Transcriptomic Sequencing Analysis on Key Genes and Pathways Regulating Cadmium (Cd) in Ryegrass (Lolium perenne L.) under Different Cadmium Concentrations
Abstract
1. Introduction
2. Results
2.1. Effects of Cd Exposure on Ryegrass Growth and the Physiological Properties
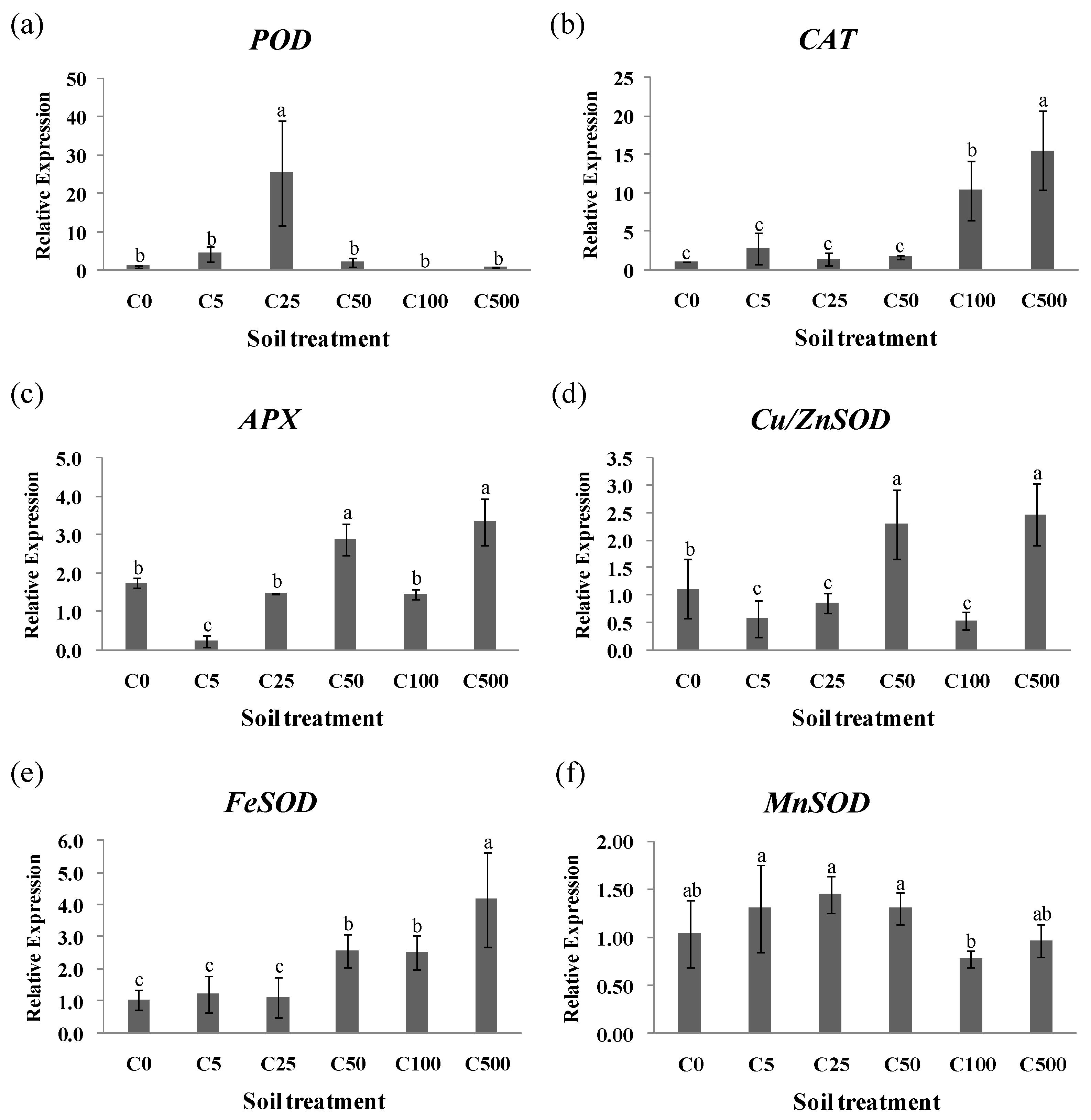
2.2. Responses of Antioxidant Enzymes of Ryegrass under Cd Stress
2.3. Transcriptional Changes of Ryegrass under Cd Stress
2.4. Validation of DEGs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Design
5.2. Physicochemical Characteristics Analysis
5.3. RNA Extraction
5.4. Gene Expression of Antioxidant Enzymes
5.5. Illumina Sequencing
5.6. Differential Expression Genes and Functional Enrichment Analysis
5.7. Quantitative Real-time PCR Validation
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev. Environ. Contam. Toxicol. 2016, 241, 73–137. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.H.; Zhang, Z.Q. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals: Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Luan, Y.N. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metal-polluted water. Int. J. Environ. Res. Public Health 2015, 12, 14958–14973. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wang, Y.J.; Khan, M.A.; Luo, W.X.; Xiang, Z.C.; Xu, W.J.; Zhong, B.; Ma, J.W.; Ye, Z.Q.; Zhu, Y.W.; et al. Effect of plant extracts and citric acid on phytoremediation of metal-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 211, 111902. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Arienzo, M.; Adamo, P.; Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 2004, 319, 13–25. [Google Scholar] [CrossRef]
- Luo, H.J.; Li, H.Y.; Zhang, X.Z.; Fu, J.M. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 2011, 20, 770–778. [Google Scholar] [CrossRef]
- Lee, J.M.; Roche, J.R.; Donaghy, D.J.; Thrush, A.; Sathish, P. Validation of reference genes for quantitative RT-PCR studies of gene expression in perennial ryegrass (Lolium perenne L.). BMC Mol. Biol. 2010, 11, 8. [Google Scholar] [CrossRef]
- Huang, L.K.; Yan, H.D.; Jiang, X.M.; Yin, G.H.; Zhang, X.Q.; Qi, X.; Zhang, Y.; Yan, Y.H.; Ma, X.; Peng, Y. Identification of candidate reference genes in perennial ryegrass for quantitative RT-PCR under various abiotic stress Conditions. PLoS ONE 2014, 9, e93724. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Hu, T.; Fu, J.M. Identification of genes associated with adaptation to NaCl toxicity in perennial ryegrass (Lolium perenne L.). Ecotoxicol. Environ. Saf. 2012, 79, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.D.; Byrne, S.; Paina, C.; Asp, T. De Novo assembly of the perennial ryegrass transcriptome using an RNA-Seq strategy. PLoS ONE 2013, 9, e103567. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Choudhary, S.; Bhardwaj, P. Comparative transcriptome profiling under cadmium stress reveals the uptake and tolerance mechanism in Brassica juncea. J. Plant. Growth Regul. 2019, 38, 1141–1152. [Google Scholar] [CrossRef]
- Qiao, K.; Liang, S.; Wang, F.H.; Wang, H.; Hu, Z.L.; Chai, T.Y. Effects of cadmium toxicity on diploid wheat (Triticum urartu) and the molecular mechanism of the cadmium response. J. Hazard. Mater. 2019, 374, 1–10. [Google Scholar] [CrossRef]
- Wang, K.H.; Liu, Y.R.; Tian, J.L.; Huang, K.Y.; Shi, T.R.; Dai, X.X.; Zhang, W.J. Transcriptional profiling and identification of heat-responsive genes in perennial ryegrass by RNA-Sequencing. Front. Plant. Sci. 2017, 8, 1032. [Google Scholar] [CrossRef]
- Li, Y.H.; Qin, Y.L.; Xu, W.H.; Chai, Y.R.; Li, T.; Zhang, C.L.; Yang, M.; He, Z.M.; Feng, D.Y. Differences of Cd uptake and expression of MT family genes and NRAMP2 in two varieties of ryegrasses. Environ. Sci. Pollut. Res. 2019, 26, 13738–13745. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhang, Y.F.; He, Y.; Cao, Q.Q.; Zhang, T.; Lou, L.Q.; Cai, Q.S. Full-length transcriptome assembly of Italian ryegrass root integrated with RNA-Seq to identify genes in response to plant cadmium stress. Int. J. Mol. Sci. 2020, 21, 1067. [Google Scholar] [CrossRef]
- Salama, A.K.; Osman, K.A.; Gouda, N.A. Remediation of lead and cadmium-contaminated soils. Int. J. Phytoremediation 2016, 18, 364–367. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, F.Z.; Xu, W.W.; Ren, J.H.; Chen, S.H.; Shen, K.; Long, Z. Enhanced phytoextraction for co-contaminated soil with Cd and Pb by ryegrass (Lolium perenne L.). Bull. Environ. Contam. Toxicol. 2019, 103, 147–154. [Google Scholar] [CrossRef]
- Li, F.L.; Qiu, Y.H.; Xu, X.Y.; Yang, F.; Wang, Z.W.; Feng, J.R.; Wang, J.D. EDTA-enhanced phytoremediation of heavy metals from sludge soil by Italian ryegrass (Lolium perenne L.). Ecotoxicol. Environ. Saf. 2020, 191, 110185. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Zhu, T.T.; Li, L.Y.; Duan, Q.X.; Liu, X.L.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant. Signal. Behav. 2021, 16, 1836884. [Google Scholar] [CrossRef]
- Jia, H.; Hou, D.Y.; O’Connor, D.; Pan, S.Z.; Zhu, J.; Bolan, N.S.; Mulder, J. Exogenous phosphorus treatment facilitates chelation-mediated cadmium detoxification in perennial ryegrass (Lolium perenne L.). J. Hazard. Mater. 2020, 389, 121849. [Google Scholar] [CrossRef]
- Bidar, G.; Pruvot, C.; Garçon, G.; Verdin, A.; Douay, S.F. Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environ. Sci. Pollut. Res. 2009, 16, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Ci, D.W.; Jiang, D.; Dai, T.B.; Jing, Q.; Cao, W.X. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef]
- Pawlak, S.; Firych, A.; Rymer, K.; Deckert, J. Cu, Zn-superoxide dismutase is differently regulated by cadmium and lead in roots of soybean seedlings. Acta Physiol. Plant 2009, 31, 741–747. [Google Scholar] [CrossRef]
- Lu, J.J.; Ma, Y.L.; Xing, G.L.; Li, W.L.; Kong, X.X.; Li, J.Y.; Wang, L.J.; Yuan, H.L.; Yang, J.S. Revelation of microalgae’s lipid production and resistance mechanism to ultra-high Cd stress by integrated transcriptome and physiochemical analyses. Environ. Pollut. 2019, 250, 186–195. [Google Scholar] [CrossRef]
- Ullah, S.; Hadi, F.; Ali, N.; Khan, S. Foliar Application of iron (Fe) improved the antioxidant defense and Cd accumulation potential of Ricinus communis under hydroponic condition. Water Air Soil Poll. 2018, 229, 284. [Google Scholar] [CrossRef]
- Huang, H.L.; Rizwan, M.; Li, M.; Song, F.R.; Zhou, S.J.; He, X.; Ding, R.; Dai, Z.H.; Yuan, Y.; Cao, M.H.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, T.; Gustot, Q.; Couder, E.; Houben, D.; Iserentant, A.; Lutts, S. Comparison of EDTA-enhanced phytoextraction and phytostabilisation strategies with Lolium perenne on a heavy metal contaminated soil. Chemosphere 2011, 85, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.L.; Nagy, I.; Pfeifer, M.; Armstead, I.; Swain, S.; Studer, B.; Mayer, K.; Campbell, J.D.; Czaban, A.; Hentrup, S.; et al. A synteny-based draft genome sequence of the forage grass Lolium perenne. Plant J. 2015, 84, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Yang, X.Y.; Yang, Z.F.; Zhong, M.Y.; Zhu, Y.Q.; Zhou, J.; Appiah, C.; Liao, Z.C.; Feng, G.Y.; Zhang, X.Q. Genome-wide investigation of the NAC transcript factor family in perennial ryegrass (Lolium perenne L.) and expression analysis under various abiotic stressor. Genomics 2020, 112, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Pakdee, O.; Songnuan, W.; Panvisavas, N.; Pokethitiyook, P.; Yokthongwattana, K.; Meetam, M. Functional characterization of metallothionein-like genes from Physcomitrella patens: Expression profiling, yeast heterologous expression, and disruption of PpMT1.2a gene. Planta 2019, 250, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Manara, A. Plant responses to heavy metal toxicity. In Plants and Heavy Metals; Springer: Dordrecht, The Netherlands, 2012; pp. 27–53. [Google Scholar]
- Samiksha, S.; Parul, P.; Rachana, S.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics and ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Domínguez-Solís, J.R.; Gutierrez-Alcala, G.; Romero, L.C.; Gotor, C. The cytosolic O-Acetylserine(thiol)lyase gene is regulated by heavy metals and can function in cadmium tolerance. J. Biol. Chem. 2001, 276, 9297–9302. [Google Scholar] [CrossRef]
- Chi, S.L.; Qin, Y.L.; Xu, W.H.; Chai, Y.R.; Feng, D.Y.; Li, Y.H.; Li, T.; Yang, M.; He, Z.M. Differences of Cd uptake and expression of OAS and IRT genes in two varieties of ryegrasses. Environ. Sci. Pollut. Res. 2019, 26, 13717–13724. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.B.; Fei, C.; Sun, H.Y.; Zhang, G.P.; Chen, Z.H.; Wu, F.B. Genome-wide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genom. 2014, 15, 611. [Google Scholar] [CrossRef] [PubMed]
- Cruz, Y.; Villar, S.; Gutiérrez, K.; Montoya-Ruiz, C.; Gallego, J.L.; Delgado, M.D.P.; Saladrriaga, J.F. Gene expression and morphological responses of Lolium perenne L. exposed to cadmium (Cd2+) and mercury (Hg2+). Sci. Rep. 2021, 11, 11257. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, X.Z.; Huang, J.J.; Ding, Y.; Wu, J.M.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L.P. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, 316–322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


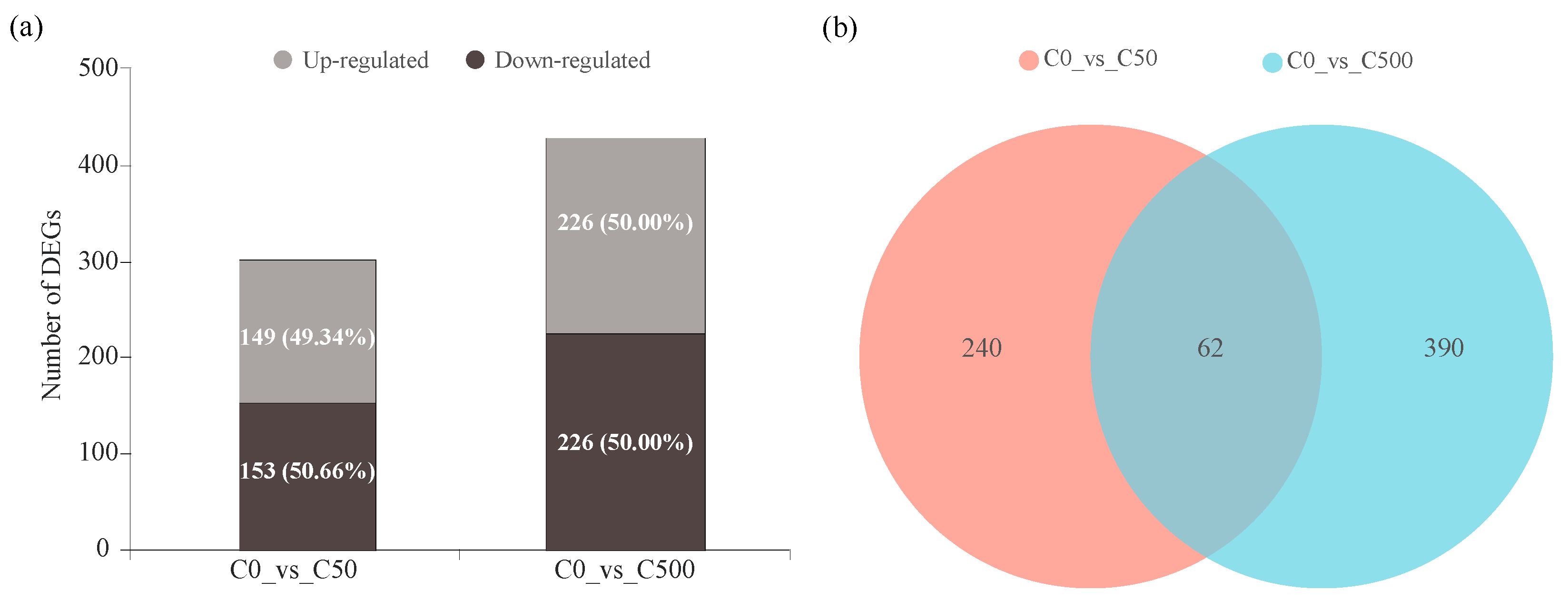
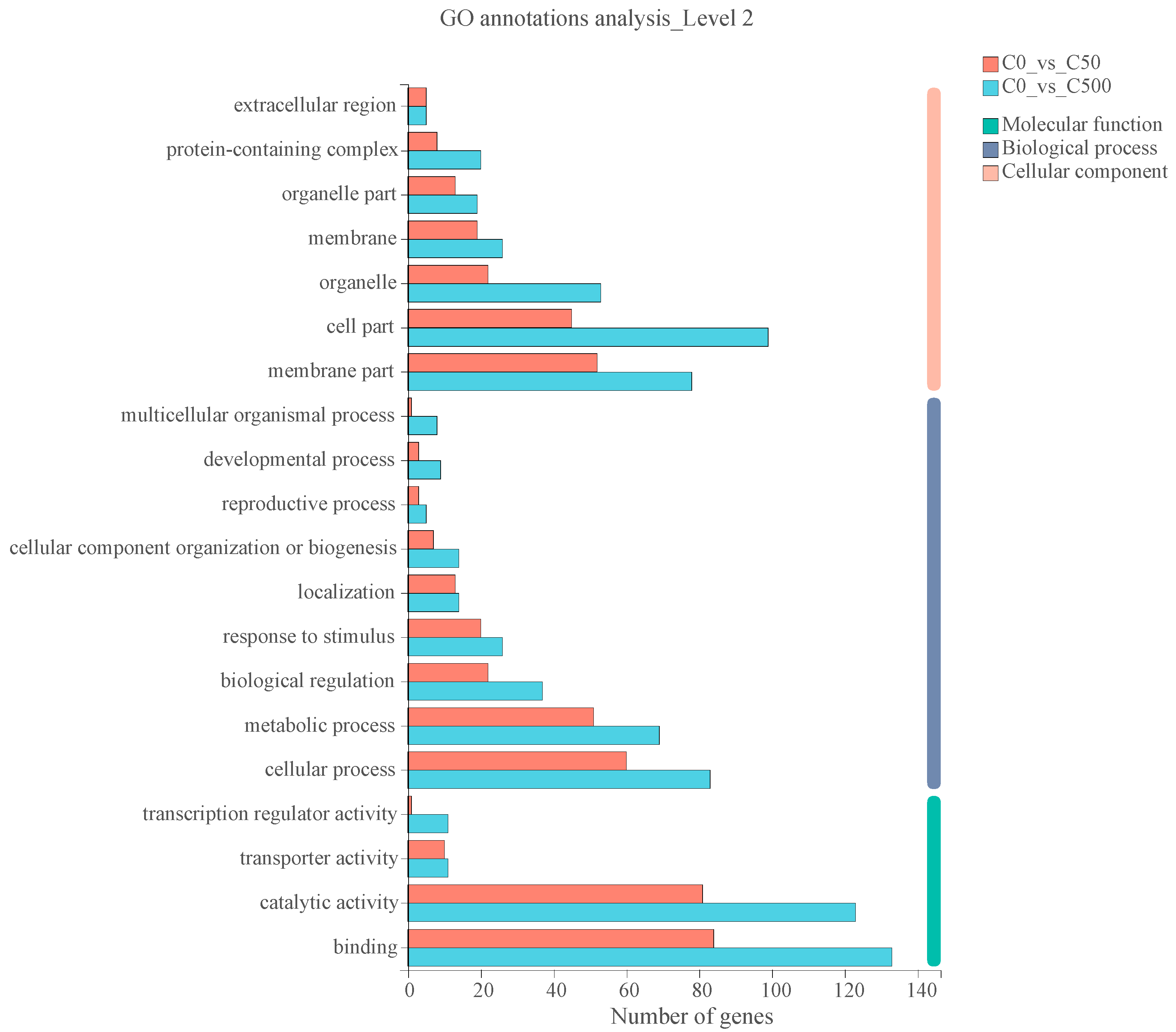

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Liu, C.; Hu, C.; Liang, S. Transcriptomic Sequencing Analysis on Key Genes and Pathways Regulating Cadmium (Cd) in Ryegrass (Lolium perenne L.) under Different Cadmium Concentrations. Toxics 2022, 10, 734. https://doi.org/10.3390/toxics10120734
Cui B, Liu C, Hu C, Liang S. Transcriptomic Sequencing Analysis on Key Genes and Pathways Regulating Cadmium (Cd) in Ryegrass (Lolium perenne L.) under Different Cadmium Concentrations. Toxics. 2022; 10(12):734. https://doi.org/10.3390/toxics10120734
Chicago/Turabian StyleCui, Bingjian, Chuncheng Liu, Chao Hu, and Shengxian Liang. 2022. "Transcriptomic Sequencing Analysis on Key Genes and Pathways Regulating Cadmium (Cd) in Ryegrass (Lolium perenne L.) under Different Cadmium Concentrations" Toxics 10, no. 12: 734. https://doi.org/10.3390/toxics10120734
APA StyleCui, B., Liu, C., Hu, C., & Liang, S. (2022). Transcriptomic Sequencing Analysis on Key Genes and Pathways Regulating Cadmium (Cd) in Ryegrass (Lolium perenne L.) under Different Cadmium Concentrations. Toxics, 10(12), 734. https://doi.org/10.3390/toxics10120734







