Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review
Abstract
1. Introduction
2. Source of Water Pollution
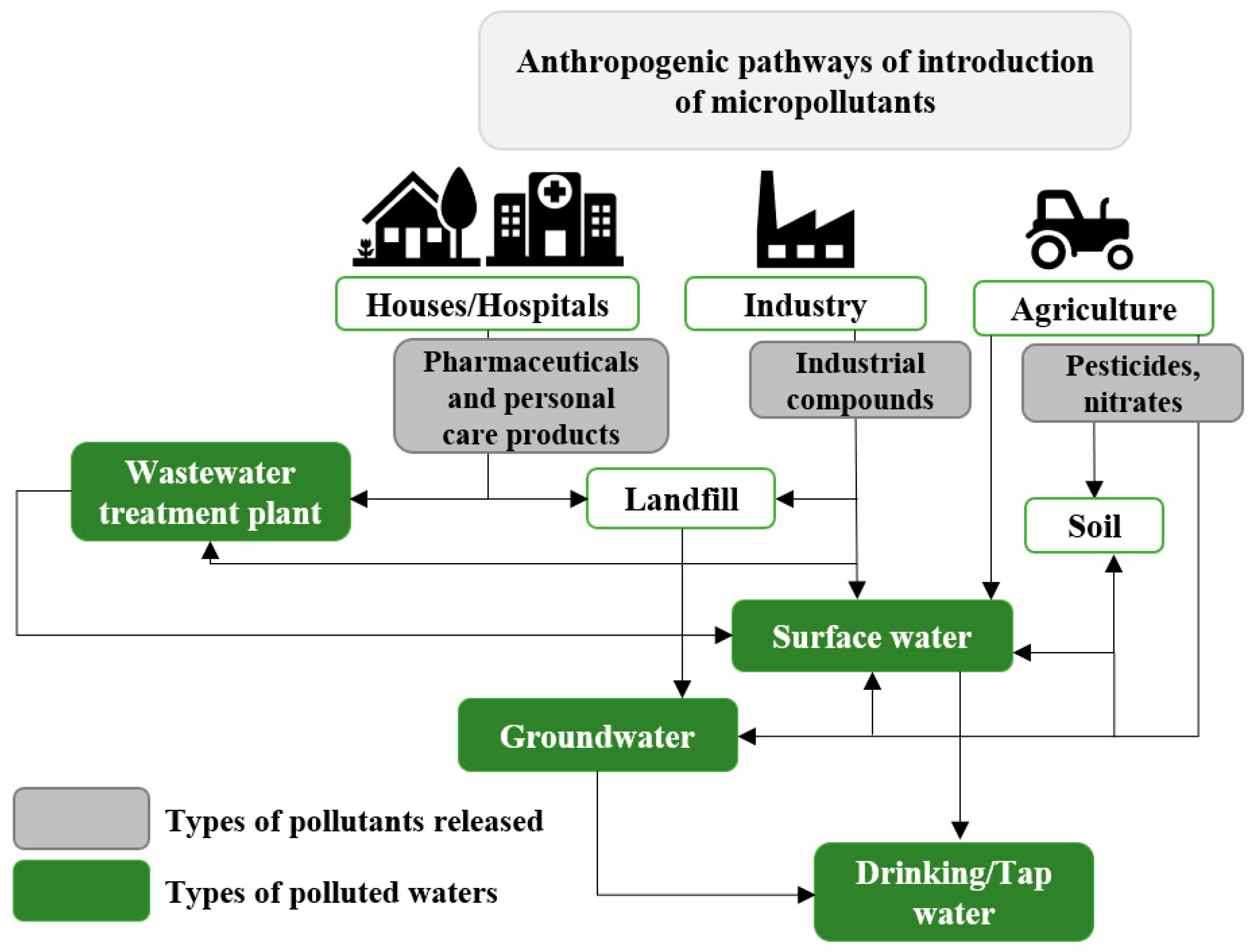
3. Toxic Effects of Pollutants
3.1. Nitrates
3.2. Dyes
3.3. PAHs, PCBs and Dioxins
3.4. Heavy Metals
3.5. Pharmaceutical and Personal Care Products
3.6. Pesticides
4. Conventional Methods of Water Treatment
| Treatment Methods | Pollutant | Sample | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Nanofiltration membrane | Dyes | Textile effluent | Effectiveness, no secondary pollution, and low energy consumption | Dependence on the membrane used | Panda et De (2015) [56] |
| Mixed matrix membranes | Heavy metals | Water | Sherugar et al. (2021) [46] | ||
| Nanofiltration membrane and reverse osmosis | Pharmaceutical compounds | Surface water | Couto et al. (2020) [47] | ||
| Chemical precipitation | Heavy metals | Water | Effectiveness, low operating cost, and simplicity | Secondary pollution | Chen et al. (2018) [49] |
| Ion exchange | Nitrate | Water | Effectiveness, low operating cost, and simplicity | Dependence on the structure of the resin and the environment of the solution | Kalaruban et al. (2016) [51] |
| Oxidation | PAHs | Water | Effectiveness, simplicity, and rapidity | Use of chemicals | Antošová et al. (2020) [53] |
| Photocatalysis + biodegradation | Dyes | Water | Low operating cost and simplicity | Establishment of a favorable environment for the development of bacteria | Waghmode et al. (2019) [55] |
| Electrochemical treatments | Heavy metals | Water | Effectiveness, low operating cost, and simplicity | Dependence on electrode materials and their high cost | Sharma et al. (2019) [54] |

5. Factors Affecting Pollutants Adsorption
5.1. Effect of Temperature
5.2. Effect of pH
5.3. Effect of Contact Time
5.4. Effect of Initial Pollutant Concentration
5.5. Effect of Initial Adsorbent Concentration
5.6. Effect of Competition with Other Pollutants
6. Kinetic and Isotherm Models
6.1. Kinetic Models
6.1.1. Adsorption Models
6.1.2. Desorption Model
- -
- Pseudo-first-orderwhere qt is the adsorption capacity (mg/g) after the contact time t, qRf is an additional parameter considering the quantity of final retained pollutant onto adsorbent at the end of the desorption process, qe (mg/g) is the amount adsorbed per mass of adsorbent at equilibrium, and is the first-order desorption rate constant (min−1) (8).
- -
- Pseudo-second-orderwhere qt is the adsorption capacity (mg/g) after the contact time t, qRf is an additional parameter considering the quantity of final retained pollutant onto adsorbent at the end of the desorption process, qe (mg/g) is the amount adsorbed per mass of adsorbent at equilibrium, and is the second-order desorption rate constant (min−1).
6.2. Isotherm Models
6.2.1. Single Pollutant
6.2.2. Multiple Pollutant
7. Adsorption Phenomena Involved in the Retention of Pollutants
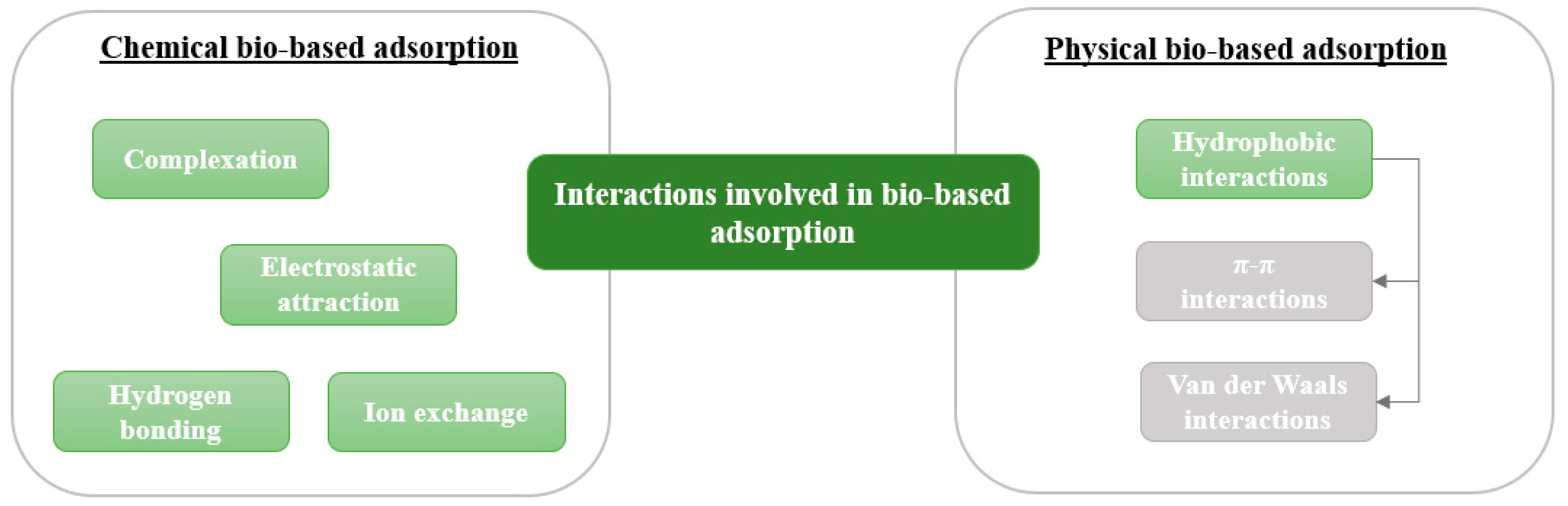
8. Bio-Based Adsorption to Remove Pollutants from Water
8.1. Agricultural Waste
8.2. Microbial Biomass
8.3. Algae
8.4. Rock and Mineral Materials
8.5. Biochar and Activated Carbon
9. Future Outlooks
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| GC-FID | Gas chromatography—flame ionization detector |
| GC–MS/MS | Gas chromatography—tandem mass spectrometry |
| HPLC | High-performance liquid chromatography |
| ICP-AES | Inductively coupled plasma atomic emission spectroscopy |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| LC-MS/MS | Liquid chromatography—tandem mass spectrometry |
| PFO | Pseudo-first order model |
| PPCPs | Pharmaceuticals and personal care products |
| PSO | Pseudo-second order model |
| Py-GC/MS | Pyrolysis–gas chromatography–mass spectrometry |
| UHPLC | Ultra high-performance liquid chromatography |
| UV-Vis | UV–visible spectrophotometry |
References
- Li, D.; Wang, M.-Q.; Lee, C. The Waste Treatment and Recycling Efficiency of Industrial Waste Processing Based on Two-Stage Data Envelopment Analysis with Undesirable Inputs. J. Clean. Prod. 2020, 242, 118279. [Google Scholar] [CrossRef]
- Speight, J.G. Sources of Water Pollution. In Natural Water Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–198. [Google Scholar] [CrossRef]
- Madhav, S.; Ahamad, A.; Singh, A.; Sharma, S.; Singh, P. Water Pollutants: Sources and Impact on the Environment and Human Health; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of Dyes from Aqueous Solution Using Fly Ash and Red Mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Yasasri, H.; de Silva, K.M.N.; de Silva, R.M. Fibrous Keratin Protein Bio Micro Structure for Efficient Removal of Hazardous Dye Waste from Water: Surface Charge Mediated Interfaces for Multiple Adsorption Desorption Cycles. Mater. Chem. Phys. 2020, 246, 122790. [Google Scholar] [CrossRef]
- Biswal, A.K. Exploring the Adsorption Efficiency of a Novel Cellulosic Material for Removal of Food Dye from Water. J. Mol. Liq. 2022, 350, 118577. [Google Scholar] [CrossRef]
- Es-sahbany, H.; El Yacoubi, A.; El Hachimi, M.L.; Boulouiz, A.; Chafik El Idrissi, B.; El Youbi, M.S. Low-Cost and Eco-Friendly Moroccan Natural Clay to Remove Many Bivalent Heavy Metal Ions: Cu2+, Co2+, Pb2+, and Ni2+. Mater. Today Proc. 2022, 58, 1162–1168. [Google Scholar] [CrossRef]
- Divband Hafshejani, L.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of Nitrate from Aqueous Solution by Modified Sugarcane Bagasse Biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, N.; Feng, C.; Hu, W. Nitrate Adsorption from Aqueous Solution Using Granular Chitosan-Fe3+ Complex. Appl. Surf. Sci. 2015, 347, 1–9. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Nazal, M.K.; Abuzaid, N. Adsorptive Removal of Polycyclic Aromatic Hydrocarbons from Contaminated Water by Biomass from Dead Leaves of Halodule Uninervis: Kinetic and Thermodynamic Studies. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Araujo, L.A.; Bezerra, C.O.; Cusioli, L.F.; Silva, M.F.; Nishi, L.; Gomes, R.G.; Bergamasco, R. Moringa Oleifera Biomass Residue for the Removal of Pharmaceuticals from Water. J. Environ. Chem. Eng. 2018, 6, 7192–7199. [Google Scholar] [CrossRef]
- Reddy, K.R.; Dastgheibi, S.; Cameselle, C. Mixed versus Layered Multi-Media Filter for Simultaneous Removal of Nutrients and Heavy Metals from Urban Stormwater Runoff. Environ. Sci. Pollut. Res. 2021, 28, 7574–7585. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Adsorption of Mixtures of Nutrients and Heavy Metals in Simulated Urban Stormwater by Different Filter Materials. J. Environ. Sci. Health Part A 2014, 49, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydraulic and Pollutant Removal Performance of Fine Media Stormwater Filtration Systems. Environ. Sci. Technol. 2008, 42, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- McLellan, S.L.; Hollis, E.J.; Depas, M.M.; Van Dyke, M.; Harris, J.; Scopel, C.O. Distribution and Fate of Escherichia Coli in Lake Michigan Following Contamination with Urban Stormwater and Combined Sewer Overflows. J. Gt. Lakes Res. 2007, 33, 566. [Google Scholar] [CrossRef]
- An, Q.; Jin, N.; Deng, S.; Zhao, B.; Liu, M.; Ran, B.; Zhang, L. Ni(II), Cr(VI), Cu(II) and Nitrate Removal by the Co-System of Pseudomonas Hibiscicola Strain L1 Immobilized on Peanut Shell Biochar. Sci. Total Environ. 2022, 814, 152635. [Google Scholar] [CrossRef] [PubMed]
- Tatarchuk, T.; Bououdina, M.; Al-Najar, B.; Bitra, R.B. Green and Ecofriendly Materials for the Remediation of Inorganic and Organic Pollutants in Water. In A New Generation Material Graphene: Applications in Water Technology; Naushad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 69–110. [Google Scholar] [CrossRef]
- Sharma, S.; Hasan, A.; Kumar, N.; Pandey, L.M. Removal of Methylene Blue Dye from Aqueous Solution Using Immobilized Agrobacterium Fabrum Biomass along with Iron Oxide Nanoparticles as Biosorbent. Environ. Sci. Pollut. Res. 2018, 25, 21605–21615. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of Azo Dyes by Anaerobic–Aerobic Sequential Biological Treatment: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Arellano-Sánchez, M.G.; Devouge-Boyer, C.; Hubert-Roux, M.; Afonso, C.; Mignot, M. Chromium Determination in Leather and Other Matrices: A Review. Crit. Rev. Anal. Chem. 2022, 52, 1537–1556. [Google Scholar] [CrossRef]
- Suman, S.; Sinha, A.; Tarafdar, A. Polycyclic Aromatic Hydrocarbons (PAHs) Concentration Levels, Pattern, Source Identification and Soil Toxicity Assessment in Urban Traffic Soil of Dhanbad, India. Sci. Total Environ. 2016, 545–546, 353–360. [Google Scholar] [CrossRef]
- Zeng, S.; Ma, J.; Ren, Y.; Liu, G.-J.; Zhang, Q.; Chen, F. Assessing the Spatial Distribution of Soil PAHs and Their Relationship with Anthropogenic Activities at a National Scale. Int. J. Environ. Res. Public Health 2019, 16, 4928. [Google Scholar] [CrossRef]
- Wolska, L.; Mechlińska, A.; Rogowska, J.; Namieśnik, J. Sources and Fate of PAHs and PCBs in the Marine Environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1172–1189. [Google Scholar] [CrossRef]
- Wang, M.; Safe, S.; Hearon, S.E.; Phillips, T.D. Strong Adsorption of Polychlorinated Biphenyls by Processed Montmorillonite Clays: Potential Applications as Toxin Enterosorbents during Disasters and Floods. Environ. Pollut. 2019, 255, 113210. [Google Scholar] [CrossRef]
- Loganathan, B.G.; Masunaga, S. PCBs, Dioxins, and Furans: Human Exposure and Health Effects. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2009; pp. 245–253. [Google Scholar] [CrossRef]
- Zare, E.N.; Fallah, Z.; Le, V.T.; Doan, V.-D.; Mudhoo, A.; Joo, S.-W.; Vasseghian, Y.; Tajbakhsh, M.; Moradi, O.; Sillanpää, M.; et al. Remediation of Pharmaceuticals from Contaminated Water by Molecularly Imprinted Polymers: A Review. Environ. Chem. Lett. 2022, 20, 2629–2664. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, Impacts and General Aspects of Pesticides in Surface Water: A Review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Della Rocca, C.; Belgiorno, V.; Meriç, S. Overview of In-Situ Applicable Nitrate Removal Processes. Desalination 2007, 204, 46–62. [Google Scholar] [CrossRef]
- Nuhoglu, A.; Pekdemir, T.; Yildiz, E.; Keskinler, B.; Akay, G. Drinking Water Denitrification by a Membrane Bio-Reactor. Water Res. 2002, 36, 1155–1166. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, J.; Song, Y.; Zhu, G.; Paerl, H.W.; Qin, B. Spatial and Temporal Distribution Characteristics of Different Forms of Inorganic Nitrogen in Three Types of Rivers around Lake Taihu, China. Environ. Sci. Pollut. Res. 2019, 26, 6898–6910. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.I.; Debik, E. Economical Approach to Nitrate Removal via Membrane Capacitive Deionization. Sep. Purif. Technol. 2019, 209, 776–781. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial Decolorization and Degradation of Synthetic Dyes: A Review. Rev. Environ. Sci. Biotechnol. 2013, 12, 75–79. [Google Scholar] [CrossRef]
- Rai, P.; Gautam, R.K.; Banerjee, S.; Rawat, V.; Chattopadhyaya, M.C. Synthesis and Characterization of a Novel SnFe2O4 @activated Carbon Magnetic Nanocomposite and Its Effectiveness in the Removal of Crystal Violet from Aqueous Solution. J. Environ. Chem. Eng. 2015, 3, 2281–2291. [Google Scholar] [CrossRef]
- Kumar, L.; Bharadvaja, N. 12—Microorganisms: A Remedial Source for Dye Pollution. In Removal of Toxic Pollutants Through Microbiological and Tertiary Treatment; Shah, M.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–333. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, H.; Zhao, Y.; Shen, J.; Chen, Z.; Chen, J. Distribution of Polycyclic Aromatic Hydrocarbons in Surface Water from the Upper Reach of the Yellow River, Northwestern China. Environ. Sci. Pollut. Res. 2015, 22, 6950–6956. [Google Scholar] [CrossRef] [PubMed]
- Misaki, K.; Takamura-Enya, T.; Ogawa, H.; Takamori, K.; Yanagida, M. Tumour-Promoting Activity of Polycyclic Aromatic Hydrocarbons and Their Oxygenated or Nitrated Derivatives. Mutagenesis 2016, 31, 205–213. [Google Scholar] [CrossRef]
- Luch, A. Nature and Nurture—Lessons from Chemical Carcinogenesis. Nat. Rev. Cancer 2005, 5, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Iqbal, J.; Islam, M.A.; Islam, A.; Khandaker, S.; Asiri, A.M.; Rahman, M.M. Ligand Based Sustainable Composite Material for Sensitive Nickel(II) Capturing in Aqueous Media. J. Environ. Chem. Eng. 2020, 8, 103591. [Google Scholar] [CrossRef]
- Denkhaus, E.; Salnikow, K. Nickel Essentiality, Toxicity, and Carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Chakraborty, S.; Das, A.K.; Manna, A.; Bhattacharyya, A.; Quah, C.K.; Fun, H.-K. Selective Colorimetric and Ratiometric Probe for Ni(ii) in Quinoxaline Matrix with the Single Crystal X-Ray Structure. RSC Adv. 2014, 4, 20922–20926. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar] [CrossRef]
- Vievard, J.; Amoikon, T.L.-S.; Coulibaly, N.A.; Devouge-Boyer, C.; Arellano-Sánchez, M.G.; Aké, M.F.D.; Djeni, N.T.; Mignot, M. Extraction and Quantification of Pesticides and Metals in Palm Wines by HS-SPME/GC–MS and ICP-AES/MS. Food Chem. 2022, 393, 133352. [Google Scholar] [CrossRef]
- Sherugar, P.; Naik, N.S.; Padaki, M.; Nayak, V.; Gangadharan, A.; Nadig, A.R.; Déon, S. Fabrication of Zinc Doped Aluminium Oxide/Polysulfone Mixed Matrix Membranes for Enhanced Antifouling Property and Heavy Metal Removal. Chemosphere 2021, 275, 130024. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing Potential of Nanofiltration, Reverse Osmosis and Membrane Distillation Drinking Water Treatment for Pharmaceutically Active Compounds (PhACs) Removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Naidu, G.; Nguyen, T.V.; Vigneswaran, S. Removing Nitrate from Water Using Iron-Modified Dowex 21K XLT Ion Exchange Resin: Batch and Fluidised-Bed Adsorption Studies. Sep. Purif. Technol. 2016, 158, 62–70. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Antošová, B.; Hrabák, P.; Antoš, V.; Wacławek, S. Chemical Oxidation of Polycyclic Aromatic Hydrocarbons in Water By Ferrates(VI). Ecol. Chem. Eng. S 2020, 27, 529–542. [Google Scholar] [CrossRef]
- Sharma, D.; Chaudhari, P.K.; Prajapati, A.K. Removal of Chromium (VI) and Lead from Electroplating Effluent Using Electrocoagulation. Sep. Sci. Technol. 2020, 55, 321–331. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.-H.; Govindwar, S.P. Sequential Photocatalysis and Biological Treatment for the Enhanced Degradation of the Persistent Azo Dye Methyl Red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.R.; De, S. Performance Evaluation of Two Stage Nanofiltration for Treatment of Textile Effluent Containing Reactive Dyes. J. Environ. Chem. Eng. 2015, 3, 1678–1690. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Truong, Q.-M.; Chen, C.-W.; Chen, W.-H.; Dong, C.-D. Pyrolysis of Marine Algae for Biochar Production for Adsorption of Ciprofloxacin from Aqueous Solutions. Bioresour. Technol. 2022, 351, 127043. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, J.; Wu, J.; Luo, Y. Removal of Phenanthrene from Coastal Waters by Green Tide Algae Ulva Prolifera. Sci. Total Environ. 2017, 609, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Foletto, V.S.; Ferreira, A.B.; da Cruz Severo, E.; Collazzo, G.C.; Foletto, E.L.; Dotto, G.L. Iron-Based Adsorbent Prepared from Litchi Peel Biomass via Pyrolysis Process for the Removal of Pharmaceutical Pollutant from Synthetic Aqueous Solution. Environ. Sci. Pollut. Res. 2017, 24, 10547–10556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Xie, T.; Cao, J. Activated Carbon Derived from Waste Tangerine Seed for the High-Performance Adsorption of Carbamate Pesticides from Water and Plant. Bioresour. Technol. 2020, 316, 123929. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The Removal of Anionic and Cationic Dyes from an Aqueous Solution Using Biomass-Based Activated Carbon. Sci. Rep. 2021, 11, 8623. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Siddiqui, Z.; Dhar, S.; Mehta, D.; Pathania, D. Adsorptive Removal of Congo Red Dye (CR) from Aqueous Solution by Cornulaca Monacantha Stem and Biomass-Based Activated Carbon: Isotherm, Kinetics and Thermodynamics. Sep. Sci. Technol. 2018, 54, 1–14. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. Pesticides Removal from Waste Water by Activated Carbon Prepared from Waste Rubber Tire. Water Res. 2011, 45, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Li, J.; Zhu, T. Adsorption Equilibrium and Thermodynamics of Acetaldehyde/Acetone on Activated Carbon. Sep. Purif. Technol. 2019, 209, 535–541. [Google Scholar] [CrossRef]
- Raghuvanshi, S.P.; Singh, R.; Kaushik, C. Kinetics Study of Methylene Blue Dye Bio-Adsorption on Bagasse. Appl. Ecol. Environ. Res. 2004, 2, 35–43. [Google Scholar] [CrossRef]
- Selim, A.Q.; Sellaoui, L.; Ahmed, S.A.; Mobarak, M.; Mohamed, E.A.; Lamine, A.B.; Erto, A.; Bonilla-Petriciolet, A.; Seliem, M.K. Statistical Physics-Based Analysis of the Adsorption of Cu2+ and Zn2+ onto Synthetic Cancrinite in Single-Compound and Binary Systems. J. Environ. Chem. Eng. 2019, 7, 103217. [Google Scholar] [CrossRef]
- Ali, M.E.M. Removal of Pharmaceutical Pollutants from Synthetic Wastewater Using Chemically Modified Biomass of Green Alga Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2018, 151, 144–152. [Google Scholar] [CrossRef]
- Kajeiou, M.; Alem, A.; Mezghich, S.; Ahfir, N.-D.; Mignot, M.; Devouge-Boyer, C.; Pantet, A. Competitive and Non-Competitive Zinc, Copper and Lead Biosorption from Aqueous Solutions onto Flax Fibers. Chemosphere 2020, 260, 127505. [Google Scholar] [CrossRef]
- Sellaoui, L.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Ávila-Camacho, B.A.; Díaz-Muñoz, L.L.; Ghalla, H.; Bonilla-Petriciolet, A.; Lamine, A.B. Understanding the Adsorption of Pb2+, Hg2+ and Zn2+ from Aqueous Solution on a Lignocellulosic Biomass Char Using Advanced Statistical Physics Models and Density Functional Theory Simulations. Chem. Eng. J. 2019, 365, 305–316. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Hussain, Z.; Chang, N.; Sun, J.; Xiang, S.; Ayaz, T.; Zhang, H.; Wang, H. Modification of Coal Fly Ash and Its Use as Low-Cost Adsorbent for the Removal of Directive, Acid and Reactive Dyes. J. Hazard. Mater. 2022, 422, 126778. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Ngo, H.H.; Guo, W.; Wen, H.; Zhang, D.; Li, C.; Qi, L. Characterization and Sulfonamide Antibiotics Adsorption Capacity of Spent Coffee Grounds Based Biochar and Hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advance in Water Pollution Research: Removal of Biological Resistant Pollutions from Wastewater by Adsorption. In Proceedings of the International Conference on Water Pollution Symposium; Oxford University Press: Oxford, UK, 1962; Volume 2, pp. 231–266. [Google Scholar]
- Njikam, E.; Schiewer, S. Optimization and Kinetic Modeling of Cadmium Desorption from Citrus Peels: A Process for Biosorbent Regeneration. J. Hazard. Mater. 2012, 213–214, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Kajeiou, M.; Alem, A.; Mezghich, S.; Ahfir, N.-D.; Mignot, M.; Pantet, A. Desorption of Zinc, Copper and Lead Ions from Loaded Flax Fibres. Environ. Technol. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H. Adsorption in Solution. Phys. Chem 1926, 57, 384–410. [Google Scholar]
- Proctor, A.; Toro-Vazquez, J.F. The Freundlich Isotherm in Studying Adsorption in Oil Processing. J. Am. Oil Chem. Soc. 1996, 73, 1627–1633. [Google Scholar] [CrossRef]
- Aksu, Z. Equilibrium Modelling of Individual and Simultaneous Biosorption of Chromium(VI) and Nickel(II) onto Dried Activated Sludge. Water Res. 2002, 36, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Apiratikul, R.; Pavasant, P. Sorption Isotherm Model for Binary Component Sorption of Copper, Cadmium, and Lead Ions Using Dried Green Macroalga, Caulerpa Lentillifera. Chem. Eng. J. 2006, 119, 135–145. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Equilibrium Modelling of Single and Binary Adsorption of Cadmium and Nickel onto Bagasse Fly Ash. Chem. Eng. J. 2006, 117, 79–91. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-Factionalized Biomass Material for Methylene Blue Dye Removal: A Comprehensive Adsorption and Mechanism Study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, Characterization and Adsorption Studies of Bamboo-Based Biochar/Montmorillonite Composite for Nitrate Removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Suo, F.; You, X.; Ma, Y.; Li, Y. Rapid Removal of Triazine Pesticides by P Doped Biochar and the Adsorption Mechanism. Chemosphere 2019, 235, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, H.; Leiviskä, T.; Heiderscheidt, E.; Postila, H.; Tanskanen, J. Removal of Metals from Industrial Wastewater and Urban Runoff by Mineral and Bio-Based Sorbents. J. Environ. Manag. 2018, 209, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.M.; El-Zawahry, M.; Emam, H.E. Efficient Removal of Organophosphorus Pesticides from Wastewater Using Polyethylenimine-Modified Fabrics. Polymer 2018, 155, 225–234. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Z.; Li, Y.; Li, C.; Ahmad, N.M.; Cheema, W.A.; Zhu, S. Removal of Hg(ii) in Aqueous Solutions through Physical and Chemical Adsorption Principles. RSC Adv. 2019, 9, 20941–20953. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A Comprehensive Review on Sources, Analysis and Toxicity of Environmental Pollutants and Its Removal Methods from Water Environment. Sci. Total Environ. 2022, 812, 152456. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Chen, B. Removal of Polycyclic Aromatic Hydrocarbons from Aqueous Solution by Raw and Modified Plant Residue Materials as Biosorbents. J. Environ. Sci. 2014, 26, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Zhu, T.; Wang, J.; Ye, M. Modeling of Dioxin Adsorption on Activated Carbon. Chem. Eng. J. 2016, 283, 1210–1215. [Google Scholar] [CrossRef]
- Abbar, B.; Alem, A.; Marcotte, S.; Pantet, A.; Ahfir, N.-D.; Bizet, L.; Duriatti, D. Experimental Investigation on Removal of Heavy Metals (Cu2+, Pb2+, and Zn2+) from Aqueous Solution by Flax Fibres. Process Saf. Environ. Prot. 2017, 109, 639–647. [Google Scholar] [CrossRef]
- Pereira, J.E.S.; Ferreira, R.L.S.; Nascimento, P.F.P.; Silva, A.J.F.; Padilha, C.E.A.; Barros Neto, E.L. Valorization of Carnauba Straw and Cashew Leaf as Bioadsorbents to Remove Copper (II) Ions from Aqueous Solution. Environ. Technol. Innov. 2021, 23, 101706. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Sánchez-Vázquez, R.; Molina, R.; Martínez, F.; Melero, J.A.; Bautista, L.F.; Iglesias, J.; Morales, G. Biological Removal of Pharmaceutical Compounds Using White-Rot Fungi with Concomitant FAME Production of the Residual Biomass. J. Environ. Manag. 2016, 180, 228–237. [Google Scholar] [CrossRef]
- Atugoda, T.; Gunawardane, C.; Ahmad, M.; Vithanage, M. Mechanistic Interaction of Ciprofloxacin on Zeolite Modified Seaweed (Sargassum Crassifolium) Derived Biochar: Kinetics, Isotherm and Thermodynamics. Chemosphere 2021, 281, 130676. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-Based Materials as Adsorbent for Antibiotics Removal: Mechanisms and Influencing Factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Chen, M.; Dong, X. KOH Activation of Wax Gourd-Derived Carbon Materials with High Porosity and Heteroatom Content for Aqueous or All-Solid-State Supercapacitors. J. Colloid Interface Sci. 2019, 537, 569–578. [Google Scholar] [CrossRef]
- Xia, M.; Shao, X.; Sun, Z.; Xu, Z. Conversion of Cotton Textile Wastes into Porous Carbons by Chemical Activation with ZnCl2, H3PO4, and FeCl3. Environ. Sci. Pollut. Res. 2020, 27, 25186–25196. [Google Scholar] [CrossRef]
- Li, J.; Lv, G.; Bai, W.; Liu, Q.; Zhang, Y.; Song, J. Modification and Use of Biochar from Wheat Straw (Triticum aestivum L.) for Nitrate and Phosphate Removal from Water. Desalination Water Treat. 2014, 4681–4693. [Google Scholar] [CrossRef]
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Ewis, D.; Hameed, B.H. A Review on Microwave-Assisted Synthesis of Adsorbents and Its Application in the Removal of Water Pollutants. J. Water Process Eng. 2021, 41, 102006. [Google Scholar] [CrossRef]
- Yagmur, E.; Turkoglu, S.; Banford, A.; Aktas, Z. The Relative Performance of Microwave Regenerated Activated Carbons on the Removal of Phenolic Pollutants. J. Clean. Prod. 2017, 149, 1109–1117. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive Removal of Different Pollutants Using Metal-Organic Framework Adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Wang, M.; You, X. Critical Review of Magnetic Polysaccharide-Based Adsorbents for Water Treatment: Synthesis, Application and Regeneration. J. Clean. Prod. 2021, 323, 129118. [Google Scholar] [CrossRef]
- Michal, J. Toxicity of Pyrolysis and Combustion Products of Poly-(Vinyl Chloride). Fire Mater. 1976, 1, 57–62. [Google Scholar] [CrossRef]
- Michal, J.; Mitera, J.; Kubát, J. Major Pyrolysis and Thermoxidative Products from Certain Polyamides. Fire Mater. 1981, 5, 1–5. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-Regeneration of Activated Carbon: A Comprehensive Review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in Decontamination of Wastewater Using Biomass-Basedcomposites: A Critical Review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef] [PubMed]
- Altun, T.; Ecevit, H. Cr(VI) Removal Using Fe2O3-Chitosan-Cherry Kernel Shell Pyrolytic Charcoal Composite Beads. Environ. Eng. Res. 2020, 25, 426–438. [Google Scholar] [CrossRef]

| Dyes | Heavy Metals | Nitrates | PAHs | Pesticides | Pharmaceuticals | |
|---|---|---|---|---|---|---|
| Electrostatic attraction | x [6,83] | x [7] | x [84] | x [85] | x [11,57] | |
| Ion exchange | x [7,13,16,86] | x [16,84] | ||||
| Complexation | x [16] | x [16] | ||||
| H-bonding | x [6,61,83] | x [85,87] | x [57] | |||
| π-π interaction | x [6,61,83] | x [10] | x [87] | x [57] | ||
| Van der Waals interaction | x [10] | x [60,85,87] |
| Adsorbent | Type of Biomass | Pollutant | Equilibrium Time | Maximum Adsorption Capacity (mg/g) | pH | Temperature (°C) | Kinetic | Isotherm | Important Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Acid-factionalized Coconut shell | AW | Methylene blue | 60 min | 50.6 | 8 | - | PSO | Freundlich | By increasing the pH, the adsorption capacity increased. | Jawad et al. (2020) [76] |
| Agrobacterium fabrum biomass | MB | Methylene blue | 60 min | 91 | 11 | 25 | PSO | Freundlich | The pH was the most influential parameter and the removal rate decreased with increasing pH. Conversely, an increase in adsorption capacity with increasing initial dye concentration was noted. | Sharma et al. (2018) [18] |
| Iron-based adsorbent from Litchi peel biomass | B | Amaranth | 180 min | 44.9 | 6.2 | No effect between 25–65 °C | PSO | BET isotherm | Modification of the raw material with iron nitrate. Low or no influence of pH and temperature on adsorption. | Foletto et al. (2017) [55] |
| Nanoadsorbent from the fruit coat of a Kendu tree | AW | Tartrazine | 125 min | 7.9 | 6 | 70 | PSO | Langmuir | Removal rate increased when the adsorbent dose and temperature were increased before reaching a plateau, but decreased when the initial dye concentration was increased. | Biswal et al. (2022) [6] |
| Powdered activated carbon from rubber seed and its shell | B | Methylene blue and Congo red | - | Methylene blue: 769.2 Congo red: 458.4 | 4 and 11 | - | PSO | Congo red: Langmuir | Pollutant removal increased with increasing contact time, and decreased with increasing initial concentration, temperature and ionic strength. | M.Nizam et al. (2021) [57] |
| Calcite, zeolite, sand, and iron filings | R | NO3−, PO₄³⁻, and 6 metals | - | - | - | - | - | Freundlich | Most of the filter materials used had lower removal efficiency when pollutants were present simultaneously. Iron filings were found to be the most effective material for removal. | Reddy et al. (2014) [13] |
| Carnauba straw (CS) and cashew leaf (CF) | AW | Cu(II) | 120 min | CS: 9.5 CL: 1.7 | 6 | - | PSO | CS: Langmuir CL: Freundlich model | The decrease in particle size allowed the increase in the adsorption rate. | Pereira et al. (2021) [82] |
| Clay | R | Cu(II), Co(II), Ni(II), and Pb(II) | 60 min | 1.1 | 8 | - | - | Freundlich and Langmuir | The adsorption capacity increased when the pH increased. | Es-sahbany et al. (2022) [7] |
| Co-system of strain L1 immobilized on peanut shell biochar (PSB) | B | Ni(II), Cr(VI), Cu(II), and NO3- | Heavy metals on PSB: 8h | Ni(II) on PSB: 24.7 | 5–8 | - | Ni(II): PSO Cr(VI): Elovich Cu(II): PFO | Ni(II) on PSB: Langmuir: | A practical application of the system to remove pollutants was simulated in a sequential batch reactor. Adsorption increased with pH for Cu(II) and Ni(II), and decreased for Cr(VI) probably because it was reduced to Cr(III). | An et al. (2022) [16] |
| Flax fibers | AW | Cu(II), Pb(II), and Zn(II) | 60 min | Cu(II): 7.8 Pb(II): 23.3 Zn(II): 4.6 | 6.4 | - | Cu(II) and Pb(II): PSO Zn(II): PFO | Langmuir | A competition effect of pollutants for adsorption sites has been demonstrated. Lead was the most adsorbed metal in the single and ternary solutions. | Kajeiou et al. (2020) [85] |
| Flax fibers | AW | Cu(II), Pb(II), and Zn(II) | 60 min | Cu(II): 9.9 Pb(II): 10.7 Zn(II): 8.4 | 4–7 | - | PSO | Langmuir | The adsorption capacity increased when the amount of adsorbent increased. | Abbar et al. (2017) [84] |
| Hydrochloric acid treated peat and citric acid-treated sawdust | AW | Zn(II), Cr(III), Ni(II), and Cu(II) | 15–30 min | Ni by hydrochloric acid treated peat: 21 | - | - | - | - | Modification of the raw material with acids (hydrochloric for peat and citric for sawdust). | Gogoi et al. (2018) [79] |
| Lignocellulosic (flamboyant) biomass biochar | B | Pb(II), Hg(II), and Zn(II) | 24h | 0.024–0.411 mmol/g | - | 40 | - | Combinaison of statistical physics models and DFT calculations | Study of the adsorption process using statistical physics models and density functional theory calculations. Antagonistic adsorption for all heavy metals. | Sellaoui et al. (2019) [66] |
| Synthetic cancrinite | R | Cu(II) and Zn(II) | - | Single solution: 118.3 and 67.0 for Cu(II) and Zn(II) | - | 50 | PSO | Langmuir | Cancrinite was synthesized from crude muscovite via activation with sodium hydroxide and is more efficient for the removal of Cu(II). Adsorbed amount decreased from a single solution to a binary solution, showing a competition effect. | Selim et al. (2019) [63] |
| Bamboo-based biochar/montmorillonite composite | B | NO3− | 100 min | Biochar: 5 Composite: 9 | 4 | - | - | Langmuir | Nitrate removal was rapid (10 min), and then the adsorption rate gradually decreased with time. | Viglašová et al. (2018) [77] |
| Biochar from wheat straw | B | NO3- and PO₄3- | - | NO3−: 2.5 PO₄³⁻: 16.6 | NO3−: 3 PO₄³⁻: 6 | - | - | Langmuir | Chloridic acid treatment of wheat straw resulted in a higher surface area and pore volume. | Li et al. (2014) [90] |
| Sugarcane Bagasse-derived biochar | B | NO3- | 60 min | 28.2 | 4.6 | - | PSO | Langmuir | Modification of biochar with epichlorohydrin, N,N-dimethylformamide, ethylenediamine, and trimethylamine. By increasing the pH and introducing the nitrate in the presence of coexisting ions, the adsorption decreased. On the contrary, it increased by increasing the adsorbent dosage and the temperature. | Divband Hafshejani et al. (2016) [8] |
| Modified natural fabrics based on cotton (MC) and wool (MW) | AW | Pirimiphos-methyl and monocrotophos | 2h | MC: 333.3–454.6 MW: 500.0–625.0 | - | - | PSO | Langmuir | The fabrics were modified with the synthetic polymer polyethyleneimine, which increased the adsorption capacity of wood for both pesticides. | Abdelhameed, El-Zawahry and E. Emamc (2018) [80] |
| Nanoadsorbent from the fruit coat of a Kendu tree | AW | Tartrazine | 125 min | 7.9 | 6 | 70 | PSO | Langmuir | Removal rate increased when the adsorbent dose and temperature were increased before reaching a plateau, but decreased when the initial dye concentration was increased. | Biswal et al. (2022) [6] |
| P-doped biochar from corn straw | B | 6 pesticides | Atrazine: 20 min | Atrazine: 79.6 | - | - | PSO | Freundlich | Activation with phosphoric acid resulted in improved adsorption performance of the biochar. Adsorption rates of the six pesticides increased with increasing adsorbent dosage. | Suo et al. (2019) [78] |
| Tangerine seed-derived biochar | B | Bendiocarb, metolcarb, isoprocarb, pirimicarb, carbaryl, and methiocarb | 12 min | 7.97–93.5 | 7 | 20 | PSO | Langmuir | Increasing the carbonization temperature and time resulted in an increase in biochar pore width and pesticide removal efficiency, respectively. | Wang et al. (2020) [56] |
| Waste rubber tire-derived biochar | B | Methoxychlor, methyl parathion, and atrazine | 60 min | 88.9–112.0 | 2 | 25 | PFO | Langmuir | A direct relationship was found between the adsorption capacity and the octanol–water partition coefficient values of the pollutants. By increasing the pH, the adsorption decreased. | Gupta et al. (2011) [59] |
| Biochar from algae | B | Ciprofloxacin | - | Brown algae-derived biochar: 250 | 7 | 25 | PSO | Langmuir | Different types of products were generated during pyrolysis: aromatics, hydrocarbons, phenols, acids, alcohols, furans, nitrogenous chemicals. | Nguyen et al. (2022) [53] |
| Modified biomass of green alga Scenedesmus obliquus | A | Tramadol | 45 min | 140.2 | 7 | - | Tramadol: PSO | Tramadol: Freundlich | Modification of the raw material with a sodium hydroxide solution that increased the removal. Competitive adsorption occurred between the pharmaceutical pollutants. | Ali et al. (2018) [64] |
| Moringa oleifera seed husk biomass | AW | Diclofenac | 1080 min | 28.7 | 5 | - | PSO | Freundlich | Chemical modification of the raw material with methyl alcohol and nitric acid solution, followed by physical modification in a muffle for 1 h at 300 °C. Adsorption decreased with increasing pH. | Araujo et al. (2018) [11] |
| White-rot fungi (Trametes versicolor and Ganoderma lucidum) | MB | 13 pharmaceutical pollutants | - | - | - | - | - | Individual and combined fungal bioassays were tested to produce a raw material for the production of biodiesel via the valorization of fungal sludge generated during the disposal process have been carried out. | Vasiliadou et al. (2016) [86] | |
| Green tide algae Ulva prolifera | A | Phenanthrene | - | - | - | 30 | Two-stage PFO | - | An increase in nutrients, temperature, and initial pollutant concentration resulted in an increase in the rate of phenanthrene removal. | Zhang et al. (2017) [54] |
| Coconut shell activated carbon | B | Dioxins | - | 600 | - | - | - | - | Adsorption capacity determined according to linear relationships between gas properties and adsorption behaviors. | Guo et al. (2016) [91] |
| Processed montmorillonite clays | R | PCBs | - | - | - | 26–37 | - | Langmuir | Steric hindrance limited the access of the pollutant to the montmorillonite clay surfaces, reducing the adsorption capacity | Wang et al. (2019) [24] |
| Raw and modified plant residues | AW | Naphthalene, acenaphthene, phenanthrene, and pyrene | 24–50h | - | - | - | PSO | Freundlich | Acid hydrolysis was used to modify the raw material. Sorption coefficients were negatively correlated with polarity and positively correlated with adsorbent aromaticity. | Xi and Chen (2014) [83] |
| Seagrass leaf powder | AW | Acenaphthylene (A), phenanthrene (P), and fluoranthene (F) | F: 6h P: 24h A: 120h | F: 2.2 P: 2.1 A: 1.1 | - | - | PSO | Freundlich | Removal efficiency increased with increasing amount of adsorbent while maximum adsorption capacity decreased, presumably since saturation could not be reached due to increasing dosage. | Akinpelu et al. (2021) [10] |
| Wood waste-derived biochar | B | 19 PAHs, 23 Nitro-PAHS, and 9 Oxygenated-PAHs | - | 2.0 | - | - | PSO | PAHs: Langmuir N-PAHs: category IV O-PAHs: category II | A molecular model was used to simulate the fundamental properties of the biochar. Destruction of micro-pores and formation of meso-pores in the biochar was observed following acid treatment. | Zhou et al. (2021) [92] |
| Biomass | Gas Used | Gradient of Temperature (°C/min) | Maximum Temperature (°C) | Carbonization Time (min) | Chemical Activating Agent | Reference |
|---|---|---|---|---|---|---|
| Peanut shell | N2 | 10 | 500 | 120 | - | An et al. (2022) [16] |
| Marine algae | N2 | 10 | 700 | 120 | ZnCl2 | Nguyen et al. (2022) [57] |
| Bamboo biomass | N2 | - | 460 | 120 | - | Viglašová et al. (2018) [84] |
| [Litchi peels | N2 | 10 | 800 | 120 | Foletto et al. (2017) [59] | |
| Lignocellulosic biomass | N2 | 10 | 600 | 120 | - | Sellaoui et al. (2019) [70] |
| Corn straw and corncob | - | - | 300 | 120 | H3PO4 | Suo et al. (2019) [85] |
| Rubber seed and shell | - | - | 800 | 480 | H2SO4 (after the pyrolysis) | M.Nizam et al. (2021) [61] |
| Tangerine seed | - | 10 | 600 | 240 | H3PO4 | Wang et al. (2020) [60] |
| Waste rubber tire | - | - | 900 | 120 | KOH | Gupta et al. (2011) [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vievard, J.; Alem, A.; Pantet, A.; Ahfir, N.-D.; Arellano-Sánchez, M.G.; Devouge-Boyer, C.; Mignot, M. Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics 2023, 11, 404. https://doi.org/10.3390/toxics11050404
Vievard J, Alem A, Pantet A, Ahfir N-D, Arellano-Sánchez MG, Devouge-Boyer C, Mignot M. Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics. 2023; 11(5):404. https://doi.org/10.3390/toxics11050404
Chicago/Turabian StyleVievard, Juliette, Abdellah Alem, Anne Pantet, Nasre-Dine Ahfir, Mónica Gisel Arellano-Sánchez, Christine Devouge-Boyer, and Mélanie Mignot. 2023. "Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review" Toxics 11, no. 5: 404. https://doi.org/10.3390/toxics11050404
APA StyleVievard, J., Alem, A., Pantet, A., Ahfir, N.-D., Arellano-Sánchez, M. G., Devouge-Boyer, C., & Mignot, M. (2023). Bio-Based Adsorption as Ecofriendly Method for Wastewater Decontamination: A Review. Toxics, 11(5), 404. https://doi.org/10.3390/toxics11050404









