Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Soil
2.3. Screening of S. bicolor Varieties Resistant to Petroleum Pollution
2.4. Phytoremediation Test
2.5. Data Processing
3. Results
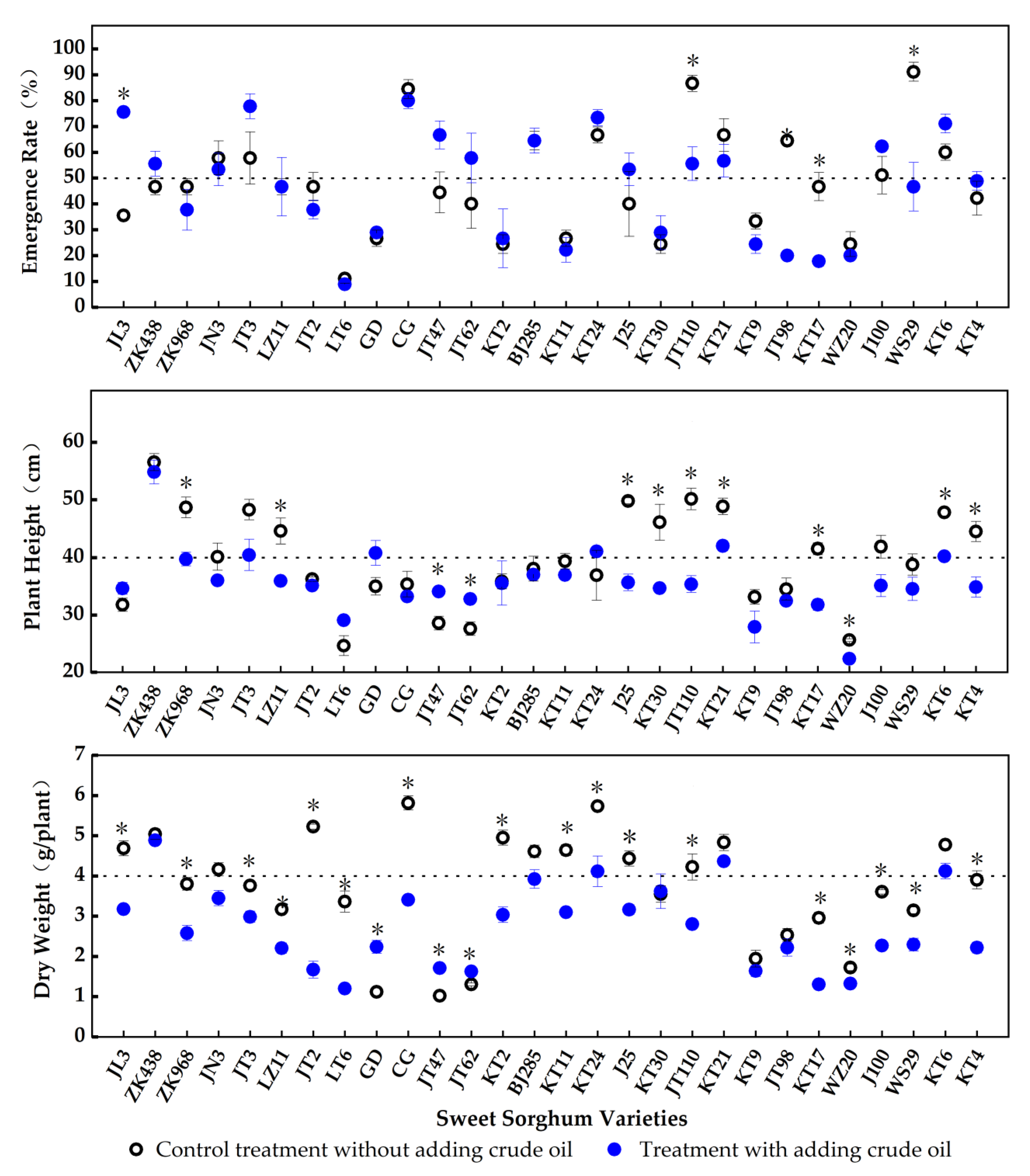
3.1. Parameters of the Plants in Petroleum-Polluted Salinized Soils
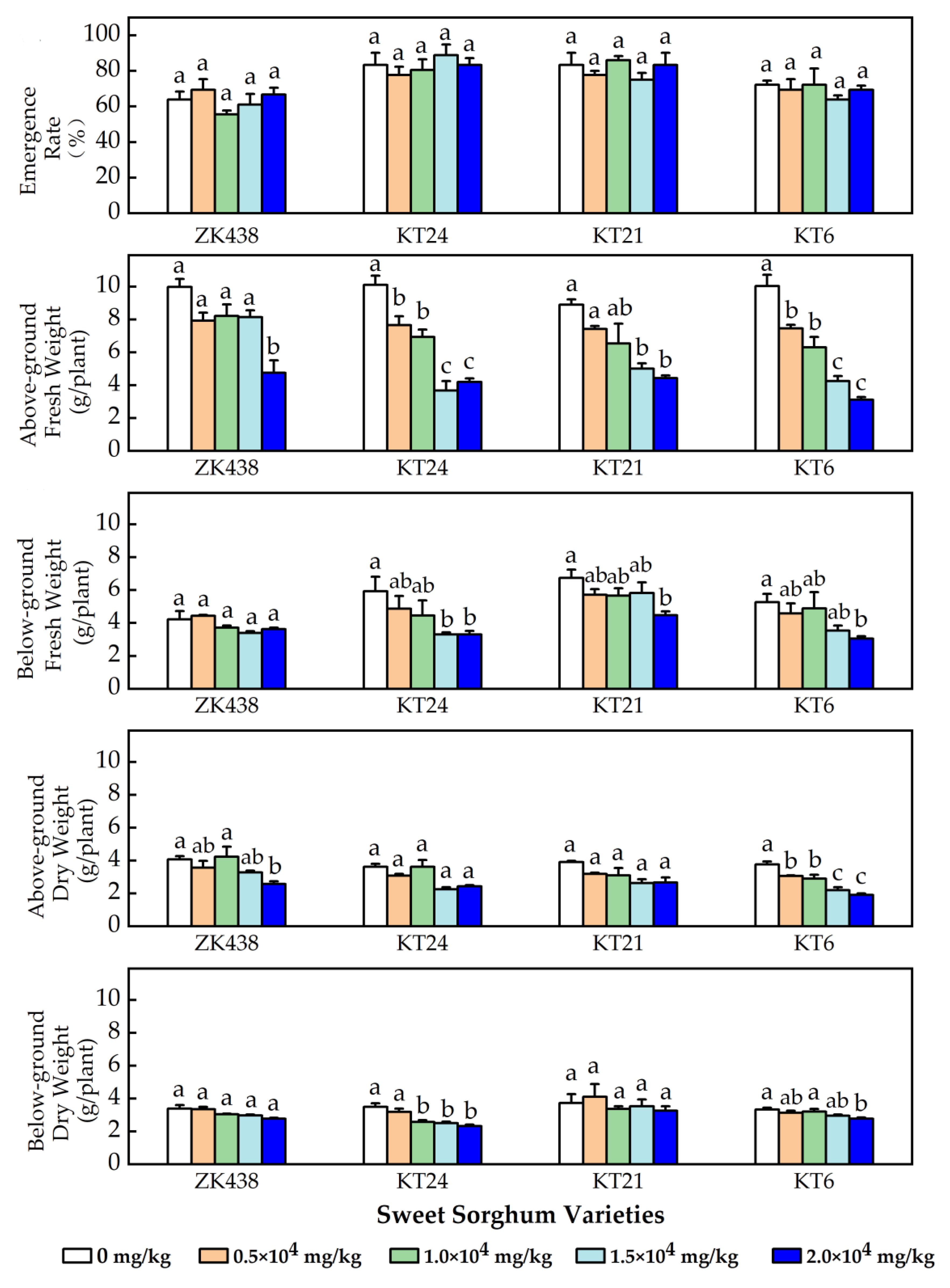
3.2. Growth of the Four Well-Performed Varieties
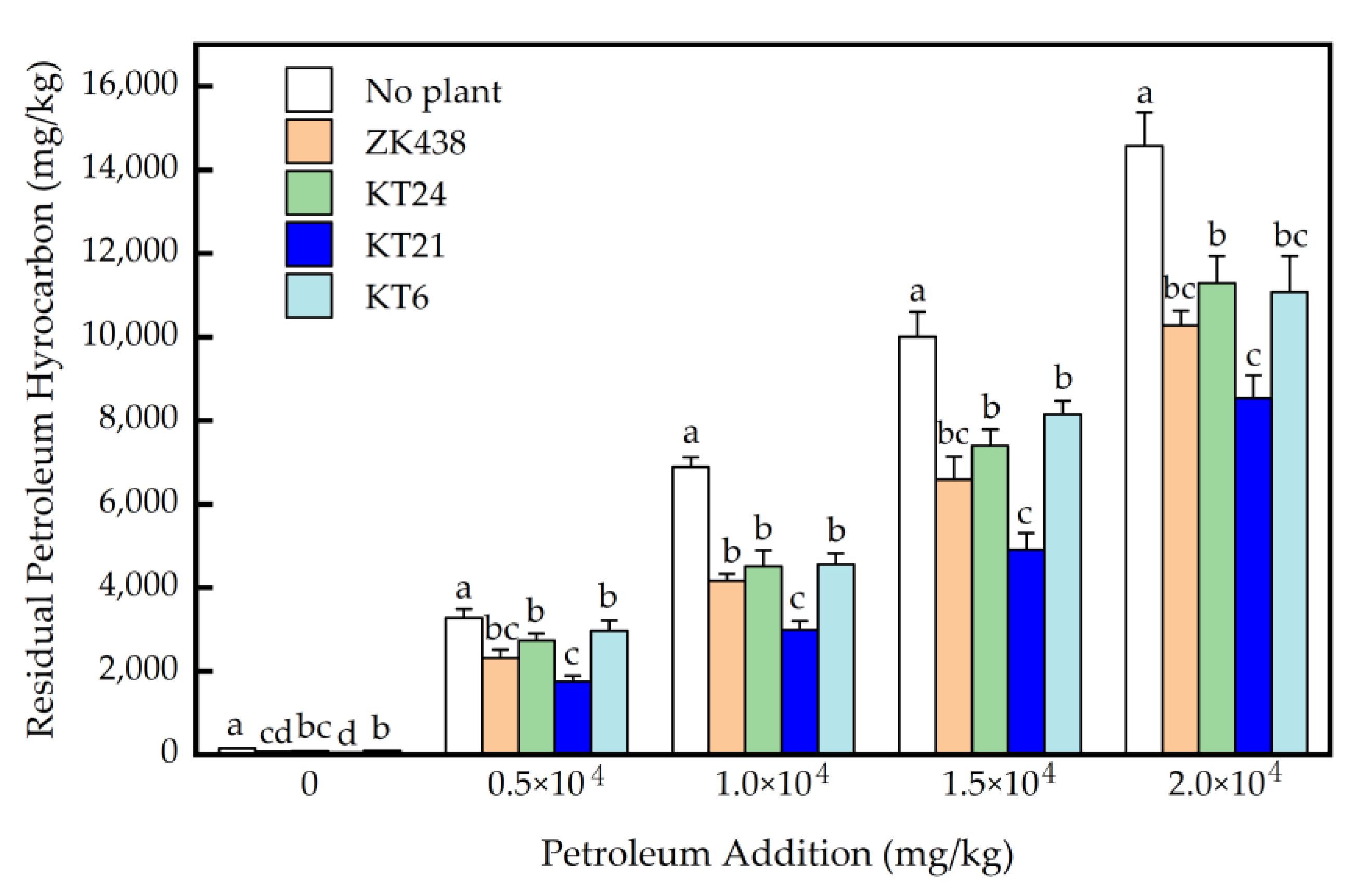
3.3. Removal of Petroleum Hydrocarbons in Soils after the Planting of Four S. bicolor Varieties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons, and their reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Dong, Q.; Xiao, M.; Li, B.; Topalović, O.; Tao, Q.; Tang, X.; Huang, R.; Chen, G.; et al. Remediation of petroleum hydrocarbons-contaminated soil: Analysis based on Chinese patents. Chemosphere 2022, 297, 134173. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yuan, L.; Du, J.; Wang, H.; Yang, X.; Duan, L.; Zheng, L.; Bahar, M.M.; Zhao, Q.; Zhang, W.; et al. Bacterial community profile of the crude oil-contaminated salinized soil in the Yellow River Delta Natural Reserve, China. Chemosphere 2022, 289, 133207. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, A.; Sima, N.A.K.; Olamaee, M.; Hashemi, M.; Nasrabadi, R.G. Remediation of salinized soils contaminated with crude oil using the halophyte Salicornia persica in conjunction with hydrocarbon-degrading bacteria. J. Environ. Manage. 2018, 219, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells. J. Hazard. Mater. 2015, 283, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Luqueño, F.; Cabrera-Lázaro, G.; Corlay-Chee, L.; López-Valdez, F.; Dendooven, L. Dissipation of phenanthrene and anthracene from soil with increasing salt content amended with wastewater sludge. Pol. J. Environ. Stud. 2017, 26, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Rosatelli, A.; Prasad, S.; Gomez, F.H.; Vaccari, M. Ex-situ bioremediation of petroleum hydrocarbon contaminated soil using mixed stimulants: Response and dynamics of bacterial community and phytotoxicity. J. Environ. Chem. Eng. 2022, 10, 108814. [Google Scholar] [CrossRef]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sust. Energ. Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Yao, S.; Liu, C.; Yin, Y.; Li, H.; Li, N. A real filed phytoremediation of multi-metals contaminated soils by selected hybrid sweet sorghum with high biomass and high accumulation ability. Chemosphere 2019, 237, 124536. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Are we ready to cultivate sweet sorghum as a bioenergy feedstock? A review on field management practices. Biomass Bioenerg. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, D. A review of sweet sorghum as a viable renewable bioenergy crop and its techno-economic analysis. Renew. Energ. 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Yin, R.; Liu, R.; Mei, Y.; Fei, W.; Sun, X. Characterization of bio-oil and bio-char obtained from sweet sorghum bagasse fast pyrolysis with fractional condensers. Fuel 2013, 112, 96–104. [Google Scholar] [CrossRef]
- Calviño, M.; Messing, J. Sweet sorghum as a model system for bioenergy crops. Curr. Opin. Biotech. 2012, 23, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant growth-promoting actinobacterial inoculant assisted phytoremediation increases cadmium uptake in Sorghum bicolor under drought and heat stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef]
- Xiao, M.Z.; Sun, R.; Du, Z.Y.; Yang, W.B.; Sun, Z.; Yuan, T.Q. A sustainable agricultural strategy integrating Cd-contaminated soils remediation and bioethanol production using sorghum cultivars. Ind. Crop. Prod. 2021, 162, 113299. [Google Scholar] [CrossRef]
- Soudek, P.; Petrová, Š.; Vaňková, R.; Song, J.; Vaněk, T. Accumulation of heavy metals using Sorghum sp. Chemosphere 2014, 104, 15–24. [Google Scholar] [CrossRef]
- Asiabadi, F.I.; Mirbagheri, S.A.; Najafi, P.; Moatar, F. Concentrations of petroleum hydrocarbons at different depths of soil following phytoremediation. Environ. Eng. Manag. J. 2018, 17, 2129–2135. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China; pp. 265–267.
- Canadian Council of Ministers of the Environment (CCME). Canada-Wide Standard for Petroleum Hydrocarbons (PHC) in Soil: Scientific Rationale. Available online: http://registry.mvlwb.ca/Documents/MV2010L1-0001/MV2010L1-0001%20-%20Canada%20Wide%20Standard%20for%20Petroleum%20HydroCarbons%20PHC%20in%20Soil%20-%20May12-10.pdf (accessed on 21 January 2022).
- Okalhoma Department of Environmental Quality. Land Risk-Based Cleanup Levels for Total Petroleum Hydrocarbons (TPH). Available online: https://emeraldcoast.surfrider.org/wp-content/uploads/2011/05/tph.pdf (accessed on 21 January 2022).
- Ministry of Ecology and Environment (MEE). Soil Environmental Quality Risk Control Standard for Soil Contamination of Development Land (GB 36600-2018). Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/W020190626596188930731.pdf (accessed on 21 January 2022).
- Sandermann, H., Jr. Higher plant metabolism of xenobiotics: The′green liver’concept. Pharmacogenet. 1994, 4, 225–241. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Niu, X.; Zhou, Q. Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil. Tillage Res. 2010, 110, 87–93. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Steliga, T.; Kluk, D. Assessment of the suitability of Melilotus officinalis for phytoremediation of soil contaminated with petroleum hydrocarbons (TPH and PAH), Zn, Pb and Cd based on toxicological tests. Toxics 2021, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.D.; El-Alawi, Y.; Gurska, J.; Glick, B.R.; Greenberg, B.M. A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem. J. 2005, 81, 139–147. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A review of germination and early growth as a proxy for plant fitness under petrogenic contamination—knowledge gaps and recommendations. Sci. Total Environ. 2017, 603, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Maila, M.P.; Cloete, T.E. Germination of Lepidium sativum as a method to evaluate polycyclic aromatic hydrocarbons (PAHs) removal from contaminated soil. Int. Biodeter. Biodegr. 2002, 50, 107–113. [Google Scholar] [CrossRef]
- Smith, M.J.; Flowers, T.H.; Duncan, H.J.; Alder, J. Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 2006, 141, 519–525. [Google Scholar] [CrossRef]
- Marques, M.; Rosa, G.S.; Aguiar, C.R.C.; Correia, S.M.; Carvalho, E.M. Seedling emergence and biomass growth of oleaginous and other tropical species in oil contaminated soil. Open Waste Manag. J. 2010, 3, 26–32. [Google Scholar] [CrossRef]
- Gaskin, S.E.; Bentham, R.H. Rhizoremediation of hydrocarbon contaminated soil using Australian native grasses. Sci. Total Environ. 2010, 408, 3683–3688. [Google Scholar] [CrossRef]
- Klokk, T. Effects of oil pollution on the germination and vegetative growth of five species of vascular plant. Oil Petrochem. Pollut. 1984, 2, 25–30. [Google Scholar] [CrossRef]
- Robson, D.B.; Germida, J.J.; Farrell, R.E.; Knight, D.J. Hydrocarbon tolerance correlates with seed mass and relative growth rate. Bioremediat. J. 2004, 8, 185–199. [Google Scholar] [CrossRef]
- Gaskin, S.; Soole, K.; Bentham, R. Screening of Australian native grasses for rhizoremediation of aliphatic hydrocarbon-contaminated soil. Int. J. Phytoremediat. 2008, 10, 378–389. [Google Scholar] [CrossRef]
- Graj, W.; Lisiecki, P.; Szulc, A.; Chrzanowski, Ł.; Wojtera-Kwiczor, J. Bioaugmentation with petroleum-degrading consortia has a selective growth-promoting impact on crop plants germinated in diesel oil-contaminated soil. Water Air Soil Pollut. 2013, 224, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Macek, T.; Macková, M.; Káš, J. Exploitation of plants for the removal of organics in environmental remediation. Biotechnol. Adv. 2000, 18, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kaimi, E.; Mukaidani, T.; Tamaki, M. Screening of twelve plant species for phytoremediation of petroleum hydrocarbon-contaminated soil. Plant Prod. Sci. 2007, 10, 211–218. [Google Scholar] [CrossRef]
- Aprill, W.; Sims, R.C. Evaluation of the use of prairie grasses for stimulating polycyclic aromatic hydrocarbon treatment in soil. Chemosphere 1990, 20, 253–265. [Google Scholar] [CrossRef]
- Meng, L.; Qiao, M.; Arp, H.P.H. Phytoremediation efficiency of a PAH-contaminated industrial soil using ryegrass, white clover, and celery as mono-and mixed cultures. J. Soil. Sediment. 2011, 11, 482–490. [Google Scholar] [CrossRef]
- Miya, R.K.; Firestone, M.K. Enhanced phenanthrene biodegradation in soil by slender oat root exudates and root debris. J. Environ. Qual. 2001, 30, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lee, J.H.; Lee, S.H.; Lee, S.W.; Jeon, J.H.; Lee, S.H.; Kim, S.O. Revitalization of Total Petroleum Hydrocarbon Contaminated Soil Remediated by Landfarming. Toxics 2022, 10, 147. [Google Scholar] [CrossRef]
- Li, J.; Ma, N.; Hao, B.; Qin, F.; Zhang, X. Coupling biostimulation and phytoremediation for the restoration of petroleum hydrocarbon-contaminated soil. Int. J. Phytoremediation 2022. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Cai, Z.; Liu, J.; Yu, B.; Zhou, Q. Phytoremediation of petroleum hydrocarbon-contaminated saline-alkali soil by wild ornamental Iridaceae species. Int. J. Phytorem. 2017, 19, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Ma, J.; Yan, G.; Dai, X.; Li, M.; Guo, S. Comparison of phytoremediation, bioaugmentation and natural attenuation for remediating saline soil contaminated by heavy crude oil. Biochem. Eng. J. 2016, 112, 170–177. [Google Scholar] [CrossRef]
- Fang, C.; Radosevich, M.; Fuhrmann, J.J. Atrazine and phenanthrene degradation in grass rhizosphere soil. Soil Biol. Biochem. 2001, 33, 671–678. [Google Scholar] [CrossRef]
- Olson, P.E.; Castro, A.; Joern, M.; DuTeau, N.M.; Pilon-Smits, E.A.; Reardon, K.F. Comparison of plant families in a greenhouse phytoremediation study on an aged polycyclic aromatic hydrocarbon–contaminated soil. J. Environ. Qual. 2007, 36, 1461–1469. [Google Scholar] [CrossRef]
- Hutchinson, S.L.; Schwab, A.P.; Banks, M.K. Biodegradation of petroleum hydrocarbons in the rhizosphere. In Phytoremediation: Transformation and Control of Contaminants; McCutcheon, S.C., Schnoor, J.L., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2003; pp. 355–386. [Google Scholar]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
| Variety | Number | Variety | Number | Variety | Number | Variety | Number |
|---|---|---|---|---|---|---|---|
| Jiliang No. 3 | JL3 | Liaotian No. 6 | LT6 | Ketian No. 11 | KT11 | JT No. 98 | JT98 |
| Zhong Ketian No. 438 | ZK438 | Gao Dancao | GD | Ketian No. 24 | KT24 | Ketian No. 17 | KT17 |
| Zhong Ketian No. 968 | ZK968 | Cao Gaoliang | CG | J No. 25 | J25 | 2031 | WZ20 |
| Jinnuo No. 3 | JN3 | JT No. 47 | JT47 | Ketian No. 30 | KT30 | J100 | J100 |
| Jitian No. 3 | JT3 | JT No. 62 | JT62 | JT No. 110 | JT110 | WSC29 | WS29 |
| Longza No. 11 | LZ11 | Ketian No. 2 | KT2 | Ketian No. 21 | KT21 | Ketian No. 6 | KT6 |
| Ji Tianza No. 2 | JT2 | BJ No. 285 | BJ285 | Ketian No. 9 | KT9 | Ketian No. 4 | KT4 |
| pH (H2O) | Salinity (%) | Organic C (g/kg) | Total N (g/kg) | Total P (g/kg) | Available N (mg/kg) | Available P (mg/kg) | Petroleum Hydrocarbon (mg/kg) |
|---|---|---|---|---|---|---|---|
| 7.85 | 0.31 | 10.78 | 0.79 | 0.87 | 35.53 | 29.09 | 146.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Xu, J.; Zhou, J.; Ren, L.; Li, J.; Zhang, Z.; Xia, J.; Xie, H.; Wu, T. Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments. Toxics 2023, 11, 208. https://doi.org/10.3390/toxics11030208
Ma D, Xu J, Zhou J, Ren L, Li J, Zhang Z, Xia J, Xie H, Wu T. Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments. Toxics. 2023; 11(3):208. https://doi.org/10.3390/toxics11030208
Chicago/Turabian StyleMa, Di, Jie Xu, Jipeng Zhou, Lili Ren, Jian Li, Zaiwang Zhang, Jiangbao Xia, Huicheng Xie, and Tao Wu. 2023. "Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments" Toxics 11, no. 3: 208. https://doi.org/10.3390/toxics11030208
APA StyleMa, D., Xu, J., Zhou, J., Ren, L., Li, J., Zhang, Z., Xia, J., Xie, H., & Wu, T. (2023). Using Sweet Sorghum Varieties for the Phytoremediation of Petroleum-Contaminated Salinized Soil: A Preliminary Study Based on Pot Experiments. Toxics, 11(3), 208. https://doi.org/10.3390/toxics11030208





