Ferroptosis as a Potential Therapeutic Target of Traditional Chinese Medicine for Mycotoxicosis: A Review
Abstract
1. Introduction
2. Mechanisms of Ferroptosis
2.1. Iron Metabolism
2.2. Lipid Metabolism
2.3. System Xc-/GPX4 Pathway Regulation
2.4. Mitochondrion-Mediated Ferroptosis
3. Role of Ferroptosis in Mycotoxicosis Diseases
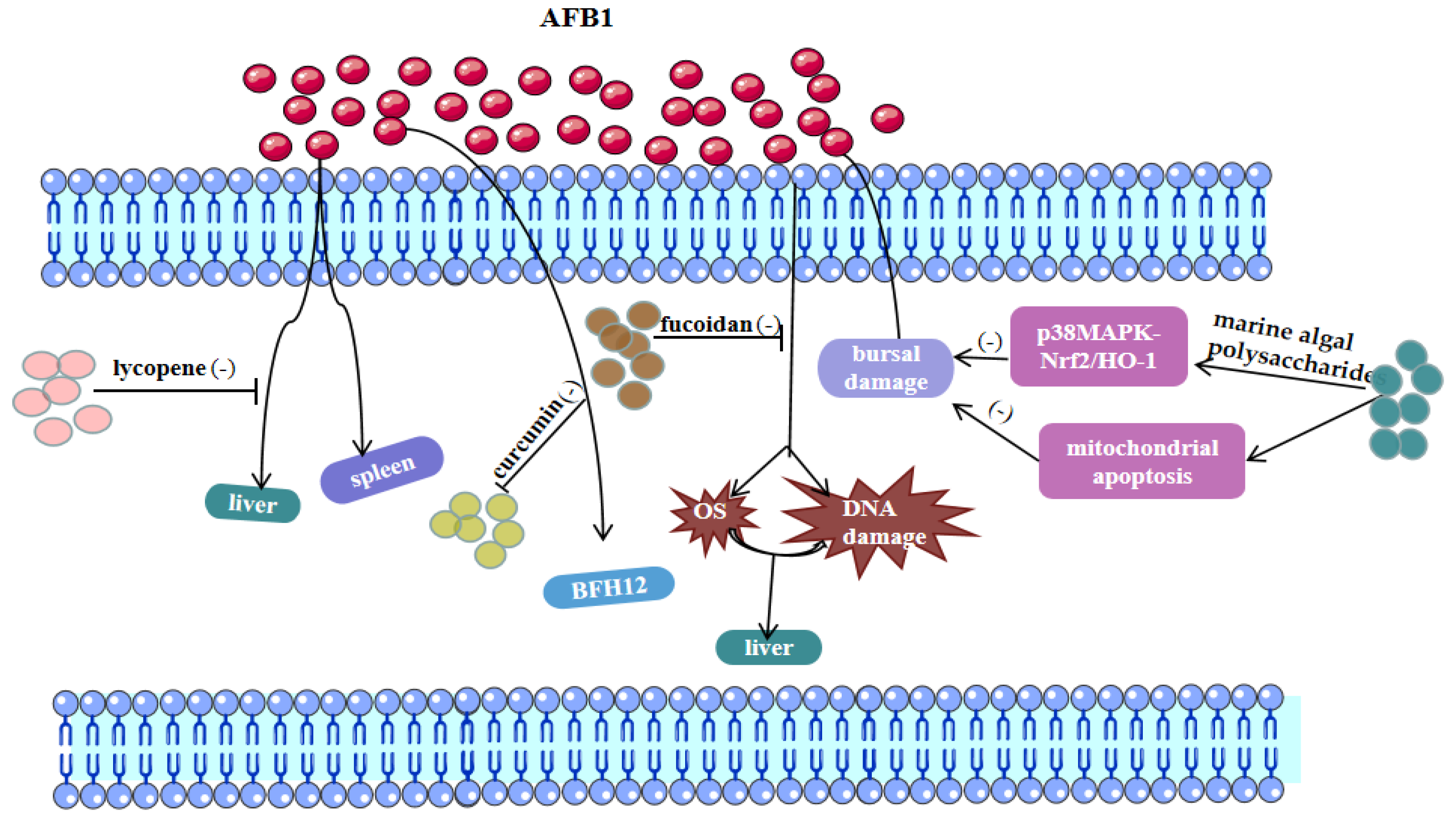
3.1. AFB1 and Ferroptosis
3.2. Zearalenone and Ferroptosis
3.3. T-2 Toxin and Ferroptosis
3.4. Patulin and Ferroptosis
4. Chinese Herbal Medicine for Ferroptosis-Mediated Mycotoxin Poisoning
4.1. Regulation of Ferroptosis by Chinese Herbal Medicines
4.2. Treatment of Mycotoxin Poisoning by Chinese Herbal Medicine
4.3. Chinese Herbal Medicine for the Treatment of Mycotoxin Poisoning Mediated by the Ferroptosis Pathway
5. Conclusions and Perspective Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Beier, R.C. T-2 toxin, a trichothecene mycotoxin: Review of toxicity, metabolism, and analytical methods. J. Agric. Food Chem. 2011, 59, 3441–3453. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, P.; Cui, Y. Review of the Reproductive Toxicity of T-2 Toxin. J. Agric. Food Chem. 2020, 68, 727–734. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Guo, H.; Wang, P.; Liu, C.; Zhou, T.; Chang, J.; Yin, Q.; Wang, L.; Jin, S.; Zhu, Q.; Lu, F. Effects of Compound Mycotoxin Detoxifier on Alleviating Aflatoxin B1-Induced Inflammatory Responses in Intestine, Liver and Kidney of Broilers. Toxins 2022, 14, 665. [Google Scholar] [CrossRef]
- Yue, K.; Liu, K.L.; Zhu, Y.D.; Ding, W.L.; Xu, B.W.; Shaukat, A.; He, Y.F.; Lin, L.X.; Zhang, C.; Huang, S.C. Novel Insights into Total Flavonoids of Rhizoma Drynariae against Meat Quality Deterioration Caused by Dietary Aflatoxin B1 Exposure in Chickens. Antioxidants 2023, 30, 83. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Lin, L.X.; Xu, T.T.; Lu, Y.; Zhang, C.D.; Yue, K.; Huang, S.C.; Dong, H.J.; Jian, F.C. Aflatoxin B1 alters meat quality associated with oxidative stress, inflammation, and gut-microbiota in sheep. Ecotoxicol. Environ. Saf. 2021, 225, 112754. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lin, L.; Wang, S.; Ding, W.; Zhang, C.; Shaukat, A.; Xu, B.; Yue, K.; Zhang, C.; Liu, F. Total Flavonoids of Rhizoma Drynariae Mitigates Aflatoxin B1-Induced Liver Toxicity in Chickens via Microbiota-Gut-Liver Axis Interaction Mechanisms. Antioxidants 2023, 12, 819. [Google Scholar] [CrossRef]
- Guo, Y.; Balasubramanian, B.; Zhao, Z.H.; Liu, W.C. Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers. Poult. Sci. 2021, 100, 844–857. [Google Scholar] [CrossRef]
- Yin, S.; Liu, X.; Fan, L.; Hu, H. Mechanisms of cell death induction by food-borne mycotoxins. Crit. Rev. Food Sci. Nutr. 2018, 58, 1406–1417. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, S.; Jiang, L.; Sun, X.; Li, J.; Wang, N.; Liu, X.; Yao, X.; Zhang, C.; Deng, H.; et al. Patulin Induces Acute Kidney Injury in Mice through Autophagy-Ferroptosis Pathway. J. Agric. Food Chem. 2022, 70, 6213–6223. [Google Scholar] [CrossRef]
- Lin, J.; Zuo, C.; Liang, T. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Ashutosh, B.; Myunghee, K. The effects of mycotoxin patulin on cells and cellular components. Trends. Food Sci. Tech. 2019, 83, 99–113. [Google Scholar]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1α/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J.; et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019, 26, 2284–2299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Y.; Xu, Z.J.; Zhang, N.Y.; Zhang, W.P.; Zuo, G.; Khalil, M.M.; Sun, L.H. Selenium mitigated aflatoxin B1-induced cardiotoxicity with potential regulation of 4 selenoproteins and ferroptosis signaling in chicks. Food Chem. Toxicol. 2021, 154, 112320. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, Y.; Wang, R.; Li, X. Role of Ferroptosis in Fibrotic Diseases. J. Inflamm. Res. 2022, 15, 3689–3708. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cao, Y.; Cao, W.; Jia, Y.; Lu, N. The Application of Ferroptosis in Diseases. Pharmacol. Res. 2020, 159, 104919. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Grosse, Y.; Chekir-Ghedira, L.; Huc, A.; Obrecht-Pflumio, S.; Dirheimer, G.; Bacha, H.; Pfohl-Leszkowicz, A. Retinol, ascorbic acid and alpha-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins ochratoxin A and zearalenone. Cancer Lett. 1997, 114, 225–229. [Google Scholar] [CrossRef]
- Lee, S.E.; Campbell, B.C.; Molyneux, R.J.; Hasegawa, S.; Lee, H.S. Inhibitory effects of naturally occurring compounds on aflatoxin B(1) biotransformation. J. Agric. Food Chem. 2001, 49, 5171–5177. [Google Scholar] [CrossRef]
- Xue, M.; Tian, Y.; Sui, Y.; Zhao, H.; Gao, H.; Liang, H.; Qiu, X.; Sun, Z.; Zhang, Y.; Qin, Y. Protective effect of fucoidan against iron overload and ferroptosis-induced liver injury in rats exposed to alcohol. Biomed. Pharm. 2022, 153, 113402. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Hirschhorn, T.; Stockwell, B.R. The development of the concept of ferroptosis. Free Radic. Biol. Med. 2019, 133, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, T.; Zhao, Y.; Meng, X.; Ding, W.; Wang, Q.; Liu, C.; Deng, H. Flavonoid 4,4’-dimethoxychalcone induced ferroptosis in cancer cells by synergistically activating Keap1/Nrf2/HMOX1 pathway and inhibiting FECH. Free Radic. Biol. Med. 2022, 188, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Shashank, M.; Ashley, I.B.; David, D.; Anne Sophie, R.; Caroline, M. Striking while the iron is hot: Iron metabolism and Ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar]
- Walter, P.B.; Knutson, M.D.; Paler-Martinez, A.; Lee, S.; Xu, Y.; Viteri, F.E.; Ames, B.N. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl. Acad. Sci. USA 2002, 99, 2264–2269. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Liu, X.Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef]
- Das, U.N. Saturated Fatty Acids, MUFAs and PUFAs Regulate Ferroptosis. Cell Chem. Biol. 2019, 26, 309–311. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Heinrich, L.; Booijink, R.; Khurana, A.; Weiskirchen, R.; Bansal, R. Lipoxygenases in chronic liver diseases: Current insights and future perspectives. Trends Pharmacol. Sci. 2022, 43, 188–205. [Google Scholar] [CrossRef]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Conrad, M.; Friedmann Angeli, J.P. Glutathione peroxidase 4 (Gpx4) and ferroptosis: What’s so special about it? Mol. Cell Oncol. 2015, 2, e9950. [Google Scholar] [CrossRef]
- Valdovinos-Flores, C.; Limón-Pacheco, J.H.; León-Rodríguez, R.; Petrosyan, P.; Garza-Lombó, C.; Gonsebatt, M.E. Systemic L-Buthionine -S-R-Sulfoximine Treatment Increases Plasma NGF and Upregulates L-cys/L-cys2 Transporter and γ-Glutamylcysteine Ligase mRNAs Through the NGF/TrkA/Akt/Nrf2 Pathway in the Striatum. Front. Cell Neurosci. 2019, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, A.A.; Wu, H.C.; Farquharson, A.; Grant, I.; Arthur, J.R.; Rotondo, D.; Choe, S.N.; Wahle, K.W. Regulation of selenoprotein GPX4 expression and activity in human endothelial cells by fatty acids, cytokines and antioxidants. Atherosclerosis 2003, 171, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadée, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef]
- Chen, S.D.; Yang, D.I.; Lin, T.K.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Khan, A.; Kuriachan, G.; Mahalakshmi, R. Cellular Interactome of Mitochondrial Voltage-Dependent Anion Channels: Oligomerization and Channel (Mis)Regulation. ACS Chem. Neurosci. 2021, 12, 3497–3515. [Google Scholar] [CrossRef]
- Zinghirino, F.; Pappalardo, X.G.; Messina, A.; Nicosia, G.; De Pinto, V.; Guarino, F. VDAC. Genes Expression and Regulation in Mammals. Front. Physiol. 2021, 12, 708695. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef] [PubMed]
- Liew, W.P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Xiao, H.; Shao, F.; Wu, M.; Ren, W.; Xiong, X.; Tan, B.; Yin, Y. The application of antimicrobial peptides as growth and health promoters for swine. J. Anim. Sci. Biotechnol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Asare, G.A.; Bronz, M.; Naidoo, V.; Kew, M.C. Interactions between aflatoxin B1 and dietary iron overload in hepatic mutagenesis. Toxicology 2007, 234, 157–166. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, T.; Peng, S.; Xu, D.; Liu, Z.; Li, H.; Hu, L.; Mo, H. Fe2+ protects postharvest pitaya (Hylocereus undulatus britt) from Aspergillus. flavus infection by directly binding its genomic DNA. Food Chem. 2022, 5, 100135. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Song, C.G.; Ding, G.; Yang, J.; Wu, J.R.; Wu, G.X.; Zhang, M.Z.; Song, C.L.; Guo, L.P.; Qin, J.C.; et al. High-Performance Functional Fe-MOF for Removing Aflatoxin B1 and Other Organic Pollutants. Adv. Mater. Interfaces 2022, 9, 2102480. [Google Scholar] [CrossRef]
- Peng, S.; Li, K.; Wang, Y.X.; Li, L.; Cheng, Y.H.; Xu, Z. Porphyrin NanoMOFs as a catalytic label in nanozyme-linked immunosorbent assay for Aflatoxin B1 detection. Anal. Biochem. 2022, 655, 114829. [Google Scholar] [CrossRef]
- Macdougall, I.C. Intravenous iron therapy in patients with chronic kidney disease: Recent evidence and future directions. Clin. Kidney J. 2017, 10 (Suppl. S1), i16–i24. [Google Scholar] [CrossRef]
- Gruber, D.C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Cui, H. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yang, D.; Huang, Y.; Wang, S. Study on the apoptosis mechanism induced by T-2 toxin. PLoS ONE 2013, 8, e83105. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qin, S.; Zheng, Y.; Xia, C.; Zhang, P.; Zhang, L.; Yao, J.; Yi, Y.; Deng, L. T-2 Toxin Induces Ferroptosis by Increasing Lipid Reactive Oxygen Species (ROS) and Downregulating Solute Carrier Family 7 Member 11 (SLC7A11). J. Agric. Food Chem. 2021, 69, 15716–15727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Chen, H.; Cao, L.; Han, K.; Zhang, H.; Cui, J.; Ma, X.; Zhao, S.; Zhao, C.; Yin, S.; Fan, L.; et al. Patulin disrupts SLC7A11-cystine-cysteine-GSH antioxidant system and promotes renal cell ferroptosis both in vitro and in vivo. Food Chem. Toxicol. 2022, 166, 113255. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Ates, M.B.; Ortatatli, M. The effects of Nigella sativa seeds and thymoquinone on aflatoxin phase-2 detoxification through glutathione and glutathione-S-transferase alpha-3, and the relationship between aflatoxin B1-DNA adducts in broilers. Toxicon 2021, 193, 86–92. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Y.X.; Li, S.; He, W.; Wang, Z.R.; Zhan, T.P.; Lv, C.Y.; Liu, Y.P.; Yang, Y.; Zeng, X.X. Progress in traditional Chinese medicine and natural extracts for the treatment of lupus nephritis. Biomed. Pharmacother. 2022, 149, 112799. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets 2017, 18, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Wei, G.; Guo, G.; Yu, H.; Zhang, Y.; Ishfaq, M.; Fazilani, S.A.; Zhang, X. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol. Environ. Saf. 2021, 208, 111725. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhong, M.; Liu, Y.; Xiong, Y.; Gao, Z.; Ma, J.; Zhuang, G.; Hong, X. Application of natural products for inducing ferroptosis in tumor cells. Biotechnol. Appl. Biochem. 2022, 69, 190–197. [Google Scholar] [CrossRef]
- Dai, X.Y.; Zhu, S.Y.; Li, M.Z.; Talukder, M.; Zhao, Y.; Li, J.L. Potential Role of Lycopene in the Inhibition of Di(2-ethylhexyl) Phthalate-Induced Ferroptosis in Spleen Via Modulation of Iron Ion Homeostasis. ACS Pharmacol. Transl. Sci. 2021, 4, 386–395. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Tu, J.; He, M.; Jiang, Y.; Yang, B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020, 315, 126236. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017, 11, 254–262. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin suppresses tumorigenesis by ferroptosis in breast cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C. Role of Flavonoids in the reatment of Iron Overload. Front. Cell Dev. Biol. 2021, 9, 685364. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Bian, Y.; Yao, J.; Wang, D.; Liu, X.; Guo, Z.; Zhang, S.; Peng, L. Targeting Cell Death: Pyroptosis, Ferroptosis, Apoptosis and Necroptosis in Osteoarthritis. Front. Cell Dev. Biol. 2022, 9, 789948. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.Y.; Li, Y.F. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods. 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Xu, F.; Wang, P.; Yao, Q.; Shao, B.; Yu, H.; Yu, K.; Li, Y. Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct. 2019, 10, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M.; Mazloomi, S.M. Therapeutic activity of fucoidan and carrageenan as marine algal polysaccharides against viruses. Biotech 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J.O. Immunomodulatory effects of polysaccharides from marine algae for treating cancer, infectious disease, and inflammation. Phytother. Res. 2022, 36, 761–777. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abdeen, A.; Jalouli, M.; Abdelkader, A.; Megahed, A.; Alkahtane, A.; Almeer, R.; Alhoshani, N.M.; Al-Johani, N.S.; Alkahtani, S.; et al. Fucoidan supplementation modulates hepato-renal oxidative stress and DNA damage induced by aflatoxin B1 intoxication in rats. Sci. Total Environ. 2021, 768, 144781. [Google Scholar] [CrossRef]
- Din, M.; Nailia, C.; Sarzamin, K.; Asad, S.; Mohammad, M.; Rafiullah. Hepatoprotective role of milk thistle (Silybum marianum) in meat type chicken fed aflatoxin B1 contaminated feed. Pak. Vet. J. 2012, 32, 443–446. [Google Scholar]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin. Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-inflammatory Pathways: A Whole Transcriptomic In Vitro Study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef]


| Chinese Herbal Medicines | Active Ingredient | Organ/Cell | Disease Model/Target | References |
|---|---|---|---|---|
| Lycopene | Carotenoids | Jejunal tissues | Murine model of multiple-mycotoxin exposure | Lin et al. [16] |
| Fucoidan | Polysaccharides | Liver tissues | Murine model of alcohol exposure | Xue et al. [29] |
| Flavonoid 4,4′-dimethoxychalcone | Flavonoids | A549 cells and 786-O cells and 293T cells | Keap1/Nrf2/HMOX1 pathway | Yang et al. [34] |
| Terpenoid | Artemisinin | Hepatocellular carcinoma cells and glioblastoma cells | GSH or GPX4 | Wu et al. [84] |
| Lycopene | Carotenoids | Spleen tissues | Murine model of DEHP exposure | Dai et al. [85] |
| morachalcone D | Flavonoids | HT22 cells | Erastin-induced cell death | Wen et al. [86] |
| Artesunate | Artemisinin | HNC cells | Nrf2–ARE antioxidant signaling pathway | Roh et al. [87] |
| Curcumin Nanoparticles | Polyphenols | MDCK cells and HT22 cells | Murine model of Intracerebral Hemorrhage | Yang et al. [88] |
| Curcumin | Polyphenols | Human BC cell lines | Murine model of breast cancer | Cao et al. [89] |
| Chinese Herbal Medicines | Active Ingredient | Mycotoxins | Mechanisms | Organ/Cell | References |
|---|---|---|---|---|---|
| Marine algal polysaccharides | Polysaccharides | AFB1 | p38MAPK-Nrf2/HO-1 and mitochondrial apoptotic signaling pathway | Bursa of Fabricius in broilers | Guo et al. [13] |
| Curcumin | Polyphenols | AFB1 | Upregulation of Nrf2/HO-1 signaling pathway and suppression of ROS and AFB1-adducts production | Liver in chickens | Li et al. [48] |
| AFB1 | Involve the expression of LncRNAs | Liver in broilers | Li et al. [83] | ||
| Lycopene | Carotenoids | AFB1 | Enhance antioxidant capacity by activating Nrf2 signaling pathway | Liver in murine | Xu et al. [90] |
| AFB1 | Enhancement of spleen antioxidant ability and inhibition of lymphocytes apoptosis | Spleen in murine | Xu et al. [91] | ||
| Fucoidan | Polysaccharides | AFB1 | Restore the hepatorenal markers and enhance the antioxidant enzyme activity | Liver and kidneys in murine | Abdel-Daim et al. [94] |
| Milk thistle | Flavonoids | AFB1 | Restore the hepatic markers | Liver in meat-type chicken | Din et al. [95] |
| Curcumin | Polyphenols | AFB1 | Relate to antioxidant response, defense response, and inflammation | Bovine fetal hepatocytes | Pauletto et al. [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Lin, L.; Yue, K.; He, Y.; Xu, B.; Shaukat, A.; Huang, S. Ferroptosis as a Potential Therapeutic Target of Traditional Chinese Medicine for Mycotoxicosis: A Review. Toxics 2023, 11, 395. https://doi.org/10.3390/toxics11040395
Ding W, Lin L, Yue K, He Y, Xu B, Shaukat A, Huang S. Ferroptosis as a Potential Therapeutic Target of Traditional Chinese Medicine for Mycotoxicosis: A Review. Toxics. 2023; 11(4):395. https://doi.org/10.3390/toxics11040395
Chicago/Turabian StyleDing, Wenli, Luxi Lin, Ke Yue, Yanfeng He, Bowen Xu, Aftab Shaukat, and Shucheng Huang. 2023. "Ferroptosis as a Potential Therapeutic Target of Traditional Chinese Medicine for Mycotoxicosis: A Review" Toxics 11, no. 4: 395. https://doi.org/10.3390/toxics11040395
APA StyleDing, W., Lin, L., Yue, K., He, Y., Xu, B., Shaukat, A., & Huang, S. (2023). Ferroptosis as a Potential Therapeutic Target of Traditional Chinese Medicine for Mycotoxicosis: A Review. Toxics, 11(4), 395. https://doi.org/10.3390/toxics11040395







