Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach
Abstract
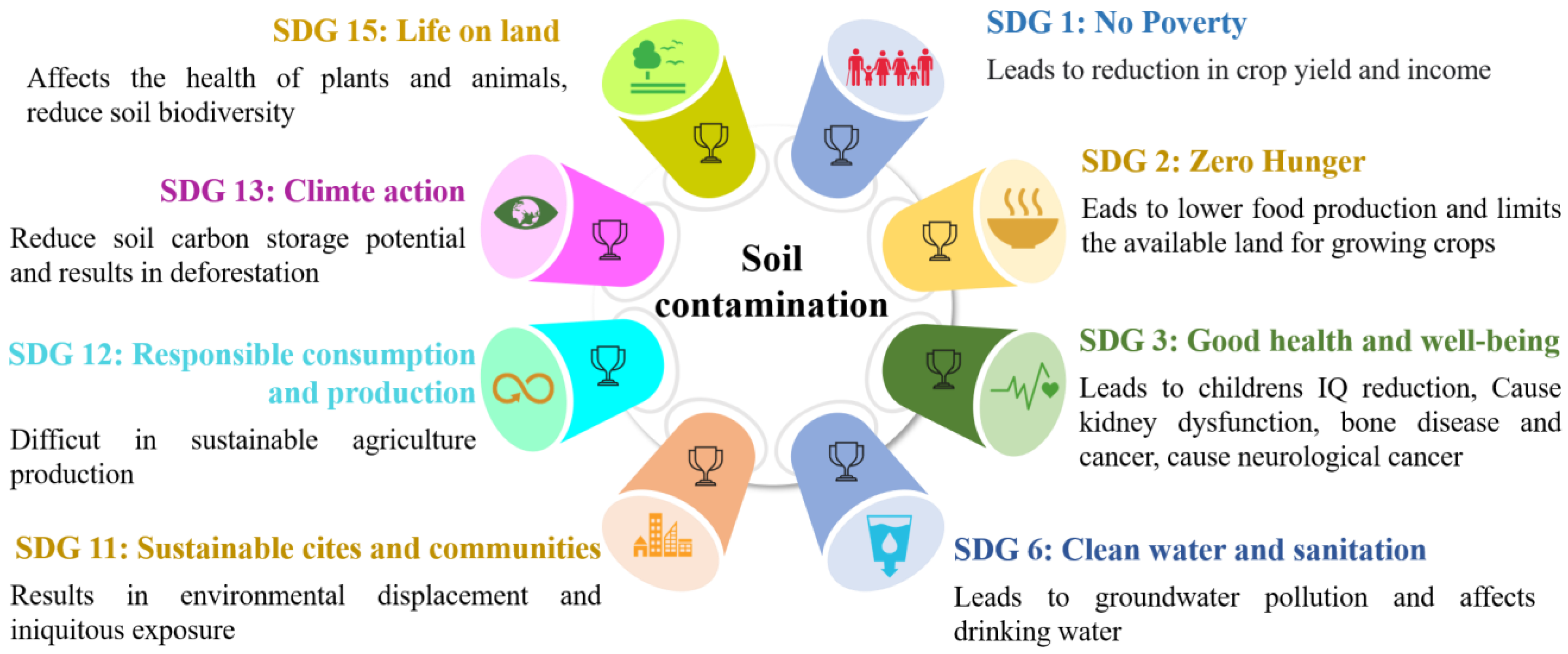
1. Introduction
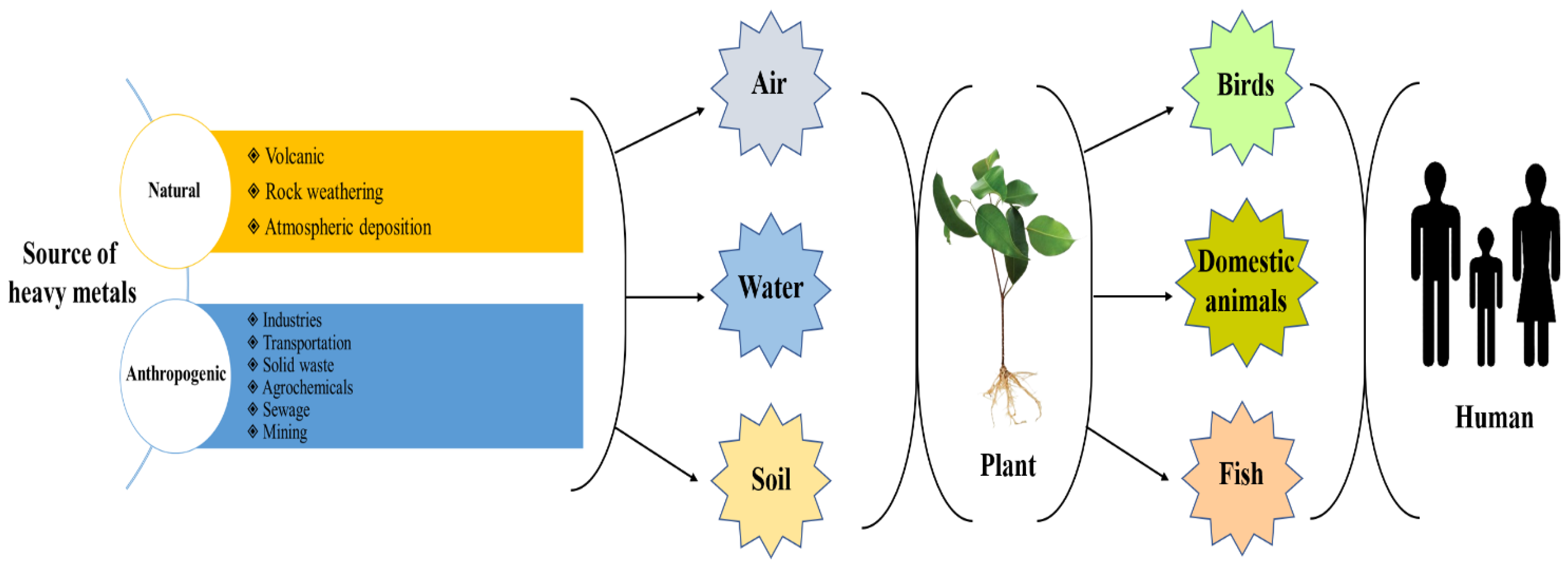
2. Heavy Metal Sources in the Environment
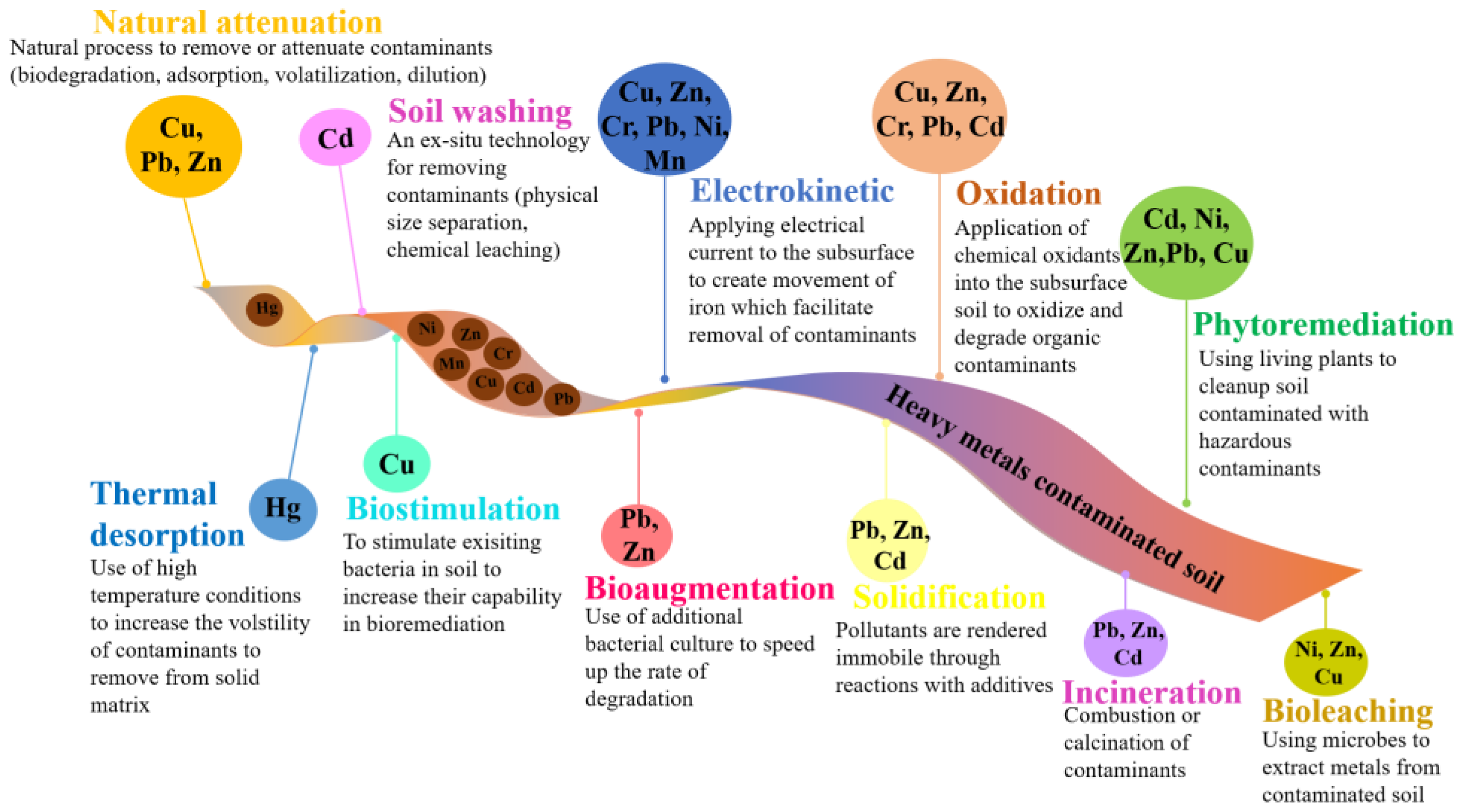
3. Recent Developments in Heavy Metal Remediation Strategies
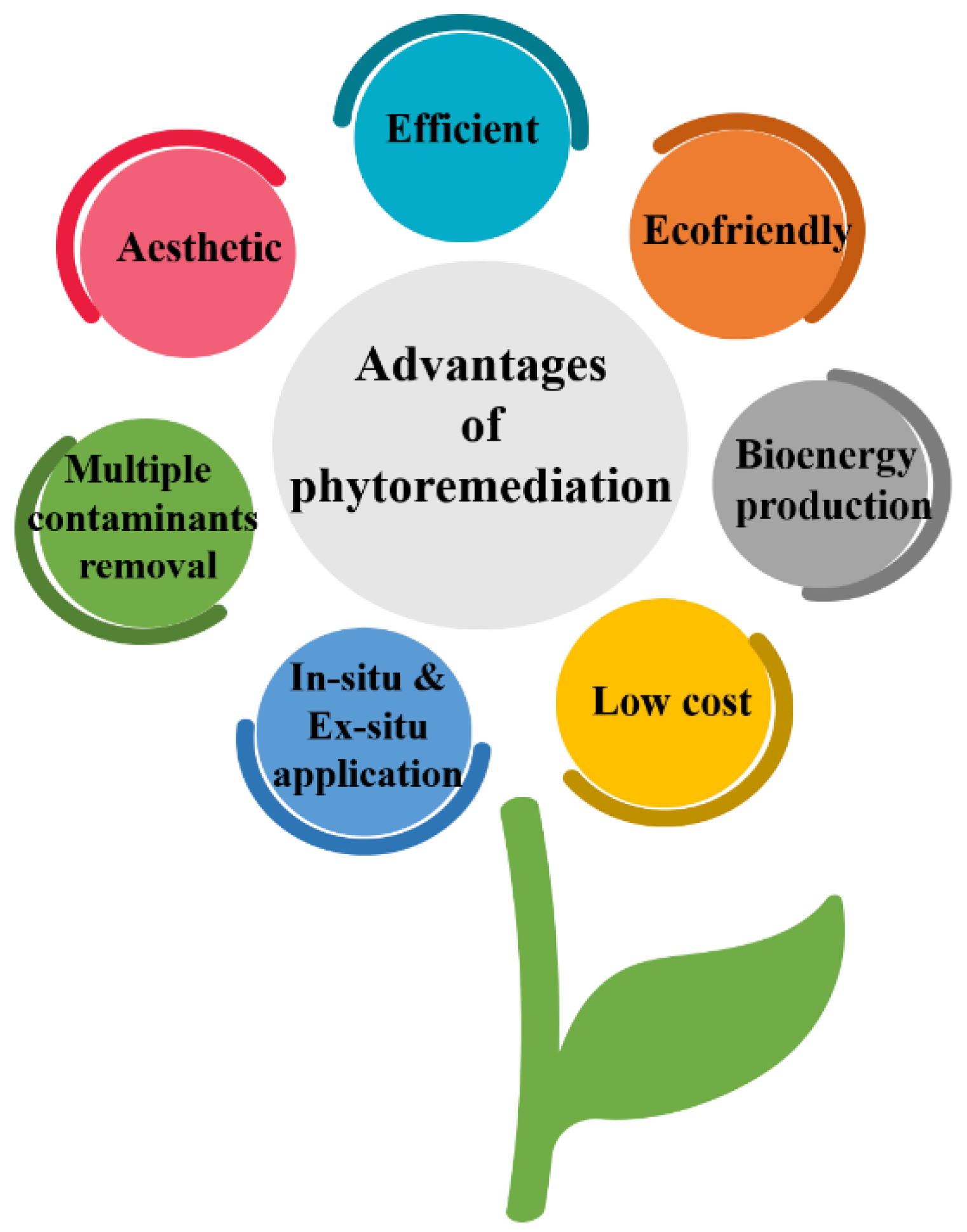
4. Heavy Metal Removal from Contaminated Soil by Phytoremediation
4.1. Phytoextraction
4.2. Phytostabilization
4.3. Phytovolatilization
4.4. Rhizofiltration
4.5. Rhizodegradation
4.6. Phytodesalination
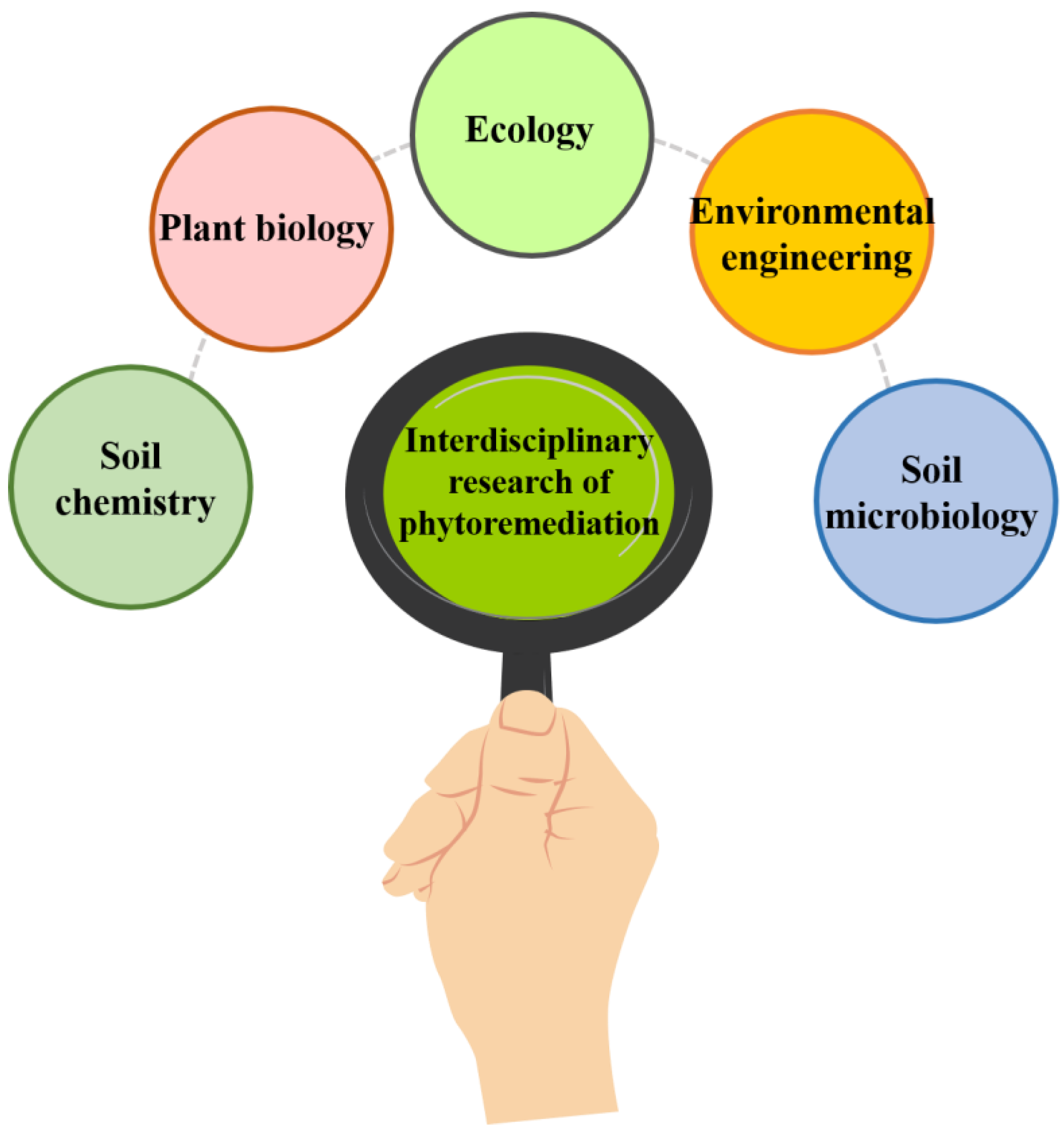
5. Potential Biotechnological Approaches for Phytoremediation
6. Factors Affecting Phytoremediation Potential
- Plant species: Different plant species have varying abilities to accumulate and remove contaminants from soil. Hyperaccumulator plants are particularly effective in absorbing heavy metals from soil.
- Contaminant type and concentration: The type and concentration of the contaminant in the soil can affect the plant’s ability to absorb and remove it. Some contaminants, such as heavy metals, can be more difficult to remove than others.
- Soil properties: Soil properties, such as pH, organic matter content, and nutrient availability, can affect the ability of plants to grow and absorb contaminants.
- Climate and weather conditions: Climate and weather conditions, such as temperature, precipitation, and sunlight, can affect plant growth and the rate of contaminant removal.
- Soil moisture: The moisture content of the soil can affect the growth and health of the plants, as well as the availability of the contaminants for uptake.
- Plant growth stage: The growth stage of the plant can affect its ability to absorb contaminants, as well as the biomass produced for removal.
- Duration of treatment: The duration of phytoremediation treatment can affect the effectiveness of contaminant removal. More extended treatment periods may be necessary for some contaminants and soil types.
- Management practices: Proper management practices, such as soil amendments and fertilization, can improve plant growth and the effectiveness of phytoremediation. In addition, the local microbial area in the rhizosphere can improve phytoremediation by influencing the accessibility and versatility of heavy metals in soils [99]. Hence, studying microbial ecology and its interactions with plants and soils is necessary to use phytoremediation effectively. Therefore, it is essential to carefully assess phytoremediation’s potential risks and benefits before its implementation in contaminated sites [100].
7. Phytoremediation: Challenges and Difficulties
7.1. Application of Phytoremediation Techniques Needs to Be Accelerated
7.2. Lack of Effective Methods to Remove Contaminated Biomass
8. Advancements in Research to Address the Problems and Challenges
8.1. Techniques Used to Increase the Effectiveness of Phytoremediation
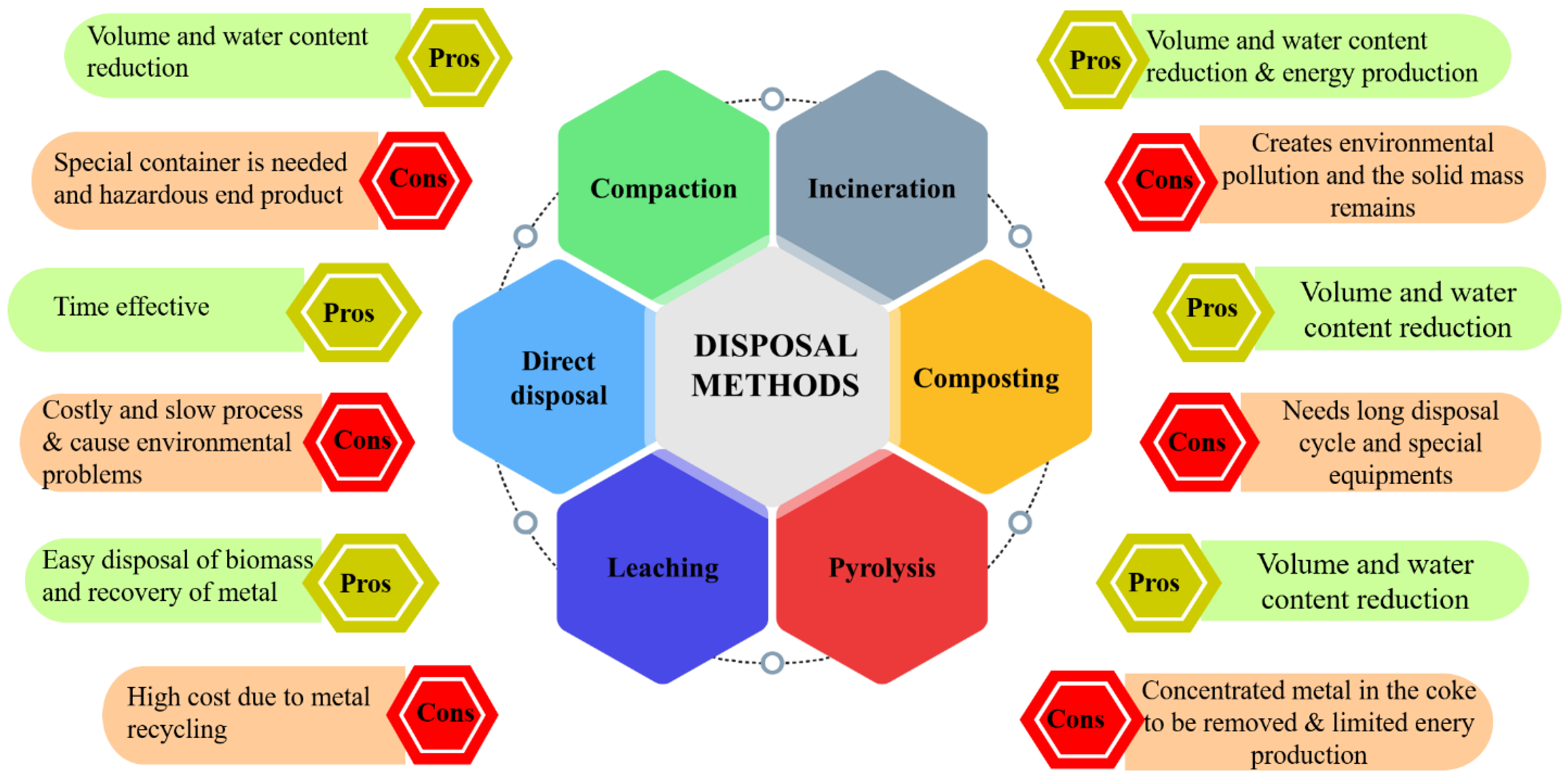
8.2. Disposal of Harmful Plant Waste after Phytoremediation
9. Challenges and Future Recommendations
- It is a slow process which may take several years to achieve significant results, especially in highly contaminated soils. The time required for remediation depends on several factors, such as the type and concentration of contaminants, the plant species used, soil properties, and environmental conditions.
- It can sometimes be less effective due to some hyperaccumulator plants’ slow growth rates and lower levels of biomass production. These factors can limit the amounts of heavy metals removed from contaminated soil within a given period. Additionally, some plants may only accumulate specific types of heavy metals, which may not be the most predominant contaminants in the soil. There may also be contaminants with lower activation abilities or plants with lower absorption potential because of a few firmly bound metal particles. Thus, there is a risk of only partial removal of pollutants from the contaminated site in the absence of appropriate consideration.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of Irrigation with Wastewater on Accumulation of Heavy Metals in Soil and Crops in the Region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Sandeep, G.; Vijayalatha, K.R.; Anitha, T. Heavy Metals and Its Impact in Vegetable Crops. Int. J. Chem. Stud. 2019, 7, 1612–1621. [Google Scholar]
- Hadia-e-Fatima, A.A. Heavy Metal Pollution–A Mini Review. J. Bacteriol. Mycol. Open Access 2018, 6, 179–181. [Google Scholar]
- Kapoor, D.; Singh, M.P. Heavy Metal Contamination in Water and Its Possible Sources. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 179–189. [Google Scholar]
- Priya, A.K.; Nagan, S. Bioremediation of Dye Effluent and Metal Contaminated Soil: Low-Cost Method for Environmental Clean up by Microbes. J. Environ. Sci. Eng. 2015, 57, 109–119. [Google Scholar]
- Devi, P.; Kumar, P. Concept and Application of Phytoremediation in the Fight of Heavy Metal Toxicity. J. Pharm. Sci. Res. 2020, 12, 795–804. [Google Scholar]
- Zhao, H.; Huang, X.; Liu, F.; Hu, X.; Zhao, X.; Wang, L.; Gao, P.; Li, J.; Ji, P. Potential of a Novel Modified Gangue Amendment to Reduce Cadmium Uptake in Lettuce (Lactuca sativa L.). J. Hazard. Mater. 2021, 410, 124543. [Google Scholar] [CrossRef]
- Beiyuan, J.; Fang, L.; Chen, H.; Li, M.; Liu, D.; Wang, Y. Nitrogen of EDDS Enhanced Removal of Potentially Toxic Elements and Attenuated Their Oxidative Stress in a Phytoextraction Process. Environ. Pollut. 2021, 268, 115719. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and Sources of Heavy Metals in Suspended Particulate Matter in a Tropical Catchment, Northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Ke, B.; Nguyen, H.; Bui, X.-N.; Bui, H.-B.; Choi, Y.; Zhou, J.; Moayedi, H.; Costache, R.; Nguyen-Trang, T. Predicting the Sorption Efficiency of Heavy Metal Based on the Biochar Characteristics, Metal Sources, and Environmental Conditions Using Various Novel Hybrid Machine Learning Models. Chemosphere 2021, 276, 130204. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation Technology and Food Security Impacts of Heavy Metal Contaminated Soils: A Review of Literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Flores, L.C. Plant Mediated Detoxification of Mercury and Lead. Arab. J. Chem. 2017, 10, S2335–S2342. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. What Are Heavy Metals? Long-Standing Controversy over the Scientific Use of the Term ‘Heavy Metals’–Proposal of a Comprehensive Definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chemother. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Priya, A.K.; Bhatnagar, A.; Gnanasekaran, L.; Rajendran, S.; Ahmed, A.; Luque, R. Bioremediation: A Sustainable Remediation Approach for the Bioeconomy. Available SSRN 4202192. Available online: https://dx.doi.org/10.2139/ssrn.4202192 (accessed on 1 April 2023).
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Pueyo, J.J. Uptake and Effects of Lead and Zinc on Alfalfa (Medicago sativa L.) Seed Germination and Seedling Growth: Role of Plant Growth Promoting Bacteria. S. Afr. J. Bot. 2019, 124, 573–582. [Google Scholar] [CrossRef]
- Fang, L.; Ju, W.; Yang, C.; Duan, C.; Cui, Y.; Han, F.; Shen, G.; Zhang, C. Application of Signaling Molecules in Reducing Metal Accumulation in Alfalfa and Alleviating Metal-Induced Phytotoxicity in Pb/Cd-Contaminated Soil. Ecotoxicol. Environ. Saf. 2019, 182, 109459. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Y.; Fu, X.; Ji, L.; Li, T.; Wang, J.; Chen, G.; Qi, Z.; Zhang, Q. Biochemical Mechanisms of Rhizospheric Bacillus Subtilis-Facilitated Phytoextraction by Alfalfa under Cadmium Stress–Microbial Diversity and Metabolomics Analyses. Ecotoxicol. Environ. Saf. 2021, 212, 112016. [Google Scholar] [CrossRef]
- Das, U.; Rahman, M.A.; Ela, E.J.; Lee, K.-W.; Kabir, A.H. Sulfur Triggers Glutathione and Phytochelatin Accumulation Causing Excess Cd Bound to the Cell Wall of Roots in Alleviating Cd-Toxicity in Alfalfa. Chemosphere 2021, 262, 128361. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Cui, W.; Zhang, Y.; Hu, H.; Yu, X.; Wang, Q.; Shen, W. Methane Alleviates Alfalfa Cadmium Toxicity via Decreasing Cadmium Accumulation and Reestablishing Glutathione Homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Kang, Y.; Liu, J.; Wang, Y.; Sun, H.; Ao, T.; Chen, W. Selenium Alleviates Toxicity in Amaranthus Hypochondriacus by Modulating the Synthesis of Thiol Compounds and the Subcellular Distribution of Cadmium. Chemosphere 2022, 291, 133108. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Shi, T.; He, L.; Hu, W.; Wu, G. Assessing Toxic Metal Chromium in the Soil in Coal Mining Areas via Proximal Sensing: Prerequisites for Land Rehabilitation and Sustainable Development. Geoderma 2022, 405, 115399. [Google Scholar] [CrossRef]
- Xue, Z.-F.; Cheng, W.-C.; Wang, L.; Hu, W. Effects of Bacterial Inoculation and Calcium Source on Microbial-Induced Carbonate Precipitation for Lead Remediation. J. Hazard. Mater. 2022, 426, 128090. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S. Hazardous Heavy Metals Contamination of Vegetables and Food Chain: Role of Sustainable Remediation Approaches—A Review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Rajendran, S.; Naushad, M.; Vo, D.-V.N.; Lichtfouse, E. Inorganic Materials for Energy, Medicine and Environmental Remediation; Springer: Cham, Switzerland, 2022; ISBN 3030798992. [Google Scholar]
- Priya, A.K.; Jalil, A.A.; Vadivel, S.; Dutta, K.; Rajendran, S.; Fujii, M.; Soto-Moscoso, M. Heavy Metal Remediation from Wastewater Using Microalgae: Recent Advances and Future Trends. Chemosphere 2022, 305, 135375. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Yang, Y.; Li, S.; Wang, T.; Oleksak, P.; Chrienova, Z.; Wu, Q.; Nepovimova, E.; Zhang, X.; et al. Phytoremediation of Heavy Metal Pollution: Hotspots and Future Prospects. Ecotoxicol. Environ. Saf. 2022, 234, 113403. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Phytoremediation of Toxic Metals Present in Soil and Water Environment: A Critical Review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860. [Google Scholar] [CrossRef]
- Verma, R.K.; Sankhla, M.S.; Jadhav, E.B.; Parihar, K.; Awasthi, K.K. Phytoremediation of Heavy Metals Extracted from Soil and Aquatic Environments: Current Advances as Well as Emerging Trends. Biointerface Res. Appl. Chem. 2022, 12, 5486–5509. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Zuo, Y.; Aioub, A.A.A.; Hu, Z. Biochemical and Phytoremediation of Plantago major L. to Protect Tomato Plants from the Contamination of Cypermethrin Pesticide. Environ. Sci. Pollut. Res. 2021, 28, 43992–44001. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Ul Haq, S.A.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of Heavy Metals in Soil and Water: An Eco-Friendly, Sustainable and Multidisciplinary Approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Tatu, G.L.A.; Vladut, N.V.; Voicea, I.; Vanghele, N.A.; Pruteanu, M.A. Removal of Heavy Metals from a Contaminated Soil Using Phytoremediation. MATEC Web Conf. 2020, 305, 00061. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal Contamination and Bioremediation of Agricultural Soils for Food Safety and Sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A Review on Various Electrochemical Techniques for Heavy Metal Ions Detection with Different Sensing Platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Nejad, Z.D.; Jung, M.C.; Kim, K.-H. Remediation of Soils Contaminated with Heavy Metals with an Emphasis on Immobilization Technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.; Bosneaga, A.; Georgescu, L. Determination of Heavy Metals in Soils Using XRF Technique. Rom. J. Phys. 2010, 55, 815–820. [Google Scholar]
- Vhahangwele, M.; Mugera, G.W. The Potential of Ball-Milled South African Bentonite Clay for Attenuation of Heavy Metals from Acidic Wastewaters: Simultaneous Sorption of Co2+, Cu2+, Ni2+, Pb2+, and Zn2+ Ions. J. Environ. Chem. Eng. 2015, 3, 2416–2425. [Google Scholar] [CrossRef]
- Friedlander, L.R.; Weisbrod, N.; Garb, Y.J. Climatic and Soil-Mineralogical Controls on the Mobility of Trace Metal Contamination Released by Informal Electronic Waste (e-Waste) Processing. Chemosphere 2019, 232, 130–139. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of Contaminated Soils by Heavy Metals and PAHs. A Brief Review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Méndez, A.; Reichman, S.M. Soil Pollution and Remediation. Int. J. Environ. Res. Public Health 2018, 15, 1657. [Google Scholar] [CrossRef]
- Arantza, S.J.; Hiram, M.R.; Erika, K.; Chávez-Avilés, M.N.; Valiente-Banuet, J.I.; Fierros-Romero, G. Bio- and Phytoremediation: Plants and Microbes to the Rescue of Heavy Metal Polluted Soils. SN Appl. Sci. 2022, 4, 59. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of Phytoremediation Combined with Other Approaches for Remediation of Heavy Metal-Polluted Soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hesham, A.E.L.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on Soil Microbial Community Structure and Activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Singh, B.S.M.; Singh, D.; Dhal, N.K. Enhanced Phytoremediation Strategy for Sustainable Management of Heavy Metals and Radionuclides. Case Stud. Chem. Environ. Eng. 2022, 5, 100176. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 78. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of Heavy Metals by Four Aquatic Macrophytes and Their Potential Use as Contamination Indicators: A Comparative Assessment. Environ. Sci. Pollut. Res. 2020, 27, 12138–12151. [Google Scholar] [CrossRef]
- Latif, A.; Abbas, A.; Iqbal, J.; Azeem, M.; Asghar, W.; Ullah, R.; Bilal, M.; Arsalan, M.; Khan, M.; Latif, R.; et al. Remediation of Environmental Contaminants Through Phytotechnology. Water. Air Soil Pollut. 2023, 234, 139. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A Critical Review on the Phytoremediation of Heavy Metals from Environment: Performance and Challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef] [PubMed]
- Bortoloti, G.A.; Baron, D. Phytoremediation of Toxic Heavy Metals by Brassica Plants: A Biochemical and Physiological Approach. Environ. Adv. 2022, 8, 100204. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ramli, N.N.; Said, N.S.M.; Alias, J.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Hasan, H.A. Practical Limitations of Bioaugmentation in Treating Heavy Metal Contaminated Soil and Role of Plant Growth Promoting Bacteria in Phytoremediation as a Promising Alternative Approach. Heliyon 2022, 8, e08995. [Google Scholar] [CrossRef]
- Gurajala, H.K.; Cao, X.; Tang, L.; Ramesh, T.M.; Lu, M.; Yang, X. Comparative Assessment of Indian Mustard (Brassica juncea L.) Genotypes for Phytoremediation of Cd and Pb Contaminated Soils. Environ. Pollut. 2019, 254, 113085. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Pető, A.; Molnár, Á.; Rácz, É.; Ördög, A.; Erdei, L.; Kolbert, Z.; Laskay, G. Comparing the Effects of Excess Copper in the Leaves of Brassica juncea (L. Czern) and Brassica napus (L.) Seedlings: Growth Inhibition, Oxidative Stress and Photosynthetic Damage. Acta Biol. Hung. 2015, 66, 205–221. [Google Scholar] [CrossRef]
- Chaudhry, H.; Nisar, N.; Mehmood, S.; Iqbal, M.; Nazir, A.; Yasir, M. Indian Mustard Brassica juncea Efficiency for the Accumulation, Tolerance and Translocation of Zinc from Metal Contaminated Soil. Biocatal. Agric. Biotechnol. 2020, 23, 101489. [Google Scholar] [CrossRef]
- Ruttens, A.; Boulet, J.; Weyens, N.; Smeets, K.; Adriaensen, K.; Meers, E.; Van Slycken, S.; Tack, F.; Meiresonne, L.; Thewys, T. Short Rotation Coppice Culture of Willows and Poplars as Energy Crops on Metal Contaminated Agricultural Soils. Int. J. Phytoremediation 2011, 13, 194–207. [Google Scholar] [CrossRef]
- Fulekar, M.H. Phytoremediation of Heavy Metals by Helianthus Annuus in Aquatic and Soil Environment. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 392–404. [Google Scholar] [CrossRef]
- Nouri, J.; Khorasani, N.; Lorestani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of Heavy Metals in Soil and Uptake by Plant Species with Phytoremediation Potential. Environ. Earth Sci. 2009, 59, 315–323. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and Prospects Concerning the Phytoremediation of Heavy Metal Polluted Soils: A Review. Eurasian Soil Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Sakakibara, M.; Ohmori, Y.; Ha, N.T.H.; Sano, S.; Sera, K. Phytoremediation of Heavy Metal-contaminated Water and Sediment by Eleocharis Acicularis. CLEAN–Soil Air Water 2011, 39, 735–741. [Google Scholar] [CrossRef]
- Al-Khafaji, M.S.; Al-Ani, F.H.; Ibrahim, A.F. Removal of Some Heavy Metals from Industrial Wastewater by Lemmna Minor. KSCE J. Civ. Eng. 2018, 22, 1077–1082. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of Cadmium (Cd) and Uranium (U) Contaminated Soils by Brassica juncea L. Enhanced with Exogenous Application of Plant Growth Regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef]
- Angle, J.S.; Baker, A.J.M.; Whiting, S.N.; Chaney, R.L. Soil Moisture Effects on Uptake of Metals by Thlaspi, Alyssum, and Berkheya. Plant Soil 2003, 256, 325–332. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Mousavinia, F.; Foroughifard, S.; Karimzadeh, A. Nano Modification of NZVI with an Aquatic Plant Azolla Filiculoides to Remove Pb (II) and Hg (II) from Water: Aging Time and Mechanism Study. J. Colloid Interface Sci. 2017, 486, 296–308. [Google Scholar] [CrossRef]
- Majid, N.M.; Islam, M.M.; Riasmi, Y. Heavy Metal Uptake and Translocation by ‘Jatropha curcas’ L. in Sawdust Sludge Contaminated Soils. Aust. J. Crop Sci. 2012, 6, 891–898. [Google Scholar]
- Zhuang, P.; Ye, Z.H.; Lan, C.Y.; Xie, Z.W.; Shu, W.S. Chemically Assisted Phytoextraction of Heavy Metal Contaminated Soils Using Three Plant Species. Plant Soil 2005, 276, 153–162. [Google Scholar] [CrossRef]
- Yang, X.E.; Long, X.X.; Ye, H.B.; He, Z.L.; Calvert, D.V.; Stoffella, P.J. Cadmium Tolerance and Hyperaccumulation in a New Zn-Hyperaccumulating Plant Species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The Influence of PH and Organic Matter Content in Paddy Soil on Heavy Metal Availability and Their Uptake by Rice Plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Xin, L.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P.; Feng, W.; Xu, W. Feasibility of Anaerobic Digestion on the Release of Biogas and Heavy Metals from Rice Straw Pretreated with Sodium Hydroxide. Environ. Sci. Pollut. Res. 2019, 26, 19434–19444. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Deng, H.; Li, M.S. Manganese Uptake and Accumulation in a Woody Hyperaccumulator, Schima Superba. Plant Soil Environ. 2008, 54, 441–446. [Google Scholar] [CrossRef]
- Yanitch, A.; Kadri, H.; Frenette-Dussault, C.; Joly, S.; Pitre, F.E.; Labrecque, M. A Four-Year Phytoremediation Trial to Decontaminate Soil Polluted by Wood Preservatives: Phytoextraction of Arsenic, Chromium, Copper, Dioxins and Furans. Int. J. Phytoremediation 2020, 22, 1505–1514. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- De Souza, T.D.; Borges, A.C.; Braga, A.F.; Veloso, R.W.; de Matos, A.T. Phytoremediation of Arsenic-Contaminated Water by Lemna Valdiviana: An Optimization Study. Chemosphere 2019, 234, 402–408. [Google Scholar] [CrossRef]
- Kumar, U.; Singh, R.S.; Mandal, J.; Nayak, A.K.; Jha, A.K. Removal of As (III) and Cr (VI) from Aqueous Solutions by Bixa Orellana Leaf Biosorbent and As (III) Removal Using Bacterial Isolates from Heavy Metal Contaminated Site. J. Indian Chem. Soc. 2022, 99, 100334. [Google Scholar] [CrossRef]
- Hernández, A.; Loera, N.; Contreras, M.; Fischer, L.; Sánchez, D. Comparison Between Lactuca sativa L. and Lolium perenne: Phytoextraction Capacity of Ni, Fe, and Co from Galvanoplastic Industry. In Energy Technology 2019: Carbon Dioxide Management and Other Technologies; Springer: Cham, Switzerland, 2019; pp. 137–147. [Google Scholar]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of Heavy Metal Contaminated Soils: Phytoremediation as a Potentially Promising Clean-up Technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Gerwing, P.D.; Greenberg, B.M. Opinion: Taking Phytoremediation from Proven Technology to Accepted Practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From Phytoremediation of Soil Contaminants to Phytomanagement of Ecosystem Services in Metal Contaminated Sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic Phytovolatilization and Epigenetic Modifications in Arundo Donax L. Assisted by a PGPR Consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef]
- Ely, C.S.; Smets, B.F. Bacteria from Wheat and Cucurbit Plant Roots Metabolize PAHs and Aromatic Root Exudates: Implications for Rhizodegradation. Int. J. Phytoremediation 2017, 19, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Zorrig, W.; Rabhi, M.; Ferchichi, S.; Smaoui, A.; Abdelly, C. Phytodesalination: A Solution for Salt-Affected Soils in Arid and Semi-Arid Regions. J. Arid Land Stud. 2012, 22, 299–302. [Google Scholar]
- Sakai, Y.; Ma, Y.; Xu, C.; Wu, H.; Zhu, W.; Yang, J. Phytodesalination of a Salt-Affected Soil with Four Halophytes in China. J. Arid Land Stud. 2012, 22, 17–20. [Google Scholar]
- Ravindran, K.C.; Venkatesan, K.; Balakrishnan, V.; Chellappan, K.P.; Balasubramanian, T. Restoration of Saline Land by Halophytes for Indian Soils. Soil Biol. Biochem. 2007, 39, 2661–2664. [Google Scholar] [CrossRef]
- Yan, Y.-Y.; Yang, B.; Lan, X.-Y.; Li, X.-Y.; Xu, F.-L. Cadmium Accumulation Capacity and Resistance Strategies of a Cadmium-Hypertolerant Fern—Microsorum Fortunei. Sci. Total Environ. 2019, 649, 1209–1223. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Zuo, Y.; Li, Y.; Qie, X.; Zhang, X.; Essmat, N.; Wu, W.; Hu, Z. Transcriptome Analysis of Plantago Major as a Phytoremediator to Identify Some Genes Related to Cypermethrin Detoxification. Environ. Sci. Pollut. Res. 2021, 28, 5101–5115. [Google Scholar] [CrossRef]
- Willscher, S.; Jablonski, L.; Fona, Z.; Rahmi, R.; Wittig, J. Phytoremediation Experiments with Helianthus Tuberosus under Different PH and Heavy Metal Soil Concentrations. Hydrometallurgy 2017, 168, 153–158. [Google Scholar] [CrossRef]
- Bastos, E.; Schneider, M.; de Quadros, D.P.C.; Welz, B.; Batista, M.B.; Horta, P.A.; Rörig, L.R.; Barufi, J.B. Phytoremediation Potential of Ulva Ohnoi (Chlorophyta): Influence of Temperature and Salinity on the Uptake Efficiency and Toxicity of Cadmium. Ecotoxicol. Environ. Saf. 2019, 174, 334–343. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Hussain, B.; Gurajala, H.K.; Yang, X. Characterization of Fava Bean (Vicia faba L.) Genotypes for Phytoremediation of Cadmium and Lead Co-Contaminated Soils Coupled with Agro-Production. Ecotoxicol. Environ. Saf. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- DalCorso, G.; Martini, F.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Enhancement of Zn Tolerance and Accumulation in Plants Mediated by the Expression of Saccharomyces Cerevisiae Vacuolar Transporter ZRC1. Planta 2021, 253, 117. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, X.; Wang, Z.; Pei, C.; Cao, M.; Luo, J. Influence of Planting Density on the Phytoremediation Efficiency of Festuca Arundinacea in Cd-Polluted Soil. Bull. Environ. Contam. Toxicol. 2021, 107, 154–159. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Hayat, K.; Zhang, D.; Zhou, P. Small Structures with Big Impact: Multi-Walled Carbon Nanotubes Enhanced Remediation Efficiency in Hyperaccumulator Solanum nigrum L. under Cadmium and Arsenic Stress. Chemosphere 2021, 276, 130130. [Google Scholar] [CrossRef]
- Manori, S.; Shah, V.; Soni, V.; Dutta, K.; Daverey, A. Phytoremediation of Cadmium-Contaminated Soil by Bidens pilosa L.: Impact of Pine Needle Biochar Amendment. Environ. Sci. Pollut. Res. 2021, 28, 58872–58884. [Google Scholar] [CrossRef]
- He, L.; Zhu, Q.; Wang, Y.; Chen, C.; He, M.; Tan, F. Irrigating Digestate to Improve Cadmium Phytoremediation Potential of Pennisetum Hybridum. Chemosphere 2021, 279, 130592. [Google Scholar] [CrossRef]
- Li, Y.; Xie, T.; Zha, Y.; Du, W.; Yin, Y.; Guo, H. Urea-Enhanced Phytoremediation of Cadmium with Willow in Pyrene and Cadmium Contaminated Soil. J. Hazard. Mater. 2021, 405, 124257. [Google Scholar] [CrossRef]
- Chen, L.; Beiyuan, J.; Hu, W.; Zhang, Z.; Duan, C.; Cui, Q.; Zhu, X.; He, H.; Huang, X.; Fang, L. Phytoremediation of Potentially Toxic Elements (PTEs) Contaminated Soils Using Alfalfa (Medicago sativa L.): A Comprehensive Review. Chemosphere 2022, 293, 133577. [Google Scholar] [CrossRef]
- Desai, M.; Haigh, M.; Walkington, H. Phytoremediation: Metal Decontamination of Soils after the Sequential Forestation of Former Opencast Coal Land. Sci. Total Environ. 2019, 656, 670–680. [Google Scholar] [CrossRef]
- Mench, M.; Schwitzguébel, J.-P.; Schroeder, P.; Bert, V.; Gawronski, S.; Gupta, S. Assessment of Successful Experiments and Limitations of Phytotechnologies: Contaminant Uptake, Detoxification and Sequestration, and Consequences for Food Safety. Environ. Sci. Pollut. Res. 2009, 16, 876–900. [Google Scholar] [CrossRef]
- Wiangkham, N.; Prapagdee, B. Potential of Napier Grass with Cadmium-Resistant Bacterial Inoculation on Cadmium Phytoremediation and Its Possibility to Use as Biomass Fuel. Chemosphere 2018, 201, 511–518. [Google Scholar] [CrossRef]
- Kovacs, H.; Szemmelveisz, K. Disposal Options for Polluted Plants Grown on Heavy Metal Contaminated Brownfield Lands—A Review. Chemosphere 2017, 166, 8–20. [Google Scholar] [CrossRef]
- Al Chami, Z.; Amer, N.; Smets, K.; Yperman, J.; Carleer, R.; Dumontet, S.; Vangronsveld, J. Evaluation of Flash and Slow Pyrolysis Applied on Heavy Metal Contaminated Sorghum Bicolor Shoots Resulting from Phytoremediation. Biomass Bioenergy 2014, 63, 268–279. [Google Scholar] [CrossRef]
- Kovacs, H.; Szemmelveisz, K.; Palotas, A.B. Solubility Analysis and Disposal Options of Combustion Residues from Plants Grown on Contaminated Mining Area. Environ. Sci. Pollut. Res. 2013, 20, 7917–7925. [Google Scholar] [CrossRef]
- Samsuri, A.W.; Tariq, F.S.; Karam, D.S.; Aris, A.Z.; Jamilu, G. The Effects of Rice Husk Ashes and Inorganic Fertilizers Application Rates on the Phytoremediation of Gold Mine Tailings by Vetiver Grass. Appl. Geochem. 2019, 108, 104366. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Bai, S.H.; Zhang, Y.; Teng, Y.; Xu, Z. Assisted Phytoremediation of a Co-Contaminated Soil with Biochar Amendment: Contaminant Removals and Bacterial Community Properties. Geoderma 2019, 348, 115–123. [Google Scholar] [CrossRef]
- Drozdova, I.; Alekseeva-Popova, N.; Dorofeyev, V.; Bech, J.; Belyaeva, A.; Roca, N. A Comparative Study of the Accumulation of Trace Elements in Brassicaceae Plant Species with Phytoremediation Potential. Appl. Geochem. 2019, 108, 104377. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on Growth, Physiological Response, Cd Subcellular Distribution and Chemical Forms of Koelreuteria Paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Zhang, X.; Su, C.; Liu, X.; Liu, Z.; Liang, X.; Zhang, Y.; Feng, Y. Effect of Plant-Growth-Promoting Rhizobacteria on Phytoremediation Efficiency of Scirpus Triqueter in Pyrene-Ni Co-Contaminated Soils. Chemosphere 2020, 241, 125027. [Google Scholar] [CrossRef]
- Pena, M.S.B.; Rollins, A. Environmental Exposures and Cardiovascular Disease: A Challenge for Health and Development in Low-and Middle-Income Countries. Cardiol. Clin. 2017, 35, 71–86. [Google Scholar] [CrossRef]
- Singh, H.; Pant, G. Phytoremediation: Low Input-Based Ecological Approach for Sustainable Environment. Appl. Water Sci. 2023, 13, 85. [Google Scholar] [CrossRef]



| S. No. | Plant Species | Contaminants Removed | Ref. |
|---|---|---|---|
| 1 | Brassica juncea L. | Cd, Cu, Zn | [57,58,59] |
| 2 | Populus sp. | Cd | [60] |
| 3 | Helianthus annuus | Zn | [61] |
| 4 | Melica jacquemontii Poaceae | Fe | [62] |
| 5 | Medicago sativa, Brassica nigra | Pb | [63] |
| 6 | Eleocharis acicularis | Cu | [64] |
| 7 | Lemna minor | Pb, Cd, Ni, Cr | [65] |
| 8 | Brassica rapa L. | U | [66] |
| 9 | Alyssum murale, Berkheya coddii | Ni | [67] |
| 10 | Azolla filiculoides | Hg (II), Pb (II) | [68] |
| 11 | Jatropha curcas | Al, Cd, Fe, Cr, Pb, Zn, Ni, Cu | [69] |
| 12 | Viola bashanensis | Zn | [70] |
| 13 | Aeollanthus subacaulis | Cu | [71] |
| 14 | Oryza sativa | Cd, Zn, Fe, Cu, Pb, Cr, Mn | [72,73] |
| 15 | Schima superba | Mn | [74] |
| Plants | Target Medium | Inducing Factor | Observation | Ref. |
|---|---|---|---|---|
| Helianthus tuberosus | Soil | Based on metal and its concentration and pH | Helianthus tuberosus showed adequate growth in the presence of minor metal fixations and a pH range of 5 to 6. With the addition of metals in the soil at pH 5, the grouping of metals in shoots grew. | [90] |
| Lemna valdiviana | Water | pH (3.94–9.02) P (0–0.14 mmol·L−1) N (0.09–13.71 mmol·L−1) | Lemna valdiviana collect more significant amounts of As (1190 mg kg−1) when the pH is between 6.30 and 9, the P concentration is 0.05 mmol L−1, and the N concentration is 7.90 mmol L−1. | [77] |
| Ulva ohnoi | Water | Salinity and temperature | Ulva ohnoi continued to develop favorably between 18 and 25 °C, S35. The focus factor with the highest value was 81.30% of Cd added at 0.63 gL−1 to 18 C and S15. | [91] |
| Vicia faba L. | Soil | Genotype | According to all indications, the genotype LXYC was the most suitable one for phytoremediation in soil that had been moderately or slightly depleted in Pb and Cd. | [92] |
| Arabidopsis thaliana and Populus alba | Soil/water | Genetic modification | Plants that communicate with ScZRC1, such as poplar and A. thaliana, could accumulate more Zn. | [93] |
| Festuca arundinacea | Soil | Planting density | At D20, the biomass and Cd accumulation peaked (13.30 g Cd m−2). | [94] |
| Solanum nigrum L. | Soil | Carbon nanotubes with multiple walls | At 5.23% to 27.97%, multi-walled carbon nanotubes could increase plant biomass. | [95] |
| Bidens pilosa L. | Soil | Activated carbon (biochar) | Under treatments containing 100 and 200 mg kg−1 biochar, respectively, the accumulation of Cd increased by 16.44 and 39.37%. | [96] |
| Pennisetum hybridum | Soil | Digestate | The growth in digestate could increase compact Cd fixation in the stems (7.94–42.39%), leaves (12.53–74.11%), and roots (18.59–57.94%). | [97] |
| Willow | Soil | Urea | Under the treatment with Cd and urea, the individual Cd convergences of the roots, xylem, bark, and leaves were 8.30, 8.15, 26.79, and 33.04 mg kg−1. | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. https://doi.org/10.3390/toxics11050422
Priya AK, Muruganandam M, Ali SS, Kornaros M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics. 2023; 11(5):422. https://doi.org/10.3390/toxics11050422
Chicago/Turabian StylePriya, A. K., Muthiah Muruganandam, Sameh S. Ali, and Michael Kornaros. 2023. "Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach" Toxics 11, no. 5: 422. https://doi.org/10.3390/toxics11050422
APA StylePriya, A. K., Muruganandam, M., Ali, S. S., & Kornaros, M. (2023). Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics, 11(5), 422. https://doi.org/10.3390/toxics11050422








