Unknown Organofluorine Mixtures in U.S. Adult Serum:Contribution from Pharmaceuticals?
Abstract
1. Introduction
2. Materials and Methods
2.1. Organofluorine Pharmaceutical Selection
2.2. Pharmacokinetic Estimates
2.3. Serum Procurement and Sampling Protocol
2.4. Sample Handling
2.5. Sample Extraction
2.6. Instrumental Analysis and Quantification
2.7. Organofluorine Mass Balance
2.8. Statistical Analysis
3. Results
3.1. Pharmacokinetic Estimates of Serum Fluorine
3.2. Characteristics of Study Serum Donors and PFAS Concentrations
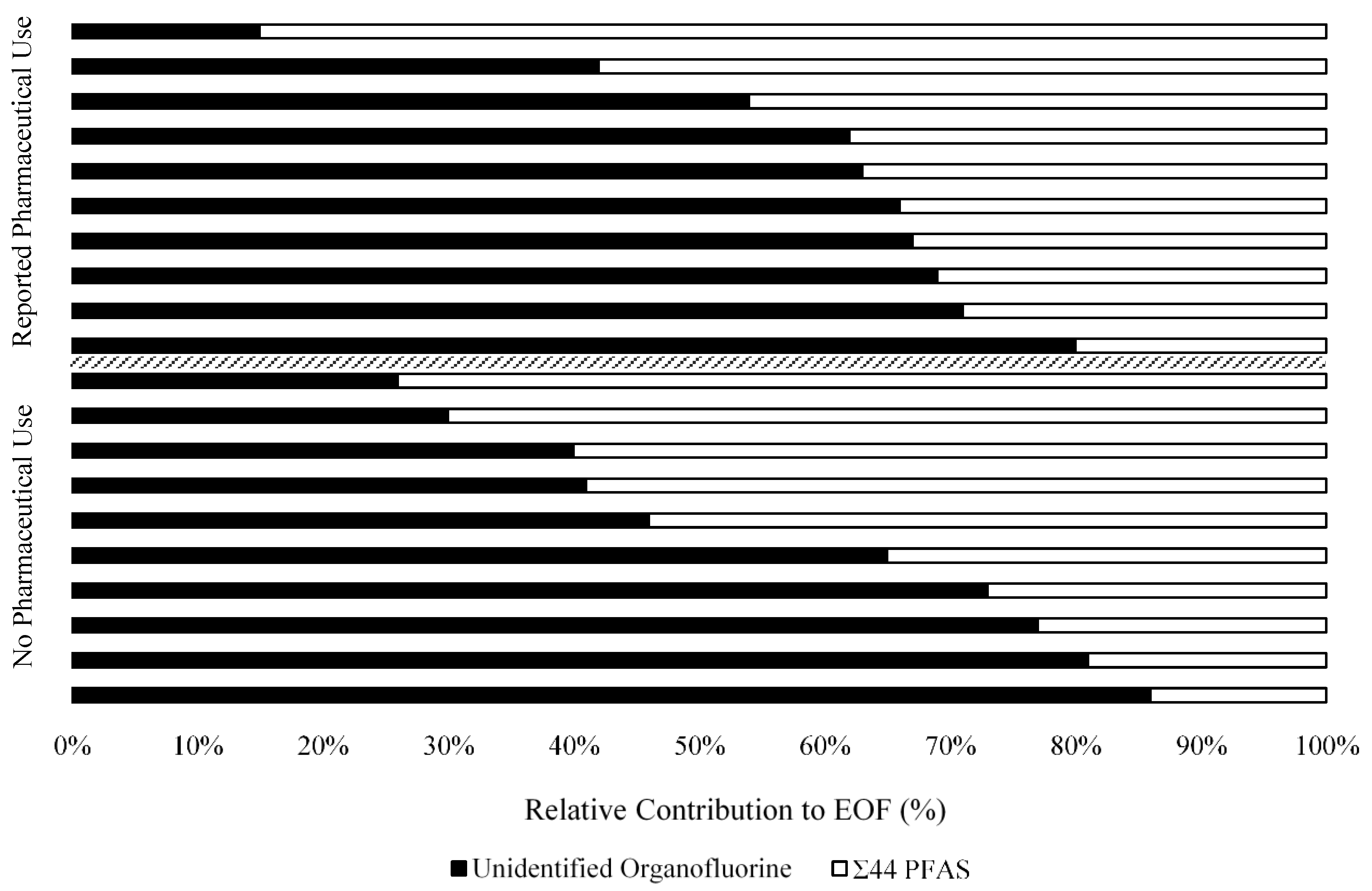
3.3. Extractable Organofluorine in Serum
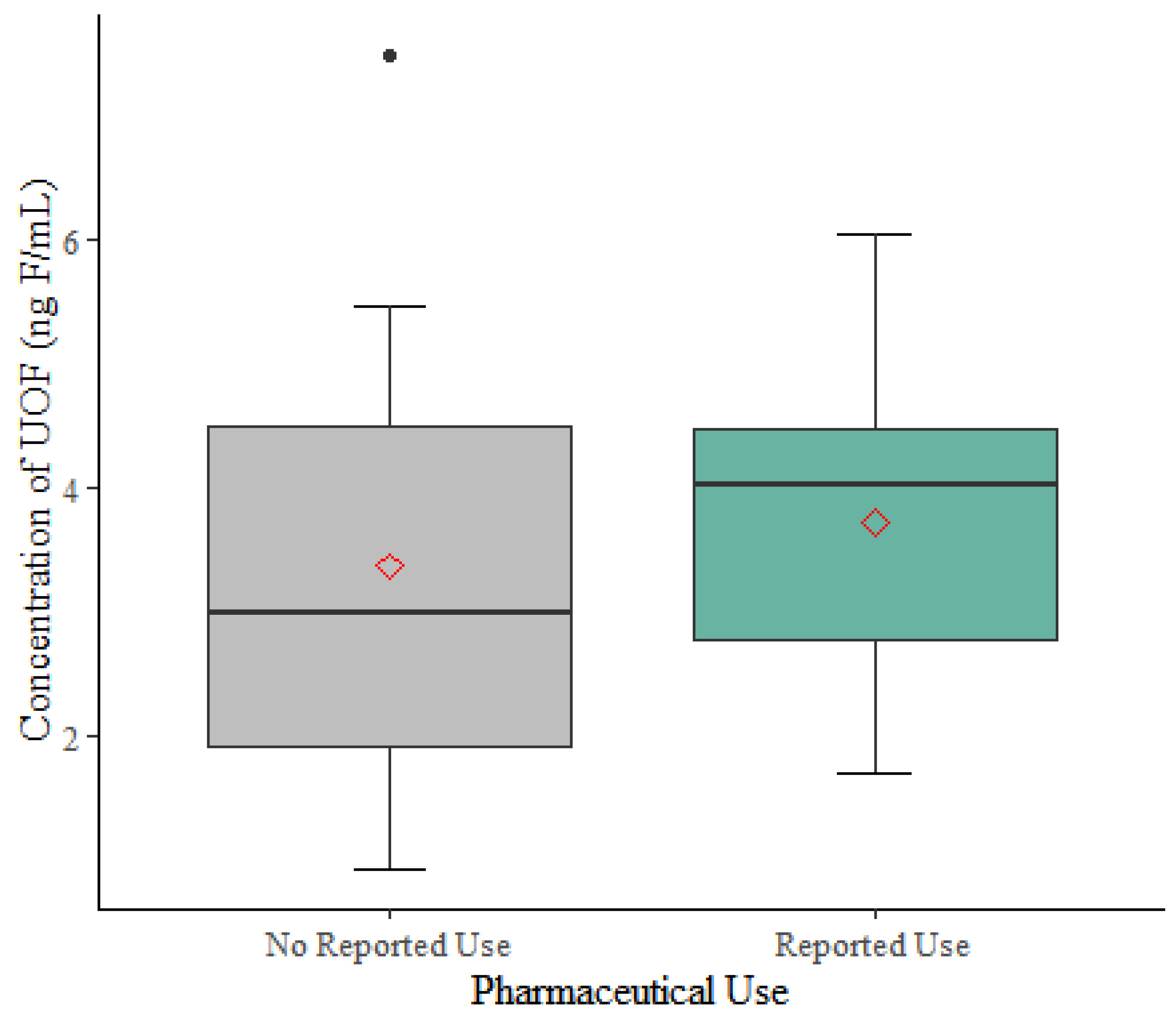
3.4. Unexplained Organofluorine in Serum
3.5. Linear Regression of UOF on Pharmaceutical Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, N.M.; Minucci, K.M.; Mullikin, A.; Slover, R.; Cohen Hubal, E.A. Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: A systematic review. Environ. Int. 2022, 162. [Google Scholar] [CrossRef]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 2008, 28, 2. [Google Scholar] [CrossRef]
- Strynar, M.; Lindstrom, A.B. Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ. Sci. Technol. 2008, 42, 3751–3756. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Early Release: Per- and Polyfluorinated Substances (PFAS) Tables, NHANES 2011-2018, Dec. 16. 2021. Available online: https://www.cdc.gov/exposurereport/pfas_early_release.html (accessed on 7 February 2022).
- Grandjean, P.; Budtz-Jørgensen, E. Immunotoxicity of perfluorinated alkylates: Calculation of benchmark doses based on serum concentrations in children. Environ. Heal. 2013, 12, 35. [Google Scholar] [CrossRef]
- NTP. Toxicity Report Tables & Curves: Perfluorinated Compounds: Carboxylates; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- NTP. Toxicity Report Tables & Curves: Perfluorinated Compounds: Sulfonates; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Blossom, S.J.; Schaider, L.A. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: Epidemiological and toxicological evidence. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 148–156. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef]
- Nelson, J.W.; Hatch, E.E.; Webster, T.F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010, 118, 197–202. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Biomonitoring Summary | CDC. 2017. Available online: https://www.cdc.gov/biomonitoring/PFAS_BiomonitoringSummary.html (accessed on 22 October 2022).
- USEPA. CompTox Chemicals Dashboard, PFASMASTER Chemical List. 2021. Available online: https://comptox.epa.gov/dashboard/chemical_lists/PFASMASTER (accessed on 13 August 2021).
- OECD. Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance. Available online: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/terminology-per-and-polyfluoroalkyl-substances.pdf (accessed on 29 July 2021).
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; DeWitt, J.C.; Knappe, D.R.U.; Maffini, M.V.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef]
- TURI. TURA Science Advisory Board. 2021. Available online: https://www.turi.org/Our_Work/Policy/Toxics_Use_Reduction_Act/Councils_and_Committees/TURA_Science_Advisory_Board (accessed on 31 October 2021).
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Library of Congress. S.1790—116th Congress (2019-2020): National Defense Authorization Act for Fiscal Year 2020, Dec. 20. 2019. Available online: https://www.congress.gov/bill/116th-congress/senate-bill/1790 (accessed on 5 March 2022).
- Hammel, E.; Webster, T.F.; Gurney, R.; Heiger-Bernays, W. Implications of PFAS Definitions Using Fluorinated Pharmaceuticals. iScience 2022, 25, 104020. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. PFAS in the US Population. 2020. Available online: https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html (accessed on 5 January 2022).
- Taves, D.R. Evidence that there are two forms of fluoride in human serum. Nature 1968, 217, 1050–1051. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Clemen, L.A.; Ellefson, M.E.; Johnson, H.O. Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 2001, 35, 766–770. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamashita, N.; So, M.K.; Rostkowski, P.; Taniyasu, S.; Lam, P.K.; Kannan, K. Trace analysis of total fluorine in human blood using combustion ion chromatography for fluorine: A mass balance approach for the determination of known and unknown organofluorine compounds. J. Chromatogr. A 2007, 1154, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.; Miyake, Y.; Taniyasu, S.; Wang, Y.; Yu, H.; So, M.K.; Jiang, G.; Wu, Y.; Li, J.; Giesy, J.P.; et al. Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China. Environ. Sci. Technol. 2008, 42, 8140–8145. [Google Scholar] [CrossRef]
- Aro, R.; Eriksson, U.; Kärrman, A.; Yeung, L.W.Y. Organofluorine Mass Balance Analysis of Whole Blood Samples in Relation to Gender and Age. Environ. Sci. Technol. 2021, 55, 13142–13151. [Google Scholar] [CrossRef]
- Miaz, L.T.; Plassmann, M.M.; Gyllenhammar, I.; Bignert, A.; Sandblom, O.; Lignell, S.; Glynn, A.; Benskin, J.P. Temporal trends of suspect-and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996-2017. Environ. Sci. Process. Impacts 2020, 22, 1071–1083. [Google Scholar] [CrossRef]
- Butt, C.M.; Muir, D.; Mabury, S.A. Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: A. review. Environ. Toxicol. Chem. 2014, 33, 243–267. [Google Scholar] [CrossRef]
- Yin, T.; Te, S.H.; Reinhard, M.; Yang, Y.; Chen, H.; He, Y.; Gin, K.Y.-H. Biotransformation of Sulfluramid (N-ethyl perfluorooctane sulfonamide) and dynamics of associated rhizospheric microbial community in microcosms of wetland plants. Chemosphere 2018, 211, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.R.; Cascella, M. Steady State Concentration; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553132/ (accessed on 7 November 2022).
- KEGG. KEGG Drug Database, New Drug Approvals in the U.S. 2021. Available online: https://www.genome.jp/kegg/drug/br08319.html (accessed on 6 August 2021).
- Agency for Healthcare Research and Quality (AHRQ). Medical Expenditure Panel Survey (MEPS) 2013–2020. 2022. Available online: https://clincalc.com/DrugStats/Top200Drugs.aspx (accessed on 7 November 2022).
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. Drugbank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef]
- Lins, R.L.; E Matthys, K.; A Verpooten, G.; Peeters, P.C.; Dratwa, M.; Stolear, J.-C.; Lameire, N.H. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 967–976. [Google Scholar] [CrossRef]
- Mayo Clinic Laboratories. Test Catalog. Mayo Foundation for Medical Education and Research. 2022. Available online: https://www.mayocliniclabs.com/test-catalog (accessed on 8 November 2022).
- Li, X.-N.; Xu, H.-R.; Chen, W.-L.; Chu, N.-N.; Zhu, J.-R. Pharmacokinetics of rosuvastatin in healthy Chinese volunteers living in China: A randomized, open-label, ascending single- and multiple-dose study. Clin. Ther. 2010, 32, 575–587. [Google Scholar] [CrossRef]
- Martin, P.D.; Mitchell, P.D.; Schneck, D.W. Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br. J. Clin. Pharmacol. 2002, 54, 472–477. [Google Scholar] [CrossRef]
- BioIVT. Biofluids (Blood-Derived), Serum. 2022. Available online: https://bioivt.com/biofluids-blood-derived/serum (accessed on 25 October 2022).
- Genuis, S.J.; Curtis, L.; Birkholz, D. Gastrointestinal Elimination of Perfluorinated Compounds Using Cholestyramine and Chlorella pyrenoidosa. ISRN Toxicol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Aro, R.; Eriksson, U.; Kärrman, A.; Jakobsson, K.; Yeung, L.W. Extractable organofluorine analysis: A way to screen for elevated per- and polyfluoroalkyl substance contamination in humans? Environ. Int. 2022, 159, 107035. [Google Scholar] [CrossRef]
- Eriksson, U.; Haglund, P.; Kärrman, A. Contribution of precursor compounds to the release of per- and polyfluoroalkyl substances (PFASs) from waste water treatment plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey Data 2017–2018. National Center for Health Statistics (NCHS). 2020. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspxBeginYear=2017 (accessed on 11 September 2022).
- Calafat, A.M.; Wong, L.-Y.; Kuklenyik, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the national health and nutrition examination survey (NHANES) 2003-2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef]
- Ruyle, B.J.; Pickard, H.M.; LeBlanc, D.R.; Tokranov, A.K.; Tchackray, C.P.; Hu, X.C.; Vecitis, C.D.; Sunderland, E.M. Isolating the AFFF signature in coastal watersheds using oxidizable PFAS precursors and unexplained organofluorine. Environ. Sci. Technol. 2021, 55, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.W.Y.; Mabury, S.A.; Mabury, S.A. Are humans exposed to increasing amounts of unidentified organofluorine? Environ. Chem. 2015, 13, 102–110. [Google Scholar] [CrossRef]
- Yeung, L.; Miyake, Y.; Wang, Y.; Taniyasu, S.; Yamashita, N.; Lam, P. Total fluorine, extractable organic fluorine, perfluorooctane sulfonate and other related fluorochemicals in liver of Indo-Pacific humpback dolphins (Sousa chinensis) and finless porpoises (Neophocaena phocaenoides) from South China. Environ. Pollut. 2009, 157, 17–23. [Google Scholar] [CrossRef]
- Schultes, L.; Vestergren, R.; Volkova, K.; Westberg, E.; Jacobson, T.; Benskin, J.P. Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: Implications for environmental emissions and human exposure. Environ. Sci. Process. Impacts 2018, 20, 1680–1690. [Google Scholar] [CrossRef]
- Schultes, L.; Peaslee, G.F.; Brockman, J.D.; Majumdar, A.; McGuinness, S.R.; Wilkinson, J.T.; Sandblom, O.; Ngwenyama, R.A.; Benskin, J.P. Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform? Environ. Sci. Technol. Lett. 2019, 6, 73–78. [Google Scholar] [CrossRef]
- MacLeod, J.; Hickman, M.; Smith, G.D. Reporting bias and self-reported drug use. Addiction 2005, 100, 562–563. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. U.S. FDA Drug Database: Prozac (Fluoxetine Hydrochloride). 2017. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=018936 (accessed on 28 October 2022).
- Kaiser, A.-M.; Aro, R.; Kärrman, A.; Weiss, S.; Hartmann, C.; Uhl, M.; Forsthuber, M.; Gundacker, C.; Yeung, L.W.Y. Comparison of extraction methods for per- and polyfluoroalkyl substances (PFAS) in human serum and placenta samples—Insights into extractable organic fluorine (EOF). Anal. Bioanal. Chem. 2020, 413, 3. [Google Scholar] [CrossRef]
- Backe, W.J.; Day, T.C.; Field, J.A. Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by nonaqueous large-volume injection HPLC-MS/MS. Environ. Sci. Technol. 2013, 47, 5226–5234. [Google Scholar] [CrossRef]
- Chappell, M.; Payne, S. Pharmacokinetics. Biosyst. Biorobotics 2002, 24, 61–72. [Google Scholar] [CrossRef]
- Pizzurro, D.M.; Seeley, M.; Kerper, L.E.; Beck, B.D. Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul. Toxicol. Pharmacol. 2019, 106, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Young, A.S.; Pickard, H.M.; Sunderland, E.M.; Allen, J.G. Organic Fluorine as an Indicator of Per- and Polyfluoroalkyl Substances in Dust from Buildings with Healthier versus Conventional Materials. Environ. Sci. Technol. 2022, 56, 17090–17099. [Google Scholar] [CrossRef] [PubMed]
- Furlong, E.T.; Batt, A.L.; Glassmeyer, S.T.; Noriega, M.C.; Kolpin, D.W.; Mash, H.; Schenck, K.M. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Sci. Total Environ. 2017, 579, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
| Pharmaceutical Information | Chemical Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Generic Name | Brand Name | Therapeutic Use | U.S. Prescriptions (2020) | Molecular Formula | Molecular Weight (g/mol) | Dissociation Constant (pka) a | #F | Fraction F | Estimated Serum Levels (ng/mL) b | Estimated Organofluorine (ng F/mL) |
| Atorvastatin | Lipitor | statin | 114,509,814 | C33H35FN2O5 | 558.65 | 4.31 | 1 | 0.034 | 2.4–11.2 d | 0.1–0.4 |
| Escitalopram | Lexapro | SSRI | 30,605,646 | C20H21FN2O | 324.40 | 9.78 | 1 | 0.059 | 15–80 e | 0.9–4.7 |
| Rosuvastatin | Crestor | Statin | 29,750,488 | C22H28FN3O6S | 481.54 | 4.00 | 1 | 0.039 | 1.8–7.4 f, g | 0.1–0.3 |
| Pantoprazole | Protonix | Proton-pump inhibitor | 26,604,040 | C16H15F2N3O4S | 383.37 | 3.92 (SB)8.19 (SA) | 2 | 0.099 | 39.9–87.9 | 4.0–8.7 |
| Fluticasone c | Flonase | intranasal corticosteroid | 24,777,490 | C22H27F3O4S | 444.51 | −3.4 (SB)13.56 (SA) | 3 | 0.128 | N/A | N/A |
| Fluoxetine | Prozac | SSRI | 23,403,050 | C17H18F3NO | 309.33 | 9.80 | 3 | 0.184 | 91–302 | 15.8–55.6 |
| Citalopram | Celexa | SSRI | 18,549,176 | C20H21FN2O | 324.40 | 9.78 | 1 | 0.059 | 50–100 e | 2.9–6.4 |
| Sitagliptin | Januvia | anti-diabetic | 9,885,657 | C16H18F6N5O5P | 407.32 | 8.78 | 6 | 0.280 | 165 | 46.3 |
| Paroxetine | Paxil | SSRI | 9,029,667 | C19H20FNO3 | 329.37 | 9.77 | 1 | 0.058 | 30–120 e | 1.7–6.9 |
| No Reported Pharma Use (n = 10) | Reported Pharma Use (n = 10) | |
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Male | 5(50) | 5(50) |
| Female | 5(50) | 5(50) |
| Race | ||
| Black | 4(40) | 4(40) |
| Hispanic | 6(60) | 6(60) |
| Age | ||
| mean (±SD) | 45 (±11.8) | 50 (±13.4) |
| median | 49 | 48.5 |
| range | 28–59 | 32–74 |
| No Reported Pharma Use (n = 10) | Reported Pharma Use (n = 10) | |
|---|---|---|
| Concentration of Σ44 PFAS (ng/mL) | ||
| mean (±SD) | 6.54 (±3.55) | 9.51 (±7.35) |
| median | 5.87 | 7.49 |
| range | 3.16–14.90 | 2.88–26.24 |
| Concentration of EOF (ng F/mL) a | ||
| mean (±SD) | 6.10 (±2.59) | 6.93 (±2.76) |
| median | 6.45 | 6.26 |
| range | 2.02–10.04 | 2.67–11.22 |
| Concentration of UOF (ng F/mL) b | ||
| mean (±SD) | 3.37 (±2.04) | 3.73 (±1.31) |
| median | 2.99 | 4.02 |
| range | 0.94–7.48 | 1.70–6.05 |
| Variable | Coefficients (95% CI) | Standard Error |
|---|---|---|
| Intercept | 2.65 (1.36 to 3.94) | 0.61 |
| Organofluorine Pharmaceutical Use | 0.36 (−1.14 to 1.85) | 0.71 |
| Age a | 1.43 (−0.06 to 2.93) | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennoyer, E.H.; Heiger-Bernays, W.; Aro, R.; Yeung, L.W.Y.; Schlezinger, J.J.; Webster, T.F. Unknown Organofluorine Mixtures in U.S. Adult Serum:Contribution from Pharmaceuticals? Toxics 2023, 11, 416. https://doi.org/10.3390/toxics11050416
Pennoyer EH, Heiger-Bernays W, Aro R, Yeung LWY, Schlezinger JJ, Webster TF. Unknown Organofluorine Mixtures in U.S. Adult Serum:Contribution from Pharmaceuticals? Toxics. 2023; 11(5):416. https://doi.org/10.3390/toxics11050416
Chicago/Turabian StylePennoyer, Emily H., Wendy Heiger-Bernays, Rudolf Aro, Leo W. Y. Yeung, Jennifer J. Schlezinger, and Thomas F. Webster. 2023. "Unknown Organofluorine Mixtures in U.S. Adult Serum:Contribution from Pharmaceuticals?" Toxics 11, no. 5: 416. https://doi.org/10.3390/toxics11050416
APA StylePennoyer, E. H., Heiger-Bernays, W., Aro, R., Yeung, L. W. Y., Schlezinger, J. J., & Webster, T. F. (2023). Unknown Organofluorine Mixtures in U.S. Adult Serum:Contribution from Pharmaceuticals? Toxics, 11(5), 416. https://doi.org/10.3390/toxics11050416






