The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Experimental Procedure
2.4. Blood Biochemistry: Hepatotoxicity
2.4.1. Alanine Aminotransferase (ALT)
2.4.2. Aspartate Aminotransferase (AST)
2.5. Blood Biochemistry: Nephrotoxicity
2.5.1. Serum Creatinine (SCr)
2.5.2. Blood Urea Nitrogen (BUN)
2.6. Brain Tissue Harvesting
2.7. Tissue Preparation
2.8. Antioxidant Status
2.8.1. Uric Acid (UA)
2.8.2. Superoxide Dismutase (SOD)
2.9. Real-Time, Quantitative PCR
2.10. Statistical Analysis
3. Results
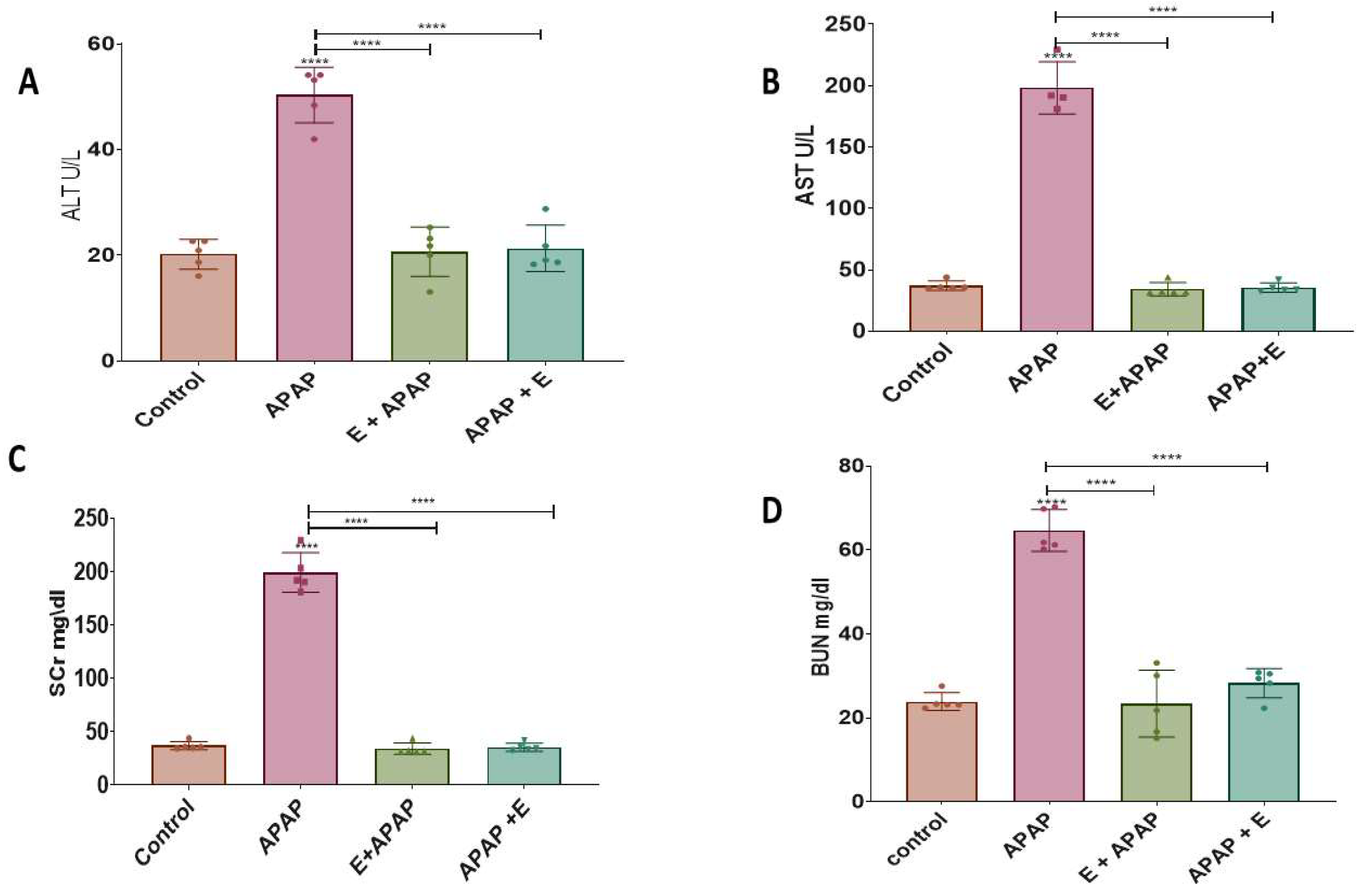
3.1. Blood Biochemistry: Hepatotoxicity
3.1.1. Alanine Aminotransferase (ALT)
3.1.2. Aspartate Aminotransferase (AST)
3.2. Blood Biochemistry: Nephrotoxicity
3.2.1. Serum Creatinine (SCr)
3.2.2. Blood Urea Nitrogen (BUN)
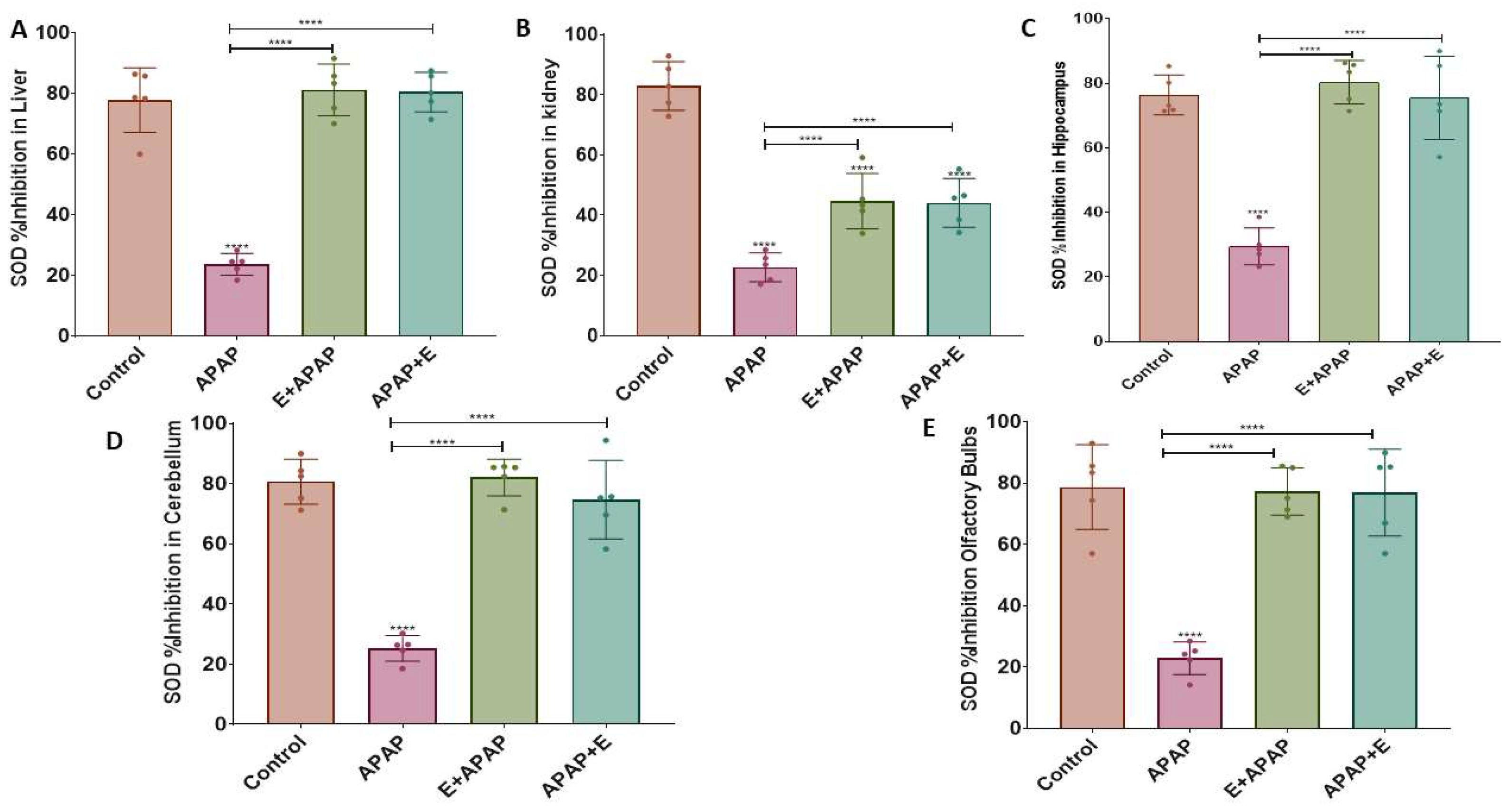
3.3. Antioxidant Status
3.3.1. Uric Acid (UA)
3.3.2. Superoxide Dismutase (SOD)
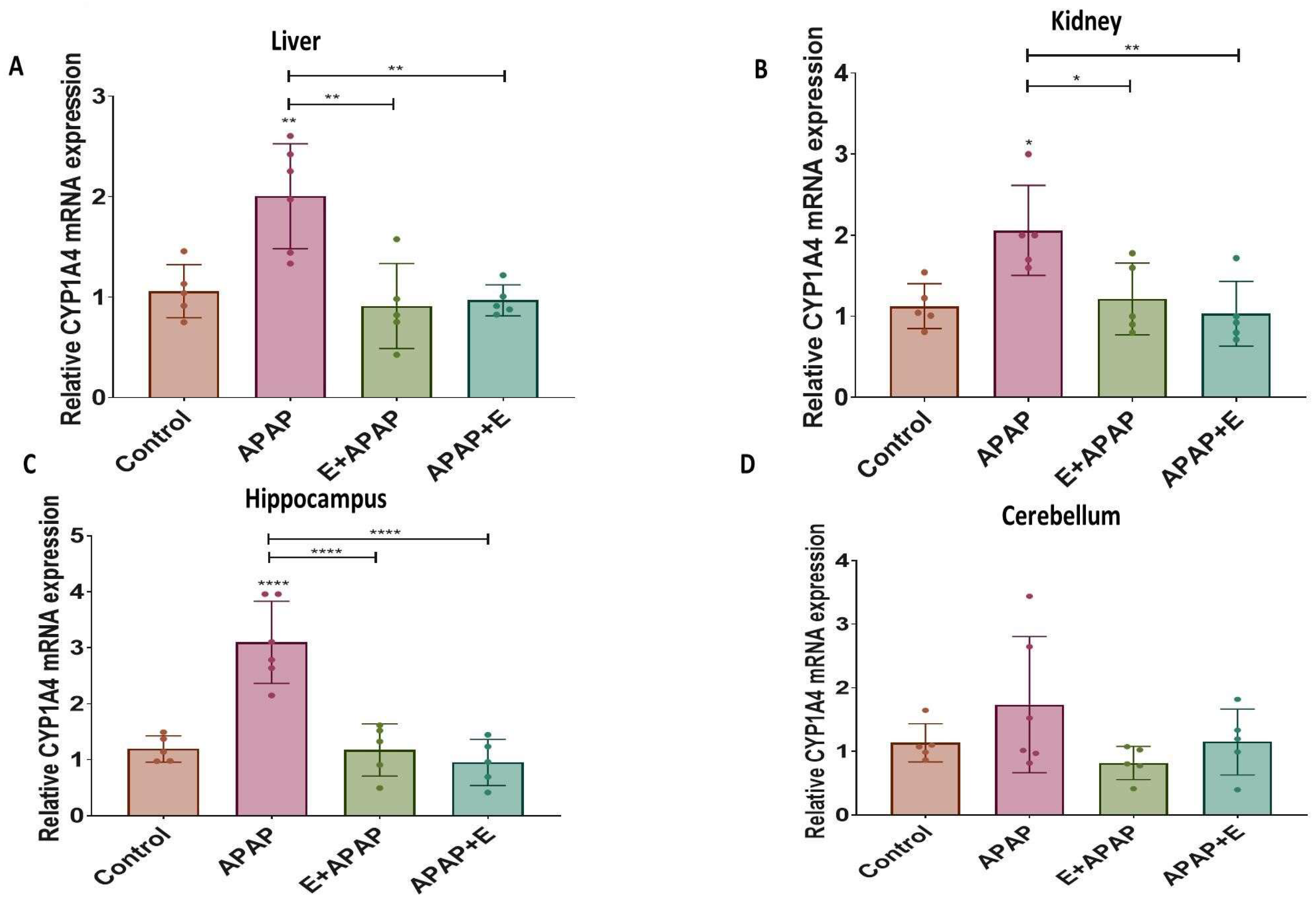
3.4. Real-Time Quantitative PCR Results:
3.4.1. Cyp1a4
3.4.2. Cyp2d6
3.4.3. Nat2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elayeh, E.; Akour, A.; Haddadin, R.N. Prevalence and predictors of self-medication drugs to prevent or treat COVID-19: Experience from a Middle Eastern country. Int. J. Clin. Pract. 2021, 75, e14860. [Google Scholar] [CrossRef] [PubMed]
- Malak, M.-Z.; AbuKamel, A. Self-medication Practices among University Students in Jordan. Malays. J. Med. Health Sci. 2019, 15, 112–119. [Google Scholar]
- Breu, A.C.; Patwardhan, V.R.; Nayor, J.; Ringwala, J.N.; Devore, Z.G.; Ganatra, R.B.; Hathorn, K.E.; Horton, L.; Iriana, S.; Tapper, E.B. A multicenter study into causes of severe acute liver injury. Clin. Gastroenterol. Hepatol. 2019, 17, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.; Tanoli, O.; Légaré, G.; Dubé, P.-A.; Habel, Y.; Lesage, A.; Low, N.C.; Lamarre, S.; Singh, S.; Rahme, E. Over-the-counter drugs and other substances used in attempted suicide presented to emergency departments in Montreal, Canada. Crisis 2018, 40, 166–175. [Google Scholar] [CrossRef]
- Moore, N.; Duret, S.; Grolleau, A.; Lassalle, R.; Barbet, V.; Duong, M.; Thurin, N.; Droz-Perroteau, C.; Gulmez, S.E. Previous drug exposure in patients hospitalised for acute liver injury: A case-population study in the French National Healthcare Data System. Drug Saf. 2019, 42, 559–572. [Google Scholar] [CrossRef]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ndetan, H.; Evans, M.W., Jr.; Singal, A.K.; Brunner, L.J.; Calhoun, K.; Singh, K.P. Light to moderate drinking and therapeutic doses of acetaminophen: An assessment of risks for renal dysfunction. Prev. Med. Rep. 2018, 12, 253–258. [Google Scholar] [CrossRef]
- Wilkes, J.M.; Clark, L.E.; Herrera, J.L. Acetaminophen Overdose in Pregnancy. South. Med. J. 2005, 98, 1118–1122. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Swan, S.H.; Kriebel, D.; Liew, Z.; Taylor, H.S.; Bornehag, C.-G.; Andrade, A.M.; Olsen, J.; Jensen, R.H.; Mitchell, R.T. Paracetamol use during pregnancy—A call for precautionary action. Nat. Rev. Endocrinol. 2021, 17, 757–766. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; De Meyts, E.R.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Apmis 2001, 109, S22–S30. [Google Scholar] [CrossRef]
- Albert, O.; Desdoits-Lethimonier, C.; Lesné, L.; Legrand, A.; Guillé, F.; Bensalah, K.; Dejucq-Rainsford, N.; Jégou, B. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum. Reprod. 2013, 28, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.M.; Mazaud-Guittot, S.; Gaudriault, P.; Lesné, L.; Serrano, T.; Main, K.M.; Jégou, B. Analgesic use—Prevalence, biomonitoring and endocrine and reproductive effects. Nat. Rev. Endocrinol. 2016, 12, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, F.S.; Mazaud-Guittot, S.; Jégou, B.; Kristensen, D.M. EDC IMPACT: Is exposure during pregnancy to acetaminophen/paracetamol disrupting female reproductive development? Endocr. Connect. 2018, 7, 149. [Google Scholar] [CrossRef]
- Rossitto, M.; Ollivier, M.; Déjardin, S.; Pruvost, A.; Brun, C.; Marchive, C.; Nguyen, A.L.; Ghettas, A.; Keime, C.; De Massy, B. In utero exposure to acetaminophen and ibuprofen leads to intergenerational accelerated reproductive aging in female mice. Commun. Biol. 2019, 2, 310. [Google Scholar] [CrossRef]
- Thiele, K.; Solano, M.E.; Huber, S.; Flavell, R.A.; Kessler, T.; Barikbin, R.; Jung, R.; Karimi, K.; Tiegs, G.; Arck, P.C. Prenatal acetaminophen affects maternal immune and endocrine adaptation to pregnancy, induces placental damage, and impairs fetal development in mice. Am. J. Pathol. 2015, 185, 2805–2818. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015, 4, 60–73. [Google Scholar] [CrossRef]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New Vistas of an Old Drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H.J. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef]
- Pingili, R.B.; Pawar, A.K.; Challa, S.R. Effect of chrysin on the formation of N-acetyl-p-benzoquinoneimine, a toxic metabolite of paracetamol in rats and isolated rat hepatocytes. Chem.-Biol. Interact. 2019, 302, 123–134. [Google Scholar] [CrossRef]
- Rothen, J.-P.; Haefeli, W.; Meyer, U.A.; Todesco, L.; Wenk, M. Acetaminophen is an inhibitor of hepatic N-acetyltransferase 2 in vitro and in vivo. Pharmacogenetics 1998, 8, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xie, G.; Su, M.; Wu, X.; Lu, X.; Wu, K.; Wei, C. Characterization of acetaminophen-induced cytotoxicity in target tissues. Am. J. Transl. Res. 2016, 8, 4440. [Google Scholar] [PubMed]
- Wang, X.; Wu, Q.; Liu, A.; Anadón, A.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Paracetamol: Overdose-induced oxidative stress toxicity, metabolism, and protective effects of various compounds in vivo and in vitro. Drug Metab. Rev. 2017, 49, 395–437. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-induced liver injury: Cascade of events leading to cell death, apoptosis or necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef]
- Ozatik, F.Y.; Teksen, Y.; Kadioglu, E.; Ozatik, O.; Bayat, Z. Effects of hydrogen sulfide on acetaminophen-induced acute renal toxicity in rats. Int. Urol. Nephrol. 2019, 51, 745–754. [Google Scholar] [CrossRef]
- Kennon-McGill, S.; McGill, M.R. Extrahepatic Toxicity of Acetaminophen: Critical Evaluation of the Evidence and Proposed Mechanisms. J. Clin. Transl. Res. 2018, 3, 5. [Google Scholar] [CrossRef]
- Moshaei-Nezhad, P.; Hosseini, S.M.; Yahyapour, M.; Iman, M.; Khamesipour, A. Protective effect of ivy leaf extract on paracetamol-induced oxidative stress and nephrotoxicity in mice. J. Herbmed Pharmacol. 2019, 8, 64–68. [Google Scholar] [CrossRef]
- Abu, I.F.; Mat, A.C.; Zulkifli, M.; Juliana, N.; Mohamad, M.H.N. Improvement of kidney histological morphology in nephrotoxic paracetamol-induced rats by Cassia alata treatment. Int. J. Res. Pharm. Sci. 2018, 9, 6–11. [Google Scholar]
- Upadhya, S.C.; Tirumalai, P.S.; Boyd, M.R.; Mori, T.; Ravindranath, V. Cytochrome P4502E (Cyp2e) in Brain: Constitutive Expression, Induction by Ethanol and Localization by Fluorescence in Situ Hybridization. Arch. Biochem. Biophys. 2000, 373, 23–34. [Google Scholar] [CrossRef]
- Howard, L.A.; Miksys, S.; Hoffmann, E.; Mash, D.; Tyndale, R.F. Brain Cyp2e1 is induced by nicotine and ethanol in rat and is higher in smokers and alcoholics. Br. J. Pharmacol. 2003, 138, 1376–1386. [Google Scholar] [CrossRef]
- Joshi, M.; Tyndale, R.F. Induction and recovery time course of rat brain Cyp2e1 after nicotine treatment. Drug Metab. Dispos. 2006, 34, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Santos, P.; Blanco, A.; Muñoz-Fernández, M.; Ceña, V. Acetaminophen induces apoptosis in rat cortical neurons. PloS ONE 2010, 5, e15360. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.I.; Pérez, M.J.; Manautou, J.E.; Mottino, A.D. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol. Res. 2016, 109, 119–131. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, H.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Grady, C.C.; Crouch, R.A.; Lie, R.K.; Miller, F.G.; Wendler, D.D. The Oxford Textbook of Clinical Research Ethics; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Henneh, I.T.; Ahlidja, W.; Alake, J.; Kwabil, A.; Ahmed, M.A.; Kyei-Asante, B.; Adinortey, M.B.; Ekor, M.; Armah, F.A. Ziziphus abyssinica root bark extract ameliorates paracetamol-induced liver toxicity in rats possibly via the attenuation of oxidative stress. Toxicol. Rep. 2022, 9, 1929–1937. [Google Scholar] [CrossRef]
- Doğan, N.; Akçam, M.; Koca, T.; Doğuç, D.K.; Özgöçmen, M. The protective effect of Capparis ovata in acute hepatotoxicity induced by paracetamol. Turk. J. Med. Sci. 2016, 46, 561–566. [Google Scholar] [CrossRef]
- Iyanda, A.A.; Adeniyi, F.A.A. Biochemical and histologic presentations of female Wistar rats administered with different doses of paracetamol/methionine. Niger. J. Physiol. Sci. 2011, 26, 151–160. [Google Scholar]
- Tras, B.; Faki, H.E.; Kutahya, Z.O.; Bahcivan, E.; Dik, B.; Uney, K. The effects of dexamethasone and minocycline alone and combined with N-acetylcysteine and vitamin E on serum matrix metalloproteinase-9 and coenzyme Q10 levels in aflatoxin B1 administered rats. Pol. J. Vet. Sci. 2022, 25, 419–427. [Google Scholar]
- Raeeszadeh, M.; Saleh Hosseini, S.M.; Amiri, A.A. Impact of Co-Administration of N-Acetylcysteine and Vitamin E on Cyclophosphamide-Induced Ovarian Toxicity in Female Rats. J. Toxicol. 2022, 2022, 9073405. [Google Scholar] [CrossRef]
- Sudheesh, N.; Ajith, T.; Janardhanan, K. Hepatoprotective effects of DL-α-lipoic acid and α-Tocopherol through amelioration of the mitochondrial oxidative stress in acetaminophen challenged rats. Toxicol. Mech. Methods 2013, 23, 368–376. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- El-Boshy, M.; BaSalamah, M.A.; Ahmad, J.; Idris, S.; Mahbub, A.; Abdelghany, A.H.; Almaimani, R.A.; Almasmoum, H.; Ghaith, M.M.; Elzubier, M. Vitamin D protects against oxidative stress, inflammation and hepatorenal damage induced by acute paracetamol toxicity in rat. Free. Radic. Biol. Med. 2019, 141, 310–321. [Google Scholar] [CrossRef]
- Jafri, M.; Subhani, M.J.; Javed, K.; Singh, S. Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J. Ethnopharmacol. 1999, 66, 355–361. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Budin, S.B.; Jie, N.W.; Hamid, A.; Husain, K.; Mohamed, J. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J. Zhejiang Univ. Sci. B 2012, 13, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Kelleher, J.; Dixon, M.; Losowsky, M. Vitamin E protection of the liver from paracetamol in the rat. Clin. Sci. Mol. Med. 1974, 47, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Trumper, L.; Girardi, G.; Elías, M.M. Acetaminophen nephrotoxicity in male Wistar rats. Arch. Toxicol. 1992, 66, 107–111. [Google Scholar] [CrossRef]
- Cekmen, M.; Ilbey, Y.; Ozbek, E.; Simsek, A.; Somay, A.; Ersoz, C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem. Toxicol. 2009, 47, 1480–1484. [Google Scholar] [CrossRef]
- Şener, G.; Şehirli, A.Ö.; Ayanoğlu-Dülger, G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice: A comparative study. J. Pineal Res. 2003, 35, 61–68. [Google Scholar] [CrossRef]
- Sathishkumar, T.; Baskar, R. Renoprotective effect of Tabernaemontana heyneana Wall. leaves against paracetamol-induced renotoxicity in rats and detection of polyphenols by high-performance liquid chromatography–diode array detector–mass spectrometry analysis. J. Acute Med. 2014, 4, 57–67. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Harbi, M.S. Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver with silymarin and Nigella sativa extract supplements. Asian Pac. J. Trop. Biomed. 2015, 5, 521–531. [Google Scholar] [CrossRef]
- da Silva, M.H.; da Rosa, E.J.F.; de Carvalho, N.R.; Dobrachinski, F.; da Rocha, J.B.T.; Mauriz, J.L.; González-Gallego, J.; Soares, F.A.A. Acute brain damage induced by acetaminophen in mice: Effect of diphenyl diselenide on oxidative stress and mitochondrial dysfunction. Neurotox. Res. 2012, 21, 334–344. [Google Scholar] [CrossRef]
- Mohammed, E.; Safwat, G. Assessment of the ameliorative role of selenium nanoparticles on the oxidative stress of acetaminophen in some tissues of male albino rats. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 80–85. [Google Scholar] [CrossRef]
- Manyike, P.T.; Kharasch, E.D.; Kalhorn, T.F.; Slattery, J.T. Contribution of Cyp2e1 and Cyp3a to acetaminophen reactive metabolite formation. Clin. Pharmacol. Ther. 2000, 67, 275–282. [Google Scholar] [CrossRef]
- Rumack, B.H. Acetaminophen misconceptions. Hepatology 2004, 40, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pickering, G. Paracetamol metabolism and related genetic differences. Drug Metab. Rev. 2011, 43, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H. Acetaminophen Associated Neurotoxicity and Its Relevance to Neurodevelopmental Disorders; University of South Florida: Tampa, FL, USA, 2017. [Google Scholar]
- Dressler, W.E.; Appelqvist, T. Plasma/blood pharmacokinetics and metabolism after dermal exposure to para-aminophenol or para-phenylenediamine. Food Chem. Toxicol. 2006, 44, 371–379. [Google Scholar] [CrossRef]
- Estrada, L.; Kanelakis, K.C.; Levy, G.N.; Weber, W.W. Tissue-and Gender-Specific Expression ofN-Acetyltransferase 2 (Nat2*) during Development of the Outbred Mouse Strain CD-1. Drug Metab. Dispos. 2000, 28, 139–146. [Google Scholar]
- Jiang, Y.; Fan, X.; Wang, Y.; Chen, P.; Zeng, H.; Tan, H.; Gonzalez, F.J.; Huang, M.; Bi, H. Schisandrol B protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of liver regeneration. Toxicol. Sci. 2015, 143, 107–115. [Google Scholar] [CrossRef]
- Pattarachotanant, N.; Prasansuklab, A.; Tencomnao, T. Momordica charantia L. Extract Protects Hippocampal Neuronal Cells against PAHs-Induced Neurotoxicity: Possible Active Constituents Include Stigmasterol and Vitamin E. Nutrients 2021, 13, 2368. [Google Scholar] [CrossRef]
- Nirala, S.K.; Bhadauria, M.; Mathur, R.; Mathur, A. Amelioration of beryllium induced alterations in hepatorenal biochemistry and ultramorphology by co-administration of tiferron and adjuvants. J. Biomed. Sci. 2007, 14, 331–345. [Google Scholar] [CrossRef]
- Landes, N.; Pfluger, P.; Kluth, D.; Birringer, M.; Rühl, R.; Böl, G.F.; Glatt, H.; Brigelius-Flohé, R. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 2003, 65, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hodgman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef] [PubMed]



| Component | Sample (µL) | Blank 1 (µL) | Blank 2 (µL) | Blank 3 (µL) |
|---|---|---|---|---|
| Sample solution | 20 | 0 | 20 | 0 |
| ddH2O | 0 | 20 | 0 | 20 |
| WST working solution | 200 | 200 | 200 | 200 |
| Enzyme working solution | 20 | 20 | 0 | 0 |
| Dilution Buffer | 0 | 0 | 20 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, A.M.; Shawaqfeh, B.; Hikmat, S.; Al-Qirim, T.; Hamadneh, L.; Al-Kouz, S.; Awad, M.M.; Hall, F.S. The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats. Toxics 2023, 11, 368. https://doi.org/10.3390/toxics11040368
Hammad AM, Shawaqfeh B, Hikmat S, Al-Qirim T, Hamadneh L, Al-Kouz S, Awad MM, Hall FS. The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats. Toxics. 2023; 11(4):368. https://doi.org/10.3390/toxics11040368
Chicago/Turabian StyleHammad, Alaa M., Baraa Shawaqfeh, Suhair Hikmat, Tariq Al-Qirim, Lama Hamadneh, Sameer Al-Kouz, Mariam M. Awad, and Frank S. Hall. 2023. "The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats" Toxics 11, no. 4: 368. https://doi.org/10.3390/toxics11040368
APA StyleHammad, A. M., Shawaqfeh, B., Hikmat, S., Al-Qirim, T., Hamadneh, L., Al-Kouz, S., Awad, M. M., & Hall, F. S. (2023). The Role of Vitamin E in Protecting against Oxidative Stress, Inflammation, and the Neurotoxic Effects of Acute Paracetamol in Pregnant Female Rats. Toxics, 11(4), 368. https://doi.org/10.3390/toxics11040368







