Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf)
Abstract
1. Introduction
2. Portunus pelagicus: Description, Phylogeny, Distribution and Habitat, Life Cycle, and Physiological Capacities
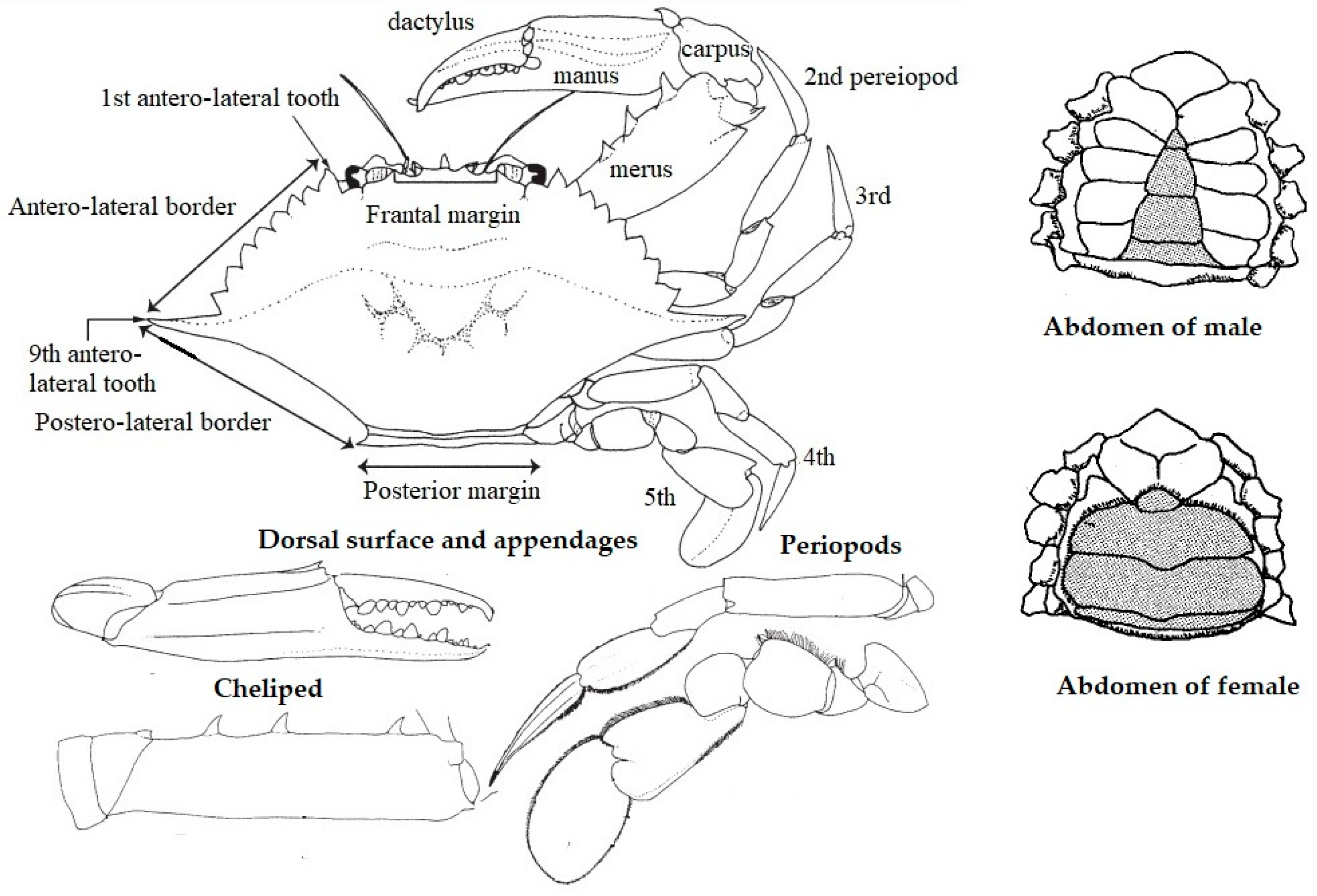
2.1. Description of Portunus pelagicus
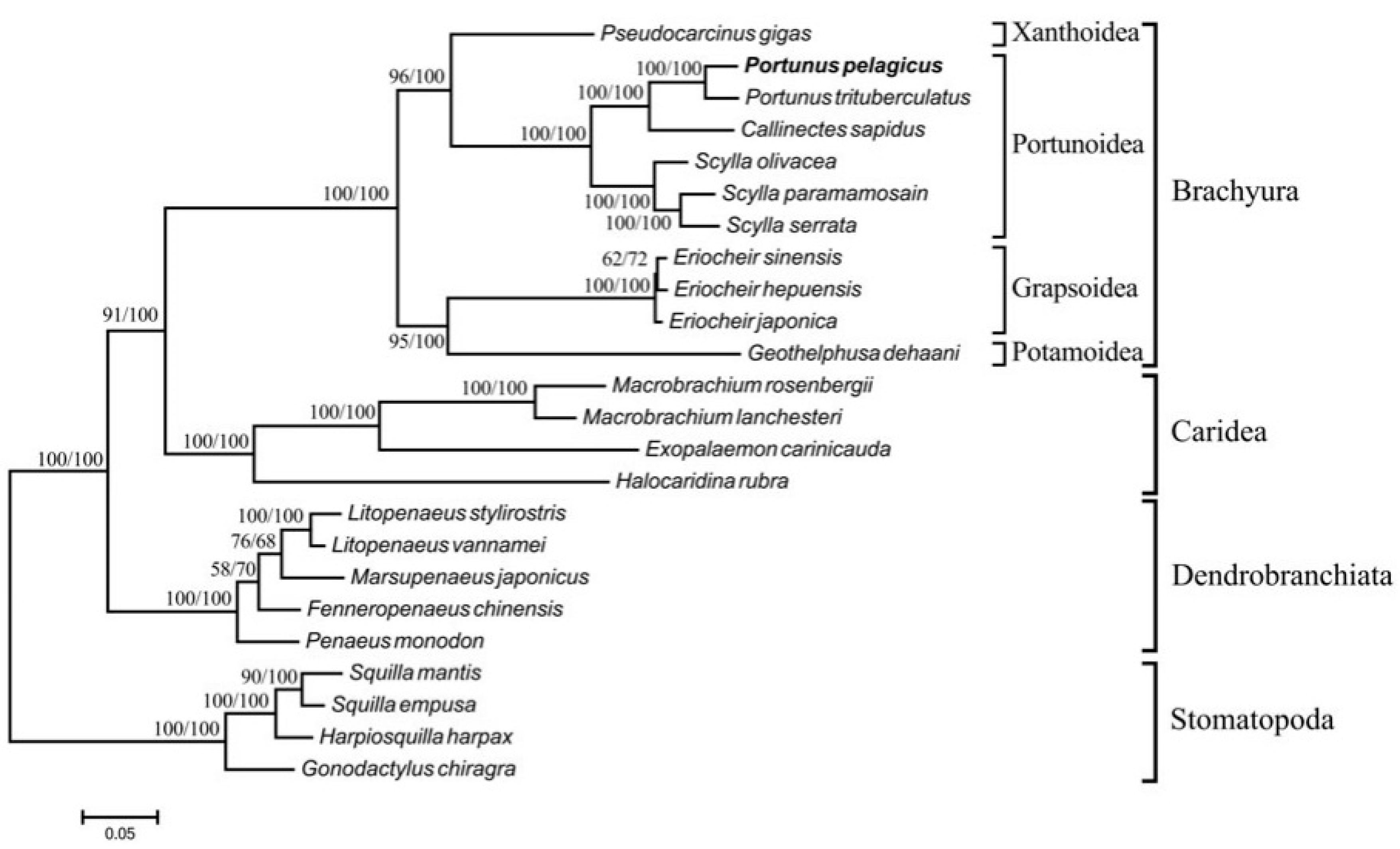
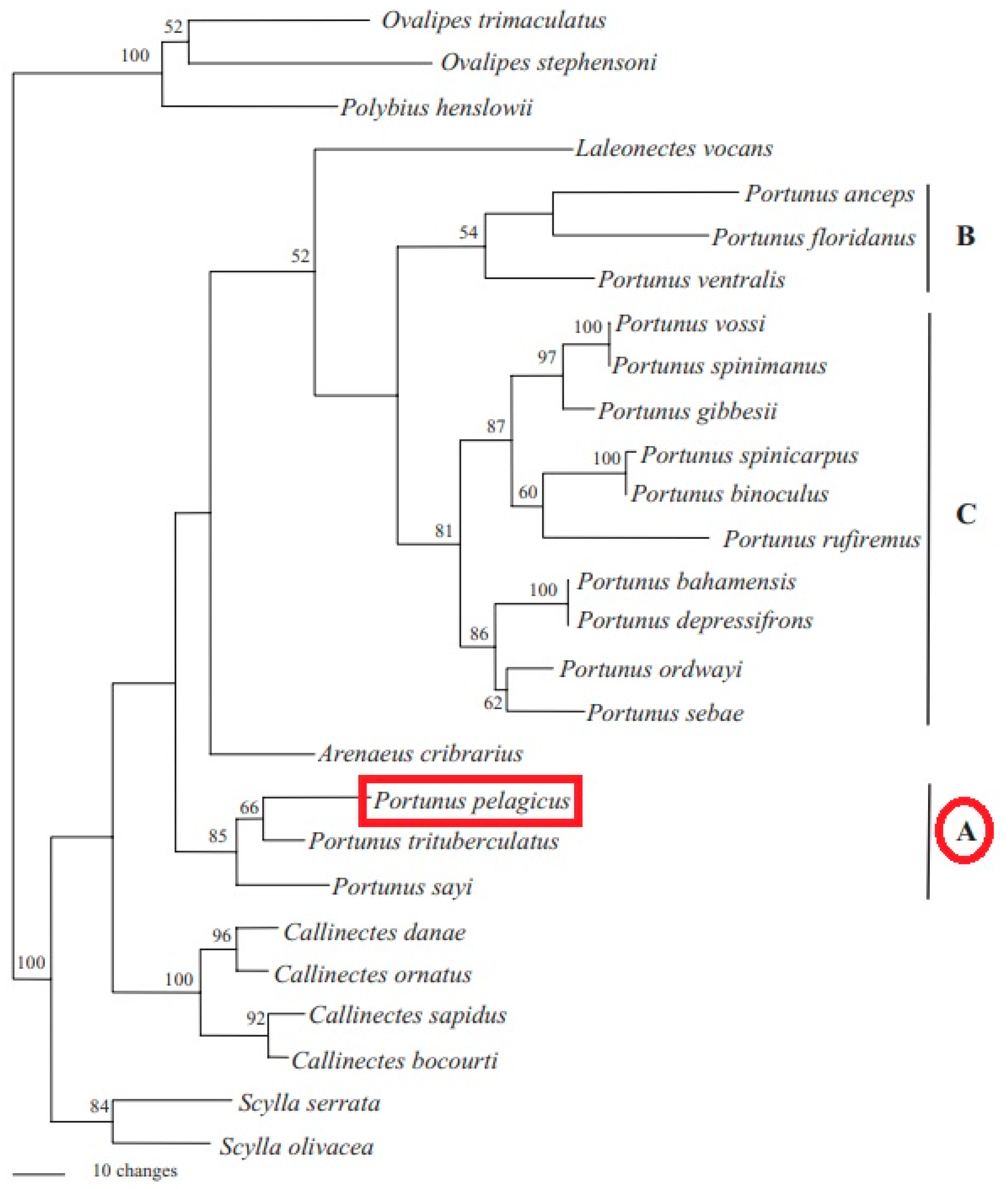
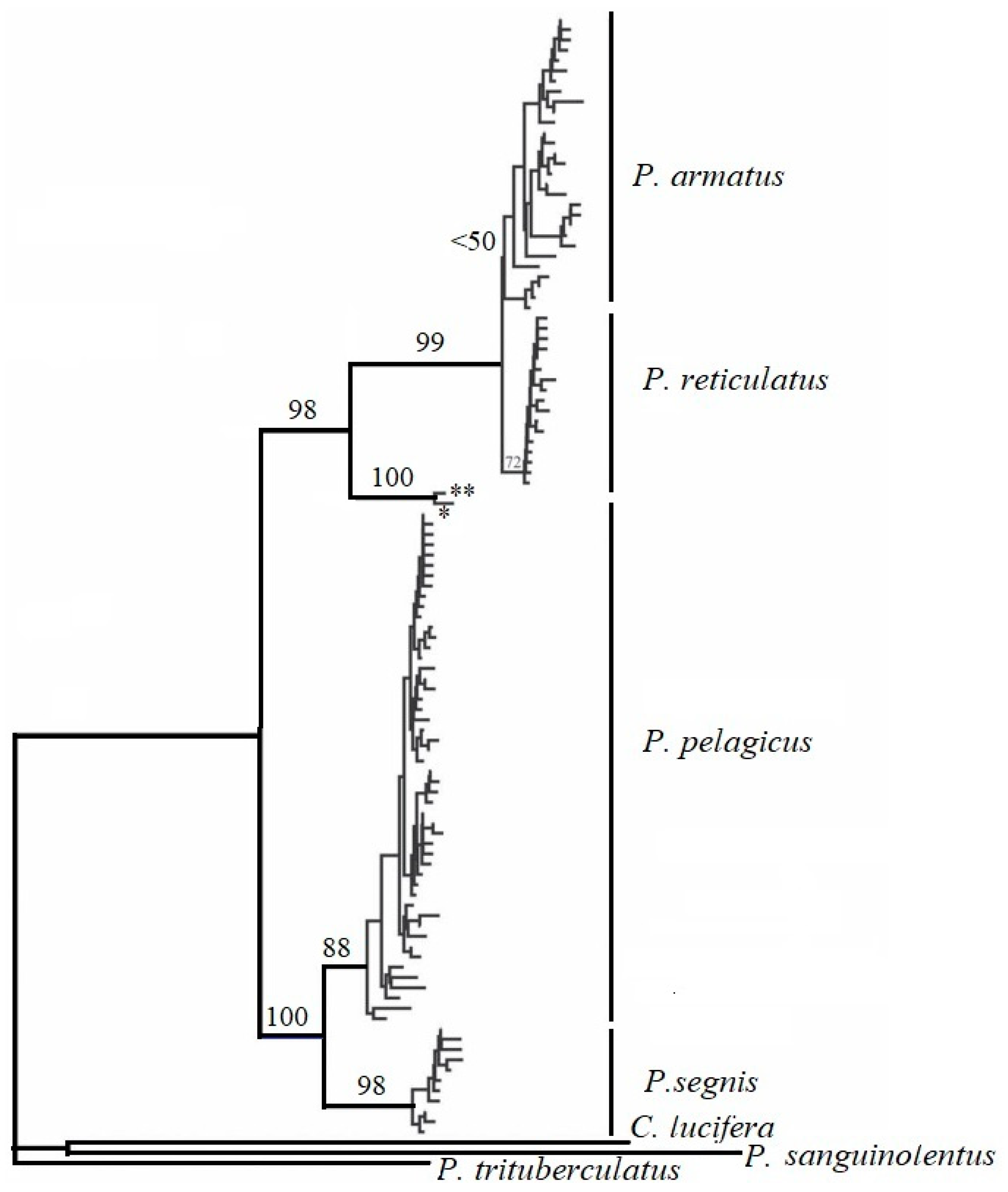
2.2. Phylogeny
2.3. Distribution and Habitat
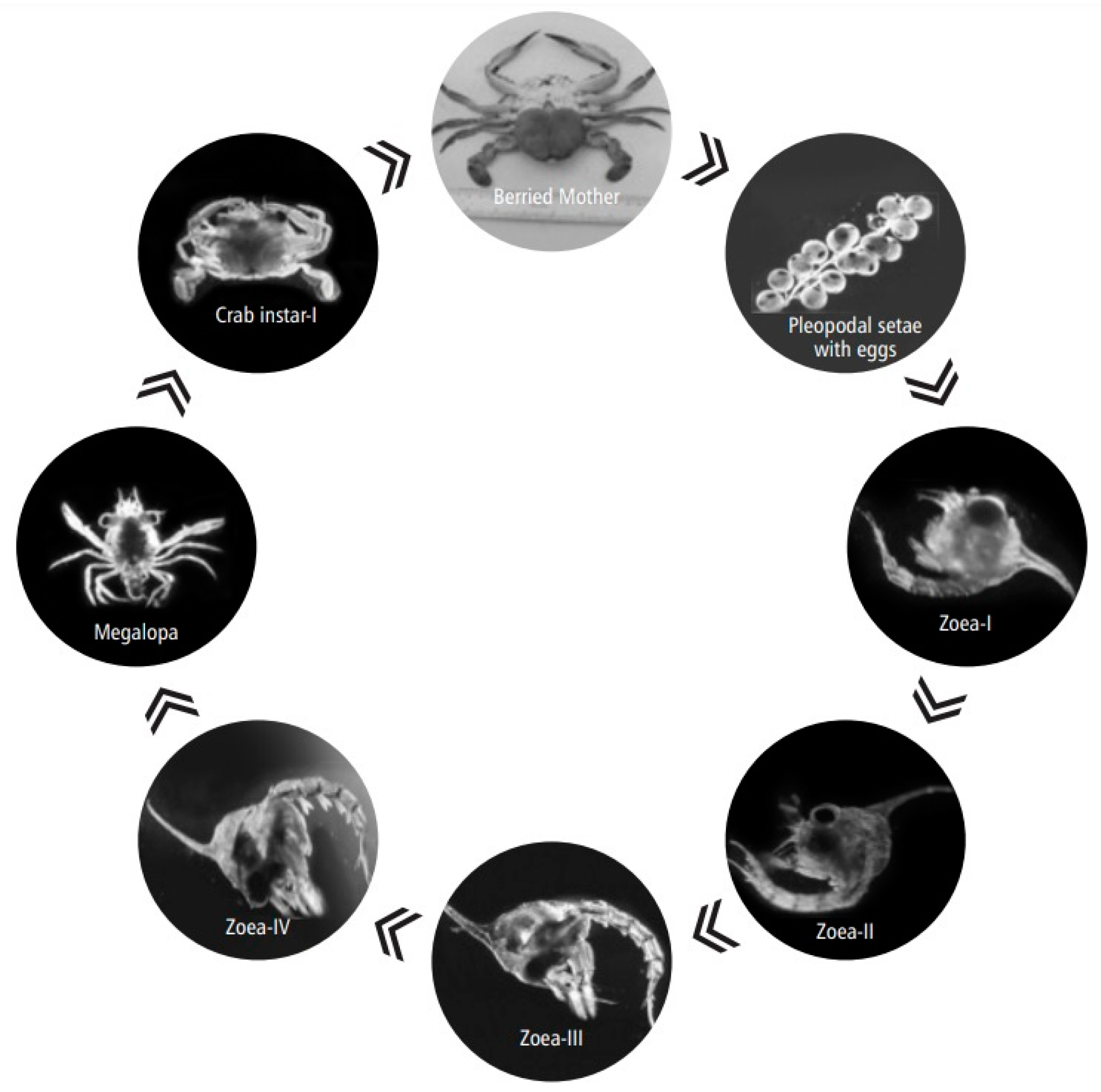
2.4. Life Cycle
2.5. Physiological Capacities
3. Portunus pelagicus as a Sentinel Species (Quantitative Bioindicator)
4. Case of Kuwait Bay (Northwestern Arabian Gulf) and Metal Pollution
4.1. Metal Concentrations in Crab Tissues
4.2. Metal Concentrations in Sediments
4.3. Metal Concentrations in Seawater
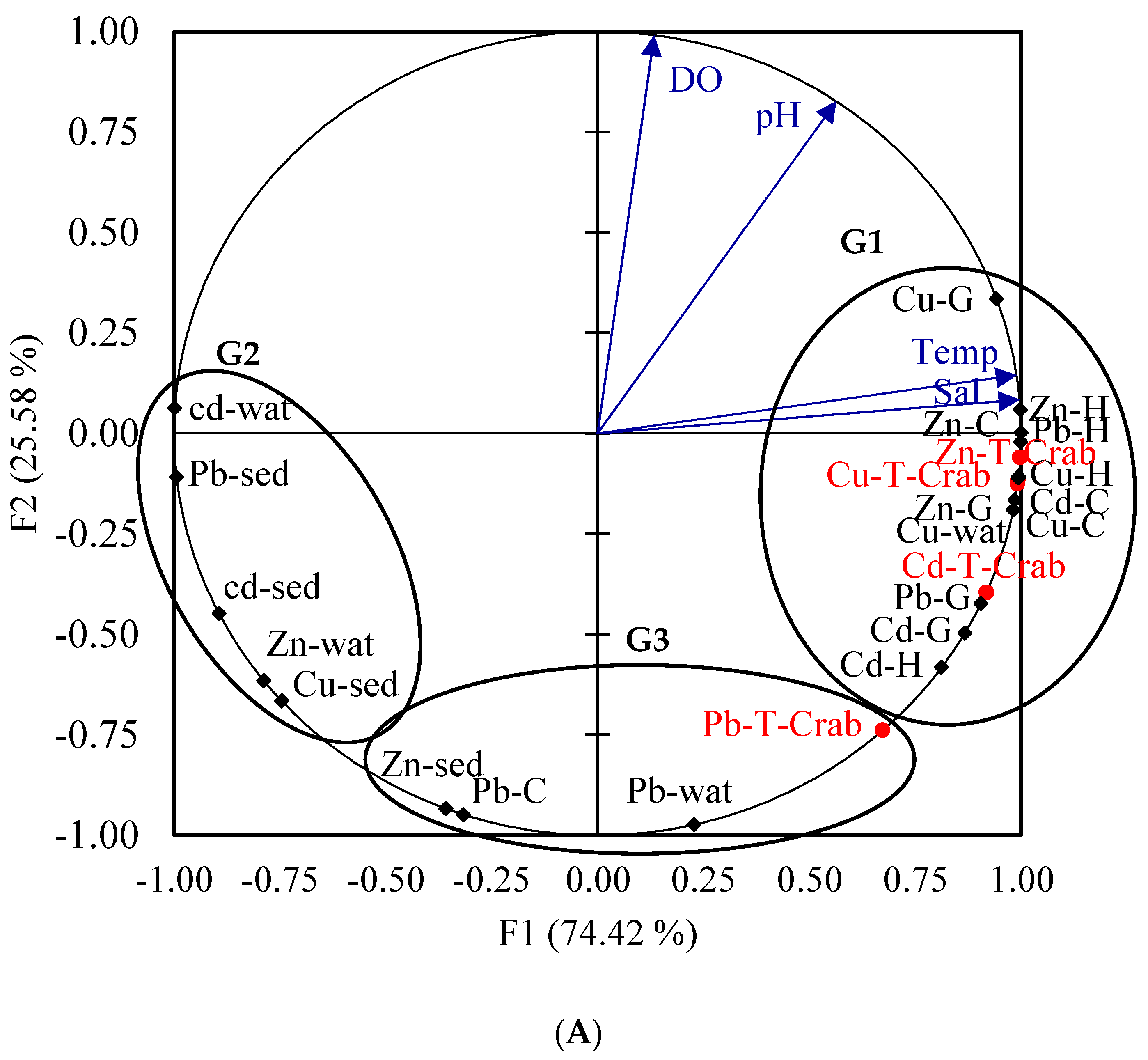
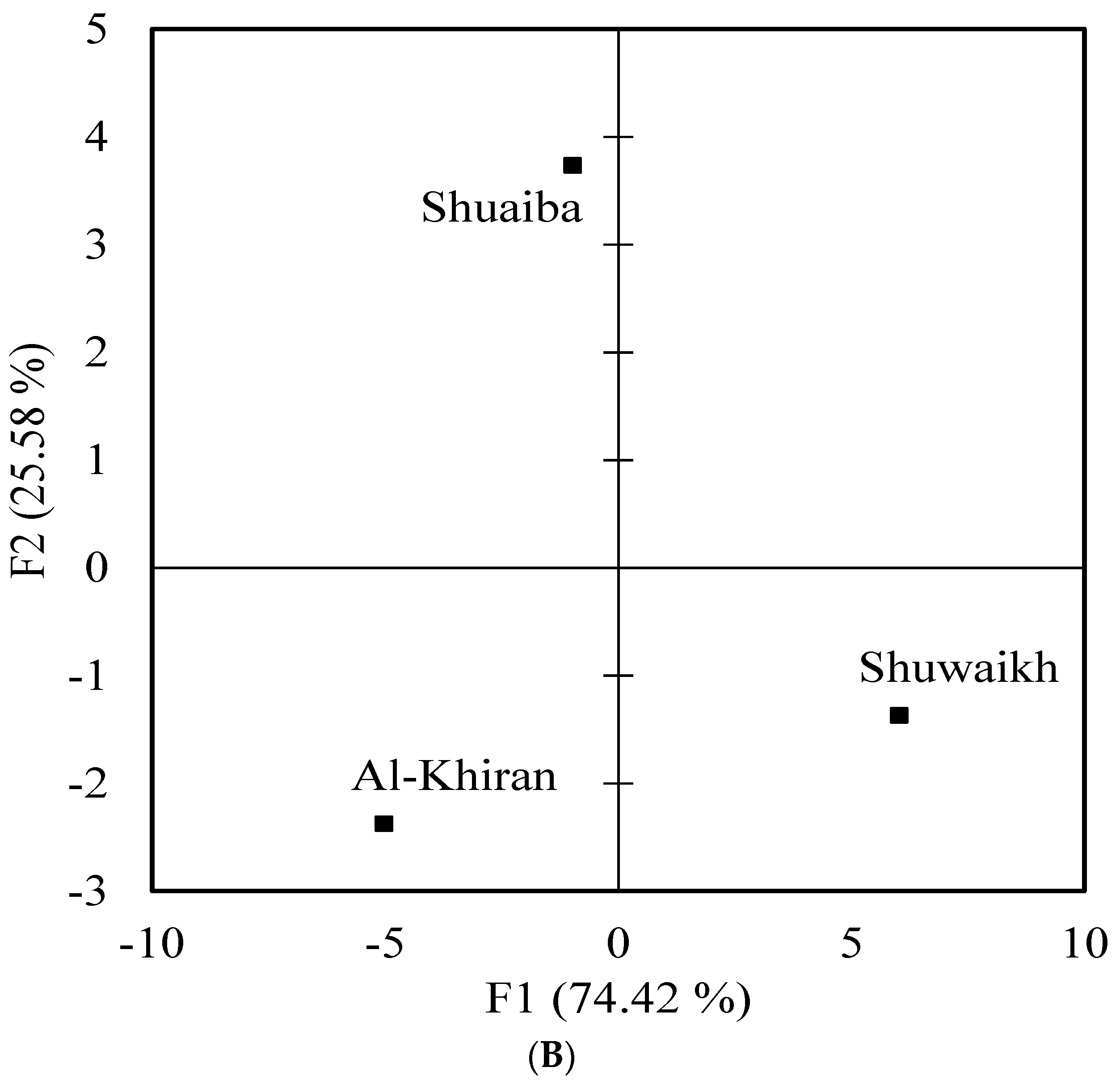
4.4. Multivariate Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koolkarnkhai, P.; Intakham, C.; Sangthong, P.; Surat, W.; Wonnapinij, P. Portunus Pelagicus MtDNA Heteroplasmy Inheritance and Its Effect on the Use of MtCR and MtCOI Sequence Data. Mitochondrial DNA Part A 2019, 30, 848–860. [Google Scholar] [CrossRef]
- Nitiratsuwan, T.; Nitithamyong, C.; Chiayvareesajja, S.; Somboonsuke, B. Distribution of Blue Swimming Crab (Portunus Pelagicus Linnaeus, 1758) in Trang Province. Songklanakarin J. Sci. Technol. 2010, 32, 207–212. [Google Scholar]
- Song, A.M. How to Capture Small-Scale Fisheries’ Many Contributions to Society?—Introducing the ‘Value-Contribution Matrix’ and Applying It to the Case of a Swimming Crab Fishery in South Korea. In Social Wellbeing and the Values of Small-Scale Fisheries; Johnson, D.S., Acott, T.G., Stacey, N., Urquhart, J., Eds.; MARE Publication Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 17, pp. 125–146. ISBN 978-3-319-60749-8. [Google Scholar]
- Obregón, C.; Christensen, J.; Zeller, D.; Hughes, M.; Tweedley, J.R.; Gaynor, A.; Loneragan, N.R. Local Fisher Knowledge Reveals Changes in Size of Blue Swimmer Crabs in Small-Scale Fisheries. Mar. Policy 2022, 143, 105144. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Zhang, Z.; Ren, T.; Lu, M.; Lei, M.; Dan, S.F.; Lan, Z.; Ma, Z.; Fang, H.; et al. Molecular Characterization of Three Peroxiredoxin Genes in Portunus Pelagicus Expressed in Response to Vibrio Alginolyticus Challenge. Aquac. Rep. 2022, 27, 101391. [Google Scholar] [CrossRef]
- Hines, A.H.; Haddon, A.M.; Wiechert, L.A. Guild Structure and Foraging Impact of Blue Crabs and Epibenthic Fish in a Subestuary of Chesapeake Bay. Mar. Ecol. Prog. Ser. 1990, 67, 105–126. [Google Scholar] [CrossRef]
- Williams, M.J. Natural Food and Feeding in the Commercial Sand Crab Portunus Pelagicus Linnaeus, 1766 (Crustacea: Decapoda: Portunidae) in Moreton Bay, Queensland. J. Exp. Mar. Biol. Ecol. 1982, 59, 165–176. [Google Scholar] [CrossRef]
- Zainal, K.A.Y. Natural Food and Feeding of the Commercial Blue Swimmer Crab, Portunus Pelagicus (Linnaeus, 1758) along the Coastal Waters of the Kingdom of Bahrain. J. Assoc. Arab Univ. Basic Appl. Sci. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Jones, D.A. A Field Guide to the Sea Shores of Kuwait and the Arabian Gulf; University of Kuwait: Kuwait City, Kuwait, 1986. [Google Scholar]
- Hosseini, M.; Mohammad, S.; Nabavi, B.; Bastami, A.A.; Parsa, Y. Mercury Concentration in Tissues of Blue Swimming Crab, Portunus Pelagicus (Linnaeus, 1758) and Sediments from Persian Gulf Coasts, Iran. World Appl. Sci. J. 2012, 18, 322–327. [Google Scholar]
- Bu-Olayan, A.H.; Thomas, B.V. Combined Toxicity of Mercury and Plastic Wastes to Crustacean and Gastropod Inhabiting the Waters in Kuwait. J. Environ. Biol. 2015, 36, 1291–1296. [Google Scholar]
- Hooshmand, H.; Shabanpour, B.; Moosavi-Nasab, M.; Golmakani, M.T. Optimization of Carotenoids Extraction from Blue Crab (Portunus Pelagicus) and Shrimp (Penaeus Semisulcatus) Wastes Using Organic Solvents and Vegetable Oils. J. Food Process. Preserv. 2017, 41, e13171. [Google Scholar] [CrossRef]
- Rafinesque, C.S. Analyse de La Nature, Ou Tableau de l’univers et Des Corps Organisés; L’imprimerie de Jean Barravecchia: Palerme, Italy, 1815. [Google Scholar]
- Lai, J.C.Y.; Ng, P.K.L.; Davie, P.J.F. A Revision of the Portunus Pelagicus (Linnaeus, 1758) Species Complex (Crustacea: Brachyura: Portunidae), with the Recognition of Four Species. Raffles Bull. Zool. 2010, 58, 199–237. [Google Scholar]
- Santhanam, R. Biology and Culture of Portunid Crabs of World Seas: Biology and Ecology of Marine Life, 1st ed.; Series: Biology and Ecology of Marine Life; Apple Academic Press: Waretown, NJ, USA, 2018; ISBN 978-1-315-20581-6. [Google Scholar]
- Meng, X.; Jia, F.; Liu, P.; Li, J. The Complete Mitogenome of Blue Swimming Crab Portunus Pelagicus Linnaeus, 1766 (Crustacea: Decapoda: Portunidae). Mitochondrial DNA Part A 2016, 27, 2789–2790. [Google Scholar] [CrossRef]
- Mantelatto, F.L.; Robles, R.; Felder, D.L. Molecular Phylogeny of the Western Atlantic Species of the Genus Portunus (Crustacea, Brachyura, Portunidae). Zool. J. Linn. Soc. 2007, 150, 211–220. [Google Scholar] [CrossRef]
- Potter, I.C.; Chrystal, P.J.; Loneragan, N.R. The Biology of the Blue Manna Crab Portunus Pelagicus in an Australian Estuary. Mar. Biol. 1983, 78, 75–85. [Google Scholar] [CrossRef]
- Kailola, P.J.; Williams, M.J.; Stewart, P.C.; Reichelt, R.E.; McNee, A.; Grieve, C. Australian Fisheries Resources; Bureau of Resource Sciences: Canberra, Australia, 1993; ISBN 978-0-642-18876-2. [Google Scholar]
- Xiao, Y.; Kumar, M. Sex Ratio, and Probability of Sexual Maturity of Females at Size, of the Blue Swimmer Crab, Portunus Pelagicus Linneaus, off Southern Australia. Fish. Res. 2004, 68, 271–282. [Google Scholar] [CrossRef]
- Castriota, L.; Falautano, M.; Maggio, T.; Perzia, P. The Blue Swimming Crab Portunus Segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology 2022, 11, 1473. [Google Scholar] [CrossRef]
- Corsini-Foka, M.; Kondilatos, G.; Economidis, P.S. Occurrence of the Lessepsian Species Portunus Pelagicus (Crustacea) and Apogon Pharaonis (Pisces) in the Marine Area of Rhodes Island. Mediterr. Mar. Sci. 2004, 5, 83. [Google Scholar] [CrossRef]
- Rabaoui, L.; Arculeo, M.; Mansour, L.; Tlig-Zouari, S. Occurrence of the Lessepsian Species Portunus Segnis (Crustacea: Decapoda) in the Gulf of Gabes (Tunisia): First Record and New Information on Its Biology and Ecology. Cah. Biol. Mar. 2015, 56, 169–175. [Google Scholar]
- Edgar, G.J. Predator-Prey Interactions in Seagrass Beds. II. Distribution and Diet of the Blue Manna Crab Portunus Pelagicus Linnaeus at Cliff Head, Western Australia. J. Exp. Mar. Biol. Ecol. 1990, 139, 23–32. [Google Scholar] [CrossRef]
- Susanto, A.; Suuronen, P.; Gorgin, S.; Irnawati, R.; Riyanto, M.; Wahyudin; Nurdin, H.S.; Hamzah, A.; Supadminingsih, F.N.; Syafrie, H. Behavioral Response and Retinal Adaptation of Blue Swimming Crab (Portunus Pelagicus) Exposed to LED Lights—Led Light as a Potential Artificial Attractant in Trap Fishing. Fish. Res. 2022, 250, 106274. [Google Scholar] [CrossRef]
- Bookhout, C.G.; Costlow, J.D., Jr. Larval Development of Portunus Spinicarpus Reared in the Laboratory. Bull. Mar. Sci. 1974, 24, 20–51. [Google Scholar]
- Stuck, K.C.; Truesdale, F.M. Larval Development of the Speckled Swimming Crab, Arenaeus Cribrarius (Decapoda: Brachyura: Portunidae) Reared in the Laboratory. Bull. Mar. Sci. 1988, 42, 101–132. [Google Scholar]
- Kornienko, E.S.; Golubinskaya, D.D. The Morphology of the Mandibles of the Larval and Adult Lobster Shrimp Boasaxius Princeps and Leonardsaxius Amurensis (Decapoda: Axiidea: Axiidae). Russ. J. Mar. Biol. 2020, 46, 257–269. [Google Scholar] [CrossRef]
- Josileen, J.; Menon, N.G. Larval Stages of the Blue Swimmer Crab, Portunus Pelagicus (Linnaeus, 1758)(Decapoda, Brachyura). Crustaceana 2004, 77, 785–803. [Google Scholar]
- Menon, N.G.; Josileen, J. Growth of the Blue Swimmer Crab, Portunus Pelagicus (Linnaeus, 1758) (Decapoda, Brachyura) in Captivity. Crustaceana 2005, 78, 1–18. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. The Effects of Salinity on the Survival, Growth and Haemolymph Osmolality of Early Juvenile Blue Swimmer Crabs, Portunus Pelagicus. Aquaculture 2006, 260, 151–162. [Google Scholar] [CrossRef]
- Lignot, J.-H.; Spanings-Pierrot, C.; Charmantier, G. Osmoregulatory Capacity as a Tool in Monitoring the Physiological Condition and the Effect of Stress in Crustaceans. Aquaculture 2000, 191, 209–245. [Google Scholar] [CrossRef]
- Qari, S.A. Thermal Tolerance of the Marine Crab, Portunus Pelagicus (Brachyura, Portunidae). Crustaceana 2014, 87, 827–833. [Google Scholar] [CrossRef]
- Azra, M.N.; Chen, J.-C.; Ikhwanuddin, M.; Abol-Munafi, A.B. Thermal Tolerance and Locomotor Activity of Blue Swimmer Crab Portunus Pelagicus Instar Reared at Different Temperatures. J. Therm. Biol. 2018, 74, 234–240. [Google Scholar] [CrossRef]
- Bates, A.E.; McKelvie, C.M.; Sorte, C.J.B.; Morley, S.A.; Jones, N.A.R.; Mondon, J.A.; Bird, T.J.; Quinn, G. Geographical Range, Heat Tolerance and Invasion Success in Aquatic Species. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131958. [Google Scholar] [CrossRef]
- Kelley, A.L. The Role Thermal Physiology Plays in Species Invasion. Conserv. Physiol. 2014, 2, cou045. [Google Scholar] [CrossRef]
- Johnston, D.; Harris, D.; Caputi, N.; Thomson, A. Decline of a Blue Swimmer Crab (Portunus Pelagicus) Fishery in Western Australia—History, Contributing Factors and Future Management Strategy. Fish. Res. 2011, 109, 119–130. [Google Scholar] [CrossRef]
- Saravanan, R.; Sugumar, V.; Beema Mahin, M.I. Heavy Metal Stress Induced Hyperglycemia in Blue Swimmer Crab, Portunus Pelagicus. Acta Oceanol. Sin. 2018, 37, 47–53. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal Dependence of Locomotor Capacity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1990, 259, R253–R258. [Google Scholar] [CrossRef]
- Sumpton, W.; Smith, G. Effect of Temperature on the Emergence, Activity and Feeding of Male and Female Sand Crabs Portunus Pelagicus. Mar. Freshw. Res. 1990, 41, 545. [Google Scholar] [CrossRef]
- Phillips, D.J.H.; Rainbow, P.S. Biomonitoring of Trace Aquatic Contaminants; Springer International Publishing: Cham, Switzerland, 2013; Volume 37, ISBN 978-94-011-2122-4. [Google Scholar]
- Chiarelli, R.; Roccheri, M.C. Marine Invertebrates as Bioindicators of Heavy Metal Pollution. Open J. Met. 2014, 4, 93–106. [Google Scholar] [CrossRef]
- Uğurlu, P.; Ünlü, E.; Satar, E.İ. The Toxicological Effects of Thiamethoxam on Gammarus Kischineffensis (Schellenberg 1937) (Crustacea: Amphipoda). Environ. Toxicol. Pharmacol. 2015, 39, 720–726. [Google Scholar] [CrossRef]
- Outa, J.O.; Kowenje, C.O.; Avenant-Oldewage, A.; Jirsa, F. Trace Elements in Crustaceans, Mollusks and Fish in the Kenyan Part of Lake Victoria: Bioaccumulation, Bioindication and Health Risk Analysis. Arch. Environ. Contam. Toxicol. 2020, 78, 589–603. [Google Scholar] [CrossRef]
- Ramírez-Ayala, E.; Arguello-Pérez, M.A.; Tintos-Gómez, A.; Pérez-Rodríguez, R.Y.; Díaz-Gómez, J.A.; Borja-Gómez, I.; Sepúlveda-Quiroz, C.A.; Patiño-Barragán, M.; Lezama-Cervantes, C.; Salomé-Baylón, J. Review of the Biomonitoring of Persistent, Bioaccumulative, and Toxic Substances in Aquatic Ecosystems of Mexico: 2001–2016. Lat. Am. J. Aquat. Res. 2020, 48, 705–738. [Google Scholar] [CrossRef]
- Daly, B.J.; Eckert, G.L.; Long, W.C. Moulding the Ideal Crab: Implications of Phenotypic Plasticity for Crustacean Stock Enhancement. ICES J. Mar. Sci. 2021, 78, 421–434. [Google Scholar] [CrossRef]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The Natural Indicator of Environmental Pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Souty-Grosset, C. Management of Freshwater Biodiversity: Crayfish as Bioindicators; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; ISBN 978-0-521-51400-2. [Google Scholar]
- de Almeida Rodrigues, P.; Ferrari, R.G.; Kato, L.S.; Hauser-Davis, R.A.; Conte-Junior, C.A. A Systematic Review on Metal Dynamics and Marine Toxicity Risk Assessment Using Crustaceans as Bioindicators. Biol. Trace Elem. Res. 2022, 200, 881–903. [Google Scholar] [CrossRef] [PubMed]
- Lobato, R.O.; Nunes, S.M.; Wasielesky, W.; Fattorini, D.; Regoli, F.; Monserrat, J.M.; Ventura-Lima, J. The Role of Lipoic Acid in the Protection against of Metallic Pollutant Effects in the Shrimp Litopenaeus Vannamei (Crustacea, Decapoda). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2013, 165, 491–497. [Google Scholar] [CrossRef]
- Negro, C.L.; Iturburu, F.G.; Mendieta, J.; Menone, M.L.; Collins, P. Are Oxidative Stress Biomarkers Sensitive to Environmental Concentrations of Chlorpyrifos Exposed to the Freshwater Crab, Zilchiopsis Collastinensis (Decapoda; Trichodactylidae)? Bull. Environ. Contam. Toxicol. 2019, 103, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, J.M.; Rato, L.D.; Simões, T.; Morão, I.F.C.; Meireles, G.; Novais, S.C.; Lemos, M.F.L. Exposure to the Insecticide Sulfoxaflor Affects Behaviour and Biomarkers Responses of Carcinus Maenas (Crustacea: Decapoda). Biology 2021, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S.; Luoma, S.N. Metal Toxicity, Uptake and Bioaccumulation in Aquatic Invertebrates—Modelling Zinc in Crustaceans. Aquat. Toxicol. 2011, 105, 455–465. [Google Scholar] [CrossRef]
- McDonald, S.; Cresswell, T.; Hassell, K. Bioaccumulation Kinetics of Cadmium and Zinc in the Freshwater Decapod Crustacean Paratya Australiensis Following Multiple Pulse Exposures. Sci. Total Environ. 2020, 720, 137609. [Google Scholar] [CrossRef]
- Alberts-Hubatsch, H.; Lee, S.Y.; Meynecke, J.-O.; Diele, K.; Nordhaus, I.; Wolff, M. Life-History, Movement, and Habitat Use of Scylla Serrata (Decapoda, Portunidae): Current Knowledge and Future Challenges. Hydrobiologia 2016, 763, 5–21. [Google Scholar] [CrossRef]
- Lui, K.K.Y.; Ng, J.S.S.; Leung, K.M.Y. Spatio-Temporal Variations in the Diversity and Abundance of Commercially Important Decapoda and Stomatopoda in Subtropical Hong Kong Waters. Estuar. Coast. Shelf Sci. 2007, 72, 635–647. [Google Scholar] [CrossRef]
- El Assal, F.; Abdel Meguid, Z. Impact of Heavy Metal Pollution on Procambarus Clarkii (Crustacea: Decapoda), from Egypt. Int. J. Waste Resour. 2017, 7, 1000270. [Google Scholar] [CrossRef]
- Vinot, I.; Pihan, J.C. Circulation of Copper in the Biotic Compartments of a Freshwater Dammed Reservoir. Environ. Pollut. 2005, 133, 169–182. [Google Scholar] [CrossRef]
- El-Deeb, R.S.; Sarhan, M.; Khafage, A.R.; Abdel Razek, F.A.; Abdel-Wahab, M.; Omar, H.A. Occurrence of Penaeus Aztecus, Ives, 1891 (Crustacea: Decapoda: Penaeidae) in the Coastal Water of Alexandria, Egypt. Egypt. J. Aquat. Res. 2020, 46, 303–309. [Google Scholar] [CrossRef]
- D’Iglio, C.; Di Fresco, D.; Spanò, N.; Albano, M.; Panarello, G.; Laface, F.; Faggio, C.; Capillo, G.; Savoca, S. Occurrence of Anthropogenic Debris in Three Commercial Shrimp Species from South-Western Ionian Sea. Biology 2022, 11, 1616. [Google Scholar] [CrossRef]
- Vogt, G. How to Minimize Formation and Growth of Tumours: Potential Benefits of Decapod Crustaceans for Cancer Research. Int. J. Cancer 2008, 123, 2727–2734. [Google Scholar] [CrossRef]
- Lande, V.W.; Sangolkar, L.N.; Kankal, N.C. Ecological Changes as Consequences of Pollution. J. Glob. Biosci. 2015, 4, 2859–2863. [Google Scholar]
- Pan, L.; Zhang, H. Metallothionein, Antioxidant Enzymes and DNA Strand Breaks as Biomarkers of Cd Exposure in a Marine Crab, Charybdis Japonica. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 144, 67–75. [Google Scholar] [CrossRef]
- Abolude, D.S.; Davies, O.A.; Avong, D.W. Level of Heavy Metals in Freshwater Crab Cardisoma Guahumi Obtained from Ahmadu Bello University Reservoir, Zaria Nigeria. Int. J. Anim. Vet. Adv. 2009, 1, 54–58. [Google Scholar]
- Saher, N.U.; Kanwal, N. Some Biomonitoring Studies of Heavy Metals in Commercial Species of Crustacean along Karachi Coast, Pakistan. Int. J. Biol. Biotechnol. 2018, 15, 269–275. [Google Scholar]
- Flint, N.; Anastasi, A.; De Valck, J.; Chua, E.M.; Rose, A.K.; Jackson, E.L. Using Mud Crabs (Scylla Serrata) as Environmental Indicators in a Harbour Health Report Card. Australas. J. Environ. Manag. 2021, 28, 188–212. [Google Scholar] [CrossRef]
- Karam, Q.; Al-Wazzan, Z. Toxicity of Petroleum Hydrocarbons to Brachyuran Crabs: A Review of Deleterious Effects of Oil-Related Xenobiotics on Life Stages. J. Mar. Biol. Assoc. U. K. 2021, 101, 561–576. [Google Scholar] [CrossRef]
- Fatemi, F.; Khoramnejadian, S. Investigation of Cadmium and Arsenic Accumulation in Portunus Pelagicus along the Asalouyeh Coast, Iran. J. Earth Environ. Health Sci. 2016, 2, 34. [Google Scholar] [CrossRef]
- Viswanathan, C.; Azhaguraj, R.; Selvanayagam, M.; Raffi, S.M. Heavy Metal Levels in Different Tissues of the Blue Swimming Crab (Portunus Pelagicus, Portunidae) Collected from Ennore Estuary. Int. J. Res. Fish. Aquac. 2013, 3, 1–6. [Google Scholar]
- Anandkumar, A.; Nagarajan, R.; Prabakaran, K.; Bing, C.H.; Rajaram, R.; Li, J.; Du, D. Bioaccumulation of Trace Metals in the Coastal Borneo (Malaysia) and Health Risk Assessment. Mar. Pollut. Bull. 2019, 145, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Karar, S.; Hazra, S.; Das, S. Assessment of the Heavy Metal Accumulation in the Blue Swimmer Crab (Portunus Pelagicus), Northern Bay of Bengal: Role of Salinity. Mar. Pollut. Bull. 2019, 143, 101–108. [Google Scholar] [CrossRef]
- McPherson, R.; Brown, K. The Bioaccumulation of Cadmium by the Blue Swimmer Crab Portunus Pelagicus L. Sci. Total Environ. 2001, 279, 223–230. [Google Scholar] [CrossRef]
- Abdolahpur Monikh, F.; Hosseini, M.; Rahmanpour, S. The Effect of Size and Sex on PCB and PAH Concentrations in Crab Portunus Pelagicus. Environ. Monit. Assess. 2014, 186, 1575–1582. [Google Scholar] [CrossRef]
- Firat, Ö.; Gök, G.; Çoğun, H.Y.; Yüzereroğlu, T.A.; Kargin, F. Concentrations of Cr, Cd, Cu, Zn and Fe in Crab Charybdis Longicollis and Shrimp Penaeus Semisulcatus from the Iskenderun Bay, Turkey. Environ. Monit. Assess. 2008, 147, 117–123. [Google Scholar] [CrossRef]
- Vogt, G. Functional Cytology of the Hepatopancreas of Decapod Crustaceans. J. Morphol. 2019, 280, jmor.21040. [Google Scholar] [CrossRef]
- Truchet, D.M.; Buzzi, N.S.; Simonetti, P.; Marcovecchio, J.E. Uptake and Detoxification of Trace Metals in Estuarine Crabs: Insights into the Role of Metallothioneins. Environ. Sci. Pollut. Res. 2020, 27, 31905–31917. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, J.; Wang, E.; Zhao, C.; Hu, X.; Chu, K.H.; Wang, L. Detoxification and Recovery after Cadmium Exposure in the Freshwater Crab Sinopotamon Henanense. Environ. Sci. Pollut. Res. 2021, 28, 58050–58067. [Google Scholar] [CrossRef]
- Barath Kumar, S.; Padhi, R.K.; Satpathy, K.K. Trace Metal Distribution in Crab Organs and Human Health Risk Assessment on Consumption of Crabs Collected from Coastal Water of South East Coast of India. Mar. Pollut. Bull. 2019, 141, 273–282. [Google Scholar] [CrossRef]
- Martins, C.D.M.G.; Barcarolli, I.F.; de Menezes, E.J.; Giacomin, M.M.; Wood, C.M.; Bianchini, A. Acute Toxicity, Accumulation and Tissue Distribution of Copper in the Blue Crab Callinectes Sapidus Acclimated to Different Salinities: In Vivo and in Vitro Studies. Aquat. Toxicol. 2011, 101, 88–99. [Google Scholar] [CrossRef]
- Malik, D.S.; Maurya, P.K. Heavy Metal Concentration in Water, Sediment, and Tissues of Fish Species (Heteropneustis Fossilis and Puntius Ticto) from Kali River, India. Toxicol. Environ. Chem. 2015, 96, 1195–1206. [Google Scholar] [CrossRef]
- SUGUMAR, V. Quantification of Crustacean Hyperglycemic Hormone and Its Hemolymph Metabolites: Following Various Stresses in the Blue Swimmer Crab Portunus Pelagicus; School of Marine Sciences: Tamil Nadu, India, 2014; p. 69. [Google Scholar]
- Ketpadung, R.; Tangkrock-olan, N. Changes in Oxygen Consumption and Heart Rate of the Blue Swimming Crab, Portunus Pelagicus (Linnaeus, 1766) Following Exposure to Sublethal Concentrations of Copper. J. Environ. Biol. 2006, 27, 7–12. [Google Scholar]
- Prabawa, A.; Riani, E.; Wardiatno, Y. The The Influence of Heavy Metals Contamination to the Population Structure and Organs of the Blue Swimming Crab (Portunus Pelagicus, LINN). J. Nat. Resour. Environ. Manag. 2014, 4, 17–23. [Google Scholar] [CrossRef]
- Yogeshwaran, A.; Gayathiri, K.; Muralisankar, T.; Gayathri, V.; Monica, J.I.; Rajaram, R.; Marimuthu, K.; Bhavan, P.S. Bioaccumulation of Heavy Metals, Antioxidants, and Metabolic Enzymes in the Crab Scylla Serrata from Different Regions of Tuticorin, Southeast Coast of India. Mar. Pollut. Bull. 2020, 158, 111443. [Google Scholar] [CrossRef]
- Nour, H.E.S. Distribution and Accumulation Ability of Heavy Metals in Bivalve Shells and Associated Sediment from Red Sea Coast, Egypt. Environ. Monit. Assess. 2020, 192, 353. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xuan, R.; Li, Y.; Zhang, X.; Wang, Q.; Wang, L. Effects of Cadmium Exposure on Digestive Enzymes, Antioxidant Enzymes, and Lipid Peroxidation in the Freshwater Crab Sinopotamon Henanense. Environ. Sci. Pollut. Res. 2013, 20, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Yilmaz, L. Influences of Sex and Seasons on Levels of Heavy Metals in Tissues of Green Tiger Shrimp (Penaeus Semisulcatus de Hann, 1844). Food Chem. 2007, 101, 1664–1669. [Google Scholar] [CrossRef]
- El-Sikaily, A.; Khaled, A.; Nemr, A.E. Heavy Metals Monitoring Using Bivalves from Mediterranean Sea and Red Sea. Environ. Monit. Assess. 2004, 98, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Taylor, A. The Effect of Salinity and Temperature on the Uptake of Cadmium and Zinc by the Common Blue Mussel, Mytilus Edulis with Some Notes on Their Survival. Mesopotamian J. Mar. Sci. 2010, 25, 11–30. [Google Scholar] [CrossRef]
- Wang, W.-X.; Lu, G. Heavy Metals in Bivalve Mollusks. In Chemical Contaminants and Residues in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 553–594. ISBN 978-0-08-100674-0. [Google Scholar]
- Parrino, V.; Costa, G.; Giannetto, A.; De Marco, G.; Cammilleri, G.; Acar, Ü.; Piccione, G.; Fazio, F. Trace Elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus Galloprovincialis and Tapes Decussatus from Faro and Ganzirri Lakes (Sicily, Italy): Flow Cytometry Applied for Hemocytes Analysis. J. Trace Elem. Med. Biol. 2021, 68, 126870. [Google Scholar] [CrossRef]
- Yu, X.-J.; Pan, K.; Liu, F.; Yan, Y.; Wang, W.-X. Spatial Variation and Subcellular Binding of Metals in Oysters from a Large Estuary in China. Mar. Pollut. Bull. 2013, 70, 274–280. [Google Scholar] [CrossRef]
- Gao, S.; Wang, W.-X. Oral Bioaccessibility of Toxic Metals in Contaminated Oysters and Relationships with Metal Internal Sequestration. Ecotoxicol. Environ. Saf. 2014, 110, 261–268. [Google Scholar] [CrossRef]
- Boening, D.W. An Evaluation of Bivalves as Biomonitors of Heavy Metals Pollution in Marine Waters. Environ. Monit. Assess. 1999, 55, 459–470. [Google Scholar] [CrossRef]
- Abdel-Baki, A.S.; Abdel-Baki, M.A.; Al-Quraishy, S. Bioaccumulation of Some Heavy Metals in Tilapia Fish Relevant to Their Concentration in Water and Sediment of Wadi Hanifah, Saudi Arabia. Afr. J. Biotechnol. 2011, 10, 2541–2547. [Google Scholar]
- Beg, M.U.; Al-Muzaini, S.; Saeed, T.; Jacob, P.G.; Beg, K.R.; Al-Bahloul, M.; Al-Matrouk, K.; Al-Obaid, T.; Kurian, A. Chemical Contamination and Toxicity of Sediment from a Coastal Area Receiving Industrial Effluents in Kuwait. Arch. Environ. Contam. Toxicol. 2001, 41, 289–297. [Google Scholar] [CrossRef]
- Beg, M.U.; Al-Jandal, N.; Al-Subiai, S.; Karam, Q.; Husain, S.; Butt, S.A.; Ali, A.; Al-Hasan, E.; Al-Dufaileej, S.; Al-Husaini, M. Metallothionein, Oxidative Stress and Trace Metals in Gills and Liver of Demersal and Pelagic Fish Species from Kuwaits’ Marine Area. Mar. Pollut. Bull. 2015, 100, 662–672. [Google Scholar] [CrossRef]
- Al-Mohanna, S.Y.; Subrahmanyam, M.N.V. Flux of Heavy Metal Accumulation in Various Organs of the Intertidal Marine Blue Crab, Portunus Pelagicus (L.) from the Kuwait Coast after the Gulf War. Environ. Int. 2001, 27, 321–326. [Google Scholar] [CrossRef]
- Sun, R.; Sun, Y.; Li, Q.X.; Zheng, X.; Luo, X.; Mai, B. Polycyclic Aromatic Hydrocarbons in Sediments and Marine Organisms: Implications of Anthropogenic Effects on the Coastal Environment. Sci. Total Environ. 2018, 640–641, 264–272. [Google Scholar] [CrossRef]
- Shaiek, M.; Zaaboub, N.; Ayas, D.; Martins, M.V.A.; Romdhane, M.S. Crabs as Bioindicators of Trace Element Accumulation in Mediterranean Lagoon (Bizerte Lagoon, Tunisia). J. Sediment. Environ. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Minitab, I. 2020. Available online: http://Www.Minitab.Com/En-US/Products/Minitab/ (accessed on 29 March 2023).
- Chessel, D.; Doledec, S. ADE Software, Version 3.6. Multivariate Analyses and Graphical Display for Environmental Data; CNRS 1451, Villeurbanne. Adobe: Mountain View, CA, USA, 1992.
- Baki, M.A.; Hossain, M.M.; Akter, J.; Quraishi, S.B.; Haque Shojib, M.F.; Atique Ullah, A.K.M.; Khan, M.F. Concentration of Heavy Metals in Seafood (Fishes, Shrimp, Lobster and Crabs) and Human Health Assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Goussanou, A.; Youssao, A.K.A.; Dagan, S.B.; Youssao, A.K.I. Bioaccumulation Des Métaux Lourds (Pb, Cd, Cu, Zn, Fe, Cr, Ni, As) Par Les Crabes Cardisoma Armatum (Herklots, 1851) Dans Le Complexe « lac Nokoué-Lagune de Porto—Novo » Au Sud Bénin. Afr. Sci. 2018, 14, 255–266. [Google Scholar]
- Kamaruzzan, B.Y.; John, B.A.; Megat, M.H.A.; Zaleha, K. Bioaccumulation of Heavy Metals in Horseshoe Crabs (Tachypleus Gigas) from Pekan, Pahang, Malaysia. Res. J. Environ. Toxicol. 2011, 5, 222–228. [Google Scholar] [CrossRef]
- Ramos, R.J.; Leite, G.R. Ecdysis as an Auxiliary Route for the Removal of Heavy Metals in Crustaceans: An Experimental Analysis with Fiddler Crabs (Minuca Burgersi). BioMetals 2022, 35, 115–124. [Google Scholar] [CrossRef]
- van den Brink, N.W.; Jemec Kokalj, A.; Silva, P.V.; Lahive, E.; Norrfors, K.; Baccaro, M.; Khodaparast, Z.; Loureiro, S.; Drobne, D.; Cornelis, G.; et al. Tools and Rules for Modelling Uptake and Bioaccumulation of Nanomaterials in Invertebrate Organisms. Environ. Sci. Nano 2019, 6, 1985–2001. [Google Scholar] [CrossRef]
- Ali, M.M.; Ali, M.L.; Proshad, R.; Islam, S.; Rahman, Z.; Tusher, T.R.; Kormoker, T.; Al, M.A. Heavy Metal Concentrations in Commercially Valuable Fishes with Health Hazard Inference from Karnaphuli River, Bangladesh. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 2646–2662. [Google Scholar] [CrossRef]
- Das, I.; Das, S.; Chakraborty, I.; Ghangrekar, M.M. Ghangrekar Bio-Refractory Pollutant Removal Using Microbial Electrochemical Technologies: A Short Review. J. Indian Chem. Soc. 2019, 96, 493–497. [Google Scholar] [CrossRef]
- Orr, K.K. Spatial and Temporal Variations in Metals in the Sediment and Water of Selected Eastern Cape Estuaries, South Africa. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2007. [Google Scholar]
- Simpson, S.L.; Batley, G.E.; Chariton, A.A. Revision of the ANZECC/ARMCANZ Sediment Quality Guidelines; CSIRO Land and Water: Melbourne, Australia, 2013; p. 121. [Google Scholar]
- Ji, Z.; Zhang, Y.; Zhang, H.; Huang, C.; Pei, Y. Fraction Spatial Distributions and Ecological Risk Assessment of Heavy Metals in the Sediments of Baiyangdian Lake. Ecotoxicol. Environ. Saf. 2019, 174, 417–428. [Google Scholar] [CrossRef]
- Adeleke, B.; Robertson-Andersson, D.; Moodley, G. Comparative Analysis of Trace Metal Levels in the Crab Dotilla Fenestrata, Sediments and Water in Durban Bay Harbour, Richards Bay Harbour and Mlalazi Estuary, Kwazulu-Natal, South Africa. Heliyon 2020, 6, e04725. [Google Scholar] [CrossRef]
- Mohammad Ali, M.; Hossain, D.; Al-Imran; Suzan Khan, M.; Begum, M.; Hasan Osman, M. Environmental Pollution with Heavy Metals: A Public Health Concern. In Heavy Metals—Their Environmental Impacts and Mitigation; Khaled Nazal, M., Zhao, H., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83968-121-9. [Google Scholar]
- Brezonik, P.L.; King, S.O.; Mach, C.E. The Influence of Water Chemistry on Trace Metal Bioavailability and Toxicity to Aquatic Organisms. In Metal Ecotoxicology Concepts and Applications; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–31. ISBN 978-1-00-306997-3. [Google Scholar]
- Cyriac, M.; Gireeshkumar, T.R.; Furtado, C.M.; Fathin, K.P.F.; Shameem, K.; Shaik, A.; Vignesh, E.R.; Nair, M.; Kocherla, M.; Balachandran, K.K. Distribution, Contamination Status and Bioavailability of Trace Metals in Surface Sediments along the Southwest Coast of India. Mar. Pollut. Bull. 2021, 164, 112042. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, D.S.; Kim, Y.S. Distribution of Metal Contamination and Grain Size in the Sediments of Nakdong River, Korea. Environ. Monit. Assess. 2020, 192, 502. [Google Scholar] [CrossRef] [PubMed]
- MacKay, H.M.; Roux, D.J.; Ashton, P.J.; Van Vliet, H.R.; Jooste, S. The Development of South African Water Quality Guidelines for the Natural Aquatic Environment. Water Sci. Technol. 1995, 32, 293–299. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Sokolova, I.M. Interactive Effects of Metal Pollution and Ocean Acidification on Physiology of Marine Organisms. Curr. Zool. 2015, 61, 653–668. [Google Scholar] [CrossRef]
- Abdel Gawad, S.S. Concentrations of Heavy Metals in Water, Sediment and Mollusk Gastropod, Lanistes Carinatus from Lake Manzala, Egypt. Egypt. J. Aquat. Res. 2018, 44, 77–82. [Google Scholar] [CrossRef]
- de Orte, M.R.; Lombardi, A.T.; Sarmiento, A.M.; Basallote, M.D.; Rodriguez-Romero, A.; Riba, I.; del Valls, A. Metal Mobility and Toxicity to Microalgae Associated with Acidification of Sediments: CO2 and Acid Comparison. Mar. Environ. Res. 2014, 96, 136–144. [Google Scholar] [CrossRef]
- de Orte, M.R.; Sarmiento, A.M.; Basallote, M.D.; Rodríguez-Romero, A.; Riba, I.; del Valls, A. Effects on the Mobility of Metals from Acidification Caused by Possible CO2 Leakage from Sub-Seabed Geological Formations. Sci. Total Environ. 2014, 470–471, 356–363. [Google Scholar] [CrossRef]

| Cadmium (Cd) µg/g | Copper (Cu) µg/g | Lead (Pb) µg/g | Zinc (Zn) µg/g | |
|---|---|---|---|---|
| Water | Measured 8.94 ± 0.12 | Measured 22.2 ± 0.96 | Measured 0.51 ± 1.42 | Measured 70.02 ± 0.38 |
| Certified 9.49 ± 0.15 | Certified 22.7 ± 2.39 | Certified 0.53 ± 0.29 | Certified 60.81 ± 4.59 | |
| % Recovery 96.8 | % Recovery 112 | % Recovery 109 | % Recovery 108 | |
| Sediment | Measured 7.15 ± 1.87 | Measured 107 ± 2.64 | Measured 169 ± 3.91 | Measured 911 ± 61.67 |
| Certified 5.63 ± 0.89 | Certified 112 ± 20.41 | Certified 192 ± 22.18 | Certified 873 ± 179 | |
| % Recovery 127 | % Recovery 90.7 | % Recovery 94 | % Recovery 101 | |
| Crab | Measured 111 ± 22.9 | Measured 176 ± 44.59 | Measured 208 ± 29.6 | Measured 49.22 ± 16.8 |
| Certified 112 ±1.96 | Certified 186 ± 3.93 | Certified 198 ± 2.17 | Certified 53.04 ± 1.98 | |
| % Recovery 106 | % Recovery 96.9 | % Recovery 107 | % Recovery 92.8 |
| Heavy Metal | Cu | Zn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Area | Shuwaikh | Shuaiba | Al-Khiran | F (d.f) | p Value | Shuwaikh | Shuaiba | Al-Khiran | F (d.f) | p Value |
| Carapace | 80.5 ± 1.49 aA | 25.2 ± 1.22 bA | 16.9 ± 0.29 cA | 2836.55 (8) | <0.0001 *** | 25.9 ± 0.27 aAB | 8.92 ± 0.31 bA | 6.87 ± 0.19 cA | 4787.89 (8) | <0.0001 *** |
| Gills | 79.1 ± 2.99 aA | 53.8 ± 5.01 bB | 24.9 ± 1.01 cB | 188.81 (8) | <0.0001 *** | 29.1 ± 3.96 aB | 14.2 ± 1.39 bB | 6.82 ± 0.58 cA | 64.59 (8) | <0.0001 *** |
| Hepatopancreas | 68.6 ± 1.23 aB | 22.6 ± 0.18 bA | 14.9 ± 0.21 cA | 4774.43 (8) | <0.0001 *** | 18.9 ± 0.69 aA | 6.92 ± 0.21 bA | 4.74 ± 0.21 cB | 927.11 (8) | <0.0001 *** |
| Total Crab | 228.20 ± 5.71 aC | 101.60 ± 6.41 bC | 56.70 ± 1.51 cC | 936.97 (8) | <0.0001 *** | 73.9 ± 4.92 aC | 30.04 ± 1.91 bC | 18.43 ± 0.98 cC | 267.33 (8) | <0.0001 *** |
| F (d.f) | 1540.0 (11) | 238.68 (11) | 1315.63 (11) | 185.46 (11) | 230.12 (11) | 337.48 (11) | ||||
| p values | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | ||||
| Heavy Metal | Pb | Cd | ||||||||
| Study area | Shuwaikh | Shuaiba | Al-Khiran | F (d.f) | p value | Shuwaikh | Shuaiba | Al-Khiran | F (d.f) | p value |
| Carapace | 2.39 ± 0.11 aA | 1.27 ± 0.19 bA | 3.76 ± 0.21 cA | 151.65 (8) | <0.0001 *** | 0.39 ± 0.01 aA | 0.24 ± 0.03 bA | 0.19 ± 0.02 bA | 69.64 (8) | <0.0001 *** |
| Gills | 3.29 ± 0.29 aA | 1.59 ± 0.21 bA | 1.06 ± 0.02 cB | 94.99 (8) | <0.0001 *** | 0.28 ± 0.02 aB | 0.16 ± 0.01 bA | 0.19 ± 0.02 bA | 39.00 (8) | 0.000 *** |
| Hepatopancreas | 4.69 ± 0.29 aB | 1.60 ± 0.12 bA | 1.76 ± 0.21 aC | 191.01 (8) | <0.0001 *** | 0.43 ± 0.02 aA | 0.16 ± 0.02 bA | 0.20 ± 0.02 bA | 159.25 (8) | <0.0001 *** |
| Total Crab | 10.37 ± 0.69 aC | 4.46 ± 0.52 bB | 6.58 ± 0.44 cD | 85.82 (8) | <0.0001 *** | 1.10 ± 0.05 aC | 0.56 ± 0.06 bB | 0.58 ± 0.06 bB | 86.97 (8) | <0.0001 *** |
| F (d.f) | 234.81 (11) | 73.44 (11) | 260.23 (11) | 488.71 (11) | 87.04 (11) | 93.50 (11) | ||||
| p values | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** | ||||
| Variables | Wat-Shkh | Sed-Shkh | Tissue-Shkh | Wat-Shu | Sed-Shu | Tissue-Shu | Wat-Kh | Sed-Kh | Tissue-Kh |
|---|---|---|---|---|---|---|---|---|---|
| wat-Shkh | 1 | ||||||||
| Sed-Shkh | 0.248 | 1 | |||||||
| Tissue-Shkh | 0.913 *** | 0.313 | 1 | ||||||
| wat-shu | 0.981 *** | 0.318 | 0.971 *** | 1 | |||||
| Sed-Shu | 0.349 | 0.612 * | 0.186 | 0.331 | 1 | ||||
| Tissue-shu | 0.919 *** | 0.286 | 0.999 *** | 0.972 *** | 0.171 | 1 | |||
| wat-Kh | 0.578 * | 0.925 *** | 0.622 * | 0.641 * | 0.628 * | 0.604 * | 1 | ||
| Sed-Kh | 0.441 | 0.949 *** | 0.392 | 0.461 | 0.722 ** | 0.368 | 0.933 *** | 1 | |
| Tissu-Kh | 0.938 *** | 0.303 | 0.998 *** | 0.983 *** | 0.207 | 0.997 *** | 0.618 * | 0.403 | 1 |
| Compartiment | Sediment | Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Area | Shuwaikh | Shuaiba | Al-Khiran | F(d.f) | p Values | Shuwaikh | Shuaiba | Al-Khiran | F(d.f) | p Values |
| Cd | 2.63 ± 0.04 a | 3.29 ± 0.25 b | 6.72 ± 0.12 c | 552.77 (8) | <0.0001 *** | 2.63 ± 0.04 a | 3.29 ± 0.25 a | 6.72 ± 0.12 a | 0.87 (8) | 0.467 |
| Cu | 15.95 ± 0.84 a | 15.23 ± 0.27 a | 33.12 ± 0.44 | 949.56 (8) | <0.0001 *** | 1.95 ± 0.84 a | 15.23 ± 0.27 a | 33.12 ± 0.44 a | 1.93 (8) | 0.226 |
| Pb | 11.42 ± 0.43 | 19.42 ± 1.79 | 30.23 ± 1.27 | 160.34 (8) | <0.0001 *** | 11.42 ± 0.43 a | 19.42 ± 1.79 b | 30.23 ± 1.27 a | 14.69 (8) | 0.005 ** |
| Zn | 33.12 ± 0.79 ab | 22.02 ± 16.14 a | 49.29 ± 1.69 b | 6.41 (8) | 0.032 * | 33.12 ± 0.79 a | 22.02 ± 16.14 a | 49.29 ± 1.69 a | 126.68 (8) | <0.0001 *** |
| Areas Water Parameters | Shuwaikh | Shuaiba | Al-Khiran |
|---|---|---|---|
| Salinity (psu) | 40.9 ± 0.98 | 40.2 ± 0.26 | 39.8 ± 0.83 |
| Dissolved oxygen (mg/L) | 4.35 ± 0.56 | 4.95 ± 0.69 | 4.12 ± 0.87 |
| Temperature (°C) | 23.4 ± 0.67 | 22.2 ± 0.97 | 21.3 ± 0.54 |
| pH | 8.06 ± 0.18 | 8.24 ± 0.94 | 7.65 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karam, Q.; Guermazi, W.; Subrahmanyam, M.N.V.; Al-Enezi, Y.; Ali, M.; Leignel, V.; Annabi-Trabelsi, N. Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf). Toxics 2023, 11, 426. https://doi.org/10.3390/toxics11050426
Karam Q, Guermazi W, Subrahmanyam MNV, Al-Enezi Y, Ali M, Leignel V, Annabi-Trabelsi N. Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf). Toxics. 2023; 11(5):426. https://doi.org/10.3390/toxics11050426
Chicago/Turabian StyleKaram, Qusaie, Wassim Guermazi, M. N. V. Subrahmanyam, Yousef Al-Enezi, Mohammad Ali, Vincent Leignel, and Neila Annabi-Trabelsi. 2023. "Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf)" Toxics 11, no. 5: 426. https://doi.org/10.3390/toxics11050426
APA StyleKaram, Q., Guermazi, W., Subrahmanyam, M. N. V., Al-Enezi, Y., Ali, M., Leignel, V., & Annabi-Trabelsi, N. (2023). Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf). Toxics, 11(5), 426. https://doi.org/10.3390/toxics11050426








