Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Collection
2.2. Experimental Design
2.3. Analytical Methods
2.3.1. COD Determination
2.3.2. TOC Determination
2.3.3. Determination of N-NH3
2.3.4. Determination of Color
3. Results and Discussion
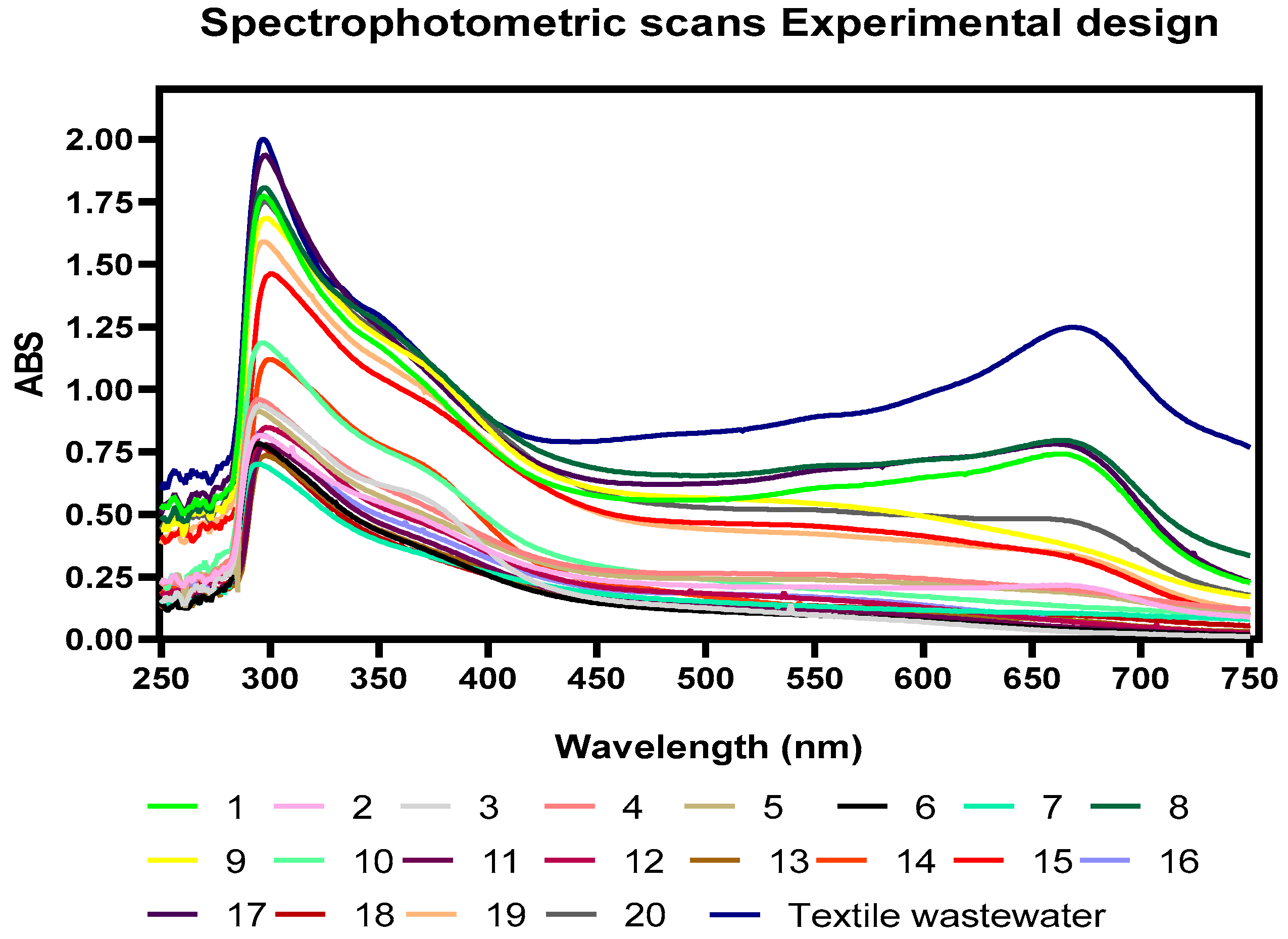
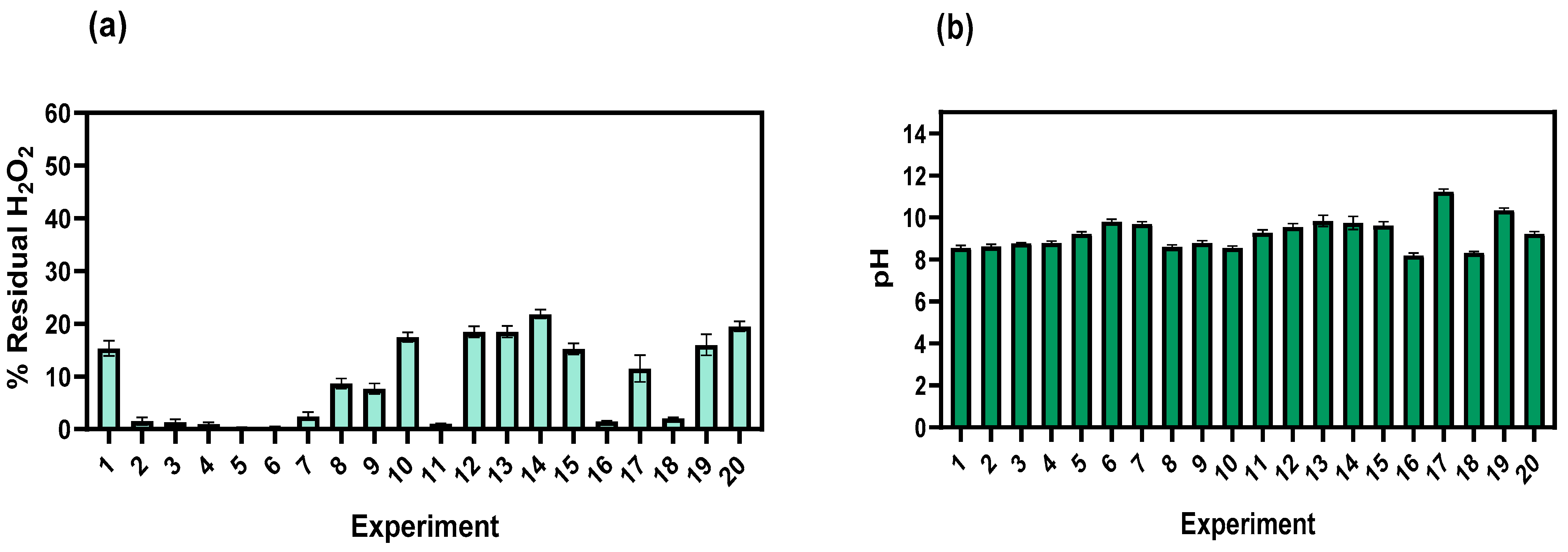
3.1. Physicochemical Characterization
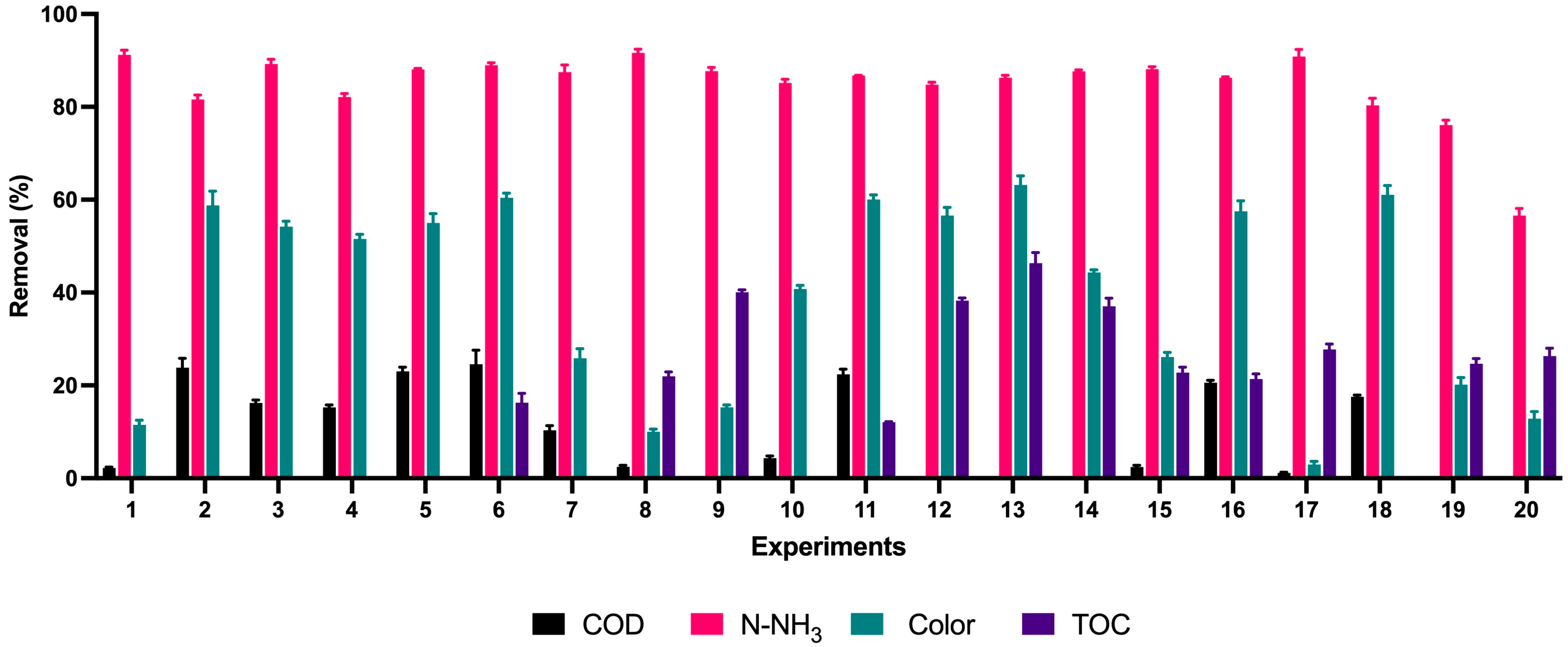
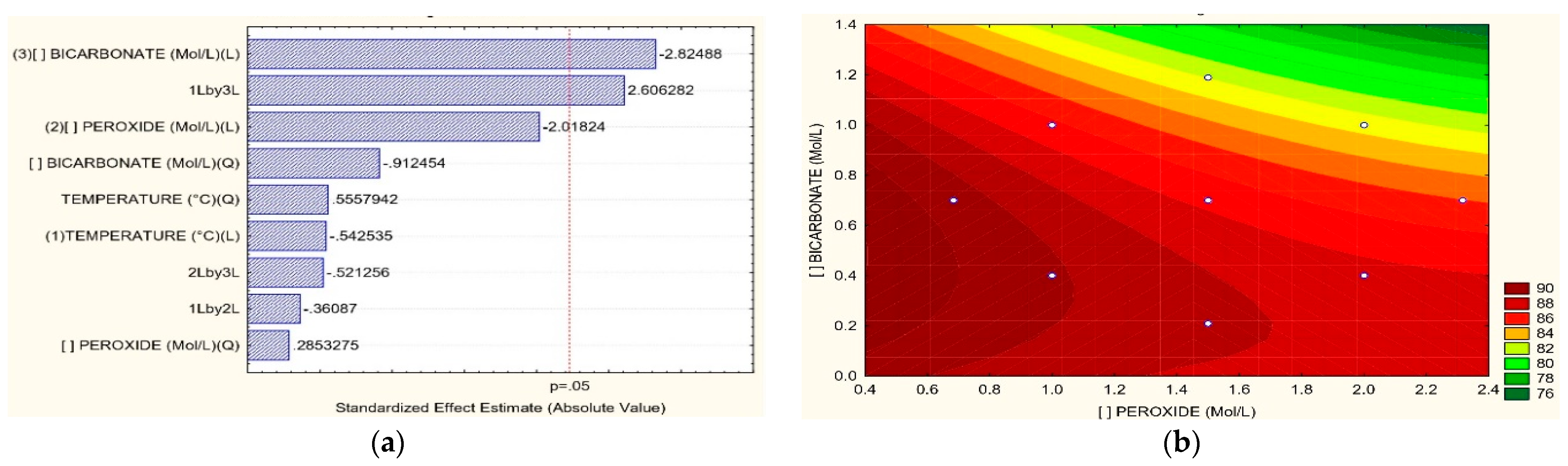
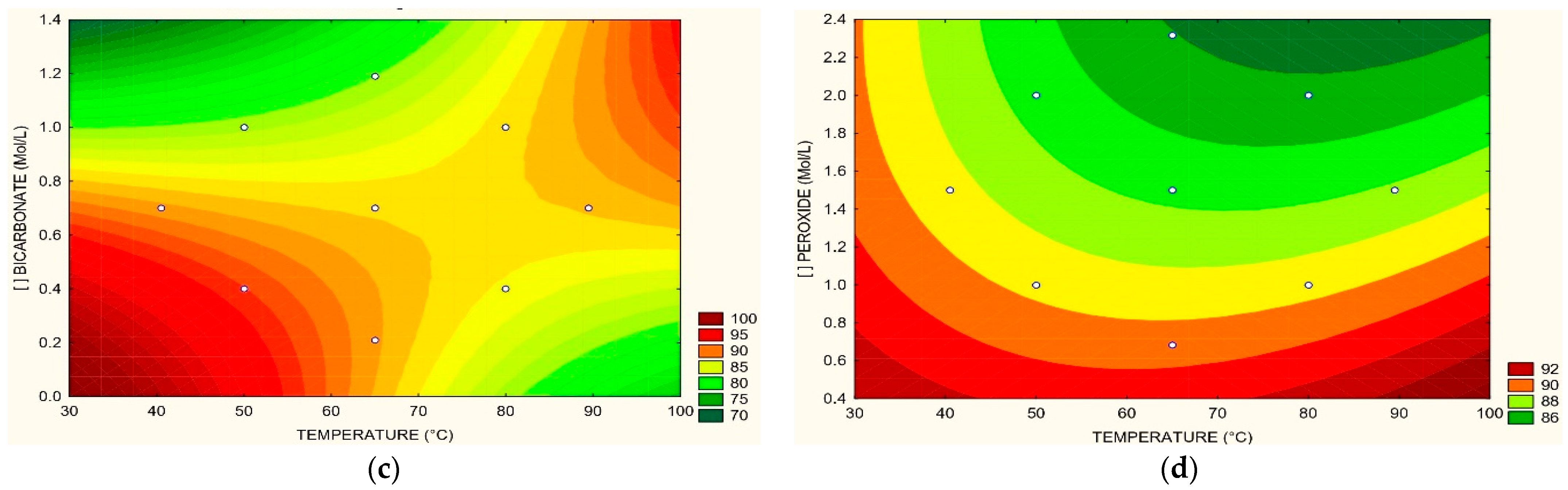
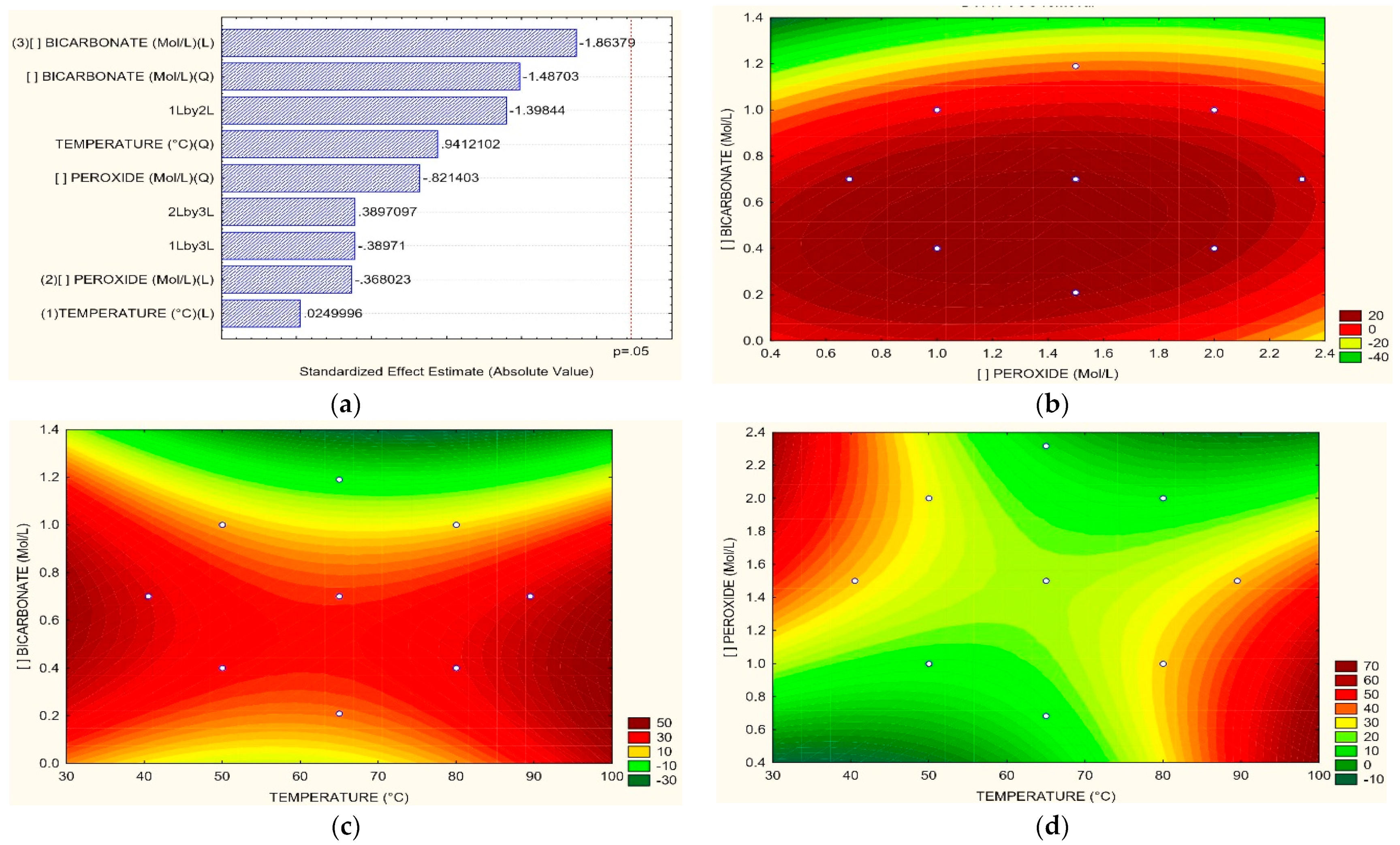
3.2. Removal of COD, TOC, Color, and N-NH3
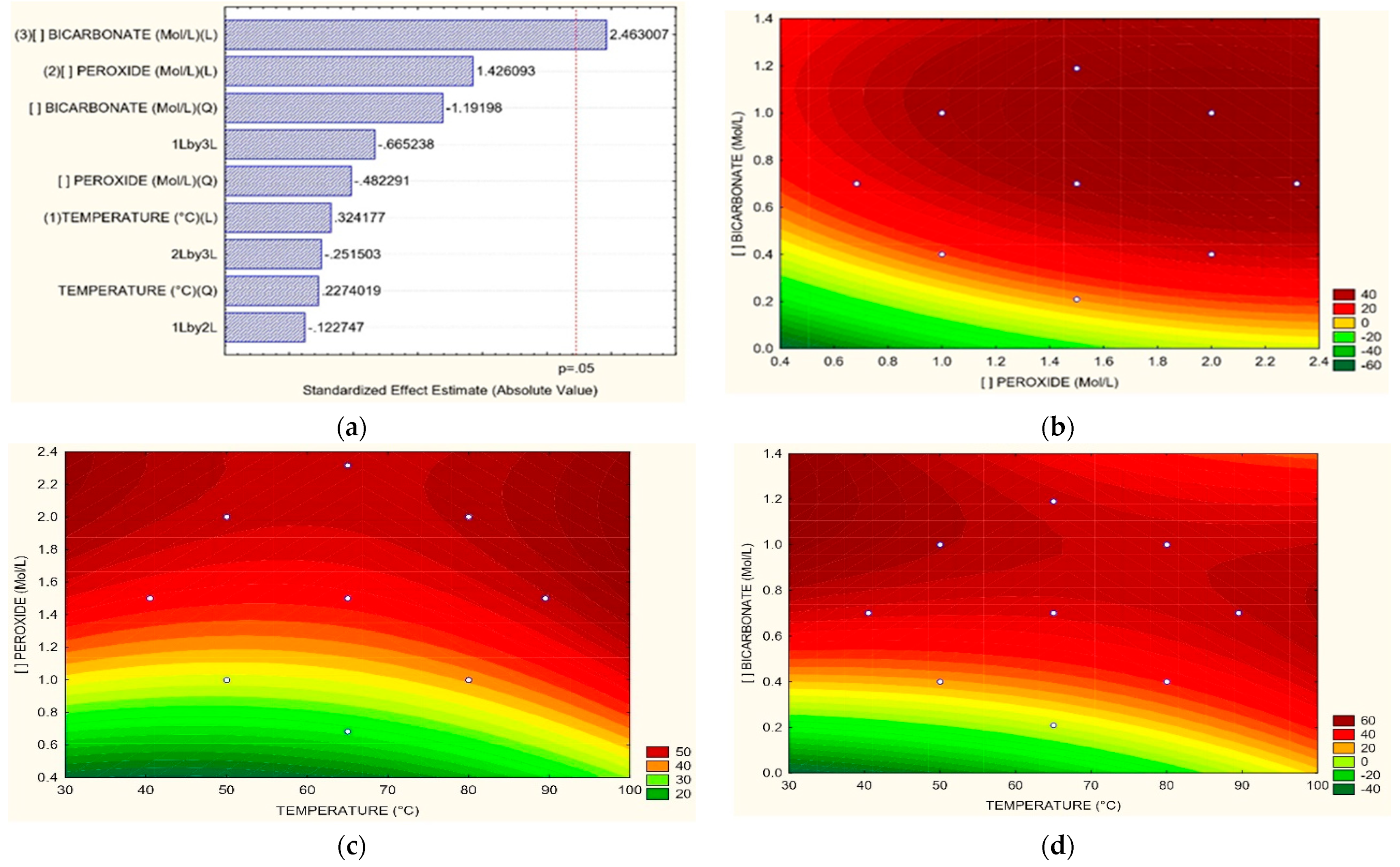
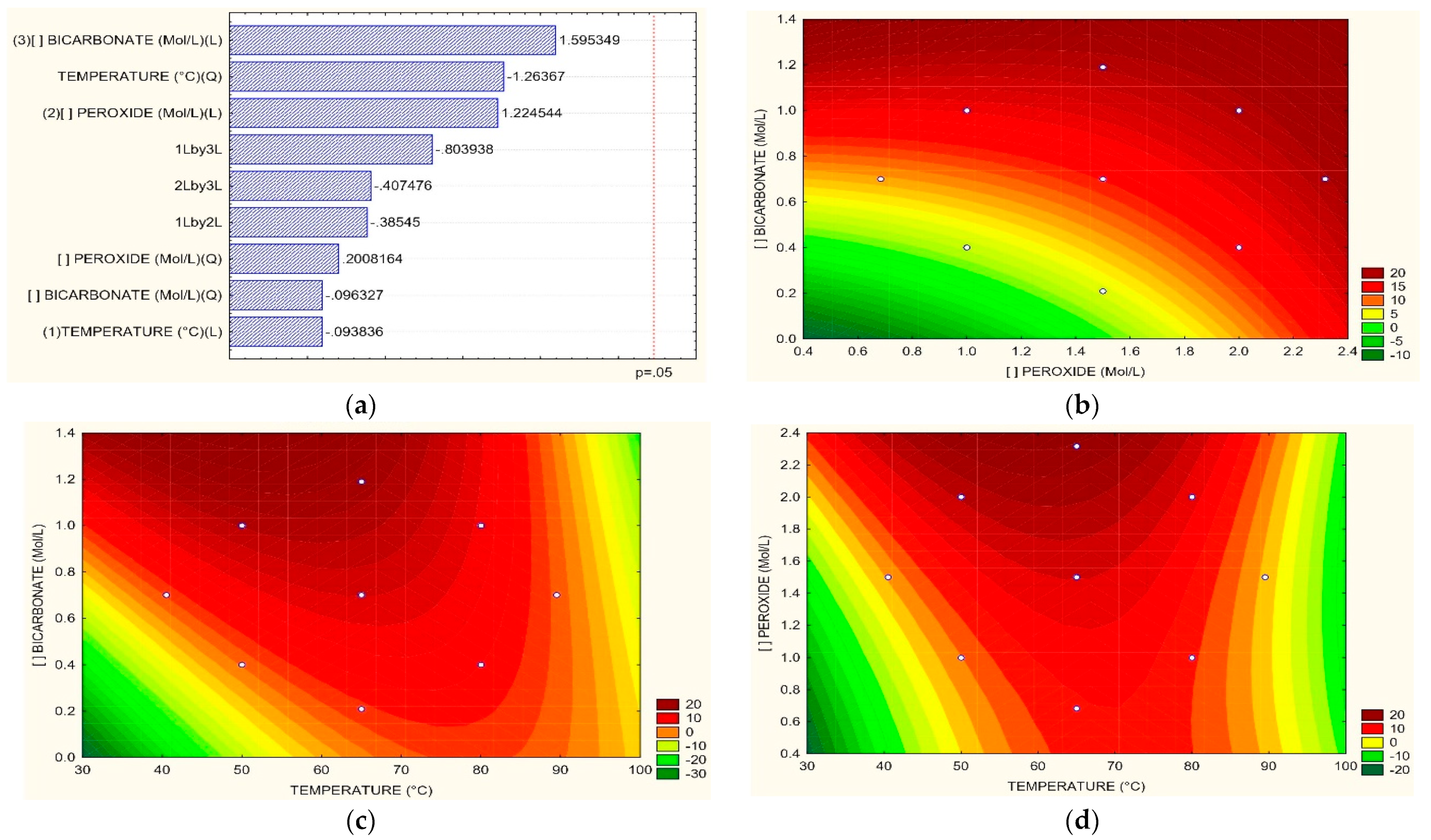
3.3. Optimum Process Conditions
| AOP | Volume Treated/Year (m3) | Labor Costs (€) | Reagent Cost (€) | Energy Costs (€) | Total Cost/Year (€) | Reference |
|---|---|---|---|---|---|---|
| O3 | 63,170 | 61,957.07 | 0 | 483,717.81 | 545,674.88 | [80] |
| O3/UV | 90,880 | 419,403.16 | 481,360.23 | |||
| H2O2/O3/UV | 49,600 | 2338.71 | 323,855.36 | 388,151.15 | ||
| H2O2/UV | 22,450 | 3178.01 | 54,488.27 | 119,623.36 | ||
| Solar/Fe(II)/H2O2 | 365,000 | 12,100 | 33,400 | 20,100 | 65,600 | [81] |
| Solar/Fe(II)/PDS | 145,300 | 177,500 | ||||
| UV/PDS | 27,300 | 50,500 | 89,900 | |||
| UV/H2O2 | 1800 | 62,100 | 76,000 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tounsadi, H.; Metarfi, Y.; Taleb, M.; El Rhazi, K.; Rais, Z. Impact of chemical substances used in textile industry on the employee’s health: Epidemiological study. Ecotoxicol. Environ. Saf. 2020, 197, 110594. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Abdollahzadeh-Sharghi, E.; Bonakdarpour, B. Anaerobic-aerobic processes for the treatment of textile dyeing wastewater containing three commercial reactive azo dyes: Effect of number of stages and bioreactor type. Chin. J. Chem. Eng. 2020, 39, 228–239. [Google Scholar] [CrossRef]
- Sarker, M.R.; Chowdhury, M.; Deb, A. Reduction of Color Intensity from Textile Dye Wastewater Using Microorganisms: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 3407–3415. [Google Scholar] [CrossRef]
- Al Prol, A.E. Study of Environmental Concerns of Dyes and Recent Textile Effluents Treatment Technology: A Review. Asian J. Fish. Aquat. Res. 2019, 3, 1–18. [Google Scholar] [CrossRef]
- Qadeer, H.A.; Fawzi Mahomoodally, M.; Nadeem, F.; Khanam, A. Wastewater Treatment and Dyes Removal Using Electrocoagulation aided by Natural Biosorbents—A Review. Int. J. Chem. Biochem. Sci. 2018, 14, 77–87. [Google Scholar]
- Al-Ghouti, M.A.; Khraisheh, M.A.M.; Allen, S.J.; Ahmad, M.N. The removal of dyes from textile wastewater: A study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef]
- Waranusantigul, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E.S. Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 2003, 125, 385–392. [Google Scholar] [CrossRef]
- Janaina, A.K.; Miguel, P.; Davi, B.G.; Barrella, W. Textile sustainability: A Brazilian etiquette issue. Environ. Sci. Policy 2020, 109, 125–130. [Google Scholar] [CrossRef]
- Amutha, K. Sustainable chemical management and zero discharges. In Sustainable Fibres and Textiles; Muthu, S.S., Ed.; Woodhead Publishing: Duxford, UK, 2017. [Google Scholar]
- Bharagava, R.N.; Chowdhary, P. Textile wastewater dyes: Toxicity profile and treatment approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R.N., Chowdhary, P., Eds.; Springer: Singapore, 2018; pp. 1–435. [Google Scholar]
- Atalay, S.; Ersöz, G. Hybrid application of advanced oxidation processes to dyes′ removal. In Green Chemistry and Water Remediation: Research and Applications; Sharma, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Machuca-Martínez, F.; Barajas-Solano, A.F. Advanced Oxidation Processes and Biotechnological Alternatives for the Treatment of Tannery Wastewater. Molecules 2021, 26, 3222. [Google Scholar] [CrossRef]
- Urbina-Suarez, N.A.; Ayala-González, D.D.; Rivera-Amaya, J.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Evaluation of the Light/Dark Cycle and Concentration of Tannery Wastewater in the Production of Biomass and Metabolites of Industrial Interest from Microalgae and Cyanobacteria. Water 2022, 14, 346. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Pan, H.; Gao, Y.; Li, N.; Zhou, Y.; Lin, Q.; Jiang, J. Recent advances in bicarbonate-activated hydrogen peroxide system for water treatment. Chem. Eng. J. 2021, 408, 127332. [Google Scholar] [CrossRef]
- Roy, M.; Saha, R. Dyes and their removal technologies from wastewater: A critical review. In Intelligent Environmental Data Monitoring for Pollution Management; Bhattacharyya, S., Mondal, N.K., Platos, J., Snášel, V., Krömer, P., Eds.; Academic Press: London, UK, 2021. [Google Scholar]
- Kan, H.; Soklun, H.; Yang, Z.; Wu, R.; Shen, J.; Qu, G.; Wang, T. Purification of dye wastewater using bicarbonate activated hydrogen peroxide: Reaction process and mechanisms. Sep. Purif. Technol. 2020, 232, 115974. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Scaria, J.; Babu, D.S.; Kumar, M.S. An overview on combined electrocoagulation-degradation processes for the effective treatment of water and wastewater. Chemosphere 2021, 263, 127907. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Soklun, H.; Qu, G.; Xia, T.; Guo, X.; Jia, H.; Zhu, L. A green strategy for simultaneous Cu(II)-EDTA decomplexation and Cu precipitation from water by bicarbonate-activated hydrogen peroxide/chemical precipitation. Chem. Eng. J. 2019, 370, 1298–1309. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Chen, Z.X.; Zhang, J.; Gong, L.; Wang, Y.X.; Zhao, H.Q.; Mu, Y. Carbonate-activated hydrogen peroxide oxidation process for azo dye decolorization: Process, kinetics, and mechanisms. Chemosphere 2018, 192, 372–378. [Google Scholar] [CrossRef]
- Puiu, M.; Galaon, T.; Bondilǎ, L.; Rǎducan, A.; Oancea, D. Feed-back action of nitrite in the oxidation of nitrophenols by bicarbonate-activated peroxide system. Appl. Catal. A Gen. 2016, 516, 90–99. [Google Scholar] [CrossRef]
- Sökmen, M.; Özkan, A. Decolourising textile wastewater with modified titania: The effects of inorganic anions on the photocatalysis. J. Photochem. Photobiol. A Chem. 2002, 147, 77–81. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; 2017APHA/AWWA/WEF; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Silva, L.G.M.; Moreira, F.C.; Cechinel, M.A.P.; Mazur, L.P.; de Souza, A.A.U.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Integration of Fenton’s reaction based processes and cation exchange processes in textile wastewater treatment as a strategy for water reuse. J. Environ. Manag. 2020, 272, 111082. [Google Scholar] [CrossRef]
- Hussain, Z.; Arslan, M.; Shacir, G.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Remediation of textile bleaching effluent by bacterial augmented horizontal flow and vertical flow constructed wetlands: A comparison at pilot scale. Sci. Total Environ. 2019, 685, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Patra, P.; Das, C.R.; Raut, S.; Raut, S. Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: An impact on its use in irrigation of wheat crop. Water Resour. Ind. 2019, 21, 100106. [Google Scholar] [CrossRef]
- Subashini, P.S.; Rajiv, P. An investigation of textile wastewater treatment using chlorella vulgaris. Orient. J. Chem. 2018, 34, 2517–2524. [Google Scholar] [CrossRef]
- Assémian, A.S.; Kouassi, K.E.; Zogbe, A.E.; Adouby, K.; Drogui, P. In-situ generation of effective coagulant to treat textile bio-refractory wastewater: Optimization through response surface methodology. J. Environ. Chem. Eng. 2018, 6, 5587–5594. [Google Scholar] [CrossRef]
- Ćurić, I.; Dolar, D.; Karadakić, K. Textile wastewater reusability in knitted fabric washing process using UF membrane technology. J. Clean. Prod. 2021, 299, 126899. [Google Scholar] [CrossRef]
- Esther Baby, J.; Jaambavi, I.; Rajeswari, G.; Akshaya, T. Optimization removal of colour and organic solid pollutants from textile industry wastewater by electrocoagulation. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 0631 de 2015; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2015.
- Guedes, A.M.F.M.; Madeira, L.M.P.; Boaventura, R.A.R.; Costa, C.A.V. Fenton oxidation of cork cooking wastewater—Overall kinetic analysis. Water Res. 2003, 37, 3061–3069. [Google Scholar] [CrossRef]
- Zhang, H.; Heung, J.C.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Medley, D.R.; Stover, E.L. Effects of Ozone on the Biodegradability of Biorefractory Pollutants on JSTOR. J. Water Pollut. Control Fed. 1983, 55, 489–494. [Google Scholar]
- Hansson, H.; Kaczala, F.; Marques, M.; Hogland, W. Photo-Fenton and Fenton oxidation of recalcitrant industrial wastewater using nanoscale zero-valent iron. Int. J. Photoenergy 2012, 2012, 531076. [Google Scholar] [CrossRef]
- Ağtaş, M.; Yılmaz, Ö.; Dilaver, M.; Alp, K.; Koyuncu, İ. Hot water recovery and reuse in textile sector with pilot scale ceramic ultrafiltration/nanofiltration membrane system. J. Clean. Prod. 2020, 256, 120359. [Google Scholar] [CrossRef]
- Jawad, A.; Li, Y.; Lu, X.; Chen, Z.; Liu, W.; Yin, G. Controlled leaching with prolonged activity for Co-LDH supported catalyst during treatment of organic dyes using bicarbonate activation of hydrogen peroxide. J. Hazard. Mater. 2015, 289, 165–173. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Chen, M.; Luo, M.; Xia, D.; Xu, A.; Zeng, Q. Fast Degradation and Biodegradability Improvement of Reactive Brilliant Red X-3B by the Cobalt(II)/Bicarbonate/Hydrogen Peroxide System. Ind. Eng. Chem. Res. 2012, 51, 11104–11111. [Google Scholar] [CrossRef]
- Balagam, B.; Richardson, D.E. The mechanism of carbon dioxide catalysis in the hydrogen peroxide N-oxidation of amines. Inorg. Chem. 2008, 47, 1173–1178. [Google Scholar] [CrossRef]
- Bennett, D.A.; Yao, H.; Richardson, D.E. Mechanism of sulfide oxidations by peroxymonocarbonate. Inorg. Chem. 2001, 40, 2996–3001. [Google Scholar] [CrossRef]
- Yao, H.; Richardson, D.E. Bicarbonate surfoxidants: Micellar oxidations of aryl sulfides with bicarbonate-activated hydrogen peroxide. J. Am. Chem. Soc. 2003, 125, 6211–6221. [Google Scholar] [CrossRef]
- Jawad, A.; Lu, X.; Chen, Z.; Yin, G. Degradation of chlorophenols by supported Co-Mg-Al layered double hydrotalcite with bicarbonate activated hydrogen peroxide. J. Phys. Chem. A 2014, 118, 10028–10035. [Google Scholar] [CrossRef]
- Zhao, T.; Li, P.; Tai, C.; She, J.; Yin, Y.; Qi, Y.; Zhang, G. Efficient decolorization of typical azo dyes using low-frequency ultrasound in presence of carbonate and hydrogen peroxide. J. Hazard. Mater. 2018, 346, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Pillai, I.M.S.; Gupta, A.K. Performance analysis of a continuous serpentine flow reactor for electrochemical oxidation of synthetic and real textile wastewater: Energy consumption, mass transfer coefficient and economic analysis. J. Environ. Manag. 2017, 193, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Ahmadi, M.; Gohari, F. Heterogeneous activation of peroxymonosulfate via nanocomposite CeO2-Fe3O4 for organic pollutants removal: The effect of UV and US irradiation and application for real wastewater. Sep. Purif. Technol. 2019, 228, 115732. [Google Scholar] [CrossRef]
- Nikravesh, B.; Shomalnasab, A.; Nayyer, A.; Aghababaei, N.; Zarebi, R.; Ghanbari, F. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater. J. Environ. Chem. Eng. 2020, 8, 104244. [Google Scholar] [CrossRef]
- Adar, E. Removal of Acid Yellow 17 from Textile Wastewater by Adsorption and Heterogeneous Persulfate Oxidation. Int. J. Environ. Sci. Technol. 2021, 18, 483–498. [Google Scholar] [CrossRef]
- Ruffino, B.; Zanetti, M. Orthophosphate vs. bicarbonate used as a buffering substance for optimizing the bromide-enhanced ozonation process for ammonia nitrogen removal. Sci. Total Environ. 2019, 692, 1191–1200. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Manenti, D.R.; Borba, F.H.; Palácio, S.M.; Colombo, A. Performance evaluation of a photo-Fenton process applied to pollutant removal from textile effluents in a batch system. J. Environ. Manag. 2012, 104, 1–8. [Google Scholar] [CrossRef]
- Jóźwiakowski, K.; Marzec, M.; Fiedurek, J.; Kamińska, A.; Gajewska, M.; Wojciechowska, E.; Wu, S.; Dach, J.; Marczuk, A.; Kowlaczyk-Juśko, A. Application of H2O2 to optimize ammonium removal from domestic wastewater. Sep. Purif. Technol. 2017, 173, 357–363. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 2015, 77, 213–248. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Osorio, V.; Perez, S.; Petrovic, M.; Zapata, A.; Malato, S.; Barceló, D.; Fatta-Kassinos, D. Solar photocatalytic treatment of trimethoprim in four environmental matrices at a pilot scale: Transformation products and ecotoxicity evaluation. Sci. Total Environ. 2012, 430, 167–173. [Google Scholar] [CrossRef]
- Hanna, P.M.; Kadiiska, M.B.; Mason, R.P. Oxygen-Derived Free Radical and Active Oxygen Complex Formation from Cobalt(II) Chelates in Vitro. Chem. Res. Toxicol. 1992, 5, 109–115. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Zhao, S. Fast degradation of Acid Orange II by bicarbonate-activated hydrogen peroxide with a magnetic S-modified CoFe2O4 catalyst. J. Taiwan Inst. Chem. Eng. 2015, 55, 90–100. [Google Scholar] [CrossRef]
- Bulca, Ö.; Palas, B.; Atalay, S.; Ersöz, G. Performance investigation of the hybrid methods of adsorption or catalytic wet air oxidation subsequent to electrocoagulation in treatment of real textile wastewater and kinetic modelling. J. Water Process Eng. 2021, 40, 101821. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K. Treatment of textile dyeing industry effluent using hydrodynamic cavitation in combination with advanced oxidation reagents. J. Hazard. Mater. 2018, 344, 1109–1115. [Google Scholar] [CrossRef]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.Á. Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Takdastan, A.; Jorfi, S.; Ghanbari, F.; Ahmadi, M.; Barzegar, G. The performance study on ultrasonic/Fe3O4/H2O2 for degradation of azo dye and real textile wastewater treatment. J. Mol. Liq. 2018, 256, 462–470. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Kiani, R.; Mirzaei, F.; Ghanbari, F.; Feizi, R.; Mehdipour, F. Real textile wastewater treatment by a sulfate radicals-Advanced Oxidation Process: Peroxydisulfate decomposition using copper oxide (CuO) supported onto activated carbon. J. Water Process Eng. 2020, 38, 101623. [Google Scholar] [CrossRef]
- Şahinkaya, S. COD and color removal from synthetic textile wastewater by ultrasound assisted electro-Fenton oxidation process. J. Ind. Eng. Chem. 2013, 19, 601–605. [Google Scholar] [CrossRef]
- Wu, T.; Englehardt, J.D. A new method for removal of hydrogen peroxide interference in the analysis of chemical oxygen demand. Environ. Sci. Technol. 2012, 46, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Pani, N.; Tejani, V.; Anantha-Singh, T.S.; Kandya, A. Simultaneous removal of COD and Ammoniacal Nitrogen from dye intermediate manufacturing Industrial Wastewater using Fenton oxidation method. Appl. Water Sci. 2020, 10, 66. [Google Scholar] [CrossRef]
- Cifcioglu-Gozuacik, B.; Ergenekon, S.M.; Ozbey-Unal, B.; Balcik, C.; Karagunduz, A.; Dizge, N.; Keskinler, B. Efficient removal of ammoniacal nitrogen from textile printing wastewater by electro-oxidation considering the effects of NaCl and NaOCl addition. Water Sci. Technol. 2021, 84, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, I.W.K.; Prajati, G.; Afifah, A.S.; Apritama, M.R. Nh3-n and cod reduction in endek (Balinese textile) wastewater by activated sludge under different do condition with ozone pretreatment. Walailak J. Sci. Technol. 2021, 18, 9127. [Google Scholar] [CrossRef]
- Eslami, A.; Mehdipour, F.; Lin, K.Y.A.; Sharifi Maleksari, H.; Mirzaei, F.; Ghanbari, F. Sono-photo activation of percarbonate for the degradation of organic dye: The effect of water matrix and identification of by-products. J. Water Process Eng. 2020, 33, 100998. [Google Scholar] [CrossRef]
- Ovhal, S.D.; Rodrigues, C.S.D.; Madeira, L.M. Photocatalytic wet peroxide assisted degradation of Orange II dye by reduced graphene oxide and zeolites. J. Chem. Technol. Biotechnol. 2021, 96, 349–359. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef]
- Muthukumar, M.; Selvakumar, N. Studies on the effect of inorganic salts on decolouration of acid dye effluents by ozonation. Dye. Pigment. 2004, 62, 221–228. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photochemical oxidation of reactive azo dye with UV–H2O2 process. Dye. Pigment. 2004, 62, 269–275. [Google Scholar] [CrossRef]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461–462, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xi, H.; Zuo, Y.; Wang, Q.; Wang, Z.; Yan, Z. Bicarbonate-activated hydrogen peroxide and efficient decontamination of toxic sulfur mustard and nerve gas simulants. J. Hazard. Mater. 2018, 344, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Chen, Y.; Zhang, K.; Luo, H.; Huang, J.; Xu, A. Catalytic degradation of Acid Orange 7 with hydrogen peroxide using Co x O y -N/GAC catalysts in a bicarbonate aqueous solution. RSC Adv. 2015, 5, 84303–84310. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; Darowna, D.; Wanag, A.; Mozia, S.; Malato, S.; Oller, I. Comparison of UV/H2O2, UV/S2O82−, solar/Fe(II)/H2O2 and solar/Fe(II)/S2O82− at pilot plant scale for the elimination of micro-contaminants in natural water: An economic assessment. Chem. Eng. J. 2017, 310, 514–524. [Google Scholar] [CrossRef]




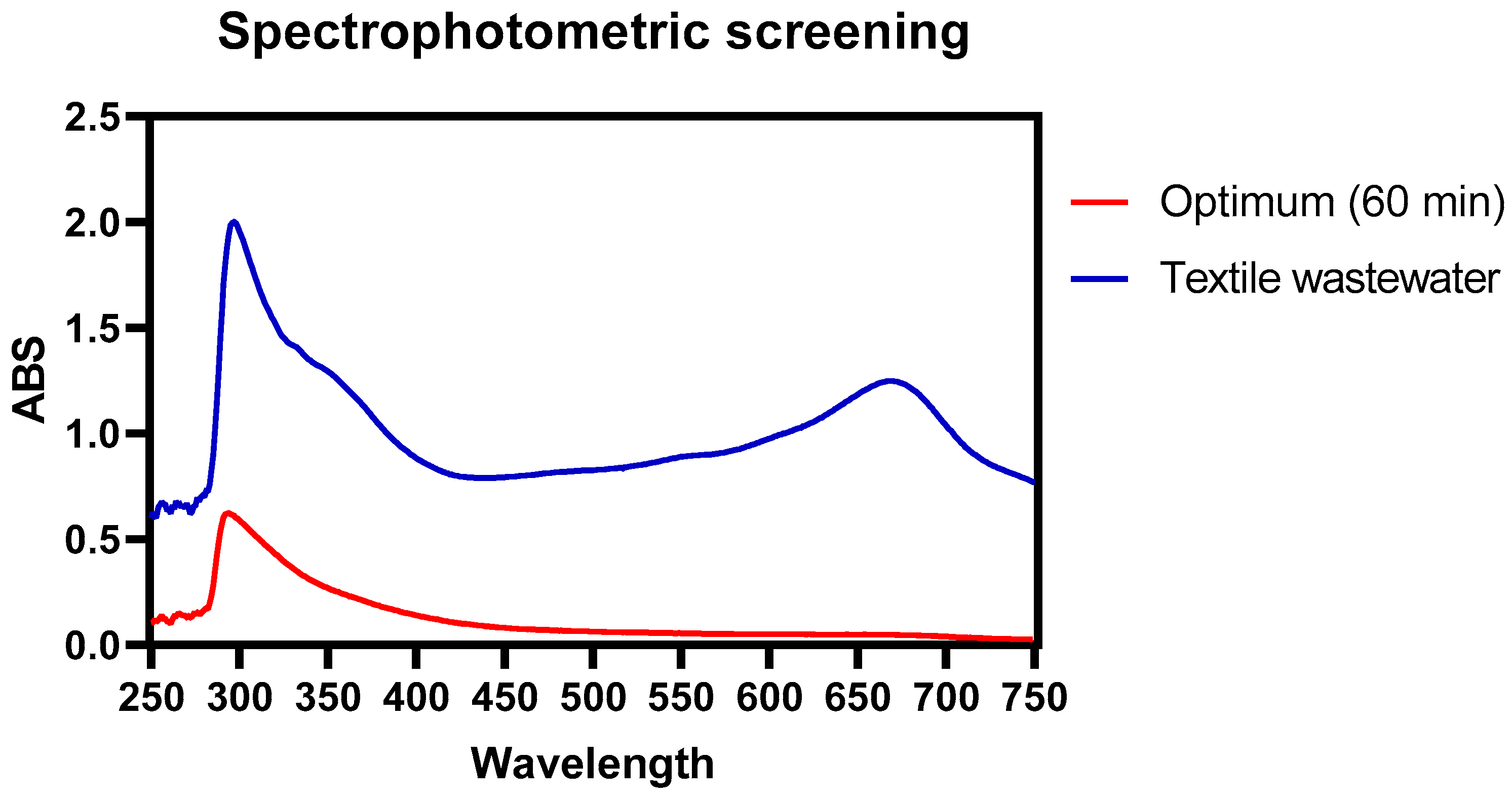
| Parameter | Units | Standard Methods Code |
|---|---|---|
| COD | mg × L−1 | 5220C |
| BOD | 5210B-4500-OG | |
| Nitrates | 4500-NO3 B | |
| Nitrites | 4500-NO2 B | |
| Ammonia nitrogen | 4500-NH3 F | |
| Phosphates | 4500-P C | |
| Total Suspended Solids (mg × L−1) | 2540D | |
| Heavy Metals Fe, Cr (mg × L−1) | 3111D | |
| Sulfides (mg × L−1) | 4500-S2 F | |
| Chlorides (mg × L−1) | 4500-ClB | |
| pH | pH units | 4500B |
| Conductivity | µS × cm−1 | 2510B |
| P | C | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | T14 | T15 | T16 | T17 | T18 | T19 | T20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 28 | 50 | 50 | 50 | 80 | 80 | 65 | 65 | 50 | 50 | 80 | 80 | 65 | 65 | 40.5 | 89.5 | 65 | 65 | 65 | 65 | 65 |
| H2O2 (M) | 0 | 1 | 1 | 2 | 1 | 2 | 1.5 | 1.5 | 1 | 2 | 1 | 2 | 1.5 | 1.5 | 1.5 | 1.5 | 0.68 | 2.31 | 1.5 | 1.5 | 1.5 |
| HCO3 (M) | 0 | 0.4 | 0.4 | 1 | 1 | 0.4 | 0.7 | 0.7 | 1 | 0.4 | 0.4 | 1 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.21 | 1.18 | 0.7 |
| Parameters | Unit | This Research | [27] | [28] | [29] | [30] | [31] | [32] | [33] |
|---|---|---|---|---|---|---|---|---|---|
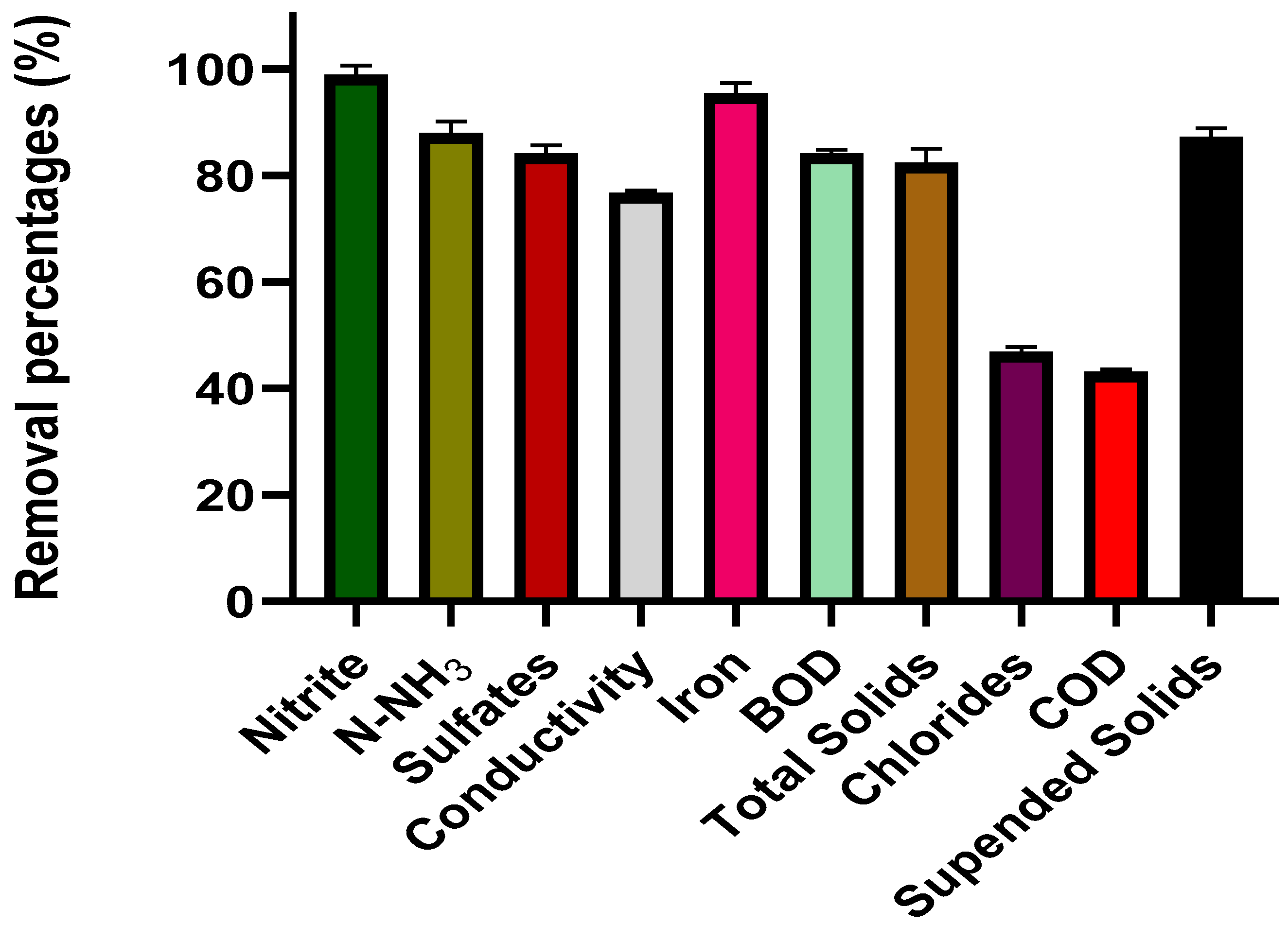
| Nitrate | mg L−1 | 65 | 9.3 | - | 29.06 ± 1.67 | - | 86.87 | - | 5.18 |
| Nitrite | mg L−1 | 1.25 | 0.004 | - | - | - | - | - | |
| N-NH3 | mg L−1 | 35.6 | - | - | - | 10 ± 4.35 | - | - | - |
| Sulfates | mg L−1 | 385 | 112 | 275 | - | - | 629 | 1384 | 8.98 |
| Conductivity | µS cm−1 | 1145 | 2269 | 5.2 | 69.0 ± 0.05 | - | 2836 | 4010 | 1675 |
| pH | - | 6.1 | 7.9 | 7.5 | 7.63 ± 0.10 | 6.25 ± 0.16 | 11.84 | 7.39 | 4.34 |
| Iron | mg L−1 | 7.5 | 0.1 | 2.35 | - | 17.77 ± 0.20 | - | - | - |
| Chrome | mg L−1 | N.D | - | - | - | - | - | - | - |
| BOD | mg L−1 | 225.56 | - | 248 | 9.33 ± 1.03 | 782 ± 8.02 | 20 | 474 | 2667 |
| Total solids | mg L−1 | 1367 | 3.6 | 4722 | - | 5483 ± 12.58 | - | - | - |
| Chlorides | mg L−1 | 850.67 | 506 | 2586 | - | 286 ± 7 | 839.58 | - | 198.39 |
| COD | mg L−1 | 598 | 65 | 689 | 18.44 ± 1.62 | 1700 ± 11.53 | 1024.6 | 1088 | 12.69 |
| Suspended solids | mg L−1 | 587.43 | - | 235 | - | - | 0.011 | 68 | - |
| Type of water | Real | Real | Real | Real | Real | Real | Real | Real |
| Relation | Equation | |
|---|---|---|
| For Color | ||
| T = 65 °C H2O2 vs. HCO3 | (7) | |
| H2O2 = 1.5 M T vs. HCO3 | (8) | |
| HCO3 = 0.7 M T vs. H2O2 | (9) | |
| For COD | ||
| T = 65 °C H2O2 vs. HCO3 | (10) | |
| H2O2 = 1.5 M T vs. HCO3 | (11) | |
| HCO3 = 0.7 M T vs. H2O2 | (12) | |
| For N-NH3 | ||
| T = 65 °C H2O2 vs. HCO3 | (13) | |
| H2O2 = 1.5 M T vs. HCO3 | (14) | |
| HCO3 = 0.7 M T vs. H2O2 | (15) | |
| For TOC | ||
| T = 65 °C H2O2 vs. HCO3 | (16) | |
| H2O2 = 1.5 M T vs. HCO3 | (17) | |
| HCO3 = 0.7 M T vs. H2O2 | (18) | |
| Parameter | % of Removal |
|---|---|
| N-NH3 | 92.35 |
| COD | 31.94 |
| Color | 68.85 |
| TOC | 35.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbina-Suarez, N.A.; Rivera-Caicedo, C.; González-Delgado, Á.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal. Toxics 2023, 11, 366. https://doi.org/10.3390/toxics11040366
Urbina-Suarez NA, Rivera-Caicedo C, González-Delgado ÁD, Barajas-Solano AF, Machuca-Martínez F. Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal. Toxics. 2023; 11(4):366. https://doi.org/10.3390/toxics11040366
Chicago/Turabian StyleUrbina-Suarez, Néstor A., Christian Rivera-Caicedo, Ángel Darío González-Delgado, Andrés F. Barajas-Solano, and Fiderman Machuca-Martínez. 2023. "Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal" Toxics 11, no. 4: 366. https://doi.org/10.3390/toxics11040366
APA StyleUrbina-Suarez, N. A., Rivera-Caicedo, C., González-Delgado, Á. D., Barajas-Solano, A. F., & Machuca-Martínez, F. (2023). Bicarbonate-Hydrogen Peroxide System for Treating Dyeing Wastewater: Degradation of Organic Pollutants and Color Removal. Toxics, 11(4), 366. https://doi.org/10.3390/toxics11040366











