QSAR Models for the Prediction of Dietary Biomagnification Factor in Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. The Literature Dataset
2.2. Regression Models Dataset
2.3. Data Curation and Calculation of the Molecular Descriptors
2.4. Multiple Linear Regression Models
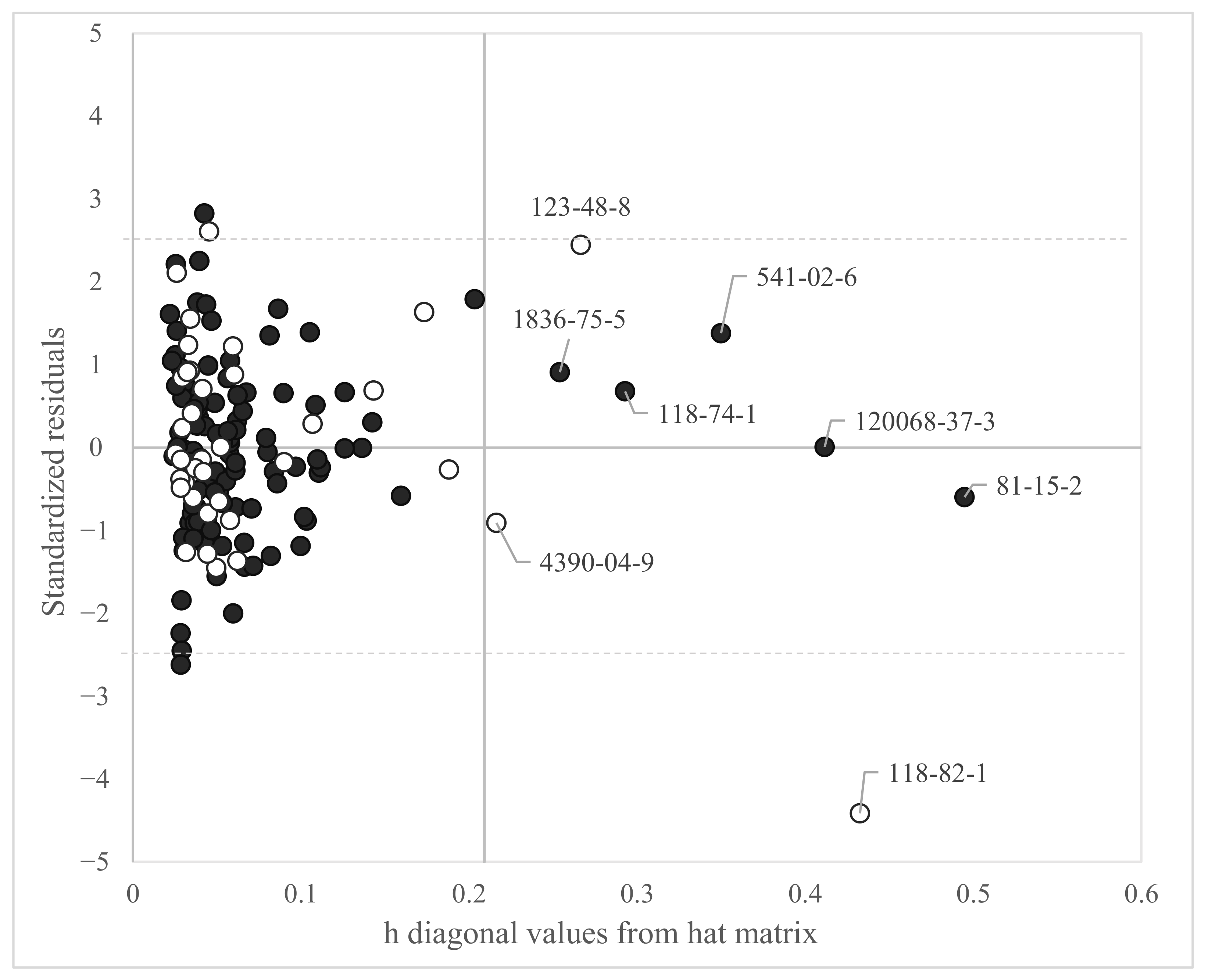
2.5. Applicability Domain
3. Results and Discussion
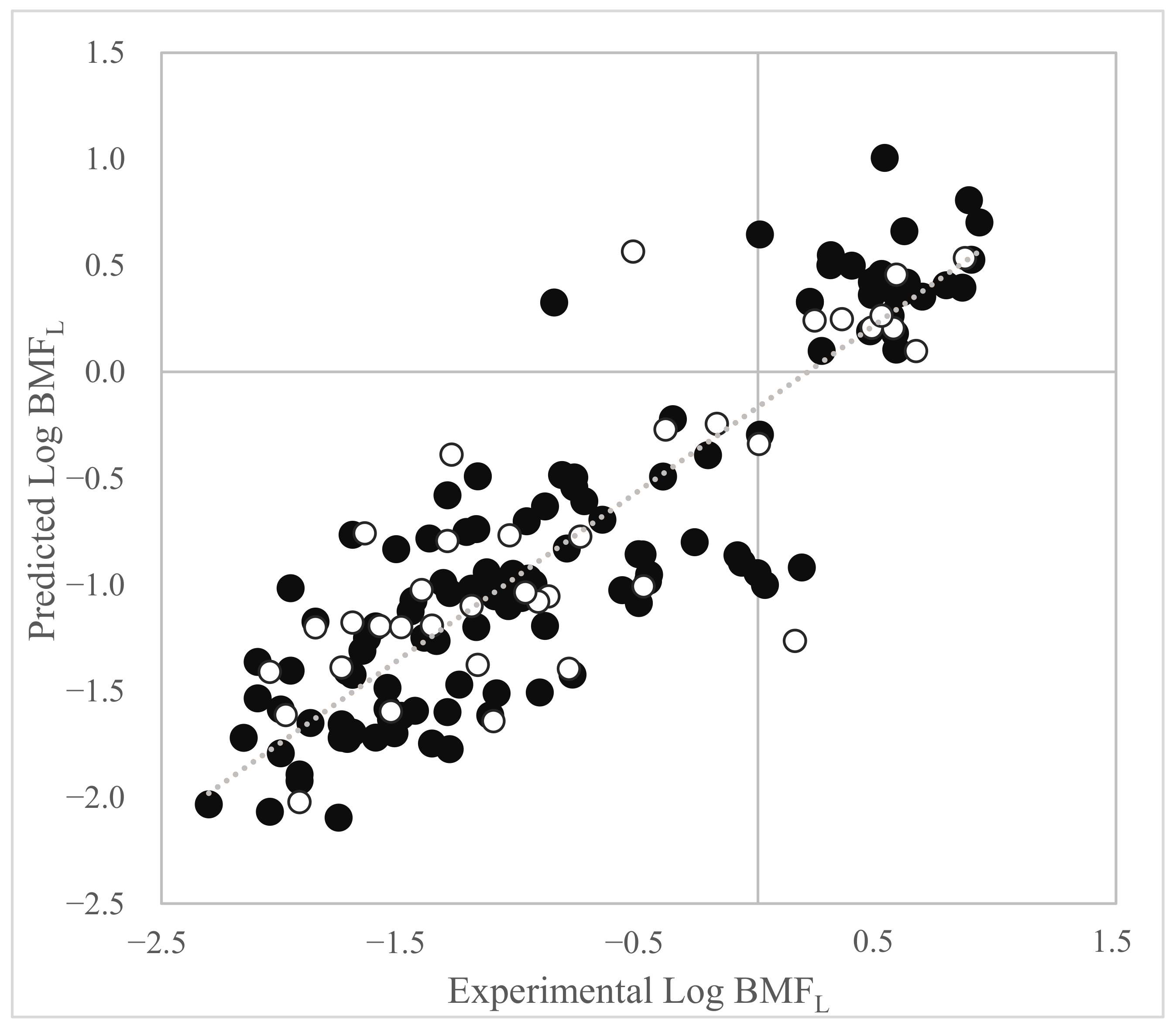
3.1. Log BMFL QSAR Based on Dataset 1
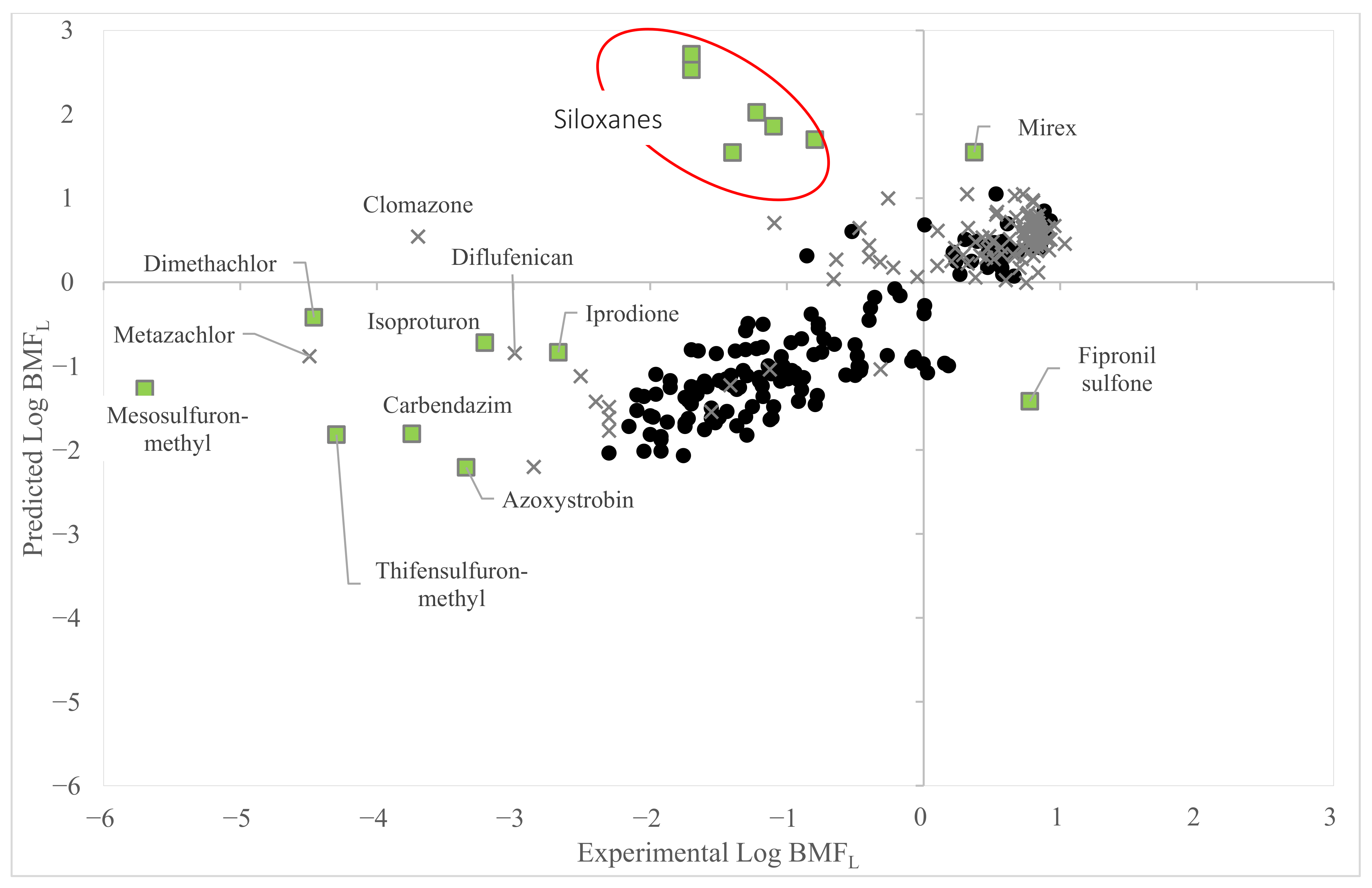
3.2. Application of the Model to Investigate Reliability of Data Identified as Low Quality (Dataset 2)
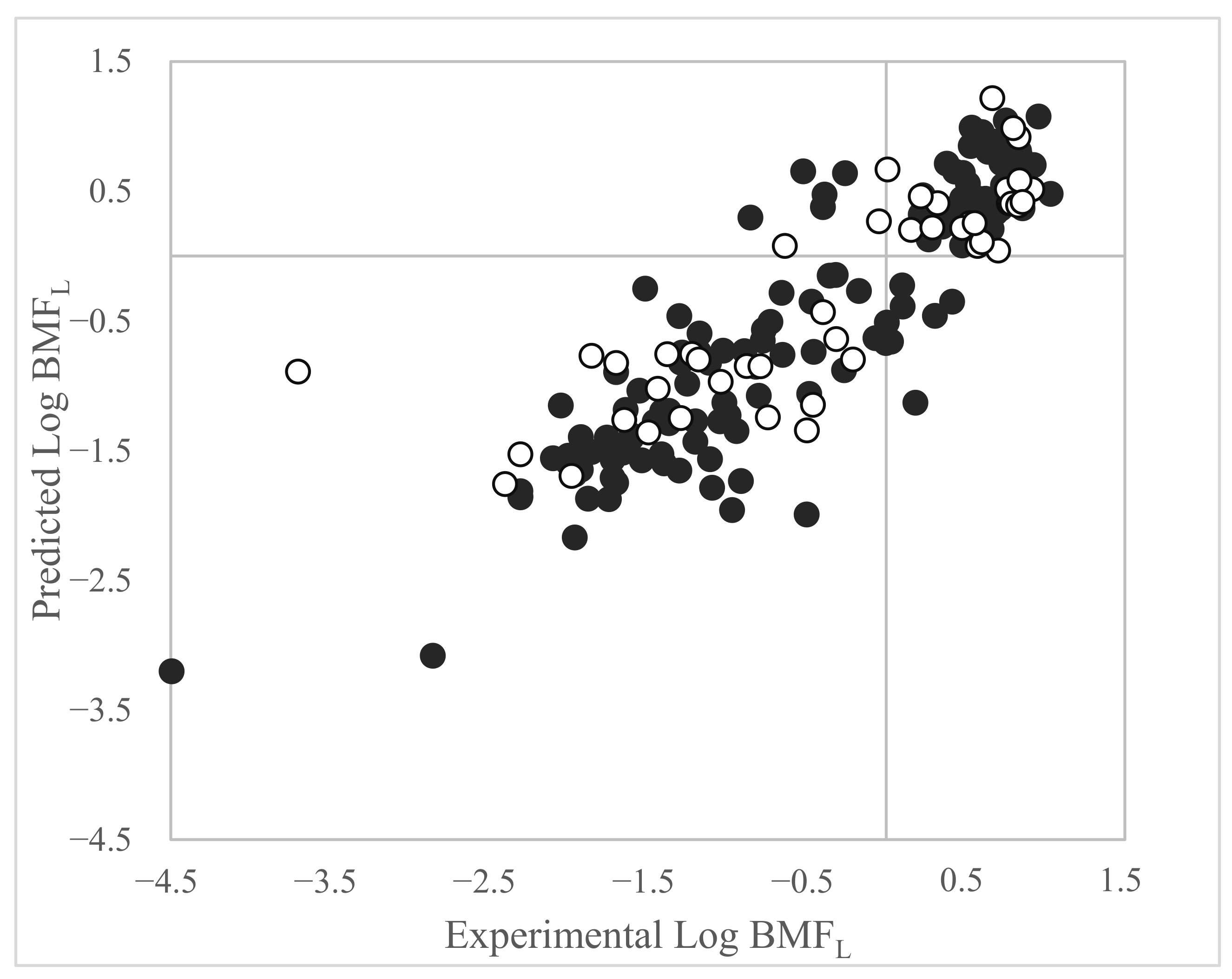
3.3. Log BMFL QSAR Based on Dataset 3
R_TpiPCTPC + 0.56 (±0.23) MLFER_S − 1.39 (±0.60) maxHother + 0.65 (±0.26) GGI5 − 4.00 × 10−3 (±2.5 × 10−3) VE3_Dt
3.4. Comparison with Existing BMFL QSAR Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment: Chapter R.7c: Endpoint Specific Guidance; European Chemicals Agency: Helsinki, Finland, 2017; Volume 3. [Google Scholar]
- OECD. Test No 305 Bioaccumulation in Fish: Aqueous and Dietary Exposure. In Test No 305 Bioaccumulation Fish Aqueous Dietary Exposure; Organisation for Economic Co-Operation and Development: Paris, France, 2012; pp. 1–72. [Google Scholar]
- Gobas, F.A.P.C.; Lee, Y.S.; Arnot, J.A. Normalizing the Biomagnification Factor. Environ. Toxicol. Chem. 2021, 40, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, N.; Inoue, Y.; Suzuki, Y.; Murakami, H.; Sumi, S.; Ishibashi, T.; Yoshida, T. Comparison of laboratory-derived biomagnification factors for hexachlorobenzene in common carp conducted under 9 test conditions. Environ. Toxicol. Chem. 2018, 37, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Gobas, F.A.P.C.; De Wolf, W.; Burkhard, L.P.; Verbruggen, E.; Plotzke, K. Revisiting bioaccumulation criteria for POPs and PBT assessments. Integr. Environ. Assess. Manag. 2009, 5, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, L.P.; Arnot, J.A.; Embry, M.R.; Farley, K.J.; Hoke, R.A.; Kitano, M.; Leslie, H.A.; Lotufo, G.R.; Parkerton, T.; Sappington, K.G.; et al. Comparing laboratory and field measured bioaccumulation endpoints. Integr. Environ. Assess. Manag. 2012, 8, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, L.P.; Cowan-Ellsberry, C.; Embry, M.R.; Hoke, R.A.; Kidd, K.A. Bioaccumulation data from laboratory and field studies: Are they comparable? Integr. Environ. Assess. Manag. 2012, 8, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Arnot, J.A.; Quinn, C.L. Development and evaluation of a database of dietary bioaccumulation test data for organic chemicals in fish. Environ. Sci. Technol. 2015, 49, 4783–4796. [Google Scholar] [CrossRef]
- Grisoni, F.; Consonni, V.; Vighi, M. Acceptable-by-design QSARs to predict the dietary biomagnification of organic chemicals in fish. Integr. Environ. Assess. Manag. 2019, 15, 51–63. [Google Scholar] [CrossRef]
- Grisoni, F.; Consonni, V.; Vighi, M. Detecting the bioaccumulation patterns of chemicals through data-driven approaches. Chemosphere 2018, 208, 273–284. [Google Scholar] [CrossRef]
- Arnot, J.A.; Toose, L.; Armitage, J.M.; Embry, M.; Sangion, A.; Hughes, L. A weight of evidence approach for bioaccumulation assessment. Integr. Environ. Assess. Manag. 2022, 1–19. [Google Scholar] [CrossRef]
- Franklin, J. How reliable are field-derived biomagnification factors and trophic magnification factors as indicators of bioaccumulation potential? Conclusions from a case study on per- and polyfluoroalkyl substances. Integr. Environ. Assess. Manag. 2016, 12, 6–20. [Google Scholar] [CrossRef]
- ECHA. Chapter R.11: PBT/vPvB assessment. In Guidance on Information Requirements and Chemical Safety Assessment; European Chemicals Agency: Helsinki, Finland, 2017. [Google Scholar] [CrossRef]
- Gramatica, P.; Papa, E. QSAR modeling of bioconcentration factor by theoretical molecular descriptors. QSAR Comb. Sci. 2003, 22, 374–385. [Google Scholar] [CrossRef]
- Zhao, C.; Boriani, E.; Chana, A.; Roncaglioni, A.; Benfenati, E. A new hybrid system of QSAR models for predicting bioconcentration factors (BCF). Chemosphere 2008, 73, 1701–1707. [Google Scholar] [CrossRef]
- Grisoni, F.; Consonni, V.; Vighi, M.; Villa, S.; Todeschini, R. Expert QSAR system for predicting the bioconcentration factor under the REACH regulation. Environ. Res. 2016, 148, 507–512. [Google Scholar] [CrossRef]
- Ivanciuc, T.; Ivanciuc, O.; Klein, D.J. Modeling the bioconcentration factors and bioaccumulation factors of polychlorinated biphenyls with posetic quantitative super-structure/activity relationships (QSSAR). Mol. Divers. 2006, 10, 133–145. [Google Scholar] [CrossRef]
- Fatemi, M.H.; Baher, E. A novel quantitative structure-activity relationship model for prediction of biomagnification factor of some organochlorine pollutants. Mol. Divers. 2009, 13, 343–352. [Google Scholar] [CrossRef]
- Chirico, N.; Bertato, L.; Papa, E. QSAR Multiple Endpoint Profiler (QSAR-ME Profiler). 2022. Available online: http://dunant.dista.uninsubria.it/qsar/ (accessed on 16 February 2023).
- NCI/CADD Group. Chemical Identifier Resolver. 2013. Available online: https://cactus.nci.nih.gov (accessed on 10 September 2020).
- Berthold Michael, R.; Cebron, N.; Dill, F. KNIME: The Konstanz Information Miner; Springer: Berlin/Heidelberg, Germany, 2007; Volume 11, pp. 58–61. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Yap, C.W. PaDEL-descriptor: A software to calculate molecular descriptors and fingerprints. J. Comput. Chem. 2010, 32, 1466–1474. [Google Scholar] [CrossRef]
- Gramatica, P.; Chirico, N.; Papa, E.; Cassani, S.; Kovarich, S. QSARINS: A new software for the development, analysis, and validation of QSAR MLR models. J. Comput. Chem. 2013, 34, 2121–2132. [Google Scholar] [CrossRef]
- Papa, E.; Kovarich, S.; Gramatica, P. Development, Validation and Inspection of the Applicability Domain of QSPR Models for Physicochemical Properties of Polybrominated Diphenyl Ethers. QSAR Comb. Sci. 2009, 28, 790–796. [Google Scholar] [CrossRef]
- Papa, E.; Villa, F.; Gramatica, P. Statistically Validated QSARs, Based on Theoretical Descriptors, for Modeling Aquatic Toxicity of Organic Chemicals in Pimephales promelas (Fathead Minnow). J. Chem. Inf. Model. 2005, 45, 1256–1266. [Google Scholar] [CrossRef]
- Leardi, R.; Boggia, R.; Terrile, M. Genetic algorithms as a strategy for feature selection. J. Chemom. 1992, 6, 267–281. [Google Scholar] [CrossRef]
- Tropsha, A.; Gramatica, P.; Gombar, V.K. The Importance of Being Earnest: Validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb. Sci. 2003, 22, 69–77. [Google Scholar] [CrossRef]
- Pearlman, R.S.; Smith, K.M. Novel software tools for chemical diversity. Perspect. Drug Discov. Des. 1998, 9, 339–353. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Bertato, L.; Taboureau, O.; Chirico, N.; Papa, E. Classification-based QSARs for predicting dietary biomagnification in fish. SAR QSAR Environ. Res. 2022, 33, 259–271. [Google Scholar] [CrossRef]
- Lavado, G.J.; Baderna, D.; Gadaleta, D.; Ultre, M.; Roy, K.; Benfenati, E. Ecotoxicological QSAR modeling of the acute toxicity of organic compounds to the freshwater crustacean Thamnocephalus platyurus. Chemosphere 2021, 280, 130652. [Google Scholar] [CrossRef]
- Galvez, J.; Garcia, R.; Salabert, M.T.; Soler, R. Charge Indexes. New Topological Descriptors. J. Chem. Inf. Comput. Sci. 1994, 34, 520–525. [Google Scholar] [CrossRef]
- Bertato, L.; Chirico, N.; Papa, E. Predicting the Bioconcentration Factor in Fish from Molecular Structures. Toxics 2022, 10, 581. [Google Scholar] [CrossRef]
- Doucette, W.J.; Shunthirasingham, C.; Dettenmaier, E.M.; Zaleski, R.T.; Fantke, P.; Arnot, J.A. A review of measured bioaccumulation data on terrestrial plants for organic chemicals: Metrics, variability, and the need for standardized measurement protocols. Environ. Toxicol. Chem. 2018, 37, 21–33. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Arnot, J. EAS-E Suite—Exposure and Safety Estimation Suite. 2021. Available online: https://arnotresearch.com/eas-e-suite/ (accessed on 16 February 2023).
- OECD. Principles for the Validation, for Regulatory Purposes, of (Quantitative) Structure-Activity Relationship Models. 2004. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/37849783.pdf (accessed on 23 February 2023).

| Model | RMSEtest | MAEtest |
|---|---|---|
| k fold_a | 0.85 | 0.61 |
| k fold_b | 1.06 | 0.78 |
| k fold_c | 0.70 | 0.55 |
| k fold_d | 0.78 | 0.52 |
| k fold_e | 0.58 | 0.48 |
| Average | 0.80 | 0.59 |
| Molecular Descriptor | Description |
|---|---|
| AATS5i | Average Broto–Moreau autocorrelation—lag 5/weighted by first ionization potential |
| BCUTw-1l | N high lowest atom weighted BCUTS |
| PubchemFP257 | ≥2 aromatic rings |
| C3SP2 | Number of doubly bounded carbons linked to 3 other carbons |
| MATS1i | Moran autocorrelation—lag 1/weighted by first ionization potential |
| GATS5m | Geary autocorrelation—lag 5/weighted by mass |
| GGI5 | Topological charge index of order 5 |
| Model | RMSEtest | MAEtest |
|---|---|---|
| k fold_a | 0.53 | 0.38 |
| k fold_b | 0.73 | 0.47 |
| k fold_c | 0.57 | 0.40 |
| k fold_d | 0.69 | 0.45 |
| k fold_e | 0.72 | 0.44 |
| Average | 0.65 | 0.43 |
| Molecular Descriptor | Description |
|---|---|
| PubchemFP503 | Cl-C:C-[#1] (where “-” matches a single, “#” matches a triple bond and “:” denotes bond aromaticity) |
| SubFPC295 | Counts of C–O, N or S bond |
| R_TpiPCTPC | Ratio of total conventional bond order (up to order 10) with total path count (up to order 10) |
| MLFER_S | Combined dipolarity/polarizability |
| maxHother | Maximum atom-type H E-State: H on aaCH, dCH2 or dsCH |
| GGI5 | Topological charge index of order 5 |
| VE3_Dt | Logarithmic coefficient sum of the last eigenvector from detour matrix |
| Authors | Method | Var. | Training | Prediction | Cross-Validation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Response Range | N° | R2 | RMSE | SE | N° | R2 | RMSE | RMSE | |||
| Fatemi and Baher, 2009 [18] | (Stepwise-MLR) MLR | 5 | −0.13, 2.49 | 35 | 0.77 (R = 0.88) | 0.24 | 7 | 0.50 (R = 0.71) | 0.25 | ||
| (Stepwise-MLR)-ANN | 0.98 (R = 0.99) | 0.03 | 0.72 (R = 0.85) | 0.11 | |||||||
| GA-MLR | 4 | 0.72 (R = 0.85) | 0.28 | 0.87 (R = 0.93) | 0.27 | ||||||
| GA-ANN | 1.0 (R = 1.0) | 0.03 | 0.83 (R = 0.91) | 0.08 | |||||||
| Grisoni et al., 2019 [9] | wNNR | 4 | −4.50, 1.10 | 160 | 0.76 | 0.52 | 54 | 0.75 | 0.54 | 0.52 | |
| MLR | 7 | 0.75 | 0.53 | 0.71 | 0.57 | 0.55 | |||||
| Consensus | 0.81 | 0.47 | 0.82 | 0.45 | 0.49 | ||||||
| Dataset 1 Equation (1) | GA-MLR | 7 | −2.30, 0.93 | 115 | 0.79 | 0.41 | 37 | 0.68 | 0.49 | 0.80 | |
| Dataset 3 Equation (3) | −4.49, 1.03 | 194 | 0.85 | 0.43 | 64 | 0.73 | 0.58 | 0.65 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertato, L.; Chirico, N.; Papa, E. QSAR Models for the Prediction of Dietary Biomagnification Factor in Fish. Toxics 2023, 11, 209. https://doi.org/10.3390/toxics11030209
Bertato L, Chirico N, Papa E. QSAR Models for the Prediction of Dietary Biomagnification Factor in Fish. Toxics. 2023; 11(3):209. https://doi.org/10.3390/toxics11030209
Chicago/Turabian StyleBertato, Linda, Nicola Chirico, and Ester Papa. 2023. "QSAR Models for the Prediction of Dietary Biomagnification Factor in Fish" Toxics 11, no. 3: 209. https://doi.org/10.3390/toxics11030209
APA StyleBertato, L., Chirico, N., & Papa, E. (2023). QSAR Models for the Prediction of Dietary Biomagnification Factor in Fish. Toxics, 11(3), 209. https://doi.org/10.3390/toxics11030209








