Multigenerational Effects of a Complex Human-Relevant Exposure during Folliculogenesis and Preimplantation Embryo Development: The FEDEXPO Study
Abstract
1. Introduction
2. Study Design
2.1. Experimental Model
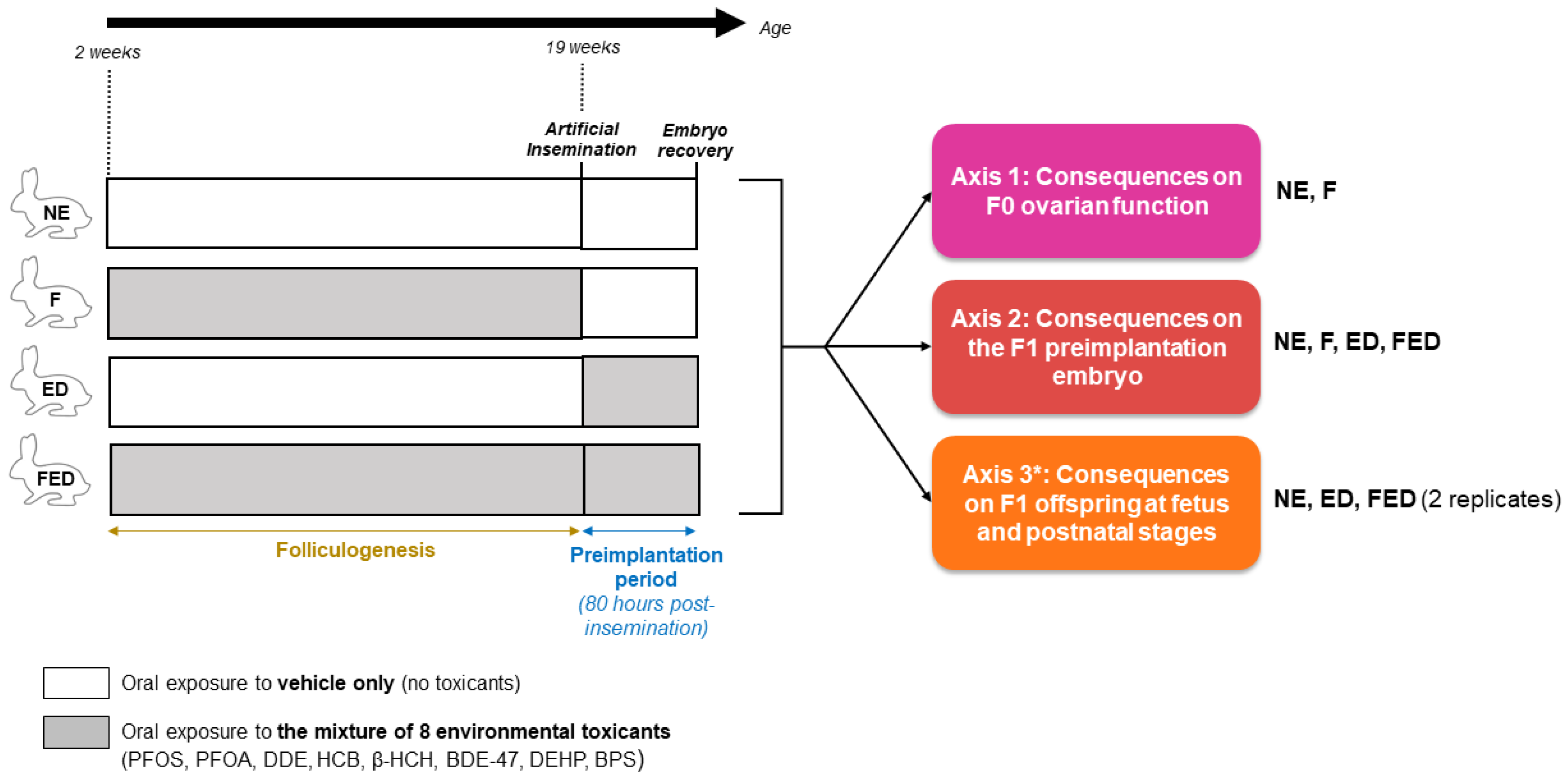
2.2. Study Groups, Objectives, and Breeding
2.3. Toxicants Mixture
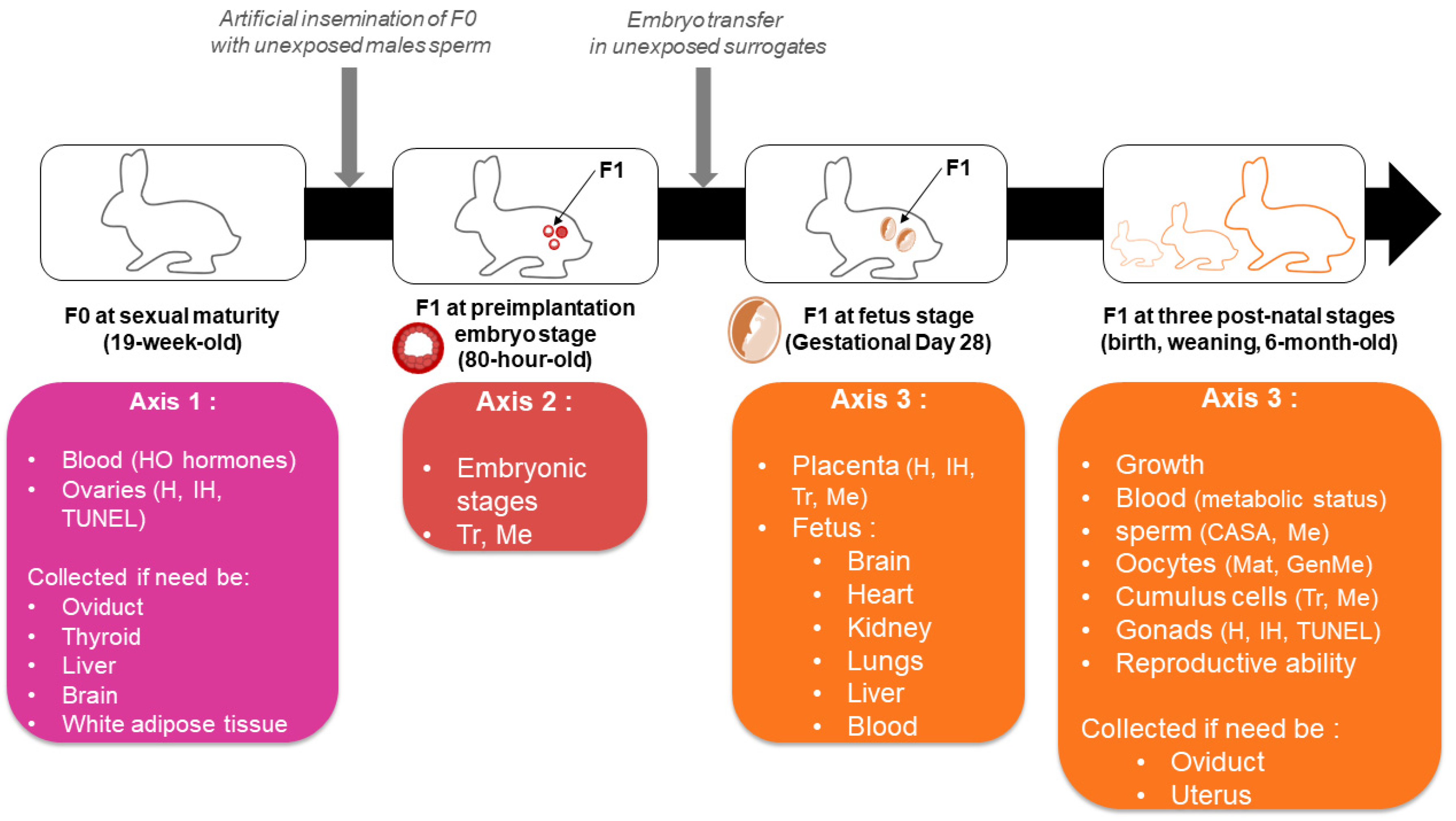
2.4. Biological Samples
2.5. Sample Size Estimation
3. Methods
3.1. F0 Mothers
3.1.1. Biochemistry of the Serum
3.1.2. Ovaries
3.2. F1 Offspring at Preimplantation Embryo Stage
3.3. F1 Offspring at Feto-Placental Stage
3.4. F1 Offspring after Birth
3.4.1. Biochemistry of the Serum
3.4.2. Gonads
3.4.3. F1 Gametes
3.4.4. Reproductive Ability
3.5. Statistical Analysis
4. Discussion
4.1. Strengths
4.2. Limits
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Basso, C.G.; de Araújo-Ramos, A.T.; Martino-Andrade, A.J. Exposure to Phthalates and Female Reproductive Health: A Literature Review. Reprod. Toxicol. 2022, 109, 61–79. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Rajakumar, G.; Shariati, M.A.; Chung, I.-M.; Thiruvengadam, M. Organopesticides and Fertility: Where Does the Link Lead To? Environ. Sci. Pollut. Res. 2021, 28, 6289–6301. [Google Scholar] [CrossRef]
- Stavridis, K.; Triantafyllidou, O.; Pisimisi, M.; Vlahos, N. Bisphenol-A and Female Fertility: An Update of Existing Epidemiological Studies. J. Clin. Med. 2022, 11, 7227. [Google Scholar] [CrossRef]
- Patel, S.; Zhou, C.; Rattan, S.; Flaws, J.A. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol. Reprod. 2015, 93, 20. [Google Scholar] [CrossRef]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine Disrupting Chemicals: Impacts on Human Fertility and Fecundity during the Peri-Conception Period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef]
- Land, K.L.; Miller, F.G.; Fugate, A.C.; Hannon, P.R. The Effects of Endocrine-disrupting Chemicals on Ovarian- and Ovulation-related Fertility Outcomes. Mol. Reprod. Dev. 2022, 89, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J.; Leandri, R.D. Environmental Pollutants, a Possible Etiology for Premature Ovarian Insufficiency: A Narrative Review of Animal and Human Data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An Emerging Threat to Female Fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Dzika, E.; Gonkowski, S.; Wojtkiewicz, J. Bisphenol S in Food Causes Hormonal and Obesogenic Effects Comparable to or Worse than Bisphenol A: A Literature Review. Nutrients 2020, 12, 532. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Rayasam, S.D.G.; Axelrad, D.A.; Bennett, D.H.; Brown, P.; Carignan, C.C.; Chartres, N.; Diamond, M.L.; Joglekar, R.; Shamasunder, B.; et al. Addressing Systemic Problems with Exposure Assessments to Protect the Public’s Health. Environ. Health 2023, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, C.; Le Magueresse-Battistoni, B.; Viguié, C.; Babajko, S.; Canivenc-Lavier, M.-C.; Chevalier, N.; Emond, C.; Habert, R.; Picard-Hagen, N.; Mhaouty-Kodja, S. Regulatory and Academic Studies to Derive Reference Values for Human Health: The Case of Bisphenol S. Environ. Res. 2022, 204, 112233. [Google Scholar] [CrossRef]
- Caporale, N.; Leemans, M.; Birgersson, L.; Germain, P.-L.; Cheroni, C.; Borbély, G.; Engdahl, E.; Lindh, C.; Bressan, R.B.; Cavallo, F.; et al. From Cohorts to Molecules: Adverse Impacts of Endocrine Disrupting Mixtures. Science 2022, 375, eabe8244. [Google Scholar] [CrossRef]
- Cattaneo, I.; Kalian, A.D.; Di Nicola, M.R.; Dujardin, B.; Levorato, S.; Mohimont, L.; Nathanail, A.V.; Carnessechi, E.; Astuto, M.C.; Tarazona, J.V.; et al. Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges. Toxins 2023, 15, 40. [Google Scholar] [CrossRef]
- Kerr, J.B.; Myers, M.; Anderson, R.A. The Dynamics of the Primordial Follicle Reserve. Reproduction 2013, 146, R205–R215. [Google Scholar] [CrossRef]
- Johansson, H.K.L.; Svingen, T.; Fowler, P.A.; Vinggaard, A.M.; Boberg, J. Environmental Influences on Ovarian Dysgenesis—Developmental Windows Sensitive to Chemical Exposures. Nat. Rev. Endocrinol. 2017, 13, 400–414. [Google Scholar] [CrossRef]
- Marcho, C.; Cui, W.; Mager, J. Epigenetic Dynamics during Preimplantation Development. Reproduction 2015, 150, R109–R120. [Google Scholar] [CrossRef]
- Clarke, H.J.; Vieux, K.F. Epigenetic Inheritance through the Female Germ-Line: The Known, the Unknown, and the Possible. Semin. Cell Dev. Biol 2015, 43, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Carlson, A.; Schwartz, J.M.; Giudice, L.C. Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: Executive Summary. Fertil. Steril. 2009, 89, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of Lifetime Health around the Time of Conception: Causes and Consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Ben Maamar, M.; McCarrey, J.R. Transgenerational Sperm DNA Methylation Epimutation Developmental Origins Following Ancestral Vinclozolin Exposure. Epigenetics 2019, 14, 721–739. [Google Scholar] [CrossRef]
- Montjean, D.; Neyroud, A.-S.; Yefimova, M.G.; Benkhalifa, M.; Cabry, R.; Ravel, C. Impact of Endocrine Disruptors upon Non-Genetic Inheritance. Int. J. Mol. Sci. 2022, 23, 3350. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Dale, B.; Elder, K. The Negative Impact of the Environment on Methylation/Epigenetic Marking in Gametes and Embryos: A Plea for Action to Protect the Fertility of Future Generations. Mol. Reprod. Dev. 2019, 86, 1273–1282. [Google Scholar] [CrossRef]
- Daniel-Carlier, N.; Harscoët, E.; Thépot, D.; Auguste, A.; Pailhoux, E.; Jolivet, G. Gonad Differentiation in the Rabbit: Evidence of Species-Specific Features. PLoS ONE 2013, 8, e60451. [Google Scholar] [CrossRef]
- Hutt, K.J.; McLaughlin, E.A.; Holland, M.K. Primordial Follicle Activation and Follicular Development in the Juvenile Rabbit Ovary. Cell Tissue Res. 2006, 326, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a Reproductive Model for Human Health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef]
- Christians, E.; Rao, V.H.; Renard, J.P. Sequential Acquisition of Transcriptional Control during Early Embryonic Development in the Rabbit. Dev. Biol. 1994, 164, 160–172. [Google Scholar] [CrossRef]
- Tesarik, J. Control of Maternal-to-Zygotic Transition in Human Embryos and Other Animal Species (Especially Mouse): Similarities and Differences. Int. J. Mol. Sci. 2022, 23, 8562. [Google Scholar] [CrossRef]
- Okamoto, I.; Patrat, C.; Thepot, D.; Peynot, N.; Fauque, P.; Daniel, N.; Diabangouaya, P.; Wolf, J.P.; Renard, J.P.; Duranthon, V.; et al. Eutherian Mammals Use Diverse Strategies to Initiate X-Chromosome Inactivation during Development. Nature 2011, 472, 370–374. [Google Scholar] [CrossRef]
- Robinson, O.; Basagana, X.; Agier, L.; de Castro, M.; Hernandez-Ferrer, C.; Gonzalez, J.R.; Grimalt, J.O.; Nieuwenhuijsen, M.; Sunyer, J.; Slama, R.; et al. The Pregnancy Exposome: Multiple Environmental Exposures in the INMA-Sabadell Birth Cohort. Environ. Sci. Technol. 2015, 49, 10632–10641. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Uria, I.; Maitre, L.; Thomsen, C.; Nieuwenhuijsen, M.J.; Chatzi, L.; Siroux, V.; Aasvang, G.M.; Agier, L.; Andrusaityte, S.; Casas, M.; et al. The Early-Life Exposome: Description and Patterns in Six European Countries. Environ. Int. 2019, 123, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Assens, M.; Frederiksen, H.; Petersen, J.H.; Larsen, T.; Skakkebaek, N.E.; Juul, A.; Andersson, A.M.; Main, K.M. Variations in Repeated Serum Concentrations of UV Filters, Phthalates, Phenols and Parabens during Pregnancy. Environ. Int. 2019, 123, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gerona, R.R.; Schwartz, J.M.; Pan, J.; Friesen, M.M.; Lin, T.; Woodruff, T.J. Suspect Screening of Maternal Serum to Identify New Environmental Chemical Biomonitoring Targets Using Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Martinez, D.; Aguilera, I.; Ballester, F.; Basterrechea, M.; Esplugues, A.; Guxens, M.; Larranaga, M.; Lertxundi, A.; Mendez, M.; et al. Socioeconomic Status and Exposure to Multiple Environmental Pollutants during Pregnancy: Evidence for Environmental Inequity? J. Epidemiol. Community Health 2012, 66, 106–113. [Google Scholar] [CrossRef]
- Gentry, P.R.; Clewell, H.J.; Clewell, R.; Campbell, J.; Van Landingham, C.; Shipp, A.M. Challenges in the Application of Quantitative Approaches in Risk Assessment: A Case Study with Di-(2-Ethylhexyl)Phthalate. Crit. Rev. Toxicol. 2011, 41, 1–72. [Google Scholar] [CrossRef]
- Gayrard, V.; Moreau, J.; Picard-Hagen, N.; Helies, V.; Marchand, P.; Antignac, J.-P.; Toutain, P.-L.; Leandri, R. Use of Mixture Dosing and Nonlinear Mixed Effect Modeling of Eight Environmental Contaminants in Rabbits to Improve Extrapolation Value of Toxicokinetic Data. Environ. Health Perspect. 2021, 129, 117006. [Google Scholar] [CrossRef]
- Tighilet, B.; Bourdet, A.; Péricat, D.; Timon-David, E.; Rastoldo, G.; Chabbert, C. SK Channels Modulation Accelerates Equilibrium Recovery in Unilateral Vestibular Neurectomized Rats. Pharmaceuticals 2021, 14, 1226. [Google Scholar] [CrossRef]
- Pérot, J.-B.; Célestine, M.; Palombo, M.; Dhenain, M.; Humbert, S.; Brouillet, E.; Flament, J. Longitudinal Multimodal MRI Characterization of a Knock-in Mouse Model of Huntington’s Disease Reveals Early Gray and White Matter Alterations. Hum. Mol. Genet. 2022, 31, 3581–3596. [Google Scholar] [CrossRef]
- Vicente, J.S.; Viudes-de-Castro, M.P.; Cedano-Castro, J.I.; Marco-Jiménez, F. Cryosurvival of Rabbit Embryos Obtained after Superovulation with Corifollitropin Alfa with or without LH. Anim. Reprod. Sci. 2018, 192, 321–327. [Google Scholar] [CrossRef]
- Valentino, S.A.; Tarrade, A.; Aioun, J.; Mourier, E.; Richard, C.; Dahirel, M.; Rousseau-Ralliard, D.; Fournier, N.; Aubrière, M.-C.; Lallemand, M.-S.; et al. Maternal Exposure to Diluted Diesel Engine Exhaust Alters Placental Function and Induces Intergenerational Effects in Rabbits. Part. Fibre Toxicol. 2015, 13, 39. [Google Scholar] [CrossRef]
- Lecarpentier, E.; Morel, O.; Tarrade, A.; Dahirel, M.; Bonneau, M.; Gayat, E.; Evain-Brion, D.; Chavatte-Palmer, P.; Tsatsaris, V. Quantification of Utero-Placental Vascularization in a Rabbit Model of IUGR with Three-Dimensional Power Doppler Angiography. Placenta 2012, 33, 769–775. [Google Scholar] [CrossRef]
- Favaron, P.O.; Mess, A.M.; de Oliveira, M.F.; Gabory, A.; Miglino, M.A.; Chavatte-Palmer, P.; Tarrade, A. Morphometric Analysis of the Placenta in the New World Mouse Necromys Lasiurus (Rodentia, Cricetidae): A Comparison of Placental Development in Cricetids and Murids. Reprod. Biol. Endocrinol. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Szendrő, Z.; Szendrő, K.; Zotte, A.D. Management of Reproduction on Small, Medium and Large Rabbit Farms: A Review. Asian-Australas. J. Anim. Sci. 2012, 25, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Mourikes, V.E.; Flaws, J.A. Effects of Chemical Mixtures on the Ovary. Reproduction 2021, 162, F91–F100. [Google Scholar] [CrossRef]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A New Chapter in the Bisphenol A Story: Bisphenol S and Bisphenol F Are Not Safe Alternatives to This Compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef]
- Kalloo, G.; Wellenius, G.A.; McCandless, L.; Calafat, A.M.; Sjodin, A.; Romano, M.E.; Karagas, M.R.; Chen, A.; Yolton, K.; Lanphear, B.P.; et al. Exposures to Chemical Mixtures during Pregnancy and Neonatal Outcomes: The HOME Study. Environ. Int. 2020, 134, 105219. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Arbuckle, T.E.; Liang, C.L.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Haines, D.; Davis, K.; Fraser, W.D. Concentrations of Persistent Organic Pollutants in Maternal and Cord Blood from the Maternal-Infant Research on Environmental Chemicals (MIREC) Cohort Study. Environ. Health 2016, 15, 59. [Google Scholar] [CrossRef]
- Huang, S.; Li, J.; Xu, S.; Zhao, H.; Li, Y.; Zhou, Y.; Fang, J.; Liao, J.; Cai, Z.; Xia, W. Bisphenol A and Bisphenol S Exposures during Pregnancy and Gestational Age—A Longitudinal Study in China. Chemosphere 2019, 237, 124426. [Google Scholar] [CrossRef]
- Keswani, C.; Dilnashin, H.; Birla, H.; Roy, P.; Tyagi, R.K.; Singh, D.; Rajput, V.D.; Minkina, T.; Singh, S.P. Global Footprints of Organochlorine Pesticides: A Pan-Global Survey. Environ. Geochem. Health 2022, 44, 149–177. [Google Scholar] [CrossRef]
- Bornman, M.S.; Aneck-Hahn, N.H.; de Jager, C.; Wagenaar, G.M.; Bouwman, H.; Barnhoorn, I.E.J.; Patrick, S.M.; Vandenberg, L.N.; Kortenkamp, A.; Blumberg, B.; et al. Endocrine Disruptors and Health Effects in Africa: A Call for Action. Environ. Health Perspect. 2017, 125, 085005. [Google Scholar] [CrossRef]
- Silver, M.J.; Saffari, A.; Kessler, N.J.; Chandak, G.R.; Fall, C.H.; Issarapu, P.; Dedaniya, A.; Betts, M.; Moore, S.E.; Routledge, M.N.; et al. Environmentally Sensitive Hotspots in the Methylome of the Early Human Embryo. Elife 2022, 11, e72031. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Schatten, H.; Sun, Q.Y. Environmental Epigenetic Inheritance through Gametes and Implications for Human Reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef]
- Sanchez-Delgado, M.; Court, F.; Vidal, E.; Medrano, J.; Monteagudo-Sanchez, A.; Martin-Trujillo, A.; Tayama, C.; Iglesias-Platas, I.; Kondova, I.; Bontrop, R.; et al. Human Oocyte-Derived Methylation Differences Persist in the Placenta Revealing Widespread Transient Imprinting. PLoS Genet. 2016, 12, e1006427. [Google Scholar] [CrossRef] [PubMed]
- Wegner, S.H.; Pinto, C.L.; Ring, C.L.; Wambaugh, J.F. High-Throughput Screening Tools Facilitate Calculation of a Combined Exposure-Bioactivity Index for Chemicals with Endocrine Activity. Environ. Int. 2020, 137, 105470. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The Exposome: From Concept to Utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Human Cohort | 95th Percentile Value | Targeted Serum Concentration |
|---|---|---|---|
| BDE-47 | HELIX [31] | 0.0156 ng/mL * | 0.156 ng/mL |
| BPS | Pregnant US women [33] | 0.09 ng/mL (Geometric mean in pregnant women) | 1 ng/mL |
| HCB | HELIX [31] | 0.24 ng/mL * | 2.4 ng/mL |
| β-HCH | INMA-Sabadell [34] (included in HELIX) [31] | 0.441 ng/mL * | 4.410 ng/mL |
| DDE | HELIX [31] | 2.118 ng/mL * | 21.18 ng/mL |
| PFOA | HELIX [31] | 5.3 ng/ml | 53 ng/mL |
| DEHP | Pregnant Danish women [32] | 5.89 * ng/mL (90th percentile in pregnant women) | 60 ng/ML ** |
| PFOS (total) | HELIX [31] | 20 ng/mL | 200 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Fouikar, S.; Duranthon, V.; Helies, V.; Jammes, H.; Couturier-Tarrade, A.; Gayrard, V.; Van Acker, N.; Frenois, F.-X.; Archilla, C.; Rousseau-Ralliard, D.; et al. Multigenerational Effects of a Complex Human-Relevant Exposure during Folliculogenesis and Preimplantation Embryo Development: The FEDEXPO Study. Toxics 2023, 11, 425. https://doi.org/10.3390/toxics11050425
El Fouikar S, Duranthon V, Helies V, Jammes H, Couturier-Tarrade A, Gayrard V, Van Acker N, Frenois F-X, Archilla C, Rousseau-Ralliard D, et al. Multigenerational Effects of a Complex Human-Relevant Exposure during Folliculogenesis and Preimplantation Embryo Development: The FEDEXPO Study. Toxics. 2023; 11(5):425. https://doi.org/10.3390/toxics11050425
Chicago/Turabian StyleEl Fouikar, Sara, Véronique Duranthon, Virginie Helies, Hélène Jammes, Anne Couturier-Tarrade, Véronique Gayrard, Nathalie Van Acker, François-Xavier Frenois, Catherine Archilla, Delphine Rousseau-Ralliard, and et al. 2023. "Multigenerational Effects of a Complex Human-Relevant Exposure during Folliculogenesis and Preimplantation Embryo Development: The FEDEXPO Study" Toxics 11, no. 5: 425. https://doi.org/10.3390/toxics11050425
APA StyleEl Fouikar, S., Duranthon, V., Helies, V., Jammes, H., Couturier-Tarrade, A., Gayrard, V., Van Acker, N., Frenois, F.-X., Archilla, C., Rousseau-Ralliard, D., Gatimel, N., & Léandri, R. (2023). Multigenerational Effects of a Complex Human-Relevant Exposure during Folliculogenesis and Preimplantation Embryo Development: The FEDEXPO Study. Toxics, 11(5), 425. https://doi.org/10.3390/toxics11050425