Surface Engineering Design of Nano FeS@Stenotrophomonas sp. by Ultrasonic Chemical Method for Efficient U(VI) and Th(IV) Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nano-FeS on Microbial Surfaces
2.3. Characterization
2.4. Adsorption Experiments
3. Results and Discussion
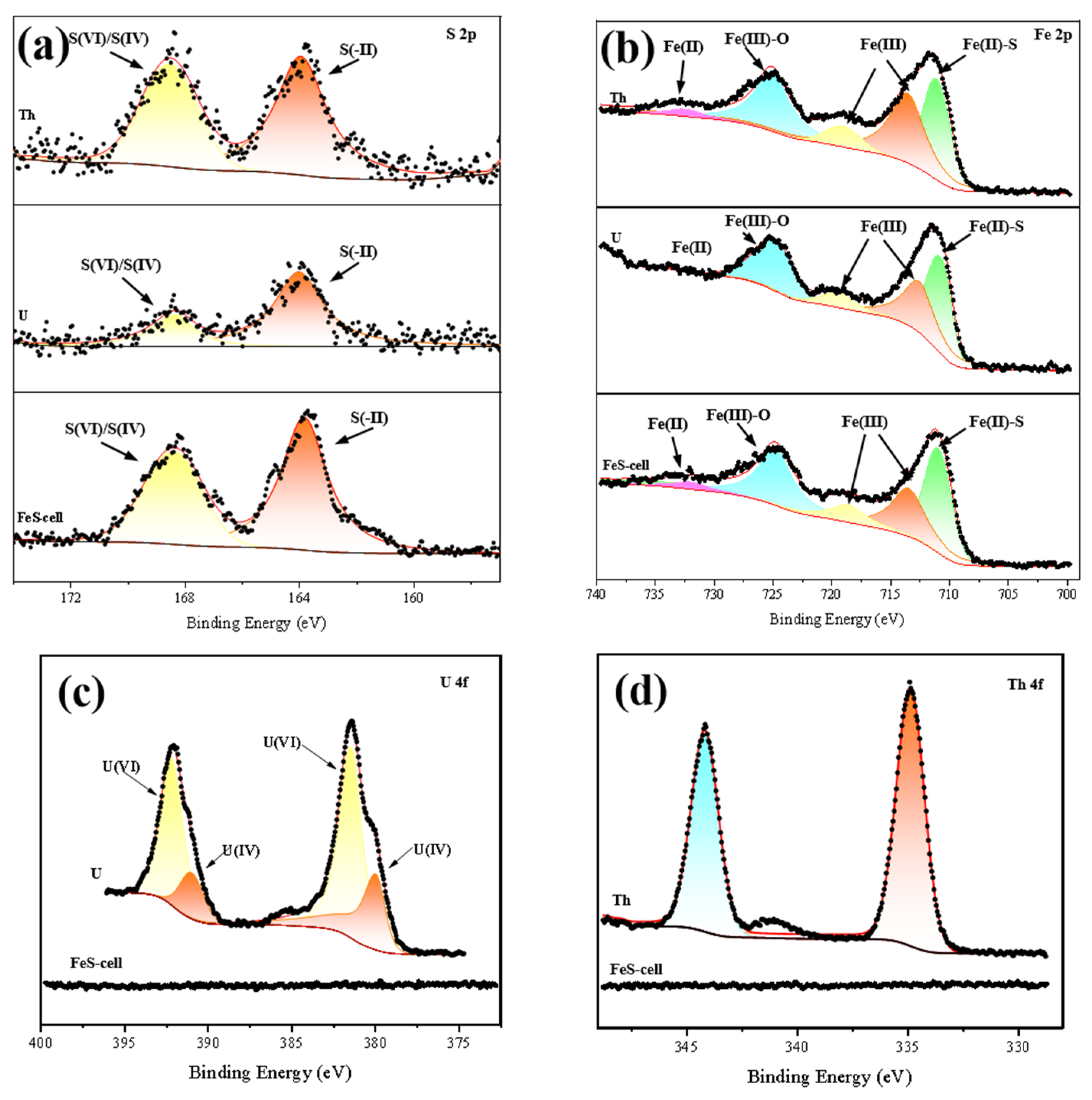
3.1. Characterization Materials
3.2. Adsorption Properties
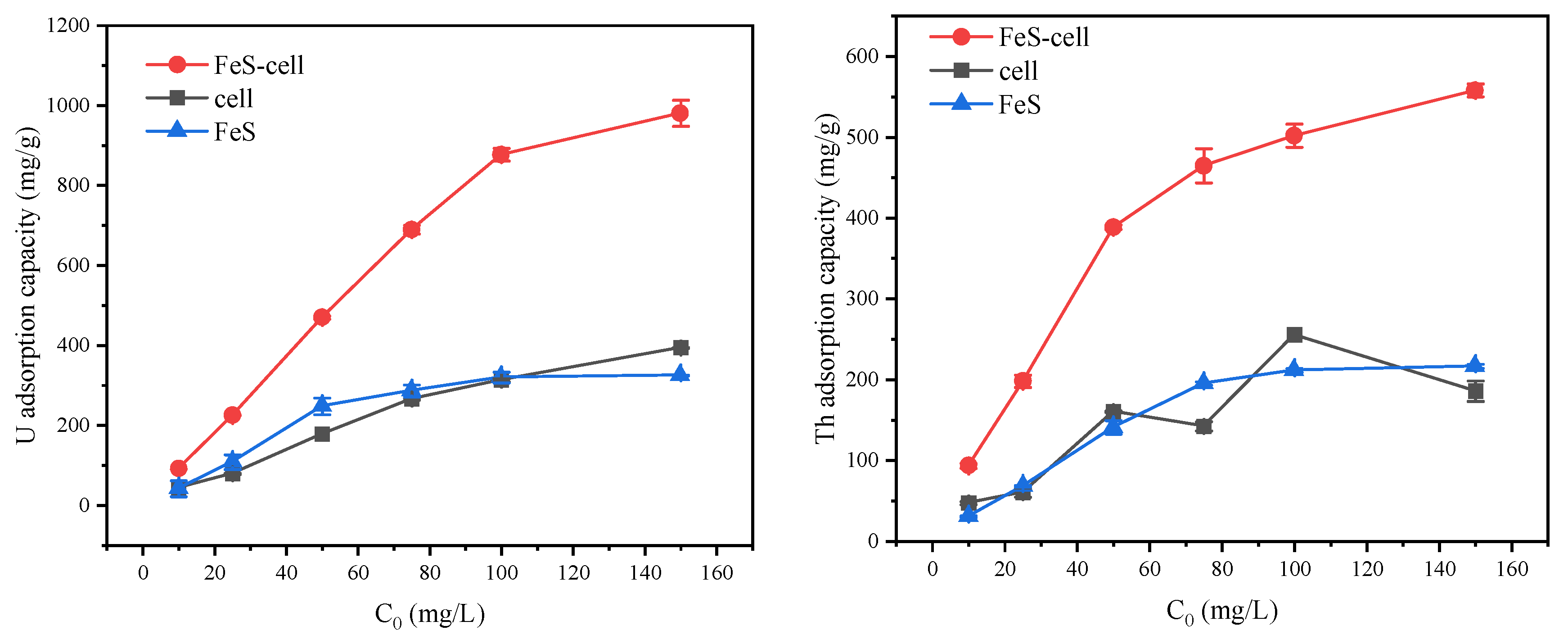
3.2.1. Comparison of the Capacities of the Cells and FeS
3.2.2. Effects of the Synthetic Ratio of Nano-FeS and the Microorganisms
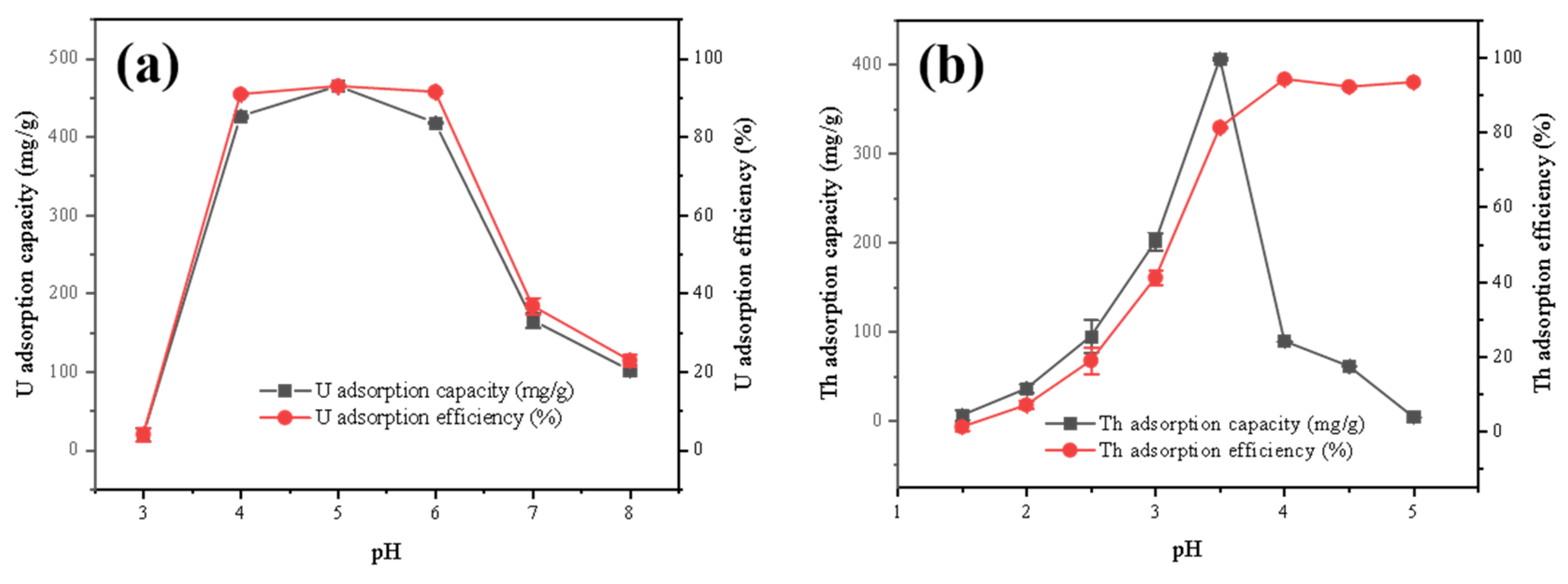
3.2.3. Effect of pH
3.2.4. Effect of Dosage
3.2.5. Effect of Ultrasonic Treatment Time
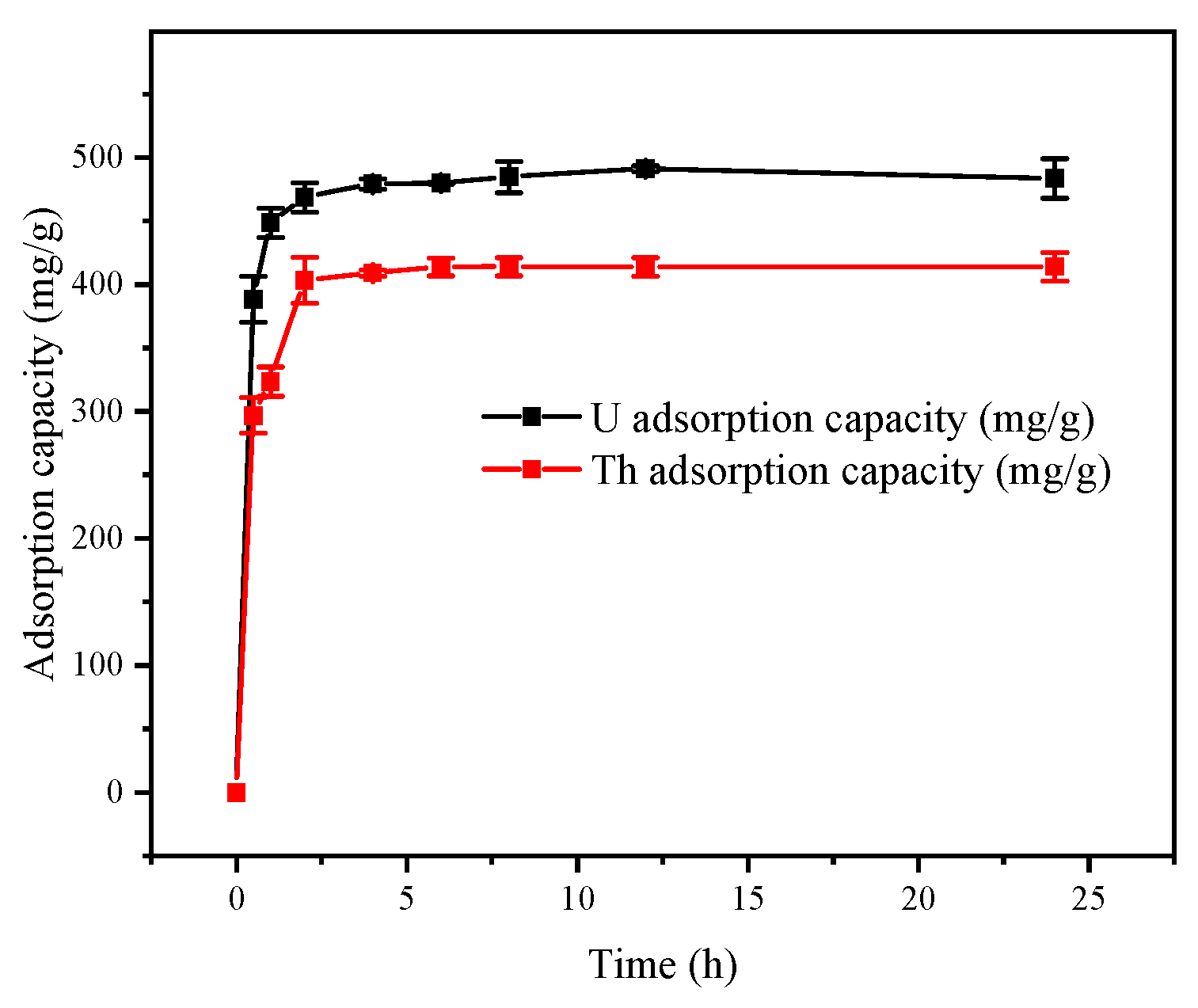
3.2.6. Effect of Adsorption Time
3.3. Adsorption Isotherms
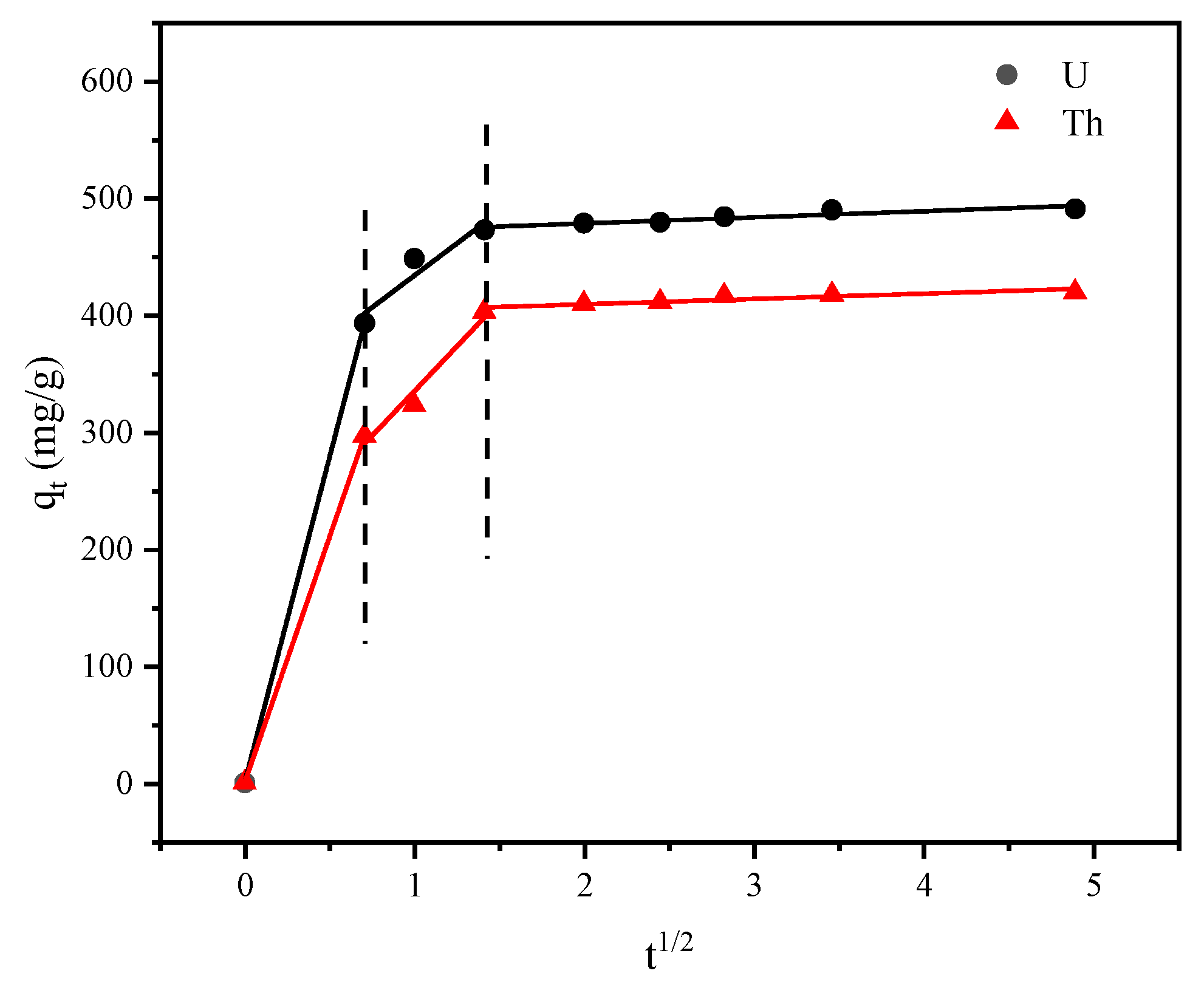
3.4. Adsorption Kinetics
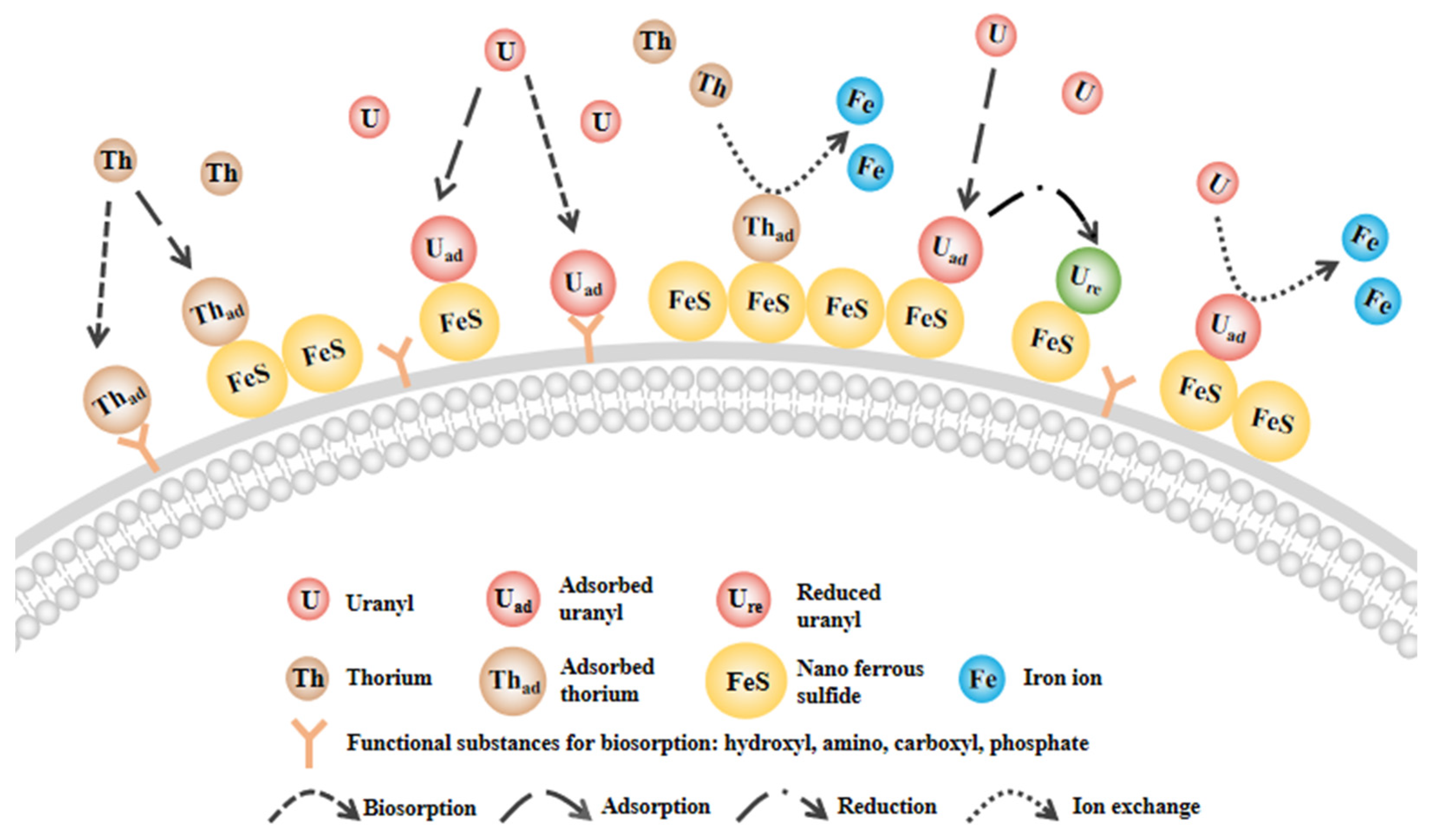
3.5. Mechanism for U and Th Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abed, A.M.; Sadaqah, R.; Kuisi, M.A. Uranium and Potentially Toxic Metals During the Mining, Beneficiation, and Processing of Phosphorite and their Effects on Ground Water in Jordan. Mine Water Environ. 2008, 27, 171–182. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Thorium biosorption by Aspergillus fumigatus, a filamentous fungal biomass. J. Hazard. Mater. 2009, 165, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Janot, N.; Lezama Pacheco, J.S.; Pham, D.Q.; O’Brien, T.M.; Hausladen, D.; Noel, V.; Lallier, F.; Maher, K.; Fendorf, S.; Williams, K.H.; et al. Physico-Chemical Heterogeneity of Organic-Rich Sediments in the Rifle Aquifer, CO: Impact on Uranium Biogeochemistry. Environ. Sci. Technol. 2016, 50, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yener, I.; Varhan Oral, E.; Dolak, I.; Ozdemir, S.; Ziyadanogullari, R. A new method for preconcentration of Th(IV) and Ce(III) by thermophilic Anoxybacillus flavithermus immobilized on Amberlite XAD-16 resin as a novel biosorbent. Ecol. Eng. 2017, 103, 43–49. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, G.; Weng, H.; Shen, W.; Huang, Z.; Lin, M. Efficient and selective separation of U(VI) and Th(IV) from rare earths using functionalized hierarchically mesoporous silica. J. Mater. Sci. 2017, 53, 3398–3416. [Google Scholar] [CrossRef]
- Findeiß, M.; Schäffer, A. Fate and Environmental Impact of Thorium Residues During Rare Earth Processing. J. Sustain. Metall. 2016, 3, 179–189. [Google Scholar] [CrossRef]
- Liesch, T.; Hinrichsen, S.; Goldscheider, N. Uranium in groundwater—Fertilizers versus geogenic sources. Sci. Total Environ. 2015, 536, 981–995. [Google Scholar] [CrossRef]
- Yang, S.K.; Tan, N.; Yan, X.M.; Chen, F.; Long, W.; Lin, Y.C. Thorium(IV) removal from aqueous medium by citric acid treated mangrove endophytic fungus Fusarium sp. #ZZF51. Mar. Pollut. Bull. 2013, 74, 213–219. [Google Scholar]
- Mahamadi, C. Will nano-biosorbents break the Achilles’ heel of biosorption technology? Environ. Chem. Lett. 2019, 17, 1753–1768. [Google Scholar] [CrossRef]
- Duan, J.; Ji, H.; Zhao, X.; Tian, S.; Liu, X.; Liu, W.; Zhao, D. Immobilization of U(VI) by stabilized iron sulfide nanoparticles: Water chemistry effects, mechanisms, and long-term stability. Chem. Eng. J. 2020, 393, 124692. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Chen, J.; Xu, L.; Sheng, G.; Fang, P.; Du, K.; Shen, C.; Guo, X. Anchoring nanoscale iron sulfide onto graphene oxide for the highly efficient immobilization of uranium (VI) from aqueous solutions. J. Mol. Liq. 2021, 332, 115910. [Google Scholar] [CrossRef]
- Qi, X.; Yin, H.; Zhu, M.; Yu, X.; Shao, P.; Dang, Z. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu(2+), Cd(2+), and Pb(2+) and its remediation efficiency on heavy metal contaminated soil. Chemosphere 2022, 294, 133733. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhou, Z.; Zhou, Y.; Zheng, L.; Guo, J.; Liu, Y.; Sun, Z.; Yang, Z.; Yu, X. Synergy of surface adsorption and intracellular accumulation for removal of uranium with Stenotrophomonas sp.: Performance and mechanisms. Environ. Res. 2022, 220, 115093. [Google Scholar] [CrossRef] [PubMed]
- Banala, U.K.; Das, N.P.I.; Toleti, S.R. Microbial interactions with uranium: Towards an effective bioremediation approach. Environ. Technol. Innov. 2021, 21, 101254. [Google Scholar] [CrossRef]
- Daneshvar, M.; Hosseini, M.R. Kinetics, isotherm, and optimization of the hexavalent chromium removal from aqueous solution by a magnetic nanobiosorbent. Environ. Sci. Pollut. Res. Int. 2018, 25, 28654–28666. [Google Scholar] [CrossRef] [PubMed]
- Ramrakhiani, L.; Ghosh, S.; Majumdar, S. Surface Modification of Naturally Available Biomass for Enhancement of Heavy Metal Removal Efficiency, Upscaling Prospects, and Management Aspects of Spent Biosorbents: A Review. Appl. Biochem. Biotechnol. 2016, 180, 41–78. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.M.; Tang, L.; Zhang, Y.; Liu, Y.Y.; Lei, X.X.; Wu, M.S.; Li, Z.; Liu, C. Cr(VI) reduction by Pseudomonas aeruginosa immobilized in a polyvinyl alcohol/sodium alginate matrix containing multi-walled carbon nanotubes. Bioresour. Technol. 2011, 102, 10733–10736. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. Sonochemical synthesis of ZnS nanolayers on the surface of microbial cells and their application in the removal of heavy metals. J. Hazard. Mater. 2020, 400, 123161. [Google Scholar] [CrossRef]
- Huang, D.; Li, B.; Ou, J.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of biosorbents and catalytic technologies for the removal of heavy metals from wastewater: Preparation, final disposal, mechanism and influencing factors. J. Environ. Manag. 2020, 261, 109879. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, P.; Li, Q.; Liu, Y.; Yin, J. Preparation of FeS@Fe3O4 core–shell magnetic nanoparticles and their application in uranyl ions removal from aqueous solution. J. Radioanal. Nucl. Chem. 2019, 321, 499–510. [Google Scholar] [CrossRef]
- He, S.; Hu, W.; Liu, Y.; Xie, Y.; Zhou, H.; Wang, X.; Chen, J.; Zhang, Y. Mechanism of efficient remediation of U(VI) using biogenic CMC-FeS complex produced by sulfate-reducing bacteria. J. Hazard. Mater. 2021, 420, 126645. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, C.; Liao, J.; Du, L.; Sun, Q.; Yang, J.; Yang, Y.; Zhang, D.; Tang, J.; Liu, N. Bioaccumulation characterization of uranium by a novel Streptomyces sporoverrucosus dwc-3. J. Environ. Sci. 2016, 41, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Yuan, Y.; Feng, L.; Sun, W.; Lin, K.; Zhang, J.; Zhang, Y.; Wang, H.; Wang, N.; Peng, Q. Highly efficient immobilization of environmental uranium contamination with Pseudomonas stutzeri by biosorption, biomineralization, and bioreduction. J. Hazard. Mater. 2022, 424, 127758. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Ren, X.; Wen, J.; Hu, S.; Xiong, J.; Jiang, T.; Wang, X.; Wang, X. Immobilization of uranium by biomaterial stabilized FeS nanoparticles: Effects of stabilizer and enrichment mechanism. J. Hazard. Mater. 2016, 302, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Volesky, B. Biosorption of uranium on Sargassum biomass. Water Res. 1999, 33, 3357–3363. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Liu, R.; Hu, B.; Qiu, M. Removal of U(vi) from aqueous solutions by an effective bio-adsorbent from walnut shell and cellulose composite-stabilized iron sulfide nanoparticles. RSC Adv. 2022, 12, 2675–2683. [Google Scholar] [CrossRef]
- Bi, Y.; Hayes, K.F. Nano-FeS inhibits UO2 reoxidation under varied oxic conditions. Environ. Sci. Technol. 2014, 48, 632–640. [Google Scholar] [CrossRef]
- Dai, Y.; Duan, L.; Du, W.; Yang, X.; Sun, S.; Xiu, Q.; Wang, S.; Zhao, S. Morphology and structure of in situ FeS affect Cr(VI) removal by sulfidated microscale zero-valent iron with short-term ultrasonication. Chemosphere 2022, 290, 133372. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Chen, J.; Liu, Z.; Wang, Z.; Na, P. Enhanced Cr(VI) removal from aqueous solutions using Ni/Fe bimetallic nanoparticles: Characterization, kinetics and mechanism. RSC Adv. 2014, 4, 50699–50707. [Google Scholar] [CrossRef]
- Pang, Y.; Ruan, Y.; Feng, Y.; Diao, Z.; Shih, K.; Hou, L.; Chen, D.; Kong, L. Ultrasound assisted zero valent iron corrosion for peroxymonosulfate activation for Rhodamine-B degradation. Chemosphere 2019, 228, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, R.; Chen, Q.; Mo, Y.; Zhang, Y. Biochar-supported starch/chitosan-stabilized nano-iron sulfide composites for the removal of lead ions and nitrogen from aqueous solutions. Bioresour. Technol. 2022, 347, 126700. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, C.; Sheng, G.; Li, M.; Linghu, W.; Huang, R. Highly efficient scavenging of uranium(VI) by molybdenum disulfide loaded ferrous sulfide composites: Kinetics, thermodynamics and mechanism aspects. J. Taiwan Inst. Chem. Eng. 2023, 142, 104614. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A.; Ahmady-Asbchin, S. Biosorption of lead (II) ions on Sargassum ilicifolium: Application of response surface methodology. Int. Biodeterior. Biodegrad. 2014, 93, 145–152. [Google Scholar] [CrossRef]
- Tunali, S.; Cabuk, A.; Akar, T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Eng. J. 2006, 115, 203–211. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saini, V.K. Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J. Colloid Interface Sci. 2007, 315, 87–93. [Google Scholar] [CrossRef]
- Han, J.; Hu, L.; He, L.; Ji, K.; Liu, Y.; Chen, C.; Luo, X.; Tan, N. Preparation and uranium (VI) biosorption for tri-amidoxime modified marine fungus material. Environ. Sci. Pollut. Res. Int. 2020, 27, 37313–37323. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.H.; Keshtkar, A.R.; Ghannadi, M.; Pahlavanzadeh, H. Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J. Hazard. Mater. 2008, 150, 612–618. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, F.; Liu, N.; Lan, T.; Yang, Y.; Zhang, T.; Liao, J.; Qing, R. A novel freeze-dried natural microalga powder for highly efficient removal of uranium from wastewater. Chemosphere 2021, 282, 131084. [Google Scholar] [CrossRef]
- Xu, L.; Li, L.; Fang, P.; Chang, K.; Chen, C.; Liao, Q. Removal of uranium (VI) ions from aqueous solution by graphitic carbon nitride stabilized FeS nanoparticles. J. Mol. Liq. 2022, 345, 117050. [Google Scholar] [CrossRef]
- Samadi, N.; Hasanzadeh, R.; Rasad, M. Adsorption isotherms, kinetic, and desorption studies on removal of toxic metal ions from aqueous solutions by polymeric adsorbent. J. Appl. Polym. Sci. 2015, 132, 41642. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, J.; Zhao, D. Application of iron sulfide particles for groundwater and soil remediation: A review. Water Res. 2016, 89, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Deng, B. Reductive Immobilization of Uranium(VI) by Amorphous Iron Sulfide. Environ. Sci. Technol. 2008, 42, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.P.; Davis, J.A.; Sun, K.; Hayes, K.F. Uranium(VI) reduction by iron(II) monosulfide mackinawite. Environ. Sci. Technol. 2012, 46, 3369–3376. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Chang, K.; Du, K.; Shen, C.; Zhou, S.; Sheng, G.; Linghu, W.; Hayat, T.; Guo, X. Graphene oxide decorated nanoscale iron sulfide for highly efficient scavenging of hexavalent chromium from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 103882. [Google Scholar] [CrossRef]
- Gong, Y.; Gai, L.; Tang, J.; Fu, J.; Wang, Q.; Zeng, E.Y. Reduction of Cr(VI) in simulated groundwater by FeS-coated iron magnetic nanoparticles. Sci. Total Environ. 2017, 595, 743–751. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Han, L.; Hu, B.; Qiu, M. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@Biochar composites. Environ. Sci. Pollut. Res. Int. 2021, 28, 55176–55185. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, W.; Wang, J.; Mirza, Z.A.; Wang, T. Optimized Synthesis of FeS Nanoparticles with a High Cr(VI) Removal Capability. J. Nanomater. 2016, 2016, 7817296. [Google Scholar] [CrossRef]










| Radioactive Element | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | R2 | Qe cal (mg/g) | KF | 1/n | R2 | |
| U6+ | 0.294 | 0.969 | 1263.7 | 351.3 | 0.375 | 0.982 |
| Th4+ | 0.128 | 0.953 | 610.9 | 157.9 | 0.304 | 0.985 |
| Radioactive Element | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | Qe exp (mg/L) | K1 (1/h) | R2 | Qe cal (mg/L) | K2 (g/(mg•h)) | h (mg/(g•h)) | R2 | |
| U6+ | 50 | 491.1 | 0.319 | 0.875 | 492.6 | 0.02145 | 5204.9 | 0.999 |
| Th4+ | 50 | 403.1 | 0.323 | 0.505 | 421.9 | 0.02174 | 3869.5 | 0.999 |
| Radioactive Element | C0 (mg/L) | Stage 1 | Stage 2 | Stage 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd1 (mg/(mg·h1/2)) | C1 | R2 | Kd2 (mg/(mg·h1/2)) | C2 | R2 | Kd3 (mg/(mg·h1/2)) | C2 | R2 | ||
| U6+ | 50 | 556.4 | 0 | 1 | 109.5 | 324.6 | 0.904 | 5.23 | 468.2 | 0.826 |
| Th4+ | 50 | 419.9 | 0 | 1 | 153 | 182 | 0.934 | 4.55 | 400.3 | 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Zhou, Z.; Guo, J.; Liu, Y.; Yang, S.; Guo, Y.; Wang, L.; Sun, Z.; Yang, Z. Surface Engineering Design of Nano FeS@Stenotrophomonas sp. by Ultrasonic Chemical Method for Efficient U(VI) and Th(IV) Extraction. Toxics 2023, 11, 297. https://doi.org/10.3390/toxics11040297
Hu Z, Zhou Z, Guo J, Liu Y, Yang S, Guo Y, Wang L, Sun Z, Yang Z. Surface Engineering Design of Nano FeS@Stenotrophomonas sp. by Ultrasonic Chemical Method for Efficient U(VI) and Th(IV) Extraction. Toxics. 2023; 11(4):297. https://doi.org/10.3390/toxics11040297
Chicago/Turabian StyleHu, Zhongqiang, Zhongkui Zhou, Jianping Guo, Yong Liu, Shunjing Yang, Yadan Guo, Liping Wang, Zhanxue Sun, and Zhihui Yang. 2023. "Surface Engineering Design of Nano FeS@Stenotrophomonas sp. by Ultrasonic Chemical Method for Efficient U(VI) and Th(IV) Extraction" Toxics 11, no. 4: 297. https://doi.org/10.3390/toxics11040297
APA StyleHu, Z., Zhou, Z., Guo, J., Liu, Y., Yang, S., Guo, Y., Wang, L., Sun, Z., & Yang, Z. (2023). Surface Engineering Design of Nano FeS@Stenotrophomonas sp. by Ultrasonic Chemical Method for Efficient U(VI) and Th(IV) Extraction. Toxics, 11(4), 297. https://doi.org/10.3390/toxics11040297






