | 5.78 | |||
| 8 | Acetamiprid | 7.32 | 107.58 | 10.19 | 9.97 | 98.11 | 2.90 | 3.11 | 94.21 | 6.35 | 7.09 | 102.36 | 4.51 | 4.64 | 99.41 | 2.48 | 2.63 |
| 9 | Acrinathrin | 25.44 | 87.18 | 12.90 | 15.57 | 96.81 | 13.01 | 14.15 | 100.45 | 8.45 | 8.86 | 98.88 | 8.67 | 9.23 | |||
| 10 | Albendazole | 10.42 | 93.43 | 7.73 | 8.70 | 104.12 | 3.76 | 3.81 | 102.98 | 4.43 | 4.52 | 103.28 | 5.39 | 5.50 | 96.07 | 2.30 | 2.52 |
| 11 | Aldicarb | 9.06 | 99.93 | 8.50 | 8.95 | 98.97 | 1.74 | 1.85 | 98.05 | 6.31 | 6.77 | 102.21 | 4.94 | 5.09 | 98.22 | 2.84 | 3.05 |
| 12 | Aldicarb-sulfone | 8.85 | 105.03 | 3.63 | 3.64 | 90.04 | 3.36 | 3.92 | 98.99 | 6.72 | 7.14 | 102.24 | 2.66 | 2.74 | |||
| 13 | Aldicarb-sulfoxide | 6.33 | 116.10 | 6.35 | 5.75 | 90.44 | 5.73 | 6.66 | 99.84 | 3.55 | 3.75 | 101.22 | 2.02 | 2.10 | |||
| 14 | Aldrin | 31.42 | 106.18 | 18.16 | 18.01 | 106.69 | 16.86 | 16.64 | 88.98 | 15.53 | 18.37 | 95.66 | 8.75 | 9.63 | 102.90 | 10.79 | 11.04 |
| 15 | Anthracene | 18.57 | 100.98 | 10.68 | 11.14 | 96.33 | 11.81 | 12.91 | 92.18 | 9.84 | 11.24 | 103.89 | 5.92 | 6.00 | |||
| 16 | Atrazine | 10.31 | 96.11 | 3.58 | 3.92 | 105.40 | 2.88 | 2.88 | 101.07 | 6.81 | 7.10 | 102.47 | 4.20 | 4.32 | 97.19 | 2.93 | 3.17 |
| 17 | Azinphos-methyl | 8.39 | 101.15 | 8.53 | 8.88 | 103.85 | 6.77 | 6.86 | 99.07 | 10.10 | 10.73 | 102.81 | 4.28 | 4.38 | 97.24 | 2.15 | 2.32 |
| 18 | Azoxystrobin | 10.50 | 98.04 | 6.27 | 6.73 | 104.37 | 2.98 | 3.01 | 97.56 | 6.32 | 6.82 | 99.06 | 5.25 | 5.58 | 100.42 | 3.61 | 3.79 |
| 19 | BDE-28 | 36.70 | 104.31 | 14.98 | 15.12 | 101.58 | 7.56 | 7.83 | 90.05 | 16.18 | 18.91 | 93.67 | 13.52 | 15.19 | 105.17 | 12.45 | 12.46 |
| 20 | BDE-47 | 32.46 | 99.47 | 11.23 | 11.88 | 111.52 | 2.64 | 2.49 | 96.16 | 15.98 | 17.49 | 97.65 | 14.95 | 16.12 | 100.18 | 11.19 | 11.75 |
| 21 | BDE-85 | 58.35 | 94.45 | 19.12 | 21.30 | 104.21 | 17.67 | 17.85 | 92.60 | 10.45 | 11.88 | 94.08 | 10.62 | 11.89 | 100.70 | 20.16 | 21.08 |
| 22 | BDE-99 | 42.81 | 104.66 | 20.57 | 20.68 | 103.83 | 13.88 | 14.08 | 90.67 | 12.91 | 14.99 | 90.83 | 10.23 | 11.86 | 105.41 | 14.66 | 14.63 |
| 23 | BDE-100 | 52.30 | 96.85 | 12.38 | 13.46 | 107.88 | 19.14 | 18.67 | 96.30 | 17.82 | 19.48 | 97.14 | 10.98 | 11.90 | 99.85 | 18.00 | 18.97 |
| 24 | BDE-153 | 36.24 | 102.12 | 18.29 | 18.85 | 104.09 | 16.37 | 16.56 | 95.49 | 18.09 | 19.94 | 99.65 | 14.59 | 15.41 | 101.80 | 12.53 | 12.96 |
| 25 | BDE-154 | 43.70 | 97.71 | 13.27 | 14.29 | 105.03 | 20.19 | 20.24 | 93.86 | 10.08 | 11.31 | 95.11 | 9.40 | 10.41 | 101.40 | 15.13 | 15.70 |
| 26 | BDE-183 | 59.57 | 108.60 | 11.54 | 11.18 | 101.10 | 23.51 | 24.48 | 89.53 | 4.87 | 5.73 | 100.61 | 15.10 | 15.80 | 101.56 | 20.65 | 21.40 |
| 27 | Benalaxyl | 11.73 | 101.52 | 14.48 | 15.01 | 101.59 | 4.49 | 4.65 | 95.51 | 4.74 | 5.22 | 100.23 | 4.63 | 4.86 | 99.99 | 4.04 | 4.25 |
| 28 | Bendiocarb | 8.45 | 105.79 | 7.77 | 7.73 | 95.96 | 4.86 | 5.33 | 97.21 | 5.10 | 5.52 | 102.51 | 5.03 | 5.16 | 98.61 | 2.73 | 2.91 |
| 29 | Bendiocarb metabolite (2, 2-dimethylbenzo-1, 3-dioxol-4-ol) | 54.51 | 96.61 | 12.05 | 13.13 | 92.35 | 5.16 | 5.89 | 102.37 | 18.93 | 19.47 | ||||||
| 30 | Benfuracarb | 52.63 | 97.77 | 12.48 | 13.43 | 112.31 | 14.78 | 13.85 | 105.31 | 20.90 | 20.89 | 95.61 | 17.44 | 19.20 | |||
| 31 | Benzo[a]anthracene | 23.00 | 100.95 | 13.34 | 13.91 | 104.58 | 7.80 | 7.85 | 86.86 | 12.31 | 14.92 | 104.46 | 13.94 | 14.04 | 97.66 | 7.66 | 8.26 |
| 32 | Benzo[a]pyrene | 18.97 | 97.86 | 14.37 | 15.46 | 105.41 | 9.73 | 9.71 | 90.28 | 17.88 | 20.85 | 96.05 | 8.26 | 9.06 | 101.85 | 6.47 | 6.68 |
| 33 | Benzo[b]fluoranthene | 37.06 | 107.89 | 12.46 | 12.16 | 100.81 | 14.27 | 14.90 | 91.94 | 9.57 | 10.95 | 91.76 | 11.46 | 13.15 | 105.88 | 12.46 | 12.39 |
| 34 | Benzo[ghi]perylene | 39.89 | 96.46 | 10.58 | 11.55 | 103.87 | 8.89 | 9.01 | 98.58 | 13.31 | 14.21 | 97.68 | 10.19 | 10.98 | 100.50 | 13.77 | 14.42 |
| 35 | Benzo[k]fluoranthene | 31.40 | 99.90 | 21.36 | 22.51 | 107.59 | 7.48 | 7.32 | 85.41 | 21.81 | 23.88 | 93.19 | 8.18 | 9.24 | 104.38 | 10.62 | 10.71 |
| 36 | Bifenthrin | 25.99 | 107.06 | 8.54 | 8.40 | 109.66 | 6.37 | 6.11 | 90.40 | 11.88 | 13.84 | 103.20 | 9.74 | 9.94 | 98.16 | 8.77 | 9.41 |
| 37 | Bitertanol | 8.81 | 101.72 | 22.45 | 23.23 | 106.23 | 12.39 | 12.28 | 90.03 | 3.62 | 4.23 | 99.49 | 5.66 | 5.99 | 101.65 | 2.84 | 2.94 |
| 38 | Boscalid (formerly nicobifen) | 47.03 | 100.63 | 19.36 | 20.25 | 104.11 | 8.22 | 8.31 | 89.07 | 12.48 | 14.74 | 95.12 | 16.20 | 17.93 | 103.61 | 16.30 | 16.56 |
| 39 | Brodifacoum | 28.30 | 84.19 | 11.76 | 14.71 | 103.80 | 13.59 | 13.78 | 94.44 | 11.45 | 12.76 | 103.36 | 10.95 | 11.15 | 98.97 | 9.67 | 10.28 |
| 40 | Bromadiolone | 25.02 | 90.11 | 18.91 | 22.09 | 95.42 | 11.38 | 12.56 | 94.54 | 4.72 | 5.25 | 102.52 | 12.54 | 12.87 | 99.43 | 8.58 | 9.09 |
| 41 | Bromopropylate | 39.80 | 103.98 | 10.96 | 11.09 | 110.51 | 9.16 | 8.73 | 86.70 | 15.43 | 18.73 | 104.76 | 15.06 | 15.13 | 99.66 | 13.68 | 14.45 |
| 42 | Bromuconazole (two isomers) | 30.91 | 106.55 | 15.23 | 15.17 | 108.81 | 10.70 | 10.20 | 87.97 | 14.45 | 17.32 | 96.38 | 14.51 | 15.79 | 103.14 | 10.58 | 10.82 |
| 43 | Bupirimate | 10.85 | 85.46 | 18.36 | 22.62 | 104.46 | 4.39 | 4.42 | 105.85 | 10.88 | 10.82 | 102.49 | 6.13 | 6.30 | 96.40 | 2.74 | 2.99 |
| 44 | Buprofezin | 18.10 | 95.66 | 3.40 | 3.74 | 107.59 | 7.25 | 7.10 | 96.26 | 5.46 | 5.97 | 99.52 | 5.86 | 6.20 | 100.45 | 6.24 | 6.54 |
| 45 | Cadusafos (ebufos) | 20.57 | 87.81 | 8.36 | 10.02 | 117.52 | 6.95 | 6.23 | 108.46 | 4.48 | 4.35 | 104.39 | 6.46 | 6.51 | 93.16 | 5.09 | 5.75 |
| 46 | Carbaryl | 11.55 | 97.61 | 7.53 | 8.12 | 97.44 | 2.60 | 2.81 | 99.40 | 7.22 | 7.65 | 101.88 | 5.76 | 5.95 | 97.76 | 3.62 | 3.90 |
| 47 | Carbendazim (azole) | 10.14 | 101.48 | 21.84 | 22.65 | 102.71 | 2.20 | 2.26 | 97.10 | 5.65 | 6.12 | 103.15 | 2.87 | 2.93 | 97.85 | 3.13 | 3.37 |
| 48 | Carbofuran | 9.94 | 93.67 | 4.44 | 4.98 | 102.64 | 3.52 | 3.61 | 100.79 | 5.85 | 6.11 | 102.98 | 3.43 | 3.51 | 96.87 | 2.61 | 2.84 |
| 49 | Carbofuran-3-hydroxy | 10.85 | 104.10 | 6.64 | 6.72 | 90.76 | 7.16 | 8.31 | 100.99 | 1.16 | 1.21 | 100.99 | 3.69 | 3.85 | |||
| 50 | Cefuroxima axetil (two isomers) | 10.45 | 107.63 | 10.02 | 9.80 | 97.71 | 3.85 | 4.14 | 92.71 | 5.91 | 6.71 | 102.92 | 5.68 | 5.81 | 99.41 | 3.57 | 3.78 |
| 51 | Chloramphenicol | 15.42 | 88.16 | 14.23 | 16.99 | 98.52 | 14.68 | 15.68 | 101.47 | 5.25 | 5.45 | ||||||
| 52 | Chlorantraniliprole | 9.55 | 108.96 | 15.78 | 15.25 | 99.65 | 6.49 | 6.86 | 95.00 | 6.38 | 7.06 | 101.51 | 3.72 | 3.85 | 99.52 | 3.26 | 3.45 |
| 53 | Chlorfenapyr | 11.15 | 96.05 | 11.01 | 12.06 | 104.19 | 12.01 | 12.14 | 95.66 | 14.47 | 15.92 | 101.29 | 3.76 | 3.91 | |||
| 54 | Chlorfenvinphos | 15.13 | 93.08 | 16.18 | 18.30 | 107.34 | 2.55 | 2.50 | 103.43 | 4.19 | 4.26 | 103.44 | 6.18 | 6.29 | 95.44 | 4.05 | 4.47 |
| 55 | Chlorobenzilate | 21.05 | 97.87 | 14.12 | 15.19 | 108.68 | 7.88 | 7.63 | 98.29 | 16.88 | 18.07 | 95.40 | 12.29 | 13.56 | 101.32 | 7.24 | 7.52 |
| 56 | Chlorophacinone | 55.65 | 94.25 | 20.33 | 24.10 | 105.39 | 13.83 | 13.81 | 100.36 | 19.20 | 20.14 | ||||||
| 57 | Chlorpropham | 39.94 | 92.73 | 11.96 | 13.57 | 110.61 | 9.51 | 9.05 | 96.09 | 13.80 | 15.12 | 98.45 | 14.97 | 16.00 | 100.46 | 13.78 | 14.44 |
| 58 | Chlorpyrifos | 39.83 | 92.57 | 15.38 | 17.49 | 105.52 | 8.87 | 8.85 | 92.26 | 16.56 | 18.89 | 96.32 | 10.16 | 11.10 | 103.09 | 13.77 | 14.06 |
| 59 | Chlorpyrifos methyl | 9.93 | 92.64 | 15.61 | 17.73 | 107.15 | 8.33 | 8.18 | 94.06 | 15.62 | 17.48 | 96.37 | 16.97 | 18.54 | 103.83 | 2.22 | 2.25 |
| 60 | Chlorthal dimethyl | 41.20 | 88.50 | 10.56 | 12.56 | 106.05 | 10.65 | 10.58 | 93.19 | 12.01 | 13.57 | 103.42 | 12.83 | 13.06 | 97.54 | 13.90 | 15.00 |
| 61 | Chrysene | 12.16 | 108.26 | 2.41 | 2.34 | 94.17 | 4.88 | 5.46 | 99.74 | 5.92 | 6.25 | 100.65 | 4.18 | 4.37 | |||
| 62 | Clindamycin | 12.54 | 72.04 | 14.13 | 20.64 | 112.63 | 14.50 | 13.56 | 108.56 | 7.37 | 7.14 | 102.46 | 5.05 | 5.19 | 95.27 | 2.77 | 3.06 |
| 63 | Clofentezine | 7.68 | 99.14 | 24.98 | 24.52 | 94.91 | 9.26 | 10.27 | 101.49 | 5.13 | 5.32 | 99.88 | 2.64 | 2.78 | |||
| 64 | Clothianidin | 10.99 | 98.28 | 11.10 | 11.89 | 97.34 | 9.80 | 10.60 | 100.77 | 5.37 | 5.61 | 99.70 | 3.77 | 3.98 | |||
| 65 | Cortiscosterone 21 acetate | 22.42 | 94.83 | 18.73 | 20.79 | 105.17 | 22.77 | 22.79 | 109.51 | 13.73 | 13.20 | 95.93 | 7.04 | 7.72 | |||
| 66 | Coumachlor | 21.24 | 107.31 | 11.49 | 11.27 | 107.08 | 7.04 | 6.92 | 103.11 | 6.14 | 6.27 | 95.47 | 6.46 | 7.12 | |||
| 67 | Coumaphos | 12.93 | 95.94 | 19.86 | 21.79 | 93.39 | 15.06 | 16.97 | 101.10 | 6.93 | 7.21 | 99.47 | 4.42 | 4.68 | |||
| 68 | Coumatetralyl | 10.65 | 100.62 | 2.32 | 2.43 | 102.04 | 11.53 | 11.89 | 102.08 | 6.66 | 6.87 | 97.23 | 3.08 | 3.34 | |||
| 69 | Cyazofamid | 13.81 | 93.47 | 12.35 | 13.91 | 99.45 | 5.13 | 5.43 | 103.02 | 6.37 | 6.51 | 101.38 | 5.49 | 5.70 | 97.05 | 4.23 | 4.59 |
| 70 | Cyflufenamid | 37.37 | 96.60 | 10.58 | 11.53 | 92.95 | 11.22 | 12.71 | 103.79 | 12.85 | 13.03 | ||||||
| 71 | Cyfluthrin (sum of four isomers) | 24.66 | 98.74 | 12.55 | 13.37 | 101.21 | 6.88 | 7.16 | 100.31 | 8.50 | 8.92 | ||||||
| 72 | Cyhalothrin (lambda isomer) | 10.29 | 107.25 | 5.73 | 5.62 | 95.42 | 6.79 | 7.49 | 101.83 | 4.31 | 4.46 | 99.67 | 3.53 | 3.73 | |||
| 73 | Cymoxanil | 36.58 | 105.21 | 4.95 | 4.95 | 94.59 | 8.18 | 9.11 | 98.88 | 16.00 | 17.03 | 101.34 | 12.65 | 13.14 | |||
| 74 | Cypermethrin (sum of four isomers) | 10.29 | 105.20 | 7.63 | 7.64 | 96.50 | 5.24 | 5.71 | 95.78 | 8.25 | 9.07 | 100.63 | 6.82 | 7.13 | 99.76 | 3.54 | 3.73 |
| 75 | Cyproconazole (two isomers) | 15.50 | 104.49 | 8.19 | 8.25 | 99.15 | 9.59 | 10.18 | 104.91 | 11.76 | 11.80 | 97.82 | 5.06 | 5.45 | |||
| 76 | Cyprodinil | 7.00 | 92.46 | 12.18 | 13.86 | 100.99 | 7.51 | 7.83 | 99.58 | 2.39 | 2.53 | ||||||
| 77 | Cyromazine | 42.82 | 103.52 | 9.90 | 10.06 | 97.41 | 24.47 | 24.44 | 116.05 | 20.00 | 18.14 | ||||||
| 78 | Danofloxacin | 23.86 | 75.98 | 14.99 | 20.77 | 117.29 | 12.71 | 11.40 | 93.72 | 15.08 | 16.94 | 105.40 | 16.03 | 16.01 | 97.73 | 7.97 | 8.58 |
| 79 | Dazomet | 13.54 | 95.56 | 19.38 | 21.35 | 103.50 | 6.07 | 6.17 | 95.26 | 12.19 | 13.47 | 99.29 | 13.17 | 13.96 | 100.39 | 4.66 | 4.89 |
| 80 | Deltamethrin | 33.46 | 93.86 | 20.04 | 22.47 | 105.04 | 15.85 | 15.88 | 92.37 | 16.09 | 18.34 | 92.78 | 13.85 | 15.71 | 109.54 | 9.91 | 9.52 |
| 81 | Demeton-S-methyl | 47.32 | 116.16 | 12.82 | 11.62 | 89.95 | 16.43 | 19.23 | 98.03 | 24.66 | 24.48 | 93.53 | 22.92 | 23.79 | |||
| 82 | Demeton-S-methyl-sulfone (Dioxydemeton) | 39.00 | 111.73 | 11.57 | 10.90 | 90.66 | 10.87 | 12.62 | 100.51 | 13.73 | 14.38 | 100.55 | 13.46 | 14.09 | |||
| 83 | Dexamethasone | 11.06 | 92.13 | 8.53 | 9.75 | 105.54 | 7.70 | 7.68 | 101.60 | 6.30 | 6.53 | 101.94 | 5.30 | 5.47 | 97.20 | 3.23 | 3.50 |
| 84 | Diazinon | 3.06 | 92.46 | 6.30 | 7.18 | 93.38 | 3.99 | 4.50 | 101.49 | 5.21 | 5.41 | 100.06 | 1.05 | 1.11 | |||
| 85 | Dibenzo[a,h]anthracene | 16.68 | 112.64 | 14.38 | 13.43 | 90.03 | 10.00 | 11.69 | 97.97 | 7.56 | 8.12 | 101.52 | 5.69 | 5.90 | |||
| 86 | Dichlorodiphenyldichloroethane (p,p′ DDD) | 37.62 | 95.53 | 16.47 | 18.15 | 107.32 | 7.25 | 7.11 | 89.48 | 15.34 | 18.05 | 90.24 | 18.81 | 21.94 | 106.76 | 12.50 | 12.32 |
| 87 | Dichlorodiphenyldichloroethylene (p,p′ DDE) | 34.11 | 106.07 | 13.76 | 13.66 | 99.57 | 8.40 | 8.88 | 94.20 | 14.12 | 15.78 | 98.30 | 8.73 | 9.35 | 101.23 | 11.79 | 12.26 |
| 88 | Dichlorodiphenyltrichloroethane (p,p′ DDT) | 14.94 | 108.60 | 4.09 | 3.97 | 90.66 | 14.41 | 16.73 | 103.97 | 14.16 | 14.34 | 99.70 | 5.13 | 5.42 | |||
| 89 | Diclofenac | 11.14 | 105.64 | 4.06 | 4.05 | 93.24 | 5.48 | 6.18 | 99.12 | 4.97 | 5.28 | 101.60 | 3.70 | 3.84 | |||
| 90 | Dicloran | 10.23 | 100.39 | 22.88 | 23.99 | 105.92 | 7.34 | 7.29 | 98.48 | 3.34 | 3.57 | ||||||
| 91 | Diclorvos | 38.24 | 111.23 | 23.60 | 22.33 | 93.47 | 17.10 | 19.26 | 94.62 | 11.43 | 12.72 | 102.13 | 13.23 | 13.64 | |||
| 92 | Dieldrin | 11.43 | 98.64 | 14.64 | 15.62 | 102.41 | 3.65 | 3.75 | 96.37 | 6.18 | 6.75 | 100.66 | 6.97 | 7.29 | 99.81 | 3.93 | 4.14 |
| 93 | Diethathyl ethyl | 14.86 | 88.24 | 7.95 | 9.48 | 108.70 | 2.58 | 2.50 | 105.93 | 3.97 | 3.95 | 102.90 | 4.63 | 4.73 | 95.31 | 3.87 | 4.27 |
| 94 | Diethofencarb | 20.11 | 102.72 | 17.08 | 17.51 | 101.57 | 3.86 | 4.00 | 99.43 | 8.58 | 9.09 | 102.32 | 9.12 | 9.38 | 97.62 | 6.64 | 7.16 |
| 95 | Difenacoum | 12.25 | 80.96 | 11.55 | 15.01 | 102.72 | 5.45 | 5.59 | 100.39 | 8.05 | 8.44 | 102.92 | 4.94 | 5.05 | 97.20 | 3.69 | 4.00 |
| 96 | Difenoconazole | 19.48 | 102.74 | 14.93 | 15.30 | 101.47 | 14.00 | 14.52 | 103.11 | 9.15 | 9.34 | 96.67 | 6.19 | 6.74 | |||
| 97 | Difethialone | 45.00 | 101.59 | 7.31 | 7.58 | 94.70 | 14.77 | 16.42 | 103.05 | 15.60 | 15.93 | ||||||
| 98 | Difloxacin | 19.08 | 90.37 | 18.36 | 21.39 | 102.44 | 15.11 | 23.80 | 105.09 | 11.28 | 11.30 | 96.33 | 5.94 | 6.49 | |||
| 99 | Diflubenzuron | 25.99 | 96.88 | 18.65 | 20.26 | 107.54 | 14.71 | 14.39 | 97.24 | 17.62 | 22.90 | 96.41 | 10.42 | 11.37 | 103.53 | 8.77 | 8.92 |
| 100 | Diflufenican | 10.75 | 96.03 | 3.56 | 3.91 | 104.44 | 1.69 | 1.70 | 97.50 | 5.98 | 6.45 | 99.88 | 5.87 | 6.18 | 100.00 | 3.70 | 3.90 |
| 101 | Dimethenamid-P (and its R-isomer) | 9.82 | 102.25 | 5.36 | 5.51 | 95.04 | 6.05 | 6.70 | 101.97 | 4.95 | 5.11 | 99.49 | 3.36 | 3.55 | |||
| 102 | Dimethoate | 12.67 | 94.43 | 16.66 | 18.57 | 100.31 | 6.33 | 6.64 | 101.63 | 9.07 | 9.40 | 101.57 | 7.88 | 8.16 | 97.78 | 4.04 | 4.35 |
| 103 | Dimethomorph (two isomers) | 37.37 | 89.48 | 12.23 | 14.39 | 111.11 | 7.12 | 6.74 | 93.84 | 17.37 | 19.49 | 95.28 | 10.47 | 11.57 | 103.95 | 12.83 | 12.99 |
| 104 | Dimethylphenylsulfamide (DMSA, metabolite of dichlofluanid) | 24.15 | 111.52 | 19.40 | 18.31 | 99.56 | 6.10 | 6.45 | 89.53 | 13.95 | 16.40 | 94.14 | 12.44 | 13.91 | 104.15 | 7.98 | 8.06 |
| 105 | Diniconazole-M | 25.63 | 98.33 | 21.68 | 23.21 | 105.75 | 11.68 | 11.62 | 102.36 | 11.88 | 12.21 | 96.91 | 8.42 | 9.15 | |||
| 106 | Dinocap | 54.01 | 106.98 | 18.75 | 18.45 | 90.90 | 9.62 | 11.14 | 104.03 | 18.77 | 18.99 | ||||||
| 107 | Diphacinone | 30.26 | 70.85 | 17.52 | 19.03 | 105.76 | 17.44 | 17.36 | 95.74 | 15.70 | 17.26 | 97.12 | 16.78 | 18.18 | 101.09 | 10.45 | 10.88 |
| 108 | Diphenylamine | 30.91 | 101.83 | 7.15 | 7.39 | 96.13 | 10.34 | 11.32 | 101.68 | 9.00 | 9.32 | 99.37 | 10.60 | 11.23 | |||
| 109 | N,N-dimethylformamidine (DMF, metabolite of amitraz) | 26.22 | 94.68 | 6.70 | 7.45 | 103.33 | 11.73 | 11.94 | 97.94 | 8.82 | 9.48 | ||||||
| 110 | Dodine | 11.59 | 110.75 | 4.01 | 3.81 | 93.52 | 7.93 | 8.93 | 100.22 | 6.75 | 7.09 | 100.39 | 3.99 | 4.18 | |||
| 111 | Endosulfan alfa | 17.85 | 98.84 | 3.86 | 4.11 | 100.61 | 4.54 | 4.75 | 101.95 | 7.04 | 7.27 | 97.48 | 5.82 | 6.28 | |||
| 112 | Endosulfan beta | 8.72 | 91.54 | 6.43 | 7.39 | 100.39 | 5.32 | 5.57 | 103.11 | 6.74 | 6.88 | 97.25 | 2.30 | 2.49 | |||
| 113 | Endosulfan sulfate | 44.34 | 102.03 | 12.59 | 12.99 | 106.47 | 16.24 | 16.06 | 91.46 | 14.51 | 16.70 | 98.19 | 11.21 | 12.02 | 102.13 | 15.37 | 15.84 |
| 114 | Endrin | 28.51 | 105.62 | 11.47 | 11.43 | 104.87 | 14.89 | 14.94 | 90.35 | 12.35 | 14.39 | 97.33 | 10.72 | 11.59 | 103.11 | 9.73 | 9.94 |
| 115 | Enrofloxacin | 49.49 | 115.11 | 10.88 | 9.95 | 92.23 | 10.15 | 11.58 | 90.60 | 16.66 | 19.36 | 101.21 | 23.80 | 24.76 | 103.66 | 24.24 | 24.62 |
| 116 | EPN | 30.69 | 118.43 | 4.97 | 4.42 | 93.36 | 21.86 | 24.65 | 96.81 | 15.31 | 16.65 | 106.31 | 9.94 | 9.84 | |||
| 117 | Epoxiconazole | 44.58 | 98.81 | 18.22 | 19.41 | 96.32 | 24.41 | 24.67 | 103.51 | 22.51 | 22.89 | ||||||
| 118 | Eprinomectin | 47.23 | 109.58 | 10.88 | 10.45 | 99.07 | 6.16 | 6.54 | 86.37 | 12.62 | 15.38 | 103.27 | 17.33 | 17.67 | 98.92 | 16.16 | 17.19 |
| 119 | Eritromicin | 10.61 | 112.06 | 10.02 | 9.42 | 95.45 | 5.64 | 6.23 | 92.98 | 7.30 | 8.26 | 101.46 | 5.40 | 5.60 | 99.72 | 3.64 | 3.85 |
| 120 | Esfenvalerate | 47.92 | 99.09 | 14.55 | 15.45 | 94.54 | 8.79 | 9.78 | 91.95 | 14.85 | 17.00 | ||||||
| 121 | Ethion (diethion) | 17.38 | 107.84 | 6.55 | 6.40 | 100.92 | 3.32 | 3.47 | 94.93 | 4.63 | 5.13 | 102.77 | 6.04 | 6.19 | 98.59 | 5.86 | 6.26 |
| 122 | Ethirimol | 28.11 | 97.99 | 14.21 | 15.26 | 94.70 | 16.88 | 18.77 | 98.41 | 12.08 | 12.92 | 101.62 | 9.70 | 10.05 | |||
| 123 | Ethofumesate | 16.21 | 91.25 | 11.64 | 13.43 | 110.47 | 4.31 | 4.11 | 106.91 | 6.92 | 6.81 | 102.24 | 5.81 | 5.98 | 95.56 | 4.56 | 5.02 |
| 124 | Ethoprophos | 10.21 | 101.69 | 2.29 | 2.37 | 92.72 | 7.46 | 8.47 | 102.49 | 4.21 | 4.32 | 99.82 | 3.51 | 3.70 | |||
| 125 | Etofenprox | 53.62 | 100.20 | 15.93 | 16.73 | 109.55 | 5.22 | 5.02 | 92.07 | 14.26 | 16.30 | 98.97 | 18.40 | 19.57 | 109.18 | 18.13 | 17.48 |
| 126 | Etoxazole | 12.52 | 82.17 | 11.50 | 14.74 | 106.77 | 4.94 | 4.87 | 103.52 | 6.52 | 6.63 | 102.93 | 5.05 | 5.17 | 95.93 | 3.20 | 3.52 |
| 127 | Famoxadone | 29.18 | 85.42 | 14.45 | 17.81 | 115.74 | 6.23 | 5.67 | 94.81 | 12.30 | 13.65 | 98.59 | 14.01 | 14.96 | 100.27 | 10.06 | 10.56 |
| 128 | Fenamidone | 34.54 | 96.20 | 9.43 | 10.31 | 106.75 | 3.34 | 3.29 | 97.38 | 5.53 | 5.98 | 97.70 | 6.43 | 6.93 | 104.34 | 11.77 | 11.87 |
| 129 | Fenamiphos | 16.04 | 103.97 | 16.68 | 16.89 | 98.60 | 13.26 | 14.16 | 103.76 | 6.17 | 6.26 | 96.77 | 4.97 | 5.40 | |||
| 130 | Fenamiphos sulfone | 21.54 | 79.80 | 11.11 | 14.66 | 104.85 | 11.91 | 11.95 | 104.61 | 13.51 | 13.59 | 102.75 | 7.74 | 7.93 | 95.11 | 6.43 | 7.12 |
| 131 | Fenamiphos sulfoxide | 13.37 | 103.04 | 14.97 | 15.29 | 97.12 | 7.01 | 7.60 | 98.03 | 12.13 | 13.02 | 100.27 | 6.16 | 6.46 | 99.91 | 4.60 | 4.85 |
| 132 | Fenarimol | 15.17 | 107.00 | 8.52 | 8.38 | 102.18 | 3.35 | 3.45 | 93.40 | 6.06 | 6.83 | 100.78 | 6.32 | 6.60 | 100.05 | 5.23 | 5.50 |
| 133 | Fenazaquin | 13.05 | 105.73 | 16.63 | 16.56 | 96.32 | 6.58 | 7.19 | 99.41 | 5.94 | 6.29 | 99.69 | 4.48 | 4.73 | |||
| 134 | Fenbendazole | 42.78 | 96.23 | 19.95 | 21.82 | 109.39 | 5.34 | 5.14 | 89.90 | 10.82 | 12.66 | 95.35 | 17.12 | 18.90 | 93.89 | 13.64 | 15.30 |
| 135 | Fenbuconazole | 19.60 | 98.06 | 10.35 | 11.12 | 102.23 | 3.41 | 3.51 | 96.79 | 6.16 | 6.70 | 98.96 | 6.15 | 6.54 | 100.65 | 6.76 | 7.07 |
| 136 | Fenbutatin oxide | 10.78 | 97.94 | 8.11 | 8.72 | 99.88 | 1.64 | 1.73 | 103.72 | 7.34 | 7.44 | 103.52 | 7.08 | 7.20 | 95.97 | 2.41 | 2.64 |
| 137 | Fenhexamid | 40.07 | 90.08 | 8.82 | 10.30 | 114.44 | 14.05 | 12.92 | 94.27 | 11.50 | 12.84 | 93.50 | 13.12 | 14.77 | 103.85 | 13.81 | 14.00 |
| 138 | Fenitrothion | 40.91 | 95.76 | 12.40 | 13.64 | 95.28 | 7.34 | 8.11 | 91.70 | 5.97 | 6.85 | 99.29 | 10.07 | 10.68 | 101.66 | 14.16 | 14.66 |
| 139 | Fenoxycarb | 12.29 | 86.84 | 13.72 | 16.63 | 91.58 | 17.17 | 19.73 | 100.48 | 9.06 | 9.49 | 99.83 | 4.23 | 4.46 | |||
| 140 | Fenpropathrin | 35.65 | 86.56 | 20.78 | 24.27 | 81.25 | 14.38 | 18.63 | 98.13 | 18.26 | 19.58 | 103.85 | 12.22 | 12.39 | |||
| 141 | Fenpropidin | 12.15 | 95.06 | 6.49 | 7.19 | 106.46 | 8.00 | 7.91 | 105.99 | 8.98 | 8.92 | 102.43 | 7.54 | 7.75 | 95.33 | 2.61 | 2.88 |
| 142 | Fenpropimorph | 35.90 | 103.28 | 13.33 | 13.58 | 90.34 | 13.75 | 16.02 | 103.55 | 6.98 | 7.09 | 97.50 | 12.08 | 13.04 | |||
| 143 | Fenpyroximate | 12.14 | 95.44 | 5.24 | 5.78 | 101.85 | 2.69 | 2.78 | 102.16 | 7.02 | 7.24 | 101.62 | 6.06 | 6.28 | 97.48 | 3.75 | 4.05 |
| 144 | Fenthion | 9.38 | 90.36 | 7.35 | 8.56 | 98.88 | 2.96 | 3.15 | 97.47 | 6.22 | 6.72 | 102.02 | 6.88 | 7.10 | 97.91 | 2.86 | 3.08 |
| 145 | Fenthion oxon | 10.55 | 84.90 | 13.69 | 16.97 | 103.86 | 2.51 | 2.55 | 104.88 | 6.93 | 6.95 | 103.80 | 6.09 | 6.18 | 95.42 | 1.75 | 1.93 |
| 146 | Fenthion oxon sulfone | 20.43 | 96.71 | 11.10 | 12.08 | 102.96 | 5.14 | 5.25 | 88.90 | 15.24 | 18.05 | 99.53 | 6.94 | 7.34 | 101.39 | 7.02 | 7.29 |
| 147 | Fenthion oxon sulfoxide | 9.90 | 85.66 | 11.30 | 13.88 | 105.85 | 4.41 | 4.39 | 101.77 | 5.58 | 5.78 | 102.66 | 5.41 | 5.54 | 96.46 | 2.33 | 2.55 |
| 148 | Fenthion sulfone | 10.05 | 107.97 | 6.70 | 6.53 | 92.79 | 4.16 | 4.71 | 100.24 | 5.77 | 6.06 | 100.92 | 3.42 | 3.57 | |||
| 149 | Fenthion sulfoxide | 7.50 | 99.96 | 4.85 | 5.10 | 92.76 | 6.52 | 7.39 | 101.15 | 5.18 | 5.39 | 99.99 | 2.58 | 2.72 | |||
| 150 | Fenvalerate | 18.02 | 106.51 | 10.34 | 10.22 | 94.20 | 4.12 | 4.60 | 100.52 | 7.73 | 8.10 | 100.12 | 6.21 | 6.53 | |||
| 151 | Fipronil | 10.85 | 102.94 | 2.32 | 2.37 | 96.57 | 4.16 | 4.54 | 101.76 | 6.99 | 7.23 | 98.21 | 3.49 | 3.74 | |||
| 152 | Fipronil sulfide | 27.97 | 100.06 | 11.14 | 11.72 | 96.61 | 12.67 | 13.81 | 98.42 | 12.29 | 13.14 | 100.61 | 9.65 | 10.10 | |||
| 153 | Flocoumafen | 32.20 | 103.31 | 18.68 | 19.03 | 106.30 | 11.28 | 11.17 | 84.22 | 19.69 | 24.61 | 92.29 | 17.76 | 20.25 | 106.01 | 10.60 | 10.52 |
| 154 | Fluazinam | 46.45 | 114.73 | 14.06 | 12.90 | 95.47 | 20.10 | 22.16 | 92.11 | 16.31 | 18.64 | 103.77 | 16.09 | 16.32 | |||
| 155 | Flubendiamide | 24.22 | 86.88 | 15.73 | 19.06 | 102.45 | 7.19 | 7.38 | 105.55 | 9.72 | 9.70 | 103.52 | 13.19 | 13.41 | 94.98 | 7.38 | 8.18 |
| 156 | Flucythrinate (two isomers) | 10.65 | 100.27 | 9.51 | 9.98 | 96.64 | 4.82 | 5.25 | 100.57 | 5.45 | 5.70 | 100.22 | 3.67 | 3.85 | |||
| 157 | Fludioxonil | 8.71 | 104.15 | 20.63 | 20.85 | 95.66 | 13.35 | 14.69 | 103.64 | 8.36 | 8.49 | 97.76 | 2.54 | 2.74 | |||
| 158 | Flufenoxuron | 33.79 | 98.86 | 17.23 | 18.38 | 109.09 | 6.89 | 6.63 | 88.18 | 11.93 | 14.25 | 96.14 | 14.12 | 15.49 | 100.17 | 11.63 | 12.25 |
| 159 | Flumequine | 38.28 | 91.35 | 16.93 | 19.51 | 109.03 | 17.09 | 16.50 | 95.00 | 11.81 | 13.08 | 96.70 | 9.83 | 10.70 | 107.39 | 12.61 | 12.36 |
| 160 | Flunixin | 27.62 | 97.46 | 6.48 | 7.00 | 107.67 | 9.38 | 9.17 | 103.82 | 9.75 | 9.89 | 95.15 | 8.67 | 9.59 | |||
| 161 | Fluopyram | 21.85 | 105.27 | 4.00 | 4.00 | 96.44 | 4.45 | 4.86 | 102.22 | 5.46 | 5.63 | 103.09 | 6.89 | 7.04 | 97.63 | 7.25 | 7.82 |
| 162 | Fluoranthene | 13.72 | 104.43 | 8.69 | 8.76 | 100.36 | 3.65 | 3.83 | 104.45 | 4.68 | 4.72 | 96.53 | 4.00 | 4.36 | |||
| 163 | Fluorene | 20.70 | 101.43 | 23.18 | 24.05 | 113.31 | 18.05 | 16.77 | 89.67 | 14.11 | 16.57 | 98.30 | 14.65 | 15.69 | 101.25 | 7.12 | 7.41 |
| 164 | Fluquinconazole | 29.95 | 95.42 | 20.55 | 22.67 | 100.08 | 8.51 | 8.95 | 90.35 | 13.57 | 15.81 | 98.39 | 14.17 | 15.16 | 101.82 | 10.33 | 10.68 |
| 165 | Flusilazole | 32.98 | 126.43 | 13.07 | 10.88 | 97.89 | 10.72 | 11.52 | 97.24 | 23.61 | 24.55 | 100.38 | 11.38 | 11.93 | |||
| 166 | Flutolanil | 60.00 | 92.66 | 12.40 | 14.08 | 101.18 | 14.94 | 15.54 | 95.13 | 19.18 | 21.22 | 93.62 | 15.30 | 17.20 | 101.91 | 20.93 | 21.62 |
| 167 | Flutriafol | 16.79 | 86.68 | 17.60 | 21.37 | 96.15 | 16.21 | 17.75 | 106.59 | 16.32 | 16.11 | 103.95 | 5.99 | 6.07 | 95.04 | 4.54 | 5.03 |
| 168 | Fluvalinate tau | 11.32 | 92.52 | 12.02 | 13.68 | 106.67 | 5.42 | 5.35 | 96.33 | 5.22 | 5.70 | 100.64 | 5.53 | 5.78 | 99.84 | 3.89 | 4.11 |
| 169 | Fonofos | 24.20 | 90.07 | 8.01 | 9.36 | 108.25 | 4.63 | 4.50 | 94.23 | 12.72 | 14.21 | 98.24 | 7.48 | 8.01 | 101.48 | 8.34 | 8.65 |
| 170 | Formetanate | 41.20 | 99.70 | 3.01 | 3.18 | 93.95 | 8.37 | 9.38 | 96.01 | 13.07 | 14.33 | 101.90 | 14.27 | 14.74 | |||
| 171 | Fosthiazate | 28.65 | 89.32 | 16.62 | 19.59 | 101.72 | 6.97 | 7.22 | 90.25 | 10.09 | 11.77 | 93.78 | 16.03 | 17.99 | 106.61 | 9.04 | 8.93 |
| 172 | Heptachlor | 56.79 | 98.56 | 14.43 | 15.41 | 104.04 | 21.87 | 22.13 | 99.77 | 19.53 | 20.61 | ||||||
| 173 | Hexachlorobencene | 10.35 | 95.58 | 6.30 | 6.94 | 101.59 | 1.16 | 1.20 | 99.57 | 6.00 | 6.34 | 102.20 | 5.31 | 5.47 | 97.65 | 3.14 | 3.38 |
| 174 | Hexachlorocyclohexane (alpha) | 29.68 | 100.23 | 18.01 | 18.91 | 101.88 | 7.48 | 7.73 | 86.97 | 13.33 | 16.13 | 97.06 | 15.73 | 17.06 | 104.25 | 10.01 | 10.10 |
| 175 | Hexachlorocyclohexane (beta) | 37.80 | 87.87 | 7.48 | 8.96 | 105.32 | 11.04 | 11.03 | 96.62 | 14.46 | 15.76 | 103.13 | 20.13 | 20.55 | 108.95 | 11.97 | 11.57 |
| 176 | Hexachlorocyclohexane (delta) | 8.40 | 92.16 | 12.43 | 14.19 | 101.83 | 9.13 | 9.44 | 89.92 | 14.21 | 16.64 | 102.61 | 14.75 | 15.13 | 100.23 | 2.89 | 3.04 |
| 177 | Hexaclorocyclohexane (gamma, lindane) | 29.92 | 100.36 | 8.08 | 8.47 | 103.61 | 10.52 | 10.69 | 96.46 | 21.78 | 23.76 | 99.08 | 20.02 | 21.28 | 102.03 | 10.31 | 10.64 |
| 178 | Hexaconazole (two isomers) | 31.46 | 100.10 | 10.03 | 10.55 | 88.50 | 11.45 | 13.62 | 97.93 | 18.94 | 20.36 | 103.54 | 10.75 | 10.93 | |||
| 179 | Hexaflumuron | 35.88 | 97.06 | 9.57 | 10.38 | 113.20 | 23.40 | 21.76 | 92.15 | 10.14 | 11.58 | 96.83 | 10.30 | 11.20 | 103.17 | 12.36 | 12.61 |
| 180 | Hexythiazox | 15.43 | 97.80 | 10.18 | 10.95 | 105.35 | 8.07 | 8.06 | 104.96 | 5.88 | 5.89 | 94.59 | 3.67 | 4.09 | |||
| 181 | Imazalil (enilconazole) | 18.45 | 97.75 | 14.01 | 15.09 | 101.04 | 5.97 | 6.22 | 100.01 | 7.81 | 8.22 | 101.55 | 6.69 | 6.94 | 97.44 | 6.02 | 6.50 |
| 182 | Imidacloprid | 7.84 | 101.35 | 22.14 | 22.99 | 98.35 | 3.04 | 3.25 | 92.25 | 5.84 | 6.66 | 101.60 | 6.37 | 6.60 | 100.02 | 2.70 | 2.84 |
| 183 | Indeno [1,2,3-cd] pyrene | 12.29 | 97.23 | 11.55 | 12.50 | 92.72 | 10.42 | 11.83 | 98.82 | 4.88 | 5.20 | 101.76 | 4.09 | 4.24 | |||
| 184 | Indoxacarb | 32.38 | 99.75 | 15.92 | 16.80 | 106.45 | 6.90 | 6.82 | 95.30 | 14.81 | 16.36 | 102.04 | 15.42 | 15.91 | 99.49 | 11.11 | 11.76 |
| 185 | Iprodione | 26.46 | 97.52 | 2.64 | 2.85 | 95.46 | 9.30 | 10.25 | 103.31 | 11.12 | 11.33 | 98.00 | 8.91 | 9.57 | |||
| 186 | Iprovalicarb | 48.25 | 103.44 | 9.99 | 10.17 | 86.34 | 15.08 | 18.38 | 101.33 | 23.37 | 24.28 | 97.77 | 16.35 | 17.60 | |||
| 187 | Isocarbophos | 16.58 | 81.00 | 9.98 | 12.97 | 111.09 | 0.64 | 0.61 | 107.48 | 7.55 | 7.39 | 104.41 | 5.94 | 5.98 | 93.67 | 3.56 | 4.00 |
| 188 | Isofenphos methyl | 26.73 | 90.40 | 17.60 | 20.50 | 113.42 | 13.97 | 12.96 | 92.51 | 16.14 | 18.37 | 95.85 | 11.95 | 13.12 | 102.59 | 9.15 | 9.39 |
| 189 | Isoprothiolane | 10.92 | 102.55 | 14.09 | 14.46 | 100.61 | 2.07 | 2.17 | 99.19 | 7.14 | 7.58 | 100.34 | 5.36 | 5.63 | 99.87 | 3.76 | 3.96 |
| 190 | Ivermectin B1a | 16.57 | 89.05 | 14.13 | 16.70 | 112.83 | 15.13 | 14.12 | 94.12 | 10.84 | 12.12 | 96.43 | 7.65 | 8.35 | 102.21 | 5.56 | 5.73 |
| 191 | Josamycin | 43.99 | 101.89 | 20.70 | 21.39 | 99.76 | 11.29 | 11.91 | 101.30 | 11.89 | 12.35 | 99.69 | 15.12 | 15.97 | |||
| 192 | Ketoprofen | 15.53 | 114.79 | 8.64 | 7.93 | 97.82 | 4.08 | 4.39 | 94.19 | 4.56 | 5.10 | 101.27 | 6.16 | 6.40 | 100.54 | 5.35 | 5.60 |
| 193 | Kresoxim methyl | 9.41 | 102.73 | 18.09 | 18.53 | 97.66 | 8.80 | 9.49 | 97.14 | 6.24 | 6.76 | 101.48 | 3.10 | 3.21 | |||
| 194 | Leptophos | 18.10 | 99.43 | 13.59 | 14.39 | 101.04 | 16.02 | 16.69 | 104.54 | 5.93 | 5.97 | 96.08 | 5.50 | 6.03 | |||
| 195 | Levamisole | 56.89 | 105.30 | 19.15 | 19.14 | 93.77 | 8.40 | 9.43 | 85.56 | 10.53 | 12.95 | 95.50 | 15.78 | 17.39 | 104.79 | 19.78 | 19.87 |
| 196 | Lincomycin | 12.57 | 93.07 | 12.69 | 14.36 | 99.86 | 11.37 | 11.98 | 98.92 | 2.42 | 2.58 | 100.26 | 4.33 | 4.55 | |||
| 197 | Linuron | 23.14 | 106.60 | 13.65 | 13.48 | 99.47 | 6.16 | 6.52 | 97.09 | 13.74 | 14.90 | 101.71 | 7.95 | 8.23 | |||
| 198 | Lufenuron | 38.43 | 96.22 | 15.91 | 17.40 | 94.36 | 21.11 | 23.55 | 93.60 | 21.32 | 23.11 | 105.14 | 13.09 | 13.10 | |||
| 199 | Malaoxon | 9.79 | 101.17 | 7.70 | 8.01 | 97.98 | 9.53 | 10.24 | 103.11 | 4.58 | 4.68 | 97.64 | 2.92 | 3.14 | |||
| 200 | Malathion | 11.22 | 82.62 | 13.11 | 16.71 | 100.25 | 6.92 | 7.26 | 105.86 | 13.54 | 13.47 | 102.32 | 7.46 | 7.68 | 95.75 | 2.46 | 2.71 |
| 201 | Mandipropamid | 12.89 | 93.28 | 5.57 | 6.29 | 105.42 | 0.28 | 0.28 | 103.46 | 5.54 | 5.63 | 102.64 | 4.44 | 4.55 | 96.42 | 3.62 | 3.95 |
| 202 | Mebendazole | 14.17 | 101.04 | 17.64 | 18.38 | 103.15 | 3.49 | 3.56 | 96.36 | 6.42 | 7.01 | 100.18 | 4.99 | 5.25 | 99.95 | 4.88 | 5.14 |
| 203 | Mefenamic acid | 13.26 | 90.24 | 9.15 | 10.67 | 108.42 | 3.34 | 3.24 | 101.01 | 6.31 | 6.57 | 103.48 | 6.31 | 6.42 | 95.81 | 3.46 | 3.80 |
| 204 | Mefenoxam (metalaxyl-M) | 10.25 | 103.36 | 12.88 | 13.12 | 102.13 | 3.34 | 3.44 | 96.29 | 5.54 | 6.06 | 101.36 | 5.62 | 5.83 | 99.72 | 3.52 | 3.72 |
| 205 | Meloxicam | 27.03 | 111.41 | 23.58 | 22.28 | 87.66 | 7.84 | 9.41 | 101.76 | 10.58 | 10.95 | 99.94 | 9.31 | 9.80 | |||
| 206 | Mepanipyrim | 13.48 | 97.45 | 11.59 | 12.52 | 104.03 | 4.06 | 4.10 | 96.00 | 5.27 | 5.78 | 100.55 | 4.75 | 4.97 | 99.83 | 4.64 | 4.89 |
| 207 | Mepiquat | 20.05 | 104.81 | 8.31 | 8.34 | 91.46 | 8.61 | 9.90 | 98.44 | 4.48 | 4.79 | 100.48 | 6.91 | 7.24 | |||
| 208 | Metaflumizone | 34.41 | 116.29 | 9.64 | 8.72 | 88.22 | 13.33 | 15.90 | 95.73 | 10.36 | 11.39 | 103.80 | 11.78 | 11.95 | |||
| 209 | Metalaxyl | 9.37 | 102.27 | 7.69 | 7.92 | 92.08 | 6.45 | 7.37 | 100.44 | 7.75 | 8.12 | 100.70 | 3.20 | 3.35 | |||
| 210 | Metaldehyde | 24.07 | 105.40 | 9.77 | 9.76 | 102.17 | 7.06 | 7.27 | 97.79 | 4.17 | 4.49 | 99.76 | 4.73 | 4.99 | 100.25 | 8.30 | 8.71 |
| 211 | Metconazole | 20.49 | 104.02 | 15.43 | 15.62 | 108.15 | 9.70 | 9.44 | 91.84 | 10.50 | 12.03 | 98.19 | 11.35 | 12.16 | 101.68 | 7.02 | 7.27 |
| 212 | Methamidophos (two isomers) | 2.94 | 110.87 | 18.67 | 17.72 | 90.48 | 6.12 | 7.12 | 100.47 | 2.55 | 2.68 | 100.92 | 0.80 | 0.83 | |||
| 213 | Methidathion | 11.98 | 97.05 | 8.53 | 9.25 | 103.75 | 4.62 | 4.69 | 99.93 | 6.66 | 7.01 | 98.82 | 4.46 | 4.75 | 100.30 | 4.13 | 4.33 |
| 214 | Methiocarb | 15.30 | 93.85 | 7.21 | 8.09 | 98.11 | 7.61 | 8.16 | 101.60 | 5.20 | 5.38 | ||||||
| 215 | Methiocarb-sufone | 8.59 | 95.91 | 4.45 | 4.88 | 101.31 | 2.62 | 2.73 | 94.97 | 7.05 | 7.81 | 100.51 | 5.19 | 5.43 | 99.78 | 2.95 | 3.11 |
| 216 | Methiocarb-sulfoxide | 13.23 | 88.78 | 5.90 | 6.99 | 104.86 | 6.82 | 6.84 | 104.54 | 6.17 | 6.21 | 101.82 | 5.30 | 5.48 | 96.86 | 3.94 | 4.28 |
| 217 | Methomyl | 7.40 | 108.31 | 5.15 | 5.01 | 93.67 | 7.23 | 8.12 | 98.37 | 5.92 | 6.33 | 101.47 | 2.36 | 2.45 | |||
| 218 | Methoxyfenozide | 9.06 | 101.82 | 3.15 | 3.25 | 91.64 | 7.20 | 8.27 | 102.14 | 3.70 | 3.81 | 100.27 | 3.12 | 3.28 | |||
| 219 | Metoxychlor | 10.18 | 99.59 | 3.79 | 4.00 | 97.39 | 6.35 | 6.86 | 102.08 | 3.77 | 3.89 | 98.23 | 3.26 | 3.49 | |||
| 220 | Metrafenone | 54.30 | 109.03 | 19.34 | 18.67 | 86.42 | 18.34 | 22.34 | 101.53 | 19.54 | 20.25 | 105.46 | 18.82 | 18.79 | |||
| 221 | Metronidazole | 11.22 | 97.19 | 11.15 | 12.07 | 103.48 | 4.54 | 4.62 | 97.54 | 5.86 | 6.33 | 99.29 | 6.04 | 6.40 | 100.33 | 3.86 | 4.05 |
| 222 | Mevinphos (phosdrin) | 14.05 | 88.35 | 12.61 | 15.02 | 114.31 | 1.58 | 1.46 | 104.46 | 7.37 | 7.42 | 103.89 | 5.30 | 5.37 | 95.13 | 3.47 | 3.83 |
| 223 | Mirex | 11.87 | 111.14 | 9.41 | 8.91 | 90.78 | 6.53 | 7.57 | 97.50 | 7.31 | 7.89 | 102.24 | 3.83 | 3.94 | |||
| 224 | Monocrotophos | 6.77 | 111.72 | 7.23 | 6.81 | 92.90 | 9.23 | 10.46 | 99.61 | 3.94 | 4.16 | 100.46 | 2.32 | 2.43 | |||
| 225 | Moxidectin | 41.02 | 98.76 | 13.82 | 14.73 | 103.02 | 14.72 | 15.04 | 88.79 | 12.89 | 15.28 | 97.74 | 17.16 | 18.48 | 99.42 | 14.08 | 14.91 |
| 226 | Myclobutanil | 6.85 | 110.18 | 3.93 | 3.76 | 92.89 | 9.45 | 10.71 | 100.33 | 5.38 | 5.65 | 100.68 | 2.32 | 2.43 | |||
| 227 | N-(2,4-dimethylphenyl)-N’-methylformamidine (DMPF, metabolite of amitraz) | 42.13 | 106.14 | 14.98 | 14.85 | 89.18 | 9.08 | 10.72 | 99.36 | 14.87 | 15.75 | 101.61 | 14.59 | 15.11 | |||
| 228 | N,N-Dimethyl-N’-p-tolylsulphamide (DMST, metabolite of tolyfluanid) | 10.56 | 97.35 | 10.02 | 10.83 | 104.27 | 7.42 | 7.49 | 98.60 | 6.49 | 6.93 | 98.66 | 6.22 | 6.64 | 100.29 | 3.64 | 3.82 |
| 229 | Naphtalene | 18.07 | 104.69 | 24.28 | 24.42 | 84.22 | 12.02 | 15.02 | 91.04 | 21.60 | 24.98 | 105.57 | 5.04 | 5.02 | |||
| 230 | Naproxen | 18.08 | 104.76 | 11.99 | 12.05 | 97.40 | 2.15 | 2.32 | 100.44 | 6.23 | 6.53 | ||||||
| 231 | Novobiocin | 23.78 | 86.15 | 21.57 | 24.36 | 101.53 | 6.13 | 6.35 | 101.37 | 8.19 | 8.51 | ||||||
| 232 | Nuarimol | 21.37 | 95.42 | 14.07 | 15.53 | 114.10 | 20.37 | 18.79 | 96.41 | 18.11 | 19.77 | 97.46 | 10.74 | 11.60 | 101.04 | 7.36 | 7.67 |
| 233 | Ofurace | 44.92 | 99.42 | 7.14 | 7.56 | 104.81 | 7.72 | 7.75 | 90.11 | 11.50 | 13.43 | 100.46 | 18.07 | 18.94 | 102.96 | 15.57 | 15.92 |
| 234 | Omethoate | 8.16 | 104.84 | 7.41 | 7.44 | 91.49 | 6.43 | 7.40 | 98.85 | 4.19 | 4.46 | 102.14 | 2.42 | 2.49 | |||
| 235 | Oxadixyl | 9.41 | 97.67 | 5.38 | 5.80 | 95.25 | 8.26 | 9.12 | 101.81 | 5.13 | 5.30 | 99.40 | 3.21 | 3.39 | |||
| 236 | Oxamyl | 4.96 | 109.00 | 5.35 | 5.17 | 99.81 | 5.00 | 5.28 | 94.77 | 5.40 | 6.00 | 102.24 | 4.17 | 4.30 | 99.23 | 1.62 | 1.72 |
| 237 | Oxfendazole | 14.43 | 112.00 | 9.85 | 9.26 | 98.20 | 2.86 | 3.06 | 93.13 | 4.89 | 5.53 | 100.75 | 7.35 | 7.68 | 100.63 | 4.97 | 5.19 |
| 238 | Oxolinic acid | 8.60 | 103.85 | 4.00 | 4.05 | 91.68 | 5.27 | 6.06 | 101.15 | 4.74 | 4.93 | 100.96 | 2.90 | 3.02 | |||
| 239 | Oxydemeton methyl | 22.47 | 102.56 | 5.85 | 6.00 | 92.13 | 4.64 | 5.30 | 99.17 | 6.15 | 6.52 | 101.58 | 7.73 | 8.01 | |||
| 240 | Oxyfluorfen | 26.60 | 107.15 | 18.84 | 18.51 | 101.78 | 18.11 | 18.73 | 93.71 | 22.54 | 24.32 | 94.68 | 10.13 | 11.27 | 104.32 | 8.85 | 8.93 |
| 241 | Paclobutrazol | 29.94 | 90.31 | 18.73 | 21.83 | 109.85 | 19.35 | 18.55 | 90.96 | 14.91 | 17.25 | 97.89 | 11.80 | 12.68 | 102.25 | 10.31 | 10.62 |
| 242 | Parathion methyl | 19.48 | 98.41 | 18.23 | 19.50 | 104.65 | 3.64 | 3.66 | 91.06 | 13.26 | 15.33 | 97.15 | 16.56 | 17.94 | 104.95 | 5.92 | 5.94 |
| 243 | PCB 28 | 30.59 | 90.80 | 12.67 | 14.68 | 106.28 | 3.43 | 3.40 | 95.75 | 18.66 | 20.51 | 97.36 | 13.88 | 15.01 | 101.58 | 10.56 | 10.95 |
| 244 | PCB 52 | 49.53 | 96.76 | 16.68 | 18.14 | 110.07 | 5.96 | 5.70 | 91.27 | 15.70 | 18.11 | 100.93 | 19.12 | 19.94 | 105.86 | 17.06 | 16.97 |
| 245 | PCB 77 | 32.20 | 103.38 | 16.60 | 16.90 | 102.27 | 10.81 | 11.12 | 98.20 | 19.63 | 21.04 | 97.88 | 11.57 | 12.44 | 102.22 | 11.11 | 11.44 |
| 246 | PCB 81 | 52.37 | 108.02 | 16.73 | 16.30 | 103.90 | 18.60 | 18.85 | 89.56 | 16.73 | 19.66 | 87.59 | 12.33 | 14.81 | 102.26 | 21.67 | 22.31 |
| 247 | PCB 101 | 42.27 | 87.04 | 19.39 | 23.45 | 109.17 | 8.10 | 7.81 | 92.00 | 20.82 | 23.83 | 95.80 | 7.97 | 8.75 | 103.43 | 14.62 | 14.87 |
| 248 | PCB 105 | 27.37 | 99.26 | 7.43 | 7.87 | 100.33 | 13.58 | 14.24 | 92.72 | 13.12 | 14.89 | 93.12 | 17.28 | 19.53 | 105.20 | 8.95 | 8.96 |
| 249 | PCB 114 | 24.13 | 103.71 | 20.87 | 21.19 | 94.46 | 12.18 | 13.57 | 91.10 | 16.36 | 18.91 | 96.99 | 17.69 | 19.20 | 102.77 | 8.20 | 8.40 |
| 250 | PCB 118 | 28.15 | 97.19 | 13.12 | 14.21 | 99.86 | 5.10 | 5.37 | 92.76 | 20.78 | 23.58 | 96.30 | 8.30 | 9.07 | 102.29 | 9.68 | 9.96 |
| 251 | PCB 123 | 31.62 | 101.08 | 13.85 | 14.43 | 100.49 | 5.75 | 6.02 | 95.08 | 19.70 | 21.81 | 94.75 | 14.61 | 16.24 | 102.61 | 10.88 | 11.16 |
| 252 | PCB 126 | 19.28 | 96.17 | 18.84 | 20.62 | 107.21 | 20.06 | 19.70 | 94.36 | 17.42 | 19.43 | 96.66 | 12.75 | 13.88 | 102.60 | 6.48 | 6.65 |
| 253 | PCB 138 | 26.55 | 96.29 | 8.95 | 9.78 | 100.71 | 15.36 | 16.05 | 93.60 | 17.01 | 19.12 | 97.66 | 12.37 | 13.33 | 100.79 | 9.16 | 9.57 |
| 254 | PCB 153 | 33.56 | 92.09 | 6.60 | 7.55 | 101.91 | 15.13 | 15.63 | 95.55 | 16.13 | 17.76 | 98.44 | 15.61 | 16.69 | 101.60 | 11.60 | 12.02 |
| 255 | PCB 156 | 55.52 | 89.30 | 5.79 | 6.82 | 104.11 | 17.82 | 18.02 | 92.53 | 17.92 | 20.38 | 101.92 | 21.37 | 22.07 | 99.35 | 18.94 | 20.07 |
| 256 | PCB 157 | 26.85 | 95.13 | 17.25 | 19.09 | 104.76 | 13.27 | 13.33 | 89.85 | 15.11 | 17.71 | 102.60 | 16.72 | 17.16 | 99.44 | 9.21 | 9.75 |
| 257 | PCB 167 | 51.81 | 113.98 | 21.88 | 20.21 | 99.57 | 17.14 | 18.12 | 87.57 | 13.30 | 15.99 | 94.35 | 18.40 | 20.53 | 93.21 | 16.57 | 18.72 |
| 258 | PCB 169 | 52.09 | 101.60 | 18.21 | 18.86 | 103.24 | 8.55 | 8.72 | 85.44 | 9.25 | 11.40 | 90.15 | 20.58 | 24.03 | 97.16 | 17.56 | 19.02 |
| 259 | PCB 180 | 13.23 | 104.96 | 14.37 | 14.41 | 106.93 | 9.30 | 9.15 | 88.67 | 14.22 | 16.88 | 93.69 | 14.56 | 16.36 | 100.90 | 4.53 | 4.73 |
| 260 | PCB 189 | 25.16 | 103.03 | 10.15 | 10.37 | 100.28 | 19.19 | 20.14 | 89.16 | 12.30 | 14.52 | 93.67 | 9.90 | 11.13 | 104.12 | 8.36 | 8.45 |
| 261 | Penconazole | 34.16 | 95.70 | 12.80 | 14.08 | 115.85 | 4.50 | 4.09 | 95.53 | 13.40 | 14.76 | 93.08 | 11.71 | 13.24 | 99.44 | 11.72 | 12.41 |
| 262 | Pencycuron | 20.48 | 98.16 | 9.03 | 9.69 | 105.55 | 4.45 | 4.43 | 96.26 | 4.83 | 5.28 | 100.53 | 7.15 | 7.49 | 100.12 | 7.06 | 7.42 |
| 263 | Pendimethalin | 17.70 | 90.91 | 14.47 | 16.76 | 105.21 | 7.43 | 7.43 | 99.02 | 3.67 | 3.90 | 102.02 | 5.04 | 5.20 | 97.75 | 5.82 | 6.27 |
| 264 | Penicilina V | 26.71 | 96.64 | 5.84 | 6.36 | 97.01 | 8.57 | 9.30 | 101.26 | 9.22 | 9.58 | ||||||
| 265 | Permethrin | 36.34 | 105.69 | 14.94 | 14.88 | 95.27 | 21.90 | 24.20 | 90.29 | 19.06 | 22.22 | 97.91 | 12.29 | 13.21 | |||
| 266 | Phenanthrene | 17.45 | 98.02 | 12.31 | 13.22 | 99.55 | 12.44 | 13.16 | 93.48 | 13.10 | 14.75 | 93.74 | 12.82 | 14.39 | 103.69 | 5.55 | 5.63 |
| 267 | Phenylbutazone | 49.13 | 91.74 | 15.73 | 18.05 | 93.57 | 19.01 | 21.39 | 105.65 | 16.94 | 16.87 | ||||||
| 268 | Phosalone | 11.29 | 100.31 | 7.80 | 8.18 | 102.56 | 5.32 | 5.47 | 94.92 | 4.35 | 4.82 | 99.26 | 7.11 | 7.54 | 100.09 | 3.89 | 4.09 |
| 269 | Phosmet | 13.71 | 104.01 | 7.48 | 7.57 | 98.61 | 6.23 | 6.65 | 103.07 | 4.47 | 4.56 | 97.45 | 4.32 | 4.67 | |||
| 270 | Phosmet-oxon | 7.06 | 97.85 | 4.69 | 5.05 | 97.35 | 8.44 | 9.13 | 102.67 | 5.14 | 5.27 | 98.02 | 1.99 | 2.13 | |||
| 271 | Pirimicarb | 10.35 | 92.13 | 7.98 | 9.12 | 108.26 | 1.33 | 1.29 | 103.45 | 4.95 | 5.04 | 103.13 | 3.94 | 4.02 | 95.99 | 2.20 | 2.42 |
| 272 | Pirimicarb-desmethyl | 8.59 | 102.89 | 7.11 | 7.27 | 101.86 | 3.08 | 3.18 | 96.80 | 6.76 | 7.35 | 101.82 | 3.15 | 3.26 | 98.62 | 2.78 | 2.97 |
| 273 | Pirimiphos ethyl | 49.06 | 92.82 | 19.20 | 21.77 | 118.12 | 2.68 | 2.39 | 87.29 | 11.06 | 13.34 | 96.72 | 14.11 | 15.36 | 110.86 | 15.95 | 15.15 |
| 274 | Pirimiphos methyl | 46.53 | 97.86 | 11.37 | 12.23 | 106.89 | 1.77 | 1.74 | 91.79 | 11.32 | 12.99 | 101.94 | 17.14 | 17.69 | 107.79 | 15.68 | 15.32 |
| 275 | Prochloraz | 11.75 | 93.09 | 13.04 | 14.74 | 109.38 | 9.57 | 9.21 | 104.39 | 6.98 | 7.03 | 103.14 | 5.34 | 5.45 | 95.40 | 2.45 | 2.71 |
| 276 | Procymidone | 47.71 | 106.77 | 7.90 | 7.79 | 88.94 | 18.04 | 21.35 | 94.09 | 11.30 | 12.65 | 96.19 | 15.88 | 17.38 | |||
| 277 | Profenofos | 19.09 | 100.50 | 11.04 | 11.56 | 101.67 | 8.80 | 9.11 | 100.12 | 6.04 | 6.35 | 101.11 | 7.81 | 8.13 | 98.72 | 6.47 | 6.90 |
| 278 | Propamocarb | 5.99 | 103.07 | 4.64 | 4.74 | 87.68 | 3.95 | 4.75 | 100.88 | 3.74 | 3.90 | 101.51 | 1.80 | 1.86 | |||
| 279 | Propargite | 20.43 | 98.90 | 9.92 | 10.56 | 102.07 | 5.44 | 5.61 | 97.64 | 5.69 | 6.14 | 98.89 | 6.67 | 7.10 | 100.75 | 7.04 | 7.36 |
| 280 | Propiconazole | 12.75 | 96.84 | 8.80 | 9.57 | 100.37 | 8.04 | 8.43 | 100.50 | 5.91 | 6.19 | 98.02 | 4.13 | 4.43 | |||
| 281 | Propoxur | 7.88 | 106.77 | 12.22 | 12.05 | 100.74 | 5.19 | 5.42 | 95.66 | 6.74 | 7.42 | 102.83 | 4.63 | 4.74 | 99.26 | 2.65 | 2.81 |
| 282 | Propyzamide (pronamide) | 7.08 | 92.65 | 19.33 | 21.96 | 95.89 | 6.00 | 6.59 | 95.61 | 6.94 | 7.65 | 100.37 | 5.71 | 5.98 | 99.85 | 2.43 | 2.57 |
| 283 | Proquinazid | 20.15 | 93.83 | 12.24 | 13.73 | 108.78 | 6.04 | 5.85 | 101.24 | 7.90 | 8.22 | 102.34 | 6.01 | 6.18 | 96.68 | 6.44 | 7.01 |
| 284 | Prothioconazol | 20.98 | 99.10 | 11.03 | 11.71 | 111.77 | 11.21 | 10.56 | 101.24 | 15.30 | 15.91 | 101.44 | 7.09 | 7.36 | 100.39 | 7.23 | 7.58 |
| 285 | Prothiophos | 54.52 | 99.76 | 20.17 | 21.29 | 115.45 | 19.52 | 17.79 | 90.43 | 16.79 | 19.54 | 92.15 | 10.78 | 12.31 | 106.16 | 18.85 | 18.69 |
| 286 | Pymetrozine | 12.11 | 104.52 | 9.45 | 9.52 | 99.96 | 6.47 | 6.81 | 98.85 | 7.40 | 7.88 | 100.89 | 4.14 | 4.32 | |||
| 287 | Pyraclostrobin | 14.46 | 92.85 | 6.84 | 7.76 | 103.95 | 2.37 | 2.40 | 97.43 | 5.66 | 6.11 | 100.22 | 6.57 | 6.90 | 100.04 | 4.98 | 5.24 |
| 288 | Pyrazophos | 13.83 | 97.27 | 7.18 | 7.77 | 104.11 | 8.90 | 9.00 | 100.36 | 6.58 | 6.90 | 100.04 | 5.66 | 5.96 | 99.70 | 4.75 | 5.02 |
| 289 | Pyrene | 35.48 | 84.48 | 4.35 | 5.42 | 118.27 | 15.20 | 13.53 | 90.78 | 10.35 | 12.00 | 95.73 | 8.21 | 9.03 | 102.81 | 12.24 | 12.53 |
| 290 | Pyridaben | 21.92 | 99.30 | 12.16 | 12.89 | 102.10 | 3.57 | 3.68 | 97.39 | 5.95 | 6.43 | 99.28 | 7.39 | 7.83 | 100.30 | 7.56 | 7.93 |
| 291 | Pyridaphenthion | 13.23 | 100.15 | 9.50 | 9.99 | 101.73 | 5.42 | 5.61 | 96.49 | 3.96 | 4.32 | 100.26 | 4.92 | 5.17 | 99.95 | 4.55 | 4.80 |
| 292 | Pyrimethanil | 21.20 | 95.66 | 16.08 | 17.69 | 105.17 | 12.00 | 12.01 | 91.20 | 11.13 | 12.84 | 94.12 | 12.46 | 13.94 | 103.96 | 6.91 | 6.99 |
| 293 | Pyriproxifen | 17.64 | 96.87 | 4.83 | 5.25 | 105.33 | 2.02 | 2.02 | 98.71 | 5.79 | 6.18 | 97.28 | 5.56 | 6.01 | 91.30 | 0.94 | 1.09 |
| 294 | Quinalfos | 11.63 | 94.48 | 10.56 | 11.76 | 96.90 | 6.47 | 7.03 | 106.43 | 6.56 | 6.49 | 101.72 | 6.64 | 6.87 | 96.67 | 3.22 | 3.51 |
| 295 | Quinoxyfen | 17.58 | 90.90 | 11.93 | 13.81 | 100.16 | 8.47 | 8.90 | 102.02 | 7.55 | 7.79 | 102.26 | 5.09 | 5.24 | 96.99 | 5.60 | 6.07 |
| 296 | Rifampicin | 38.32 | 95.11 | 8.51 | 9.42 | 99.82 | 8.14 | 8.58 | 99.31 | 13.14 | 13.93 | ||||||
| 297 | Rotenone | 38.71 | 116.09 | 14.12 | 12.80 | 101.14 | 19.83 | 20.64 | 103.06 | 11.26 | 11.50 | 95.90 | 12.74 | 13.98 | |||
| 298 | Roxithromycin | 16.85 | 105.39 | 12.55 | 12.54 | 90.66 | 7.64 | 8.87 | 96.26 | 3.89 | 4.25 | 103.64 | 5.33 | 5.41 | |||
| 299 | Simazine | 5.25 | 106.22 | 13.48 | 13.35 | 95.00 | 4.14 | 4.59 | 93.84 | 6.29 | 7.06 | 101.60 | 4.45 | 4.61 | 99.67 | 1.79 | 1.89 |
| 300 | Spinosad (two isomers) | 22.35 | 105.88 | 7.75 | 7.70 | 106.76 | 13.25 | 13.05 | 100.51 | 6.11 | 6.45 | 97.07 | 7.36 | 7.88 | |||
| 301 | Spiramycin (two isomers) | 6.56 | 95.12 | 6.27 | 6.94 | 98.30 | 4.51 | 4.83 | 101.25 | 2.10 | 2.19 | ||||||
| 302 | Spirodiclofen | 15.03 | 105.95 | 10.56 | 10.49 | 96.95 | 4.55 | 4.94 | 100.27 | 5.18 | 5.44 | ||||||
| 303 | Spiromesifen | 12.81 | 101.56 | 4.83 | 5.00 | 99.43 | 6.18 | 6.54 | 97.60 | 5.75 | 6.20 | 100.24 | 4.41 | 4.63 | |||
| 304 | Spiroxamine | 11.53 | 84.84 | 7.85 | 9.74 | 105.10 | 1.89 | 1.90 | 104.57 | 7.11 | 7.15 | 102.72 | 4.87 | 4.99 | 96.16 | 2.90 | 3.17 |
| 305 | Strychnine | 11.90 | 98.61 | 6.60 | 7.04 | 92.65 | 8.23 | 9.35 | 101.86 | 5.04 | 5.21 | 100.73 | 4.08 | 4.27 | |||
| 306 | Sulfacetamide | 18.66 | 95.38 | 12.81 | 14.13 | 101.54 | 7.67 | 7.95 | 100.85 | 6.43 | 6.71 | ||||||
| 307 | Sulfachloropiridacine | 4.66 | 96.98 | 0.49 | 0.53 | 92.42 | 5.44 | 6.19 | 99.53 | 3.02 | 3.20 | 100.56 | 1.56 | 1.64 | |||
| 308 | Sulfadiacine | 22.38 | 108.52 | 15.90 | 15.42 | 91.45 | 5.34 | 6.15 | 97.90 | 5.89 | 6.33 | 102.45 | 7.61 | 7.82 | |||
| 309 | Sulfadimetoxine | 10.71 | 110.25 | 12.55 | 11.99 | 96.34 | 4.79 | 5.24 | 96.18 | 5.92 | 6.47 | 100.38 | 6.04 | 6.34 | 100.47 | 3.68 | 3.86 |
| 310 | Sulfadoxine | 7.31 | 107.49 | 7.96 | 7.79 | 94.89 | 5.76 | 6.39 | 94.43 | 6.42 | 7.16 | 102.25 | 5.28 | 5.44 | 99.73 | 2.51 | 2.64 |
| 311 | Sulfameracine | 6.60 | 103.87 | 2.73 | 2.77 | 94.75 | 4.37 | 4.85 | 100.53 | 7.96 | 8.33 | 100.38 | 2.26 | 2.37 | |||
| 312 | Sulfametacine | 8.13 | 105.16 | 1.02 | 1.02 | 92.79 | 8.30 | 9.42 | 100.18 | 5.58 | 5.86 | 100.61 | 2.78 | 2.90 | |||
| 313 | Sulfametizole | 4.48 | 105.55 | 4.45 | 4.44 | 94.95 | 7.79 | 8.64 | 102.75 | 5.57 | 5.71 | 99.45 | 1.49 | 1.58 | |||
| 314 | Sulfametoxazole | 9.86 | 105.88 | 3.94 | 3.92 | 93.12 | 8.76 | 9.90 | 103.21 | 4.47 | 4.56 | 98.89 | 3.29 | 3.50 | |||
| 315 | Sulfametoxipiridacine | 10.11 | 103.07 | 3.90 | 3.99 | 89.72 | 6.58 | 7.72 | 101.01 | 5.53 | 5.76 | 100.77 | 3.46 | 3.61 | |||
| 316 | Sulfamonomethoxine | 30.69 | 120.10 | 11.58 | 10.15 | 93.43 | 9.85 | 11.10 | 100.65 | 5.38 | 5.63 | 100.77 | 10.60 | 11.07 | |||
| 317 | Sulfapyridine | 13.16 | 107.39 | 7.54 | 7.39 | 95.05 | 7.02 | 7.77 | 98.88 | 7.30 | 7.77 | 101.09 | 4.49 | 4.68 | |||
| 318 | Sulfaquinoxaline | 19.01 | 108.10 | 8.28 | 8.06 | 92.03 | 6.07 | 6.95 | 98.12 | 7.23 | 7.76 | 102.11 | 6.45 | 6.65 | |||
| 319 | Sulfatiazole | 13.98 | 107.25 | 18.23 | 17.89 | 91.67 | 8.92 | 10.24 | 100.18 | 10.87 | 11.42 | 100.13 | 4.82 | 5.06 | |||
| 320 | Sulfisoxazole | 6.76 | 95.64 | 5.52 | 6.08 | 90.14 | 5.37 | 6.27 | 101.96 | 3.79 | 3.91 | 99.82 | 2.32 | 2.45 | |||
| 321 | Tebuconazole | 4.02 | 96.70 | 9.65 | 10.50 | 96.71 | 8.87 | 9.65 | 100.97 | 5.13 | 5.35 | 99.07 | 1.22 | 1.30 | |||
| 322 | Tebufenocide | 9.66 | 98.70 | 9.29 | 9.90 | 103.48 | 4.98 | 5.07 | 100.37 | 9.81 | 10.29 | 98.74 | 6.14 | 6.55 | 100.66 | 3.31 | 3.46 |
| 323 | Tebufenpyrad | 54.25 | 98.40 | 19.31 | 20.65 | 103.42 | 14.26 | 14.51 | 87.22 | 16.18 | 19.53 | 96.43 | 16.30 | 17.80 | 93.97 | 17.61 | 19.73 |
| 324 | Teflubenzuron | 14.59 | 103.61 | 16.19 | 16.45 | 91.96 | 11.61 | 13.29 | 99.92 | 10.40 | 10.96 | 100.04 | 5.03 | 5.29 | |||
| 325 | Tefluthrin | 18.98 | 94.50 | 18.42 | 20.52 | 109.30 | 5.13 | 4.94 | 95.83 | 13.31 | 14.62 | 97.15 | 10.26 | 11.12 | 103.00 | 6.29 | 6.43 |
| 326 | Telodrin (isobenzan) | 31.11 | 93.25 | 19.35 | 21.84 | 116.61 | 15.64 | 14.12 | 89.63 | 17.21 | 20.21 | 94.46 | 10.00 | 11.14 | 104.86 | 10.43 | 10.47 |
| 327 | Terbufos | 17.89 | 89.87 | 18.10 | 21.20 | 101.84 | 16.58 | 17.14 | 98.77 | 16.02 | 17.07 | 99.46 | 14.72 | 15.58 | 105.00 | 5.24 | 5.25 |
| 328 | Terbuthylazine | 14.43 | 93.29 | 4.25 | 4.80 | 109.08 | 4.95 | 4.78 | 96.04 | 4.63 | 5.07 | 98.95 | 5.77 | 6.14 | 100.31 | 4.97 | 5.22 |
| 329 | Tetrachlorvinphos | 15.02 | 86.68 | 16.44 | 19.97 | 104.08 | 5.78 | 5.84 | 103.29 | 9.75 | 9.93 | 100.97 | 4.69 | 4.89 | 96.47 | 4.48 | 4.89 |
| 330 | Tetraconazole | 38.67 | 89.01 | 16.15 | 19.10 | 114.33 | 10.30 | 9.48 | 94.97 | 19.82 | 21.97 | 99.62 | 11.36 | 12.01 | 106.33 | 12.98 | 12.85 |
| 331 | Tetradifon | 21.58 | 92.25 | 17.26 | 19.70 | 108.82 | 9.05 | 8.75 | 97.26 | 10.04 | 10.87 | 98.91 | 10.35 | 11.02 | 99.23 | 7.38 | 7.83 |
| 332 | Tetramethrin | 38.36 | 110.19 | 8.77 | 8.38 | 87.06 | 11.18 | 13.51 | 99.88 | 13.15 | 13.85 | 96.19 | 12.68 | 13.88 | |||
| 333 | Thiabendazole | 6.96 | 109.34 | 8.89 | 8.56 | 99.89 | 4.64 | 4.89 | 92.53 | 7.57 | 8.61 | 102.95 | 3.30 | 3.37 | 99.44 | 2.36 | 2.50 |
| 334 | Thiacloprid | 7.62 | 100.27 | 9.33 | 9.79 | 99.72 | 2.71 | 2.86 | 96.75 | 5.67 | 6.17 | 102.32 | 4.32 | 4.44 | 98.86 | 2.49 | 2.65 |
| 335 | Thiamethoxam | 8.90 | 94.30 | 9.01 | 10.05 | 101.19 | 2.45 | 2.55 | 99.73 | 3.06 | 3.23 | ||||||
| 336 | Thiophanate methyl | 9.82 | 94.93 | 7.65 | 8.49 | 102.70 | 1.97 | 2.02 | 100.03 | 5.00 | 5.27 | 101.78 | 5.03 | 5.20 | 97.82 | 3.00 | 3.22 |
| 337 | Tolclofos methyl | 18.85 | 96.49 | 2.19 | 2.39 | 93.05 | 7.46 | 8.44 | 104.27 | 5.91 | 5.97 | ||||||
| 338 | Tolfenamic acid | 15.70 | 96.60 | 20.72 | 22.58 | 113.09 | 5.70 | 5.31 | 93.69 | 11.93 | 13.40 | 95.77 | 17.20 | 18.90 | 101.95 | 5.29 | 5.46 |
| 339 | Triadimefon | 15.86 | 103.69 | 22.53 | 24.92 | 96.37 | 21.63 | 23.62 | 97.25 | 7.36 | 7.97 | 100.42 | 5.47 | 5.73 | |||
| 340 | Triadimenol | 11.21 | 90.21 | 16.68 | 19.46 | 108.01 | 10.30 | 10.04 | 104.18 | 2.94 | 2.97 | 102.84 | 6.90 | 7.07 | 95.94 | 2.60 | 2.86 |
| 341 | Triazophos (hostathion) | 11.28 | 107.10 | 15.08 | 14.83 | 96.40 | 6.06 | 6.62 | 100.08 | 4.21 | 4.43 | 99.92 | 3.88 | 4.09 | |||
| 342 | Trichlorfon | 11.09 | 97.09 | 8.26 | 8.96 | 103.21 | 3.75 | 3.82 | 97.44 | 5.31 | 5.74 | 99.08 | 5.57 | 5.92 | 100.42 | 3.82 | 4.00 |
| 343 | Trifloxystrobin | 7.09 | 94.36 | 14.78 | 16.49 | 96.83 | 5.76 | 6.26 | 98.87 | 4.98 | 5.31 | 100.65 | 2.41 | 2.52 | |||
| 344 | Triflumizole | 14.95 | 98.38 | 11.61 | 12.43 | 99.91 | 3.85 | 4.05 | 97.81 | 6.89 | 7.41 | 99.87 | 5.89 | 6.21 | 100.10 | 5.15 | 5.42 |
| 345 | Triflumuron | 10.52 | 97.57 | 4.93 | 5.32 | 100.94 | 4.59 | 4.79 | 97.95 | 7.98 | 8.58 | 99.43 | 5.30 | 5.62 | 100.20 | 3.62 | 3.81 |
| 346 | Trifluralin | 18.05 | 94.40 | 16.87 | 18.82 | 95.90 | 6.43 | 7.05 | 103.36 | 7.85 | 8.00 | 97.05 | 5.78 | 6.27 | |||
| 347 | Trimethoprim | 47.12 | 112.07 | 12.04 | 11.31 | 96.24 | 11.78 | 12.88 | 101.74 | 20.93 | 21.65 | 108.42 | 15.79 | 15.33 | |||
| 348 | Triticonazole | 10.27 | 105.14 | 4.25 | 4.26 | 95.26 | 6.47 | 7.15 | 96.56 | 7.30 | 7.96 | 102.90 | 2.96 | 3.03 | |||
| 349 | Tylmicosin | 10.97 | 113.79 | 16.19 | 14.98 | 88.65 | 6.89 | 8.18 | 101.93 | 6.09 | 6.29 | 99.54 | 3.75 | 3.97 | |||
| 350 | Tylosin | 10.56 | 107.72 | 11.23 | 10.98 | 96.69 | 5.96 | 6.49 | 99.51 | 5.98 | 6.32 | 100.64 | 3.62 | 3.79 | |||
| 351 | Vinclozolin | 18.23 | 87.26 | 12.21 | 14.73 | 113.36 | 11.73 | 10.89 | 98.12 | 18.88 | 20.25 | 96.44 | 12.88 | 14.06 | 102.62 | 6.09 | 6.25 |
| 352 | Warfarin | 10.77 | 87.78 | 17.57 | 21.07 | 91.37 | 23.07 | 24.58 | 95.23 | 6.45 | 7.13 | 102.69 | 4.32 | 4.43 | 98.14 | 3.45 | 3.70 |
| 353 | Zoxamide | 17.19 | 80.27 | 12.78 | 16.76 | 100.65 | 7.00 | 7.33 | 106.21 | 9.24 | 9.16 | 103.64 | 7.51 | 7.62 | 94.40 | 4.35 | 4.85 |
| Blank cells were set for compounds that have a higher LOQ than the lowest concentrations tested. | |||||||||||||||||
Appendix B. Detected Concentrations (Minimum (Min), Maximum (MAX) and Median (Me) Malues, with Frequencies of Detection) of Analytes in Human Series (n = 25)
| POPs | min–MAX (ng/mL) | Me (ng/mL) | n (%) Positives |
| DDE-p,p′ | 0.36–51.4 | 3.75 | 23 (92%) |
| Naphthalene | 1.17–3.35 | 1.62 | 20 (80%) |
| HCB | 0.3–4.74 | 0.99 | 12 (48%) |
| HCH-B | 0.29–4.09 | 1.34 | 12 (48%) |
| PCB-153 | 0.31–1.14 | 0.48 | 10 (40%) |
| PCB-180 | 0.32–0.92 | 0.47 | 10 (40%) |
| PCB-138 | 0.3–0.82 | 0.44 | 8 (32%) |
| Pharmaceuticals | min–MAX (ng/mL) | Me (ng/mL) | n (%) Positives |
| Acetaminophen | 4–539.64 | 16.65 | 9 (36%) |
| Naproxen | 30.46–4058.04 | 650.33 | 3 (12%) |
| Ketoprofen | 7.41–30.93 | 16.87 | 3 (12%) |
| Levamisole | 11.98–16.37 | 14.18 | 2 (8%) |
| POP (persistent organic pollutant); DDE-p,p′ (dichlorodiphenyldichloroethylene); HCB (hexachlorobenzene); HCH-B (beta-hexachlorocyclohexane); and PCB (polychlorinated biphenyl). | |||
References
- Jia, X.; Yin, S.; Xu, J.; Li, N.; Ren, M.; Qin, Y.; Zhou, J.; Wei, Y.; Guo, Y.; Gao, M.; et al. An Efficient Method to Simultaneously Analyze Multi-Class Organic Pollutants in Human Serum. Environ. Pollut. 2019, 251, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Luzardo, O.P.; Valerón, P.F.; Zumbado, M.; Serra-Majem, L.; Camacho, M.; González-Antuña, A.; Boada, L.D. Persistent Organic Pollutants and Risk of Diabetes and Obesity on Healthy Adults: Results from a Cross-Sectional Study in Spain. Sci. Total Environ. 2017, 607–608, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ramírez, R.; Ortiz-Pérez, M.D.; Batres-Esquivel, L.; Castillo, C.G.; Ilizaliturri-Hernández, C.A.; Díaz-Barriga, F. Rapid Analysis of Persistent Organic Pollutants by Solid Phase Microextraction in Serum Samples. Talanta 2014, 123, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Croen, L.A.; Sjödin, A.; Yoshida, C.K.; Zerbo, O.; Kharrazi, M.; Windham, G.C. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ. Health Perspect. 2017, 125, 474–480. [Google Scholar] [CrossRef]
- Stockholm Convention Listing of POPs in the Stockholm Convention (Updated May 2013). Available online: http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx (accessed on 23 April 2021).
- Li, A.J.; Banjabi, A.A.; Takazawa, M.; Kumosani, T.A.; Yousef, J.M.; Kannan, K. Serum Concentrations of Pesticides Including Organophosphates, Pyrethroids and Neonicotinoids in a Population with Osteoarthritis in Saudi Arabia. Sci. Total Environ. 2020, 737, 139706. [Google Scholar] [CrossRef]
- Calaf, G.M. Role of Organophosphorous Pesticides and Acetylcholine in Breast Carcinogenesis. Semin. Cancer Biol. 2021, 76, 206–217. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, K.; Du, J.; Ou, M.; Hou, J.; Wang, D.; Wang, J.; Zhang, W.; Sun, G. Serum Concentrations of Neonicotinoids and Their Characteristic Metabolites in Elderly Population from South China: Association with Osteoporosis. Environ. Res. 2022, 203, 111772. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, S. Human Exposure to Neonicotinoids and the Associated Health Risks: A Review. Environ. Int. 2022, 163, 107201. [Google Scholar] [CrossRef]
- Luzardo, O.P.; Almeida-González, M.; Ruiz-Suárez, N.; Zumbado, M.; Henríquez-Hernández, L.A.; Meilán, M.J.; Camacho, M.; Boada, L.D. Validated Analytical Methodology for the Simultaneous Determination of a Wide Range of Pesticides in Human Blood Using GC-MS/MS and LC-ESI/MS/MS and Its Application in Two Poisoning Cases. Sci. Justice 2015, 55, 307–315. [Google Scholar] [CrossRef]
- Mokh, S.; El Khatib, M.; Koubar, M.; Daher, Z.; Al Iskandarani, M. Innovative SPE-LC-MS/MS Technique for the Assessment of 63 Pharmaceuticals and the Detection of Antibiotic-Resistant-Bacteria: A Case Study Natural Water Sources in Lebanon. Sci. Total Environ. 2017, 609, 830–841. [Google Scholar] [CrossRef]
- Gómez-Ramírez, P.; Blanco, G.; García-Fernández, A.J. Validation of Multi-Residue Method for Quantification of Antibiotics and Nsaids in Avian Scavengers by Using Small Amounts of Plasma in HPLC-MS-TOF. Int. J. Environ. Res. Public Health 2020, 17, 4058. [Google Scholar] [CrossRef] [PubMed]
- Krokos, A.; Tsakelidou, E.; Michopoulou, E.; Raikos, N.; Theodoridis, G.; Gika, H. NSAIDs Determination in Human Serum by GC-MS. Separations 2018, 5, 37. [Google Scholar] [CrossRef]
- Svarcova, A.; Lankova, D.; Gramblicka, T.; Stupak, M.; Hajslova, J.; Pulkrabova, J. Integration of Five Groups of POPs into One Multi-Analyte Method for Human Blood Serum Analysis: An Innovative Approach within Biomonitoring Studies. Sci. Total Environ. 2019, 667, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, P.; Jiménez-Montalbán, P.J.; Delgado, D.; Martínez-López, E.; María-Mojica, P.; Godino, A.; García-Fernández, A.J. Development of a QuEChERS Method for Simultaneous Analysis of Antibiotics in Carcasses for Supplementary Feeding of Endangered Vultures. Sci. Total Environ. 2018, 626, 319–327. [Google Scholar] [CrossRef]
- Lee, J.E.; Oh, H.B.; Im, H.; Han, S.B.; Kim, K.H. Multiresidue Analysis of 85 Persistent Organic Pollutants in Small Human Serum Samples by Modified QuEChERS Preparation with Different Ionization Sources in Mass Spectrometry. J. Chromatogr. A 2020, 1623, 461170. [Google Scholar] [CrossRef] [PubMed]
- Rial-Berriel, C.; Acosta-Dacal, A.; Zumbado, M.; Luzardo, O.P. Micro QuEChERS-Based Method for the Simultaneous Biomonitoring in Whole Blood of 360 Toxicologically Relevant Pollutants for Wildlife. Sci. Total Environ. 2020, 736, 139444. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Acosta-Dacal, A.; Zumbado, M.; Henríquez-Hernández, L.A.; Rodríguez-Hernández, Á.; Macías-Montes, A.; Boada, L.D.; Travieso-Aja, M.D.M.; Cruz, B.M.; Luzardo, O.P. A Method Scope Extension for the Simultaneous Analysis of Pops, Current-Use and Banned Pesticides, Rodenticides, and Pharmaceuticals in Liver. Application to Food Safety and Biomonitoring. Toxics 2021, 9, 238. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Lee, J.; Lee, J.; Kim, E.; Liu, K.H.; Lee, H.S.; Kim, J.H. Validation of a Multiresidue Analysis Method for 379 Pesticides in Human Serum Using Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 3550–3560. [Google Scholar] [CrossRef]
- Montenarh, D.; Hopf, M.; Warth, S.; Maurer, H.H.; Schmidt, P.; Ewald, A.H. A Simple Extraction and LC-MS/MS Approach for the Screening and Identification of over 100 Analytes in Eight Different Matrices. Drug Test Anal. 2015, 7, 214–240. [Google Scholar] [CrossRef]
- Soares, S.; Rosado, T.; Barroso, M.; Vieira, D.N.; Gallardo, E. Organophosphorus Pesticide Determination in Biological Specimens: Bioanalytical and Toxicological Aspects. Int. J. Leg. Med. 2019, 133, 1763–1784. [Google Scholar] [CrossRef]
- Appenzeller, B.M.R.; Hardy, E.M.; Grova, N.; Chata, C.; Faÿs, F.; Briand, O.; Schroeder, H.; Duca, R.C. Hair Analysis for the Biomonitoring of Pesticide Exposure: Comparison with Blood and Urine in a Rat Model. Arch. Toxicol. 2017, 91, 2813–2825. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, D.; Hopf, M.; Maurer, H.H.; Schmidt, P.; Ewald, A.H. Detection and Quantification of Benzodiazepines and Z-Drugs in Human Whole Blood, Plasma, and Serum Samples as Part of a Comprehensive Multi-Analyte LC-MS/MS Approach. Anal. Bioanal. Chem. 2014, 406, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, D.; Hopf, M.; Maurer, H.H.; Schmidt, P.; Ewald, A.H. Development and Validation of a Multi-Analyte LC-MS/MS Approach for Quantification of Neuroleptics in Whole Blood, Plasma, and Serum. Drug Test Anal. 2016, 8, 1080–1089. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021. 2021. Available online: https://www.accredia.it/en/documento/guidance-sante-11312-2021-analytical-quality-control-and-method-validation-procedures-for-pesticide-residues-analysis-in-food-and-feed/ (accessed on 28 May 2023).
- Scientific Working Group for Forensic Toxicology. Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology. J. Anal. Toxicol. 2013, 37, 452–474. [Google Scholar] [CrossRef]
- Manz, K.E.; Yamada, K.; Scheidl, L.; La Merrill, M.A.; Lind, L.; Pennell, K.D. Targeted and Nontargeted Detection and Characterization of Trace Organic Chemicals in Human Serum and Plasma Using QuEChERS Extraction. Toxicol. Sci. 2022, 185, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Palát, J.; Kukučka, P.; Codling, G.P.; Price, E.J.; Janků, P.; Klánová, J. Application of 96-Well Plate SPE Method for Analysis of Persistent Organic Pollutants in Low Volume Blood Serum Samples. Chemosphere 2022, 287, 132300. [Google Scholar] [CrossRef]
- Lacassie, E.; Dreyfuss, M.F.; Gaulier, J.M.; Marquet, P.; Daguet, J.L.; Lachâtre, G. Multiresidue Determination Method for Organophosphorus Pesticides in Serum and Whole Blood by Gas Chromatography-Mass-Selective Detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 759, 109–116. [Google Scholar] [CrossRef]
- Hložek, T.; Bursová, M.; Čabala, R. Fast Ibuprofen, Ketoprofen and Naproxen Simultaneous Determination in Human Serum for Clinical Toxicology by GC–FID. Clin. Biochem. 2014, 47, 109–111. [Google Scholar] [CrossRef]
- Rial-Berriel, C.; Acosta-Dacal, A.; González, F.; Pastor-Tiburón, N.; Zumbado, M.; Luzardo, O.P. Supporting Dataset on the Validation and Verification of the Analytical Method for the Biomonitoring of 360 Toxicologically Relevant Pollutants in Whole Blood. Data Brief 2020, 31, 105878. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS Sample Preparation Methods for the Analysis of Pesticide Residues in Fruits and Vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Ortiz-Andrelluchi, A.; Álvarez-Pérez, J.; Acosta-Dacal, A.; Zumbado, M.; Martínez-González, M.A.; Boada, L.D.; Salas-Salvadó, J.; Luzardo, O.P.; Serra-Majem, L. Human Biomonitoring of Persistent Organic Pollutants in Elderly People from the Canary Islands (Spain): A Temporal Trend Analysis from the PREDIMED and PREDIMED-Plus Cohorts. Sci. Total Environ. 2021, 758, 143637. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, D.; Wernet, M.P.; Hopf, M.; Maurer, H.H.; Schmidt, P.H.; Ewald, A.H. Quantification of 33 Antidepressants by LC-MS/MS–Comparative Validation in Whole Blood, Plasma, and Serum. Anal. Bioanal. Chem. 2014, 406, 5939–5953. [Google Scholar] [CrossRef]
- Pope, J.D.; Drummer, O.H.; Schneider, H.G. The Cocaine Cutting Agent Levamisole Is Frequently Detected in Cocaine Users. Pathology 2018, 50, 536–539. [Google Scholar] [CrossRef]
| No. | Compound | Class | Technique | Retention Time (min) | Polarity | Quantification | Confirmation | Fragmentor Voltage (V) | Linearity (R2) | ME (%) | LOQ (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRM (m/z) | Collision Energy (eV) | MRM (m/z) | Collision Energy (eV) | ||||||||||
| 1 | 2-Phenylphenol | P | GC | 6.28 | positive | 169.0 ➔ 115.0 | 30 | 169.0 ➔ 141.0 | 15 | 70 | 0.9802 | 127.84 | 0.6 |
| 2 | 4,4′-Dichlorobenzophenone (metabolite of dicofol) | P | GC | 10 | positive | 250.0 ➔ 139.0 | 15 | 250.0 ➔ 215.0 | 5 | 70 | 0.9762 | 90.4 | 0.3 |
| 3 | Abamectine | P | LC | 10.98 | positive | 890.5 ➔ 567.1 | 10 | 895.5 ➔ 751.4 | 45 | 160 | 0.9811 | 89.99 | 2.5 |
| 4 | Acenaphthene | POP | GC | 5.93 | positive | 153.0 ➔ 152.0 | 25 | 153.0 ➔ 151.0 | 35 | 70 | 0.9846 | 127.45 | 0.3 |
| 5 | Acenaphtylene | POP | GC | 6.14 | positive | 152.0 ➔ 151.0 | 25 | 152.0 ➔ 126.0 | 30 | 70 | 0.9673 | 143.84 | 0.6 |
| 6 | Acephate | P | LC | 1.65 | positive | 184.0 ➔ 143.0 | 15 | 143.0 ➔ 95.0 | 15 | 70 | 0.9941 | 109.14 | 2.5 |
| 7 | Acetaminophen (Paracetemol) | M | LC | 2.76 | positive | 152.1 ➔ 65.0 | 40 | 152.1 ➔ 93.0 | 20 | 150 | 0.9881 | 160.9 | 1.25 |
| 8 | Acetamiprid | P | LC | 4.44 | positive | 223.1 ➔ 126.0 | 27 | 223.1 ➔ 90.0 | 45 | 140 | 0.9981 | 95.84 | 0.3 |
| 9 | Acrinathrin | P | LC | 10.71 | positive | 559.0 ➔ 208.0 | 10 | 559.0 ➔ 181.0 | 30 | 70 | 0.9904 | 89.03 | 0.6 |
| 10 | Albendazole | M | LC | 7.26 | positive | 266.1 ➔ 234.1 | 16 | 266.1 ➔ 191.0 | 32 | 155 | 0.9966 | 90.75 | 0.15 |
| 11 | Aldicarb | P | LC | 5.17 | positive | 208.0 ➔ 116.0 | 10 | 116.0 ➔ 89.1 | 4 | 100 | 0.9979 | 84.14 | 0.15 |
| 12 | Aldicarb-sulfone | P | LC | 2.8 | positive | 240.1 ➔ 76.0 | 16 | 223.1 ➔ 86.1 | 13 | 75 | 0.9966 | 90.21 | 0.6 |
| 13 | Aldicarb-sulfoxide | P | LC | 2.75 | positive | 207.1 ➔ 131.9 | 10 | 207.1 ➔ 89.1 | 10 | 86 | 0.9972 | 92.14 | 1.25 |
| 14 | Aldrin | POP | GC | 9.89 | positive | 255.0 ➔ 220.0 | 25 | 263.0 ➔ 228.0 | 10 | 70 | 0.9840 | 116.27 | 0.3 |
| 15 | Anthracene | POP | GC | 8.4 | positive | 178.0 ➔ 176.0 | 35 | 178.0 ➔ 152.0 | 30 | 70 | 0.9868 | 133.33 | 0.6 |
| 16 | Atrazine | P | LC | 6.77 | positive | 216.0 ➔ 173.9 | 15 | 216.0 ➔ 103.8 | 30 | 130 | 0.9974 | 85.71 | 0.15 |
| 17 | Azinphos-methyl | P | LC | 7.28 | positive | 318.0 ➔ 132.1 | 8 | 340.0 ➔ 160.0 | 10 | 60 | 0.9969 | 100.46 | 0.15 |
| 18 | Azoxystrobin | P | LC | 7.59 | positive | 404.1 ➔ 372.1 | 8 | 404.1 ➔ 344.1 | 24 | 110 | 0.9985 | 89.93 | 0.15 |
| 19 | BDE-28 | POP | GC | 12.23 | positive | 406.0 ➔ 246.0 | 20 | 406.0 ➔ 167.0 | 25 | 70 | 0.9768 | 124.38 | 0.15 |
| 20 | BDE-47 | POP | GC | 14.32 | positive | 326.0 ➔ 138.0 | 45 | 484.0 ➔324.0 | 25 | 70 | 0.9824 | 128.48 | 0.3 |
| 21 | BDE-85 | POP | GC | 17.1 | positive | 564.0 ➔ 404.0 | 25 | 566.0 ➔ 406.0 | 25 | 70 | 0.9618 | 128.8 | 0.3 |
| 22 | BDE-99 | POP | GC | 16.28 | positive | 566.0 ➔ 406.0 | 25 | 564.0 ➔ 404.0 | 30 | 70 | 0.9758 | 141.83 | 0.3 |
| 23 | BDE-100 | POP | GC | 15.86 | positive | 566.0 ➔ 406.0 | 25 | 564.0 ➔ 404.0 | 25 | 70 | 0.9698 | 147.25 | 0.3 |
| 24 | BDE-153 | POP | GC | 18.06 | positive | 644.0 ➔ 484.0 | 25 | 486.0 ➔ 377.0 | 30 | 70 | 0.9792 | 141.23 | 0.15 |
| 25 | BDE-154 | POP | GC | 17.5 | positive | 644.0 ➔ 484.0 | 25 | 486.0 ➔ 377.0 | 30 | 70 | 0.9776 | 149.11 | 0.3 |
| 26 | BDE-183 | POP | GC | 20.14 | positive | 561.6 ➔ 454.7 | 40 | 563.6 ➔ 454.7 | 40 | 70 | 0.9649 | 233.14 | 0.15 |
| 27 | Benalaxyl | P | LC | 8.98 | positive | 326.2 ➔ 148.0 | 20 | 326.2 ➔ 208.0 | 12 | 90 | 0.9985 | 82.41 | 0.15 |
| 28 | Bendiocarb | P | LC | 5.92 | positive | 224.1 ➔ 166.9 | 8 | 224.2 ➔ 108.9 | 15 | 120 | 0.9977 | 101.28 | 0.3 |
| 29 | Bendiocarb metabolite (2, 2-dimethylbenzo-1, 3-dioxol-4-ol) | P | GC | 4.83 | positive | 166.0 ➔ 151.0 | 10 | 166.0 ➔ 126.0 | 20 | 70 | 0.9559 | 347.74 | 2.5 |
| 30 | Benfuracarb | P | LC | 9.73 | positive | 411.2 ➔ 190.0 | 13 | 411.2 ➔ 252.0 | 15 | 110 | 0.9304 | 22.53 | 1.25 |
| 31 | Benzo[a]anthracene | POP | GC | 13.88 | positive | 228.0 ➔ 226.0 | 40 | 228.0 ➔ 202.0 | 35 | 70 | 0.9857 | 126.76 | 0.15 |
| 32 | Benzo[a]pyrene | POP | GC | 16.91 | positive | 252.0 ➔ 250.0 | 45 | 252.0 ➔ 248.0 | 60 | 70 | 0.9863 | 138.96 | 0.15 |
| 33 | Benzo[b]fluoranthene | POP | GC | 16.27 | positive | 252.0 ➔ 248.0 | 60 | 252.0 ➔ 226.0 | 35 | 70 | 0.9796 | 141.04 | 0.3 |
| 34 | Benzo[ghi]perylene | POP | GC | 19.65 | positive | 276.0 ➔ 274.0 | 50 | 276.0 ➔ 272.0 | 60 | 70 | 0.9828 | 129.94 | 0.15 |
| 35 | Benzo[k]fluoranthene | POP | GC | 16.3 | positive | 252.0 ➔ 250.0 | 45 | 252.0 ➔ 224.0 | 40 | 70 | 0.9801 | 125.79 | 0.3 |
| 36 | Bifenthrin | P | GC | 13.89 | positive | 440.0 ➔ 181.0 | 5 | 440.0 ➔ 165.0 | 60 | 94 | 0.9893 | 99.72 | 0.15 |
| 37 | Bitertanol | P | LC | 9.22 | positive | 338.2 ➔ 70.0 | 4 | 338.2 ➔ 269.2 | 5 | 100 | 0.9961 | 102.64 | 0.3 |
| 38 | Boscalid (formerly nicobifen) | P | GC | 16.55 | positive | 3434.0 ➔ 272.0 | 30 | 343.0 ➔ 140.0 | 45 | 100 | 0.9721 | 100.39 | 0.15 |
| 39 | Brodifacoum | AR | LC | 10.64 | negative | 521.3 ➔ 79.0 | 50 | 523.3 ➔ 135.0 | 45 | 220 | 0.9879 | 36.3 | 0.3 |
| 40 | Bromadiolone | AR | LC | 9.7 | negative | 525.3 ➔ 250.0 | 40 | 527.3 ➔ 250.0 | 40 | 200 | 0.9892 | 88 | 0.3 |
| 41 | Bromopropylate | P | GC | 13.87 | positive | 341.0 ➔ 183.0 | 15 | 341.0 ➔ 157.0 | 45 | 70 | 0.9760 | 100.52 | 0.15 |
| 42 | Bromuconazole (two isomers) | P | GC | 13.81 | positive | 295.0 ➔ 173.0 | 10 | 295.0 ➔ 175.0 | 10 | 70 | 0.9764 | 97.2 | 0.3 |
| 43 | Bupirimate | P | LC | 8.4 | positive | 273.0 ➔ 108.0 | 15 | 273.0 ➔ 193.0 | 5 | 70 | 0.9953 | 96.56 | 0.3 |
| 44 | Buprofezin | P | LC | 9.88 | positive | 306.1 ➔ 201.0 | 12 | 306.1 ➔ 116.0 | 12 | 140 | 0.9975 | 79.09 | 0.15 |
| 45 | Cadusafos (ebufos) | P | LC | 9.4 | positive | 271.1 ➔ 159.0 | 16 | 271.1 ➔ 131.0 | 22 | 100 | 0.9903 | 88.95 | 0.3 |
| 46 | Carbaryl | P | LC | 6.24 | positive | 202.1 ➔ 145.1 | 4 | 202.1 ➔ 127.1 | 28 | 95 | 0.9968 | 101.8 | 0.15 |
| 47 | Carbendazim (azole) | P | LC | 3.4 | positive | 192.1 ➔ 160.1 | 4 | 202.1 ➔ 127.1 | 28 | 90 | 0.9977 | 70.73 | 0.3 |
| 48 | Carbofuran | P | LC | 5.95 | positive | 222.1 ➔ 123.1 | 20 | 222.1 ➔ 165.1 | 30 | 80 | 0.9973 | 93.77 | 0.15 |
| 49 | Carbofuran-3-hydroxy | P | LC | 4.27 | positive | 238.1 ➔ 163.1 | 10 | 238.1 ➔ 181.1 | 10 | 110 | 0.9976 | 79.76 | 0.6 |
| 50 | Cefuroxima axetil (two isomers) | M | LC | 5.4 | positive | 533.0 ➔ 447.0 | 15 | 533.0 ➔ 386.0 | 20 | 160 | 0.9970 | 197.75 | 0.3 |
| 51 | Chloramphenicol | M | LC | 4.62 | negative | 321.0 ➔ 152.1 | 4 | 323.0 ➔ 152.1 | 4 | 113 | 0.9766 | 115.61 | 2.5 |
| 52 | Chlorantraniliprole | P | LC | 7.33 | positive | 483.9 ➔ 452.9 | 16 | 483.9 ➔ 285.9 | 8 | 105 | 0.9979 | 101.23 | 0.3 |
| 53 | Chlorfenapyr | P | GC | 12.01 | positive | 247.0 ➔ 200.0 | 30 | 247.0 ➔ 227.0 | 15 | 70 | 0.9864 | 93.61 | 0.6 |
| 54 | Chlorfenvinphos | P | LC | 9.08 | positive | 361.1 ➔ 98.9 | 34 | 358.9 ➔ 155.1 | 8 | 105 | 0.9947 | 96.77 | 0.3 |
| 55 | Chlorobenzilate | P | GC | 12.14 | positive | 251.0 ➔ 111.0 | 40 | 251.0 ➔ 139.0 | 15 | 70 | 0.9866 | 93.38 | 0.15 |
| 56 | Chlorophacinone | AR | LC | 8.75 | negative | 373.2 ➔ 201.0 | 20 | 375.2 ➔ 203.0 | 20 | 160 | 0.9345 | 72.97 | 5 |
| 57 | Chlorpropham | P | GC | 7.12 | positive | 213.0 ➔ 127.0 | 15 | 153.0 ➔ 90.0 | 25 | 70 | 0.9818 | 109.49 | 0.15 |
| 58 | Chlorpyrifos | P | GC | 9.93 | positive | 314.0 ➔ 258.0 | 15 | 314.0 ➔ 286.0 | 5 | 70 | 0.9798 | 98.63 | 0.15 |
| 59 | Chlorpyrifos methyl | P | GC | 9.12 | positive | 286.0 ➔ 93.0 | 25 | 286.0 ➔ 271.0 | 15 | 70 | 0.9839 | 87.88 | 0.15 |
| 60 | Chlorthal dimethyl | POP | GC | 10.03 | positive | 300.9 ➔ 166.9 | 55 | 300.9 ➔ 222.9 | 25 | 70 | 0.9757 | 103.06 | 0.15 |
| 61 | Chrysene | M | GC | 13.95 | positive | 228.0 ➔ 226.0 | 40 | 228.0 ➔ 227.0 | 25 | 70 | 0.9800 | 123.04 | 0.3 |
| 62 | Clindamycin | P | LC | 5.65 | positive | 425.2 ➔ 126.1 | 20 | 425.2 ➔ 377.2 | 20 | 150 | 0.9969 | 98.59 | 1.25 |
| 63 | Clofentezine | P | LC | 9.2 | positive | 303.1 ➔ 138.0 | 12 | 303.1 ➔ 102.0 | 40 | 120 | 0.9940 | 92.23 | 0.3 |
| 64 | Clothianidin | M | LC | 3.9 | positive | 250.0 ➔ 169.0 | 8 | 250.0 ➔ 131.9 | 8 | 100 | 0.9959 | 109.87 | 1.25 |
| 65 | Cortiscosterone 21 acetate | P | LC | 7.89 | positive | 389.1 ➔ 329.0 | 13 | 389.1 ➔ 371.0 | 13 | 80 | 0.9968 | 89.53 | 1.25 |
| 66 | Coumachlor | P | LC | 8.6 | positive | 343.1 ➔ 162.8 | 15 | 342.1 ➔ 285.0 | 15 | 120 | 0.9849 | 94.86 | 0.6 |
| 67 | Coumaphos | AR | LC | 8.99 | positive | 363.0 ➔ 227.0 | 30 | 363.0 ➔ 306.9 | 15 | 120 | 0.9912 | 107.11 | 0.6 |
| 68 | Coumatetralyl | P | LC | 8.26 | negative | 291.1 ➔ 141.0 | 30 | 291.1 ➔ 247.0 | 20 | 140 | 0.9922 | 93.55 | 0.6 |
| 69 | Cyazofamid | P | LC | 8.48 | positive | 325.0 ➔ 108.0 | 20 | 325.0 ➔ 261.1 | 15 | 90 | 0.9946 | 90.54 | 0.6 |
| 70 | Cyflufenamid | P | LC | 9.19 | positive | 413.1 ➔ 223.1 | 33 | 413.1 ➔ 295.1 | 23 | 70 | 0.9952 | 89.99 | 0.3 |
| 71 | Cyfluthrin (sum of four isomers) | P | GC | 16.21 | positive | 226.0 ➔ 206.0 | 25 | 198.9 ➔ 170.1 | 25 | 70 | 0.9735 | 76.97 | 2.5 |
| 72 | Cyhalothrin (lambda isomer) | P | LC | 10.48 | positive | 181.1 ➔ 152.1 | 10 | 181.1 -> 127.1 | 46 | 70 | 0.9883 | 97.58 | 2.5 |
| 73 | Cymoxanil | P | LC | 4.7 | positive | 199.1 ➔ 128.0 | 4 | 199.1 ➔ 110.9 | 12 | 90 | 0.9976 | 92.87 | 1.25 |
| 74 | Cypermethrin (sum of four isomers) | P | GC | 16.54 | positive | 163.0 ➔ 109.0 | 20 | 163.0 ➔ 127.0 | 5 | 70 | 0.9786 | 66.91 | 1.25 |
| 75 | Cyproconazole (two isomers) | P | LC | 8.14 | positive | 292.2 ➔ 70.2 | 18 | 292.2 ➔ 125.1 | 24 | 100 | 0.9971 | 128.58 | 0.3 |
| 76 | Cyprodinil | P | LC | 8.57 | positive | 226.0 ➔ 93.0 | 33 | 226.0 ➔ 108 | 25 | 100 | 0.9914 | 60.14 | 0.6 |
| 77 | Cyromazine | M | LC | 1.23 | positive | 167.1 ➔ 85.0 | 16 | 167.1 ➔ 125.0 | 20 | 120 | 0.9942 | 33.21 | 2.5 |
| 78 | Danofloxacin | P | LC | 3.53 | positive | 358.2 ➔ 340.1 | 20 | 358.2 ➔ 82.1 | 50 | 159 | 0.9495 | 210.36 | 2.5 |
| 79 | Dazomet | POP | GC | 7.81 | positive | 161.9 ➔ 44.0 | 28 | 161.9 ➔ 89.0 | 5 | 70 | 0.9830 | 116.07 | 0.3 |
| 80 | Deltamethrin | POP | LC | 10.64 | positive | 523.0 ➔ 281.0 | 10 | 523.0 ➔ 506.0 | 5 | 100 | 0.9775 | 79.77 | 1.25 |
| 81 | Demeton-S-methyl | POP | LC | 6 | positive | 230.9 ➔ 88.9 | 5 | 230.9 ➔ 61.0 | 30 | 50 | 0.9968 | 88.14 | 0.15 |
| 82 | Demeton-S-methyl-sulfone (Dioxydemeton) | P | LC | 3.3 | positive | 263.0 ➔ 169.0 | 24 | 263.0 ➔ 109.0 | 12 | 120 | 0.9974 | 91.79 | 0.6 |
| 83 | Dexamethasone | P | LC | 7.16 | positive | 393.2 ➔ 373.2 | 2 | 393.2 ➔ 355.2 | 6 | 103 | 0.9924 | 102.52 | 1.25 |
| 84 | Diazinon | P | GC | 8.28 | positive | 137.1 ➔ 54.0 | 20 | 304.0 ➔ 179.0 | 15 | 70 | 0.9710 | 102.06 | 0.15 |
| 85 | Dibenzo[a,h]anthracene | M | GC | 19.18 | positive | 278.0 ➔ 276.0 | 40 | 278.0 ➔ 250.0 | 60 | 70 | 0.9866 | 131.16 | 0.15 |
| 86 | Dichlorodiphenyldichloroethane (p,p′ DDD) | P | GC | 12.32 | positive | 235.0 ➔ 165.0 | 20 | 235.0 ➔ 199.0 | 15 | 70 | 0.9898 | 127.87 | 0.15 |
| 87 | Dichlorodiphenyldichloroethylene (p,p′ DDE) | POP | GC | 11.58 | positive | 318.0 ➔ 176.0 | 60 | 318.0 ➔ 248.0 | 30 | 70 | 0.9581 | 116.04 | 0.3 |
| 88 | Dichlorodiphenyltrichloroethane (p,p′ DDT) | P | GC | 12.98 | positive | 235.0 ➔ 165.0 | 40 | 235.0 ➔ 199.0 | 15 | 70 | 0.9435 | 14.81 | 1.25 |
| 89 | Diclofenac | P | LC | 8.73 | positive | 296.0 ➔ 215.1 | 16 | 296.0 ➔ 214.1 | 48 | 103 | 0.9839 | 71.47 | 2.5 |
| 90 | Dicloran | M | GC | 7.8 | positive | 206.0 ➔ 176.0 | 10 | 206.0 ➔ 148.0 | 25 | 70 | 0.9872 | 106.98 | 0.6 |
| 91 | Dichlorvos | POP | LC | 5.79 | positive | 221.0 ➔ 79.1 | 28 | 221.0 ➔ 109.1 | 16 | 105 | 0.9971 | 42.69 | 0.6 |
| 92 | Dieldrin | P | GC | 11.67 | positive | 263.0 ➔ 228.0 | 15 | 277.0 ➔ 241.0 | 15 | 70 | 0.9724 | 111.8 | 1.25 |
| 93 | Diethathyl ethyl | P | LC | 8.73 | positive | 312.2 ➔ 238.1 | 15 | 312.2 ➔ 162.0 | 30 | 120 | 0.9974 | 84.45 | 0.15 |
| 94 | Diethofencarb | AR | LC | 7.59 | positive | 268.2 ➔ 226.1 | 5 | 268.2 ➔ 152.0 | 20 | 110 | 0.9949 | 102.38 | 0.15 |
| 95 | Difenacoum | P | LC | 10.25 | negative | 443.2 ➔ 135.0 | 40 | 443.2 ➔ 293.0 | 35 | 200 | 0.9926 | 79.52 | 0.3 |
| 96 | Difenoconazole | AR | LC | 9.41 | positive | 406.1 ➔ 250.9 | 28 | 406.1 ➔ 337.0 | 16 | 176 | 0.9963 | 118.11 | 0.3 |
| 97 | Difethialone | M | LC | 10.8 | negative | 537.3 ➔ 79.0 | 50 | 537.3 ➔ 151.0 | 45 | 220 | 0.9892 | 38.64 | 0.6 |
| 98 | Difloxacin | P | LC | 3.85 | positive | 400.2 ➔ 382.1 | 20 | 400.2 ➔ 356.1 | 16 | 149 | 0.9692 | 234.88 | 2.5 |
| 99 | Diflubenzuron | P | LC | 8.63 | positive | 311.0 ➔ 158.0 | 8 | 311.0 ➔ 141.0 | 32 | 90 | 0.9742 | 85.04 | 1.25 |
| 100 | Diflufenican | P | GC | 13.27 | positive | 394.0 ➔ 266.0 | 10 | 266.0 -> 246.0 | 10 | 70 | 0.9810 | 97.76 | 0.15 |
| 101 | Dimethenamid-P (and its R-isomer) | P | LC | 7.72 | positive | 276.1 ➔ 244.1 | 10 | 276.1 ➔ 168.1 | 20 | 125 | 0.9979 | 92.69 | 0.15 |
| 102 | Dimethoate | P | LC | 4.2 | positive | 230.0 ➔ 125.0 | 16 | 230.0 ➔ 198.8 | 20 | 70 | 0.9976 | 63.84 | 0.6 |
| 103 | Dimethomorph (two isomers) | P | LC | 7.87 | positive | 388.1 ➔ 301.1 | 20 | 388.1 ➔ 165.1 | 32 | 180 | 0.9955 | 121.84 | 0.3 |
| 104 | Dimethylphenylsulfamide (DMSA, metabolite of dichlofluanid) | P | LC | 5.24 | positive | 201.1 ➔ 92.1 | 15 | 201.1 ➔ 137.1 | 5 | 100 | 0.9954 | 63.21 | 1.25 |
| 105 | Diniconazole-M | P | GC | 12.27 | positive | 326.1 ➔ 70.0 | 15 | 328.1 ➔ 70.0 | 15 | 70 | 0.9838 | 85.44 | 0.3 |
| 106 | Dinocap | AR | LC | 10.43 | negative | 295.4 ➔ 208.9 | 30 | 295.4 ➔ 193.0 | 35 | 150 | 0.9819 | 68.11 | 1.25 |
| 107 | Diphacinone | P | LC | 8.45 | negative | 339.1 ➔ 167.0 | 25 | 339.1 ➔ 145.0 | 20 | 170 | 0.9348 | 94.92 | 5 |
| 108 | Diphenylamine | P | GC | 6.97 | positive | 168.0 ➔ 167.2 | 15 | 169.0 ➔ 66.0 | 15 | 70 | 0.9805 | 116.63 | 0.3 |
| 109 | N,N-dimethylformamidine (DMF, metabolite of amitraz) | P | LC | 5.48 | positive | 150.1 ➔ 77.0 | 40 | 149.9 ➔ 105.8 | 30 | 100 | 0.9878 | 166.59 | 1.25 |
| 110 | Dodine | P | LC | 9.1 | positive | 228.3 ➔ 43.0 | 40 | 228.3 ➔ 57.0 | 25 | 150 | 0.9965 | 154.55 | 0.6 |
| 111 | Endosulfan alfa | P | GC | 11.21 | positive | 241.0 ➔ 206.0 | 15 | 195.0 ➔ 160.0 | 10 | 70 | 0.9789 | 97.34 | 0.3 |
| 112 | Endosulfan beta | P | GC | 12.22 | positive | 241.0 ➔ 206.0 | 15 | 195.0 ➔ 159.0 | 15 | 70 | 0.9836 | 101.02 | 0.3 |
| 113 | Endosulfan sulfate | P | GC | 12.97 | positive | 270.0 ➔ 235.0 | 15 | 387.0 ➔ 289.0 | 5 | 70 | 0.9251 | 38.28 | 0.3 |
| 114 | Endrin | P | GC | 12.05 | positive | 263.0 ➔ 193.0 | 35 | 245.0 ➔ 173.0 | 25 | 70 | 0.9603 | 114.03 | 1.25 |
| 115 | Enrofloxacin | P | LC | 3.62 | positive | 360.2 ➔ 316.1 | 16 | 360.2 ➔ 245.1 | 28 | 144 | 0.9308 | 139.22 | 2.5 |
| 116 | EPN | POP | GC | 13.9 | positive | 157.0 ➔ 63.0 | 10 | 157.0 ➔ 110.0 | 15 | 70 | 0.9677 | 75.05 | 0.3 |
| 117 | Epoxiconazole | M | LC | 8.47 | positive | 330.0 ➔ 120.9 | 24 | 330.1 ➔ 100.9 | 50 | 120 | 0.9961 | 91.32 | 0.3 |
| 118 | Eprinomectin | P | LC | 10.83 | positive | 878.5 ➔ 186.0 | 15 | 936.5 ➔ 490.4 | 60 | 160 | 0.9844 | 106.34 | 2.5 |
| 119 | Eritromicin | P | LC | 6.83 | positive | 734.5 ➔ 158.1 | 32 | 734.5 ➔ 576.3 | 16 | 172 | 0.9955 | 102.43 | 0.3 |
| 120 | Esfenvalerate | M | GC | 17.56 | positive | 167.1 ➔ 125.1 | 15 | 167.1 ➔ 89.1 | 45 | 70 | 0.9855 | 59.05 | 1.25 |
| 121 | Ethion (diethion) | M | LC | 10.02 | positive | 385.0 ➔ 199.0 | 5 | 385.0 ➔ 171.0 | 10 | 100 | 0.9944 | 92.79 | 0.15 |
| 122 | Ethirimol | P | LC | 4.8 | positive | 210.2 ➔ 140.1 | 20 | 210.2 ➔ 98.1 | 28 | 160 | 0.9972 | 79.49 | 0.6 |
| 123 | Ethofumesate | P | GC | 9.59 | positive | 286.0 ➔ 207.0 | 5 | 286.0 ➔ 161.0 | 20 | 70 | 0.9801 | 102.24 | 0.15 |
| 124 | Ethoprophos | P | LC | 8.41 | positive | 243.1 ➔ 97.0 | 30 | 243.1 ➔ 130.9 | 15 | 90 | 0.9954 | 96.81 | 0.3 |
| 125 | Etofenprox | P | GC | 16.75 | positive | 163.0 ➔ 107.0 | 20 | 163.0 ➔ 135.0 | 10 | 70 | 0.9858 | 102.63 | 0.3 |
| 126 | Etoxazole | P | LC | 10.33 | positive | 360.1 ➔ 141.0 | 26 | 360.1 ➔ 304.0 | 16 | 160 | 0.9974 | 80.95 | 0.15 |
| 127 | Famoxadone | P | LC | 9.05 | positive | 392.1 ➔ 330.9 | 5 | 392.2 ➔ 238.1 | 12 | 110 | 0.9915 | 89.82 | 0.6 |
| 128 | Fenamidone | P | LC | 7.73 | positive | 392.1 ➔ 330.9 | 5 | 392.1 ➔ 238.1 | 12 | 110 | 0.9883 | 105.31 | 0.3 |
| 129 | Fenamiphos | P | LC | 8.64 | positive | 304.1 ➔ 217.1 | 20 | 304.1 ➔ 202.0 | 36 | 120 | 0.9960 | 89.88 | 0.3 |
| 130 | Fenamiphos sulfone | P | LC | 6.17 | positive | 336.1 ➔ 188.0 | 31 | 336.1 ➔ 266.0 | 23 | 120 | 0.9964 | 126.5 | 0.3 |
| 131 | Fenamiphos sulfoxide | P | LC | 5.92 | positive | 320.1 ➔ 233.0 | 20 | 320.1 ➔ 108.1 | 44 | 120 | 0.9950 | 136.43 | 1.25 |
| 132 | Fenarimol | P | GC | 15.04 | positive | 139.0 ➔ 75.0 | 30 | 139.0 ➔ 111.0 | 15 | 70 | 0.9933 | 99.43 | 0.15 |
| 133 | Fenazaquin | P | LC | 10.75 | positive | 307.2 ➔ 57.1 | 25 | 307.2 ➔ 161.1 | 16 | 90 | 0.9776 | 43.22 | 0.15 |
| 134 | Fenbendazole | P | LC | 8.09 | positive | 300.1 ➔ 268.1 | 20 | 300.1 ➔ 159.0 | 36 | 156 | 0.9955 | 88.98 | 0.15 |
| 135 | Fenbuconazole | P | GC | 16.18 | positive | 198.0 ➔ 102.0 | 30 | 198.0 ➔ 78.0 | 30 | 70 | 0.9795 | 74.4 | 0.3 |
| 136 | Fenbutatin oxide | M | LC | 11.61 | positive | 519.0 ➔ 197.0 | 55 | 517.3 ➔ 194.9 | 60 | 180 | 0.9840 | 63.62 | 0.3 |
| 137 | Fenhexamid | P | LC | 8.35 | positive | 302.1 ➔ 97.1 | 20 | 302.1 ➔ 55.1 | 40 | 130 | 0.9892 | 86.52 | 1.25 |
| 138 | Fenitrothion | P | GC | 9.57 | positive | 277.0 ➔ 109.0 | 15 | 277.0 ➔ 125.0 | 15 | 70 | 0.9591 | 90.52 | 1.25 |
| 139 | Fenoxycarb | P | LC | 8.69 | positive | 302.1 ➔ 88.0 | 20 | 302.1 ➔ 116.1 | 10 | 110 | 0.9939 | 97.48 | 0.3 |
| 140 | Fenpropathrin | P | LC | 14 | positive | 367.2 ➔ 125.0 | 16 | 350.0 ➔ 125.0 | 16 | 72 | 0.9829 | 95.37 | 0.6 |
| 141 | Fenpropidin | P | LC | 7.25 | positive | 274.3 ➔ 147.0 | 30 | 274.3 ➔ 86.0 | 25 | 170 | 0.9968 | 102.13 | 0.15 |
| 142 | Fenpropimorph | P | LC | 7.51 | positive | 304.3 ➔ 147.1 | 30 | 304.3 ➔ 130.0 | 25 | 120 | 0.9970 | 98.38 | 0.15 |
| 143 | Fenpyroximate | P | LC | 10.49 | positive | 422.2 ➔ 366.2 | 12 | 422.2 ➔ 135.0 | 36 | 160 | 0.9951 | 86.38 | 0.3 |
| 144 | Fenthion | P | GC | 9.89 | positive | 278.0 ➔ 109.0 | 15 | 278.0 -> 125.0 | 20 | 70 | 0.9909 | 100.18 | 0.15 |
| 145 | Fenthion oxon | P | LC | 7.33 | positive | 263.1 ➔ 231.2 | 16 | 263.1 ➔ 216.0 | 24 | 120 | 0.9964 | 97.32 | 0.3 |
| 146 | Fenthion oxon sulfone | P | LC | 4.5 | positive | 295.0 ➔ 217.0 | 15 | 295.0 ➔ 104.2 | 24 | 110 | 0.9973 | 109.02 | 0.6 |
| 147 | Fenthion oxon sulfoxide | P | LC | 4.25 | positive | 279.0 ➔ 264.2 | 20 | 279.0 ➔ 104.1 | 28 | 110 | 0.9977 | 84.23 | 0.6 |
| 148 | Fenthion sulfone | P | LC | 6.39 | positive | 311.0 ➔ 125.0 | 22 | 311.0 ➔ 109.0 | 28 | 140 | 0.9943 | 115.8 | 1.25 |
| 149 | Fenthion sulfoxide | P | LC | 6.16 | positive | 295.0 ➔ 108.9 | 30 | 295.0 ➔ 280.0 | 18 | 140 | 0.9966 | 118.68 | 0.6 |
| 150 | Fenvalerate | P | GC | 17.37 | positive | 167.0 ➔ 125.1 | 22 | 167.0 ➔ 89.0 | 30 | 70 | 0.9852 | 68.95 | 1.25 |
| 151 | Fipronil | P | LC | 10.63 | negative | 435.0 ➔ 330.0 | 12 | 435.0 ➔ 249.9 | 26 | 116 | 0.9740 | 94.67 | 0.15 |
| 152 | Fipronil sulfide | P | GC | 10.55 | positive | 351.0 ➔ 255.0 | 20 | 420.0 ➔ 351.0 | 25 | 70 | 0.9687 | 136.6 | 1.25 |
| 153 | Flocoumafen | P | LC | 10.35 | negative | 541.3 ➔ 382.0 | 25 | 541.3 ➔ 161.0 | 40 | 230 | 0.9865 | 63.98 | 0.3 |
| 154 | Fluazinam | P | LC | 10 | negative | 462.9 ➔ 416.0 | 10 | 462.9 ➔ 398.0 | 9 | 140 | 0.9966 | 90.09 | 0.6 |
| 155 | Flubendiamide | AR | LC | 8.79 | positive | 408.0 ➔ 274.0 | 15 | 408.0 ➔ 256.0 | 30 | 120 | 0.9924 | 117.11 | 1.25 |
| 156 | Flucythrinate (two isomers) | P | GC | 16.68 | positive | 156.9 ➔ 107.1 | 15 | 199.1 ➔ 107.1 | 25 | 70 | 0.9866 | 79.25 | 0.15 |
| 157 | Fludioxonil | P | GC | 11.52 | positive | 248.0 ➔ 127.0 | 30 | 248.1 ➔ 182.1 | 10 | 70 | 0.9891 | 90.92 | 0.15 |
| 158 | Flufenoxuron | P | LC | 10.34 | positive | 489.1 ➔ 158.0 | 20 | 489.1 ➔ 140.9 | 56 | 110 | 0.9874 | 92.41 | 0.6 |
| 159 | Flumequine | P | LC | 6.12 | positive | 262.1 ➔ 244.0 | 16 | 262.1 ➔ 202.0 | 32 | 116 | 0.9947 | 134.56 | 0.3 |
| 160 | Flunixin | P | LC | 8.1 | positive | 297.1 ➔ 279.1 | 24 | 297.1 ➔ 264.1 | 32 | 141 | 0.9957 | 30.22 | 0.6 |
| 161 | Fluopyram | M | GC | 10.62 | positive | 173.0 ➔ 95.0 | 35 | 223.0 ➔ 196.0 | 40 | 70 | 0.9866 | 96.79 | 0.15 |
| 162 | Fluoranthene | M | GC | 10.68 | positive | 202.0 ➔ 201.0 | 27 | 202.0 ➔ 152.0 | 42 | 70 | 0.9837 | 118.33 | 0.15 |
| 163 | Fluorene | P | GC | 6.8 | positive | 165.0 ➔ 163.0 | 40 | 165.0 ➔ 139.0 | 30 | 70 | 0.9736 | 121.63 | 0.6 |
| 164 | Fluquinconazole | POP | GC | 15.81 | positive | 340.0 ➔ 298.0 | 15 | 340.0 ➔ 286.0 | 25 | 70 | 0.9608 | 107.7 | 0.3 |
| 165 | Flusilazole | POP | LC | 8.65 | positive | 316.1 ➔ 247.1 | 15 | 316.1 ➔ 165.0 | 20 | 160 | 0.9899 | 86.47 | 0.3 |
| 166 | Flutolanil | P | LC | 7.93 | positive | 324.1 ➔ 262.1 | 16 | 324.1 ➔ 242.1 | 24 | 130 | 0.9979 | 92.71 | 0.15 |
| 167 | Flutriafol | P | GC | 11.26 | positive | 219.0 ➔ 95.0 | 35 | 219.0 ➔ 123.0 | 15 | 70 | 0.9910 | 95.35 | 0.3 |
| 168 | Fluvalinate tau | P | GC | 17.57 | positive | 250.1 ➔ 55.1 | 30 | 252.0 ➔ 200.0 | 20 | 70 | 0.9754 | 40.31 | 1.25 |
| 169 | Fonofos | P | GC | 8.24 | positive | 246.0 ➔ 109.0 | 15 | 246.0 ➔ 237.0 | 5 | 70 | 0.9814 | 105.24 | 0.3 |
| 170 | Formetanate | P | LC | 1.77 | positive | 222.1 ➔ 165.1 | 12 | 222.1 ➔ 46.2 | 28 | 105 | 0.9290 | 72.76 | 2.5 |
| 171 | Fosthiazate | P | LC | 6.53 | positive | 284.0 ➔ 104.0 | 20 | 284.0 ➔ 227.8 | 8 | 90 | 0.9975 | 94.08 | 0.15 |
| 172 | Heptachlor | P | GC | 9.3 | positive | 272.0 ➔ 237.0 | 15 | 274.0 ➔ 239.0 | 15 | 70 | 0.9806 | 78.64 | 0.15 |
| 173 | Hexachlorobencene | P | GC | 7.76 | positive | 284.0 ➔ 214.0 | 40 | 284.0 ➔ 249.0 | 25 | 70 | 0.9825 | 117.77 | 0.3 |
| 174 | Hexachlorocyclohexane (alpha) | POP | GC | 7.63 | positive | 219.0 ➔ 109.0 | 10 | 219.0 ➔ 183.0 | 10 | 70 | 0.9891 | 111.63 | 0.3 |
| 175 | Hexachlorocyclohexane (beta) | POP | GC | 8.03 | positive | 219.0 ➔ 109.0 | 40 | 219.0 ➔ 183.0 | 5 | 70 | 0.9772 | 92.19 | 0.3 |
| 176 | Hexachlorocyclohexane (delta) | POP | GC | 8.5 | positive | 219.0 ➔ 109.0 | 45 | 219.0 ➔ 183.0 | 5 | 70 | 0.9771 | 62.23 | 0.6 |
| 177 | Hexaclorocyclohexane (gamma, lindane) | POP | GC | 8.13 | positive | 291.0 ➔ 109.0 | 40 | 219.0 ➔183.0 | 10 | 70 | 0.9682 | 75.72 | 1.25 |
| 178 | Hexaconazole (two isomers) | POP | LC | 8.49 | positive | 314.1 ➔ 70.1 | 20 | 316.1 ➔ 70.1 | 20 | 95 | 0.9844 | 100.39 | 0.3 |
| 179 | Hexaflumuron | P | LC | 9.57 | negative | 458.8 ➔ 439.0 | 8 | 458.8 ➔ 175.0 | 30 | 100 | 0.9918 | 105.98 | 0.6 |
| 180 | Hexythiazox | P | LC | 10.18 | positive | 353.1 ➔ 227.9 | 8 | 353.1 ➔ 168.1 | 24 | 120 | 0.9946 | 92.42 | 0.15 |
| 181 | Imazalil (enilconazole) | P | LC | 6.53 | positive | 297.1 ➔ 159.0 | 20 | 297.1 ➔ 69.1 | 18 | 100 | 0.9972 | 97.09 | 0.15 |
| 182 | Imidacloprid | P | LC | 4.1 | positive | 256.0 ➔ 175.0 | 12 | 256.0 ➔ 209.0 | 12 | 110 | 0.9951 | 224 | 0.6 |
| 183 | Indeno [1,2,3-cd] pyrene | P | GC | 19.11 | positive | 276.0 ➔ 274.0 | 50 | 276.0 ➔ 272.0 | 60 | 70 | 0.9812 | 131.78 | 0.3 |
| 184 | Indoxacarb | POP | LC | 9.47 | positive | 528.1 ➔ 293.1 | 10 | 528.1 ➔ 202.8 | 48 | 140 | 0.9883 | 181.2 | 0.6 |
| 185 | Iprodione | P | GC | 13.67 | positive | 314.0 ➔ 56.0 | 20 | 314.0 ➔ 245.0 | 10 | 70 | 0.9588 | 29.79 | 1.25 |
| 186 | Iprovalicarb | P | LC | 8.2 | positive | 321.2 ➔ 119.0 | 15 | 321.2 ➔ 202.9 | 20 | 110 | 0.9913 | 101.06 | 0.3 |
| 187 | Isocarbophos | P | GC | 10.37 | positive | 230.0 ➔ 155.0 | 25 | 230.0 ➔ 198.0 | 10 | 70 | 0.9856 | 99.29 | 0.3 |
| 188 | Isofenphos methyl | P | LC | 8.82 | positive | 199.0 ➔ 121.0 | 10 | 241.0 ➔ 121.0 | 25 | 70 | 0.9974 | 84.43 | 0.15 |
| 189 | Isoprothiolane | P | GC | 11.45 | positive | 291.1 ➔ 189.0 | 30 | 291.1 ➔ 145.0 | 36 | 100 | 0.9928 | 99.83 | 0.3 |
| 190 | Ivermectin B1a | P | LC | 11.5 | positive | 897.5 ➔ 753.5 | 50 | 897.5 ➔ 329.3 | 60 | 160 | 0.9657 | 65.26 | 1.25 |
| 191 | Josamycin | M | LC | 7.48 | positive | 860.5 ➔ 173.9 | 40 | 860.5 ➔ 108.9 | 40 | 200 | 0.9964 | 86.07 | 0.3 |
| 192 | Ketoprofen | M | LC | 7.34 | positive | 255.1 ➔ 209.1 | 8 | 255.1 ➔ 77.1 | 48 | 123 | 0.9949 | 114.88 | 1.25 |
| 193 | Kresoxim methyl | M | LC | 8.8 | positive | 116.0 ➔ 89.0 | 15 | 206.0 ➔ 131.0 | 10 | 70 | 0.9906 | 80.83 | 0.6 |
| 194 | Leptophos | P | GC | 14.58 | positive | 171.1 ➔ 77.1 | 15 | 377.0 ➔ 362.0 | 20 | 70 | 0.9664 | 80.36 | 0.3 |
| 195 | Levamisole | P | LC | 3.19 | positive | 205.1 ➔ 178.1 | 20 | 205.1 ➔ 123.0 | 32 | 141 | 0.9959 | 105.79 | 0.6 |
| 196 | Lincomycin | M | LC | 3.57 | positive | 407.2 ➔ 126.1 | 24 | 407.2 ➔ 359.2 | 16 | 150 | 0.9882 | 117.63 | 1.25 |
| 197 | Linuron | M | LC | 7.56 | positive | 249.0 ➔ 160.1 | 20 | 249.0 ➔ 182.3 | 8 | 120 | 0.9963 | 93.64 | 0.6 |
| 198 | Lufenuron | POP | LC | 10.04 | negative | 509.0 ➔ 339.0 | 5 | 509.0 ➔ 326.1 | 15 | 90 | 0.9933 | 111.99 | 0.3 |
| 199 | Malaoxon | P | LC | 6.05 | positive | 315.1 ➔ 127.2 | 12 | 315.1 ➔ 99.1 | 36 | 120 | 0.9967 | 93.21 | 0.15 |
| 200 | Malathion | P | LC | 7.95 | positive | 348.0 ➔ 126.7 | 15 | 348.0 ➔ 285.0 | 8 | 100 | 0.9978 | 92.86 | 0.15 |
| 201 | Mandipropamid | P | LC | 7.9 | positive | 412.1 ➔ 328.1 | 8 | 412.1 ➔ 356.1 | 4 | 130 | 0.9950 | 101.16 | 0.15 |
| 202 | Mebendazole | P | LC | 6.7 | positive | 296.1 ➔ 264.1 | 20 | 296.1 ➔ 77.0 | 48 | 151 | 0.9978 | 96.88 | 0.3 |
| 203 | Mefenamic acid | P | LC | 9.5 | positive | 242.1 ➔ 209.1 | 28 | 242.1 ➔ 180.1 | 0 | 108 | 0.9857 | 120.65 | 0.6 |
| 204 | Mefenoxam (metalaxyl-M) | M | LC | 6.97 | positive | 280.0 ➔ 220.0 | 10 | 280.0 ➔ 192.0 | 15 | 110 | 0.9982 | 90.57 | 0.15 |
| 205 | Meloxicam | M | LC | 7.13 | positive | 352.5 ➔ 114.8 | 20 | 352.5 ➔ 140.8 | 20 | 130 | 0.9940 | 152.18 | 0.6 |
| 206 | Mepanipyrim | P | GC | 11.14 | positive | 222.0 ➔ 221.0 | 15 | 222.0 ➔ 207.0 | 15 | 70 | 0.9818 | 79.51 | 0.6 |
| 207 | Mepiquat | M | LC | 0.66 | positive | 114.0 ➔ 98.0 | 36 | 114.0 ➔ 70.0 | 45 | 100 | 0.9954 | 63.31 | 0.6 |
| 208 | Metaflumizone | P | LC | 9.92 | negative | 505.0 ➔ 302.0 | 14 | 541.0 ➔ 302.0 | 20 | 90 | 0.9974 | 108.71 | 0.3 |
| 209 | Metalaxyl | P | GC | 9.31 | positive | 234.0 ➔ 146.1 | 20 | 249.0 ➔ 146.0 | 20 | 70 | 0.9888 | 119.76 | 0.15 |
| 210 | Metaldehyde | P | LC | 3.94 | positive | 194.1 ➔ 61.9 | 5 | 194.1 ➔ 106.0 | 5 | 50 | 0.9973 | 74.2 | 1.25 |
| 211 | Metconazole | P | LC | 9.17 | positive | 320.1 ➔ 70.2 | 33 | 322.1 ➔ 70.2 | 24 | 250 | 0.9981 | 86.75 | 0.15 |
| 212 | Methamidophos (two isomers) | P | LC | 1.18 | positive | 142.0 ➔ 94.0 | 12 | 142.0 ➔ 125.0 | 12 | 85 | 0.9936 | 120.45 | 2.5 |
| 213 | Methidathion | P | LC | 7.13 | positive | 320.1 ➔ 144.8 | 8 | 320.1 ➔ 85.0 | 30 | 84 | 0.9982 | 87.88 | 0.15 |
| 214 | Methiocarb | P | LC | 7.68 | positive | 226.1 ➔ 169.0 | 4 | 226.1 ➔ 121.1 | 12 | 90 | 0.9960 | 108.04 | 0.3 |
| 215 | Methiocarb-sufone | P | LC | 4.52 | positive | 258.1 ➔ 201.1 | 8 | 258.1 ➔ 122.1 | 22 | 100 | 0.9967 | 152.19 | 1.25 |
| 216 | Methiocarb-sulfoxide | P | LC | 4.03 | positive | 242.0 ➔ 185.0 | 22 | 242.0 ➔ 122.0 | 28 | 90 | 0.9974 | 100.81 | 0.6 |
| 217 | Methomyl | P | LC | 3.22 | positive | 163.1 ➔ 88.0 | 5 | 163.0 ➔ 106.0 | 8 | 80 | 0.9977 | 67.78 | 0.6 |
| 218 | Methoxyfenozide | P | LC | 8 | positive | 369.2 ➔ 149.0 | 10 | 369.2 ➔ 313.1 | 15 | 85 | 0.9982 | 88.3 | 0.15 |
| 219 | Metoxychlor | P | GC | 13.98 | positive | 227.0 ➔ 141.0 | 20 | 227.0 ➔ 169.0 | 15 | 70 | 0.9648 | 55.26 | 1.25 |
| 220 | Metrafenone | POP | LC | 9.29 | positive | 409.1 ➔ 209.1 | 8 | 411.1 ➔ 209.1 | 12 | 108 | 0.9948 | 102.68 | 0.15 |
| 221 | Metronidazole | P | LC | 2.55 | positive | 172.1 ➔ 128.0 | 12 | 172.1 ➔ 82.1 | 24 | 98 | 0.9949 | 127.67 | 1.25 |
| 222 | Mevinphos (phosdrin) | P | LC | 4.43 | positive | 225.0 ➔ 193.1 | 15 | 225.0 ➔ 127.0 | 12 | 65 | 0.9961 | 79.85 | 1.25 |
| 223 | Mirex | M | GC | 14.8 | positive | 237.0 ➔ 143.0 | 30 | 274.0 ➔ 237.0 | 10 | 70 | 0.9774 | 84.26 | 0.3 |
| 224 | Monocrotophos | P | LC | 3.3 | positive | 224.1 ➔ 126.8 | 12 | 224.1 ➔ 98.1 | 15 | 100 | 0.9972 | 82.04 | 1.25 |
| 225 | Moxidectin | POP | LC | 11.26 | positive | 641.4 ➔ 529.2 | 5 | 641.4 ➔ 499.2 | 5 | 100 | 0.9753 | 106.29 | 1.25 |
| 226 | Myclobutanil | P | LC | 8.1 | positive | 289.1 ➔ 70.1 | 16 | 289.1 ➔ 125.1 | 32 | 110 | 0.9977 | 183.45 | 0.15 |
| 227 | N-(2,4-dimethylphenyl)-N′-methylformamidine (DMPF, metabolite of amitraz) | M | LC | 3.35 | positive | 163.1 ➔ 122.1 | 15 | 163.1 ➔ 107.1 | 15 | 100 | 0.9842 | 104.84 | 2.5 |
| 228 | N,N-Dimethyl-N′-p-tolylsulphamide (DMST, metabolite of tolyfluanid) | P | LC | 6.09 | positive | 215.1 ➔ 106.1 | 10 | 215.1 ➔ 151.1 | 4 | 90 | 0.9949 | 111.49 | 0.6 |
| 229 | Naphtalene | POP | GC | 4.45 | positive | 128.0 ➔ 127.0 | 15 | 128.0 ➔ 102.0 | 25 | 70 | 0.9717 | 160.38 | 1.25 |
| 230 | Naproxen | M | LC | 7.59 | positive | 231.0 ➔ 185.0 | 10 | 231.1 ➔ 169.9 | 21 | 120 | 0.9764 | 409.72 | 5 |
| 231 | Novobiocin | M | LC | 9.62 | positive | 613.2 ➔ 218.1 | 10 | 613.2 ➔ 396.1 | 10 | 150 | 0.9812 | 175.16 | 2.5 |
| 232 | Nuarimol | P | GC | 13.24 | positive | 235.0 ➔ 139.0 | 15 | 235.0 ➔ 111.0 | 40 | 70 | 0.9866 | 101.96 | 0.15 |
| 233 | Ofurace | P | LC | 12.75 | positive | 282.0 ➔ 159.9 | 20 | 282.0 ➔ 147.9 | 30 | 100 | 0.9720 | 57.23 | 0.3 |
| 234 | Omethoate | P | LC | 2.85 | positive | 214.1 ➔ 124.8 | 22 | 214.1 ➔ 183.0 | 5 | 100 | 0.9968 | 95.64 | 0.6 |
| 235 | Oxadixyl | P | LC | 5.46 | positive | 279.1 ➔ 219.2 | 5 | 279.1 ➔ 132.2 | 32 | 110 | 0.9973 | 88.73 | 0.6 |
| 236 | Oxamyl | P | LC | 2.87 | positive | 237.1 ➔ 72.0 | 12 | 237.1 ➔ 90.0 | 5 | 70 | 0.9984 | 88.54 | 0.3 |
| 237 | Oxfendazole | M | LC | 5.63 | positive | 316.1 ➔ 159.0 | 32 | 316.1 ➔ 191.1 | 16 | 166 | 0.9963 | 143.49 | 0.3 |
| 238 | Oxolinic acid | P | LC | 5.06 | positive | 262.1 ➔ 216.0 | 32 | 262.1 ➔ 160.0 | 36 | 110 | 0.9941 | 126.86 | 0.6 |
| 239 | Oxydemeton methyl | M | LC | 3 | positive | 247.0 ➔ 169.0 | 12 | 247.0 ➔ 109.0 | 24 | 100 | 0.9975 | 90.62 | 0.6 |
| 240 | Oxyfluorfen | P | GC | 11.69 | positive | 252.0 ➔ 146.0 | 40 | 300.0 ➔ 223.0 | 15 | 70 | 0.9829 | 76.28 | 0.3 |
| 241 | Paclobutrazol | P | LC | 11.05 | positive | 294.1 ➔ 70.1 | 16 | 294.1 ➔ 125.2 | 36 | 115 | 0.9850 | 79 | 0.15 |
| 242 | Parathion methyl | P | GC | 9.12 | positive | 263.0 ➔ 109.0 | 15 | 263.0 ➔ 79.0 | 30 | 70 | 0.9823 | 86.34 | 0.3 |
| 243 | PCB 28 | POP | GC | 9.02 | positive | 256.0 ➔ 186.0 | 25 | 256.0 ➔ 151.0 | 50 | 70 | 0.9827 | 119.38 | 0.3 |
| 244 | PCB 52 | POP | GC | 9.58 | positive | 292.0 ➔ 222.0 | 25 | 292.0 ➔ 220.0 | 25 | 70 | 0.9791 | 117.94 | 0.15 |
| 245 | PCB 77 | POP | GC | 11.74 | positive | 292.0 ➔ 220.0 | 25 | 292.0 ➔ 222.0 | 25 | 70 | 0.9828 | 119 | 0.15 |
| 246 | PCB 81 | POP | GC | 11.57 | positive | 292.0 ➔ 220.0 | 25 | 292.0 ➔ 222.0 | 25 | 70 | 0.9691 | 118.13 | 0.15 |
| 247 | PCB 101 | POP | GC | 11.08 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9773 | 145.98 | 0.15 |
| 248 | PCB 105 | POP | GC | 12.67 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9795 | 122.8 | 0.15 |
| 249 | PCB 114 | POP | GC | 12.39 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9815 | 129.03 | 0.15 |
| 250 | PCB 118 | POP | GC | 12.19 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9839 | 127.49 | 0.15 |
| 251 | PCB 123 | POP | GC | 12.14 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9802 | 133.73 | 0.3 |
| 252 | PCB 126 | POP | GC | 13.23 | positive | 326.0 ➔ 256.0 | 30 | 328.0 ➔ 256.0 | 30 | 70 | 0.9856 | 128.92 | 0.15 |
| 253 | PCB 138 | POP | GC | 13.07 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9822 | 134.56 | 0.15 |
| 254 | PCB 153 | POP | GC | 12.58 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9791 | 123.08 | 0.3 |
| 255 | PCB 156 | POP | GC | 13.97 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9608 | 129.48 | 0.15 |
| 256 | PCB 157 | POP | GC | 14.07 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9811 | 143.51 | 0.15 |
| 257 | PCB 167 | POP | GC | 13.56 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9830 | 127.89 | 0.15 |
| 258 | PCB 169 | POP | GC | 14.62 | positive | 360.0 ➔ 290.0 | 25 | 360.0 ➔ 288.0 | 25 | 70 | 0.9850 | 132.35 | 0.15 |
| 259 | PCB 180 | POP | GC | 14.25 | positive | 394.0 ➔ 324.0 | 30 | 394.0 ➔ 322.0 | 30 | 70 | 0.9920 | 139.99 | 0.15 |
| 260 | PCB 189 | POP | GC | 15.26 | positive | 394.0 ➔ 324.0 | 30 | 394.0 ➔ 322.0 | 30 | 70 | 0.9852 | 132.07 | 0.15 |
| 261 | Penconazole | P | GC | 10.51 | positive | 248.0 ➔ 157.0 | 30 | 248.0 ➔ 192.0 | 15 | 70 | 0.9881 | 101.88 | 0.15 |
| 262 | Pencycuron | P | LC | 9.32 | positive | 329.1 ➔ 125.1 | 24 | 329.1 ➔ 217.9 | 12 | 160 | 0.9975 | 81.56 | 0.15 |
| 263 | Pendimethalin | P | GC | 10.2 | positive | 252.0 ➔ 162.0 | 10 | 252.0 ➔ 191.0 | 5 | 70 | 0.9960 | 99.46 | 0.3 |
| 264 | Penicilina V | M | LC | 6.48 | positive | 383.2 ➔ 159.9 | 10 | 383.2 ➔ 113.9 | 40 | 130 | 0.9848 | 161.32 | 2.5 |
| 265 | Permethrin | P | GC | 15.7 | positive | 183.0 ➔ 128.0 | 15 | 183.1 ➔ 153.1 | 15 | 70 | 0.9796 | 95.57 | 1.25 |
| 266 | Phenanthrene | POP | GC | 8.33 | positive | 178.0 ➔ 176.0 | 35 | 178.0 ➔ 152.0 | 28 | 70 | 0.9854 | 140.09 | 0.3 |
| 267 | Phenylbutazone | M | LC | 8.24 | positive | 309.2 ➔ 160.2 | 20 | 309.2 ➔ 77.1 | 55 | 140 | 0.9492 | 43.54 | 2.5 |
| 268 | Phosalone | P | LC | 9.2 | positive | 385.1 ➔ 182.0 | 20 | 385.1 ➔ 110.9 | 55 | 80 | 0.9972 | 90.45 | 0.3 |
| 269 | Phosmet | P | LC | 7.35 | positive | 318.0 ➔ 159.9 | 16 | 318.0 ➔ 133.0 | 40 | 90 | 0.9963 | 98.52 | 0.6 |
| 270 | Phosmet-oxon | P | LC | 5.39 | positive | 302.0 ➔ 160.0 | 10 | 302.0 ➔ 133.0 | 38 | 60 | 0.9967 | 86.6 | 1.25 |
| 271 | Pirimicarb | P | LC | 5.31 | positive | 239.1 ➔ 72.1 | 20 | 239.1 ➔ 182.1 | 12 | 100 | 0.9968 | 88.23 | 0.15 |
| 272 | Pirimicarb-desmethyl | P | LC | 3.6 | positive | 225.1 ➔ 168.1 | 8 | 225.1 ➔ 72.1 | 20 | 100 | 0.9982 | 73.62 | 0.3 |
| 273 | Pirimiphos ethyl | P | GC | 10.26 | positive | 318.0 ➔ 166.0 | 15 | 318.0 ➔ 182.0 | 15 | 70 | 0.9794 | 95.24 | 0.15 |
| 274 | Pirimiphos methyl | P | GC | 9.57 | positive | 306.1 ➔ 164.0 | 20 | 306.1 ➔ 108.1 | 32 | 100 | 0.9881 | 95.62 | 0.15 |
| 275 | Prochloraz | P | LC | 9.11 | positive | 376.0 ➔ 308.0 | 10 | 376.0 ➔ 70.1 | 20 | 100 | 0.9949 | 96.56 | 0.15 |
| 276 | Procymidone | P | GC | 10.8 | positive | 283.0 ➔ 67.0 | 40 | 283.0 ➔ 68.0 | 25 | 70 | 0.9871 | 102.97 | 0.6 |
| 277 | Profenofos | P | LC | 9.75 | positive | 375.0 ➔ 305.0 | 20 | 373.0 ➔ 303.0 | 20 | 100 | 0.9948 | 84.29 | 0.3 |
| 278 | Propamocarb | P | LC | 2.93 | positive | 189.2 ➔ 102.0 | 12 | 189.2 ➔ 144.0 | 8 | 110 | 0.9969 | 124.45 | 0.6 |
| 279 | Propargite | P | LC | 10.35 | positive | 368.2 ➔ 231.1 | 4 | 368.2 ➔ 175.0 | 12 | 88 | 0.9973 | 79.42 | 0.15 |
| 280 | Propiconazole | P | LC | 9.03 | positive | 342.0 ➔ 69.0 | 21 | 342.0 ➔ 159.0 | 39 | 90 | 0.9953 | 90.91 | 0.6 |
| 281 | Propoxur | P | LC | 5.88 | positive | 210.1 ➔ 168.1 | 35 | 210.1 ➔ 65.1 | 40 | 70 | 0.9980 | 84.74 | 0.3 |
| 282 | Propyzamide (pronamide) | P | LC | 7.94 | positive | 256.1 ➔ 190.0 | 16 | 256.1 ➔ 173.0 | 25 | 90 | 0.9971 | 92.15 | 0.3 |
| 283 | Proquinazid | P | LC | 10.59 | positive | 288.0 ➔ 245.0 | 15 | 288.0 ➔ 217.0 | 30 | 70 | 0.9942 | 80.72 | 0.15 |
| 284 | Prothioconazol | P | GC | 11.86 | positive | 186.0 ➔ 49.0 | 20 | 186.0 ➔ 53.0 | 25 | 70 | 0.9923 | 96.14 | 0.15 |
| 285 | Prothiophos | P | GC | 11.46 | positive | 266.9 ➔ 221.0 | 35 | 162.0 ➔ 63.1 | 30 | 70 | 0.9875 | 95.4 | 0.3 |
| 286 | Pymetrozine | P | LC | 2.8 | positive | 218.1 ➔ 105.0 | 20 | 218.1 ➔ 78.0 | 52 | 120 | 0.9960 | 148.99 | 1.25 |
| 287 | Pyraclostrobin | P | LC | 9.15 | positive | 388.1 ➔ 193.8 | 8 | 388.1 ➔ 163.1 | 28 | 120 | 0.9980 | 84.91 | 0.15 |
| 288 | Pyrazophos | P | LC | 9.23 | positive | 374.1 ➔ 222.1 | 23 | 374.1 ➔ 194.0 | 32 | 100 | 0.9971 | 106.05 | 0.3 |
| 289 | Pyrene | POP | GC | 11.14 | positive | 202.0 ➔ 201.0 | 27 | 202.0 ➔ 200.0 | 45 | 70 | 0.9852 | 111.07 | 0.3 |
| 290 | Pyridaben | P | LC | 10.75 | positive | 365.2 ➔ 309.0 | 8 | 309.1 ➔ 147.0 | 16 | 168 | 0.9961 | 54.03 | 0.15 |
| 291 | Pyridaphenthion | P | LC | 8.11 | positive | 341.0 ➔ 189.0 | 22 | 341.0 ➔ 205.0 | 34 | 100 | 0.9980 | 101.11 | 0.3 |
| 292 | Pyrimethanil | P | GC | 8.27 | positive | 198.0 ➔ 118.0 | 40 | 198.0 ➔ 158.0 | 20 | 70 | 0.9876 | 107.48 | 0.15 |
| 293 | Pyriproxifen | P | LC | 10.08 | positive | 322.2 ➔ 96.0 | 12 | 322.2 ➔ 184.9 | 24 | 80 | 0.9981 | 82.74 | 0.15 |
| 294 | Quinalfos | P | LC | 8.75 | positive | 299.1 ➔ 96.9 | 30 | 299.1 ➔147.1 | 20 | 130 | 0.9953 | 91.26 | 0.3 |
| 295 | Quinoxyfen | P | LC | 10.13 | positive | 308.0 ➔ 197.0 | 32 | 308.2 ➔ 161.8 | 55 | 120 | 0.9954 | 81.86 | 0.3 |
| 296 | Rifampicin | M | LC | 7.99 | positive | 823.5 ➔ 791.4 | 15 | 823.5 ➔ 399.1 | 25 | 160 | 0.9758 | 75.59 | 2.5 |
| 297 | Rotenone | P | LC | 8.63 | positive | 395.1 ➔ 213.1 | 20 | 395.1 ➔ 192.1 | 25 | 150 | 0.9757 | 115.42 | 0.6 |
| 298 | Roxithromycin | M | LC | 7.75 | positive | 838.5 ➔ 158.1 | 40 | 838.5 ➔ 116.1 | 55 | 200 | 0.9940 | 113.62 | 0.6 |
| 299 | Simazine | P | LC | 5.87 | positive | 202.4 ➔ 68.1 | 30 | 202.4 ➔ 68.1 | 20 | 120 | 0.9982 | 70.91 | 0.3 |
| 300 | Spinosad (two isomers) | P | LC | 9.19 | positive | 732.4 ➔ 142.0 | 22 | 732.4 ➔ 98.0 | 60 | 130 | 0.9924 | 89.19 | 1.25 |
| 301 | Spiramycin (two isomers) | M | LC | 4.68 | positive | 439.1 ➔ 101.1 | 20 | 439.1 ➔ 88.0 | 50 | 70 | 0.9970 | 91.74 | 2.5 |
| 302 | Spirodiclofen | P | LC | 10.51 | positive | 411.1 ➔ 71.2 | 15 | 411.1 ➔ 313.0 | 5 | 110 | 0.9923 | 118.42 | 2.5 |
| 303 | Spiromesifen | P | LC | 10.28 | positive | 388.0 ➔ 273.0 | 25 | 273.0 ➔ 187.0 | 15 | 110 | 0.9956 | 75.77 | 0.6 |
| 304 | Spiroxamine | P | LC | 7.7 | positive | 298.3 ➔ 144.1 | 16 | 298.3 ➔100.1 | 32 | 120 | 0.9961 | 105.38 | 0.3 |
| 305 | Strychnine | AR | LC | 3.15 | positive | 335.1 ➔ 184.0 | 45 | 335.1 ➔ 156.0 | 40 | 105 | 0.9966 | 91.72 | 0.6 |
| 306 | Sulfacetamide | M | LC | 2.12 | positive | 215.3 ➔ 155.9 | 10 | 215.3 ➔ 92.0 | 20 | 90 | 0.9877 | 143.43 | 2.5 |
| 307 | Sulfachloropiridacine | M | LC | 3.75 | positive | 285.0 ➔ 156.0 | 12 | 285.0 ➔ 92.1 | 28 | 101 | 0.9979 | 285.3 | 0.6 |
| 308 | Sulfadiacine | M | LC | 2.86 | positive | 251.0 ➔ 92.0 | 28 | 251.0 ➔ 156.0 | 12 | 111 | 0.9918 | 229.63 | 1.25 |
| 309 | Sulfadimetoxine | M | LC | 4.81 | positive | 311.0 ➔ 92.0 | 32 | 311.0 ➔ 156.0 | 16 | 139 | 0.9976 | 229.39 | 0.3 |
| 310 | Sulfadoxine | M | LC | 4.15 | positive | 311.1 ➔ 92.0 | 32 | 311.1 ➔ 156.0 | 16 | 126 | 0.9977 | 167.2 | 0.3 |
| 311 | Sulfameracine | M | LC | 3.32 | positive | 265.0 ➔ 92.0 | 28 | 265.0 ➔ 156.0 | 12 | 126 | 0.9972 | 280.38 | 0.6 |
| 312 | Sulfametacine | M | LC | 3.7 | positive | 279.1 ➔ 186.0 | 12 | 279.1 ➔ 92.0 | 32 | 134 | 0.9970 | 293.67 | 0.6 |
| 313 | Sulfametizole | M | LC | 3.37 | positive | 271.0 ➔ 92.0 | 28 | 271.0 ➔ 155.9 | 8 | 103 | 0.9972 | 321.37 | 1.25 |
| 314 | Sulfametoxazole | M | LC | 3.96 | positive | 254.0 ➔ 92.0 | 28 | 254.0 ➔ 156.0 | 12 | 111 | 0.9968 | 235.15 | 0.6 |
| 315 | Sulfametoxipiridacine | M | LC | 3.77 | positive | 281.0 ➔ 155.9 | 12 | 281.0 ➔ 92.1 | 28 | 121 | 0.9963 | 257.12 | 0.6 |
| 316 | Sulfamonomethoxine | M | LC | 4.03 | positive | 281.1 ➔ 156.0 | 14 | 281.1 ➔ 92.1 | 32 | 120 | 0.9886 | 298.13 | 1.25 |
| 317 | Sulfapyridine | M | LC | 2.8 | positive | 250.0 ➔ 156.0 | 12 | 250.0 ➔ 92.0 | 28 | 126 | 0.9953 | 236.91 | 1.25 |
| 318 | Sulfaquinoxaline | M | LC | 5 | positive | 301.0 ➔ 156.0 | 12 | 301.0 ➔ 92.1 | 32 | 159 | 0.9945 | 257.62 | 0.6 |
| 319 | Sulfatiazole | M | LC | 3.05 | positive | 256.0 ➔ 92.0 | 28 | 256.0 ➔ 156.0 | 12 | 106 | 0.9924 | 395.5 | 1.25 |
| 320 | Sulfisoxazole | M | LC | 4.14 | positive | 268.0 ➔ 156.0 | 8 | 268.0 ➔ 92.1 | 24 | 106 | 0.9973 | 251.38 | 0.6 |
| 321 | Tebuconazole | P | LC | 8.92 | positive | 308.2 ➔ 70.2 | 22 | 308.2 ➔ 125.1 | 53 | 120 | 0.9978 | 70.16 | 0.6 |
| 322 | Tebufenocide | P | LC | 8.67 | positive | 353.1 ➔ 132.9 | 22 | 353.1 ➔ 297.1 | 20 | 90 | 0.9971 | 80.95 | 0.15 |
| 323 | Tebufenpyrad | P | LC | 14.07 | positive | 334.2 ➔ 117.0 | 47 | 334.2 ➔ 145.0 | 37 | 180 | 0.9846 | 104.68 | 0.15 |
| 324 | Teflubenzuron | P | LC | 10 | negative | 379.0 ➔ 339.0 | 15 | 379.0 ➔ 196.0 | 25 | 100 | 0.9912 | 77.79 | 1.25 |
| 325 | Tefluthrin | P | GC | 8.42 | positive | 177.0 ➔ 127.0 | 15 | 177.0 ➔ 87.0 | 15 | 70 | 0.9910 | 114.07 | 0.15 |
| 326 | Telodrin (isobenzan) | P | GC | 10.15 | positive | 310.8 ➔ 240.8 | 25 | 310.8 ➔ 274.8 | 5 | 70 | 0.9805 | 95.38 | 0.3 |
| 327 | Terbufos | P | GC | 8.15 | positive | 231.0 ➔ 97.0 | 20 | 231.0 ➔ 129.0 | 15 | 70 | 0.9904 | 102.34 | 0.15 |
| 328 | Terbuthylazine | P | GC | 7.74 | positive | 214.0 ➔ 104.0 | 20 | 214.0 ➔ 132.0 | 10 | 70 | 0.9975 | 81.85 | 0.15 |
| 329 | Tetrachlorvinphos | P | LC | 8.74 | positive | 367.0 ➔ 127.0 | 16 | 365.0 ➔ 127.0 | 16 | 110 | 0.9940 | 92.08 | 0.3 |
| 330 | Tetraconazole | P | GC | 10.03 | positive | 336.0 ➔ 204.0 | 35 | 336.0 ➔ 218.0 | 20 | 70 | 0.9793 | 102.89 | 0.3 |
| 331 | Tetradifon | P | GC | 14.36 | positive | 158.9 ➔ 111.0 | 20 | 354.0 ➔ 159.0 | 10 | 70 | 0.9910 | 104.79 | 0.15 |
| 332 | Tetramethrin | P | GC | 13.81 | positive | 164.0 ➔ 77.0 | 30 | 164.0 ➔ 107.0 | 15 | 70 | 0.9878 | 153.48 | 0.6 |
| 333 | Thiabendazole | P | LC | 3.9 | positive | 202.0 ➔ 175.0 | 24 | 202.0 ➔ 131.0 | 26 | 170 | 0.9980 | 71.17 | 0.3 |
| 334 | Thiacloprid | P | LC | 4.81 | positive | 253.0 ➔ 126.0 | 16 | 253.0 ➔ 90.0 | 40 | 140 | 0.9983 | 102.02 | 0.15 |
| 335 | Thiamethoxam | P | LC | 3.35 | positive | 292.0 ➔ 211.1 | 8 | 292.0 ➔ 132.0 | 22 | 80 | 0.9972 | 124.12 | 2.5 |
| 336 | Thiophanate methyl | P | LC | 5.89 | positive | 343.0 ➔ 151.0 | 20 | 343.0 ➔ 93.0 | 46 | 90 | 0.9976 | 191.68 | 0.3 |
| 337 | Tolclofos methyl | M | GC | 9.2 | positive | 265.0 ➔ 93.0 | 30 | 265.0 ➔ 220.0 | 25 | 70 | 0.9846 | 102.61 | 0.3 |
| 338 | Tolfenamic acid | P | LC | 9.77 | negative | 260.0 ➔ 216.1 | 8 | 260.0 ➔ 35.1 | 20 | 108 | 0.9872 | 543.45 | 1.25 |
| 339 | Triadimefon | M | LC | 8.05 | positive | 294.1 ➔ 69.3 | 20 | 294.1 ➔ 197.2 | 15 | 100 | 0.9948 | 197.11 | 0.3 |
| 340 | Triadimenol | P | LC | 8.23 | positive | 296.1 ➔ 70.0 | 10 | 298.1 ➔ 70.0 | 10 | 80 | 0.9968 | 130.33 | 0.6 |
| 341 | Triazophos (hostathion) | P | LC | 8.18 | positive | 314.1 ➔ 162.0 | 19 | 314.1 ➔ 118.9 | 35 | 100 | 0.9983 | 85.32 | 0.3 |
| 342 | Trichlorfon | P | LC | 4.05 | positive | 256.9 ➔ 109.0 | 12 | 258.9 ➔ 109.0 | 12 | 170 | 0.9966 | 191.7 | 1.25 |
| 343 | Trifloxystrobin | P | LC | 9.5 | positive | 409.1 ➔ 186.0 | 12 | 409.1 ➔ 145.0 | 52 | 110 | 0.9979 | 75.41 | 0.15 |
| 344 | Triflumizole | P | LC | 9.55 | positive | 346.1 ➔ 278.0 | 4 | 345.9 ➔ 73.0 | 15 | 80 | 0.9982 | 85.3 | 0.15 |
| 345 | Triflumuron | P | LC | 9.19 | positive | 359.0 ➔ 156.0 | 8 | 359.0 ➔ 139.0 | 32 | 120 | 0.9933 | 94.81 | 0.6 |
| 346 | Trifluralin | P | GC | 7.26 | positive | 264.0 ➔ 160.0 | 15 | 306.0 ➔ 264.0 | 5 | 70 | 0.9843 | 105.19 | 0.6 |
| 347 | Trimethoprim | P | LC | 3.08 | positive | 291.2 ➔ 123.0 | 24 | 291.2 ➔ 230.1 | 20 | 162 | 0.9959 | 85.63 | 0.6 |
| 348 | Triticonazole | M | LC | 8.4 | positive | 318.1 ➔ 70.1 | 33 | 320.1 ➔ 70.1 | 16 | 110 | 0.9760 | 119.71 | 1.25 |
| 349 | Tylmicosin | P | LC | 5.52 | positive | 869.6 ➔ 174.1 | 48 | 869.6 ➔ 696.4 | 44 | 294 | 0.9894 | 170.35 | 2.5 |
| 350 | Tylosin | M | LC | 6.85 | positive | 916.5 ➔ 174.1 | 40 | 916.5 ➔ 772.4 | 28 | 210 | 0.9966 | 92.36 | 1.25 |
| 351 | Vinclozolin | P | GC | 9.09 | positive | 212.0 ➔ 145.0 | 25 | 212.0 ➔ 109.0 | 50 | 70 | 0.9868 | 105.41 | 0.15 |
| 352 | Warfarin | AR | LC | 7.86 | negative | 307.1 ➔ 161.1 | 20 | 307.1 ➔ 250.1 | 20 | 140 | 0.9949 | 96.71 | 0.3 |
| 353 | Zoxamide | P | LC | 9.03 | positive | 336.0 ➔ 187.1 | 25 | 187.1 ➔ 88.9 | 40 | 200 | 0.9911 | 99.62 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial-Berriel, C.; Ramos-Luzardo, Á.; Acosta-Dacal, A.; Macías-Montes, A.; Fernández-Valerón, P.; Henríquez-Hernández, L.A.; Zumbado, M.; Boada, L.D.; Luzardo, O.P. Validation of a Method Scope Extension for Simple Biomonitoring of 353 Pollutants in Serum Samples. Toxics 2023, 11, 498. https://doi.org/10.3390/toxics11060498
Rial-Berriel C, Ramos-Luzardo Á, Acosta-Dacal A, Macías-Montes A, Fernández-Valerón P, Henríquez-Hernández LA, Zumbado M, Boada LD, Luzardo OP. Validation of a Method Scope Extension for Simple Biomonitoring of 353 Pollutants in Serum Samples. Toxics. 2023; 11(6):498. https://doi.org/10.3390/toxics11060498
Chicago/Turabian StyleRial-Berriel, Cristian, Álvaro Ramos-Luzardo, Andrea Acosta-Dacal, Ana Macías-Montes, Pilar Fernández-Valerón, Luis Alberto Henríquez-Hernández, Manuel Zumbado, Luis D. Boada, and Octavio P. Luzardo. 2023. "Validation of a Method Scope Extension for Simple Biomonitoring of 353 Pollutants in Serum Samples" Toxics 11, no. 6: 498. https://doi.org/10.3390/toxics11060498
APA StyleRial-Berriel, C., Ramos-Luzardo, Á., Acosta-Dacal, A., Macías-Montes, A., Fernández-Valerón, P., Henríquez-Hernández, L. A., Zumbado, M., Boada, L. D., & Luzardo, O. P. (2023). Validation of a Method Scope Extension for Simple Biomonitoring of 353 Pollutants in Serum Samples. Toxics, 11(6), 498. https://doi.org/10.3390/toxics11060498











