Embryotoxicity and Teratogenicity of Steroidal Saponin Isolated from Ophiopholis mirabilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction and Isolation of Secondary Metabolites
2.3. Chemical Structure Elucidation Procedures
2.4. Zebrafish Embryo Bioassay
2.4.1. Dose-Response to Zebrafish Embryos
2.4.2. Zebrafish Embryo and Larvae Teratogenicity
2.4.3. Zebrafish LC50 and Mortality Calculations
2.4.4. Zebrafish Hatching Rate
2.4.5. Zebrafish Deformity Rate
2.5. Statistics
3. Results and Discussion
3.1. Chemical Structure Elucidation of Asterosaponin P1
3.2. Zebrafish Mortality and Hatching Rates Treated by Asterosaponin P1
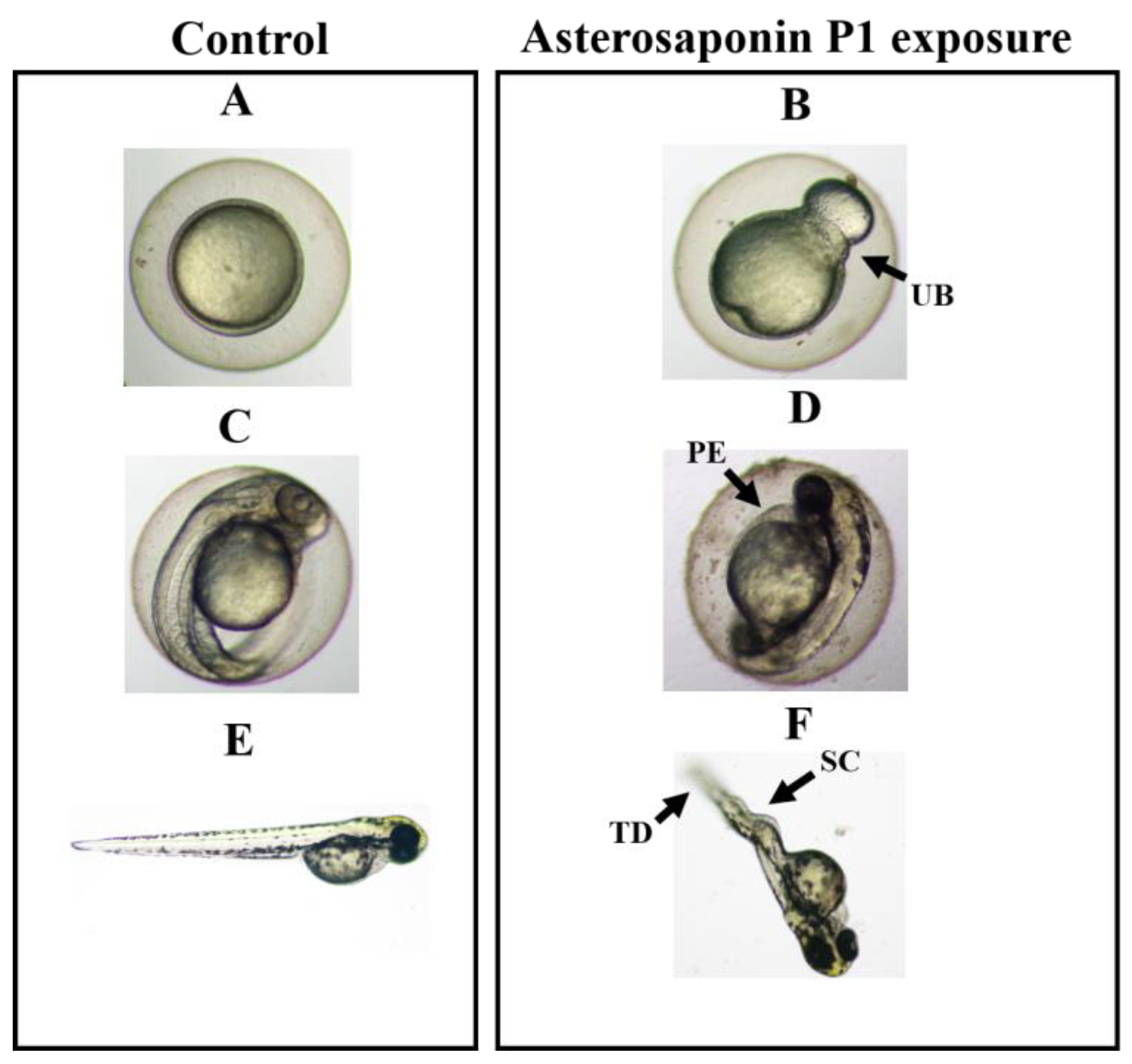
3.3. Teratogenicity of Zebrafish Embryos Treated by Asterosaponin P1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y. Fauna Sinica, Invertebrata Vol. 40: Echinodermata, Ophiuroidea; ConchBooks: Harxheim, Germany, 2004. [Google Scholar]
- Stöhr, S.; O’Hara, T.D.; Thuy, B. Global diversity of brittle stars (Echinodermata: Ophiuroidea). PLoS ONE 2012, 7, e31940. [Google Scholar] [CrossRef] [PubMed]
- Broom, D. Aggregation behaviour of the brittle-star Ophiothrix fragilis. J. Mar. Biol. Assoc. UK 1975, 55, 191–197. [Google Scholar] [CrossRef]
- Thuy, B. Exceptionally well-preserved brittle stars from the Pliensbachian (Early Jurassic) of the French Ardennes. Palaeontology 2011, 54, 215–233. [Google Scholar] [CrossRef]
- Yu, N.; Sun, S.; Zhang, G.; Zhang, F. Reproductive cycle of Ophiopholis mirabilis (Echinodermata: Ophiuroidea) in Zhangzi Island area, northern Yellow Sea. J. Oceanol. Limnol. 2021, 39, 173–184. [Google Scholar] [CrossRef]
- Yu, N.; Sun, S.; Wang, S.; Liu, Q.; Zhang, G.; Zhang, F.; Sun, X. An enhanced underwater camera apparatus for seabed observation of megabenthic epifauna in the northern Yellow Sea. J. Oceanol. Limnol. 2020, 38, 1799–1810. [Google Scholar] [CrossRef]
- Mantelatto, M.C.; Vidon, L.F.; Silveira, R.B.; Menegola, C.; Rocha, R.M.d.; Creed, J.C. Host species of the non-indigenous brittle star Ophiothela mirabilis (Echinodermata: Ophiuroidea): An invasive generalist in Brazil? Mar. Biodivers. Rec. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Alvarado, J.J.; Fernández, C. Equinodermos del Parque Nacional Marino Ballena, Pacífico, Costa Rica. Rev. De Biol. Trop. 2005, 53, 275–284. [Google Scholar]
- Dauria, M.V.; Riccio, R.; Minale, L.; Labarre, S.; Pusset, J. Novel marine steroid sulfates from Pacific ophiuroids. J. Org. Chem. 1987, 52, 3947–3952. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Levina, E.V.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Sulfated steroids from pacific brittle stars Ophiopholis aculeata, Ophiura sarsi, and Stegophiura brachiactis. J. Nat. Prod.-Lloydia 1994, 57, 1631–1637. [Google Scholar] [CrossRef]
- Dauria, M.V.; Paloma, L.G.; Minale, L.; Riccio, R.; Zampella, A. On the composition of sulfated polyhydroxysteroids in some ophiuroids and the structure determination of six new constituents. J. Nat. Prod. 1995, 58, 189–196. [Google Scholar] [CrossRef]
- Roccatagliata, A.J.; Maier, M.S.; Seldes, A.M.; Pujol, C.A.; Damonte, E.B. Antiviral sulfated steroids from the ophiuroid Ophioplocus januarii. J. Nat. Prod. 1996, 59, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Roccatagliata, A.J.; Maier, M.S.; Seldes, A.M. New sulfated polyhydroxysteroids from the Antarctic ophiuroid Astrotoma agassizii. J. Nat. Prod. 1998, 61, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Klimenko, A.; Huber, R.; Marcourt, L.; Chardonnens, E.; Koval, A.; Khotimchenko, Y.S.; Ferreira Queiroz, E.; Wolfender, J.-L.; Katanaev, V.L. A Cytotoxic Porphyrin from North Pacific Brittle Star Ophiura sarsii. Mar. Drugs 2021, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Kalinovsky, A.I.; Dmitrenok, P.S.; Stonik, V.A. Hemolytic steroid disulfates from the far eastern starfish Pteraster pulvillus. J. Nat. Prod. 2003, 66, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Iken, K.; Greer, S.P.; Amsler, C.D.; McClintock, J.B. A new antifouling bioassay monitoring brown algal spore swimming behaviour in the presence of echinoderm extracts. Biofouling 2003, 19, 327–334. [Google Scholar] [CrossRef]
- Wang, R.; Dang, Y.; Feng, J.; Wang, Q.; Dai, P.; Guo, C. Optimization of the extraction of ophiurasaponin from Ophiopholis mirabilis with the orthogonal design method. Mar. Sci. 2012, 36, 46–49. [Google Scholar]
- Jiang, B.; Wang, Q.; He, Y.; Ren, D. Extraction of collagen from brittle star Ophiopholis mirabilis. J. Dalian Ocean Univ. 2013, 28, 498–501. [Google Scholar]
- Hassan, S.M.; Moussa, E.A.; Abbott, L.C. Effects of quillaja saponin (Quillaja saponaria) on early embryonic zebrafish (Danio rerio) development. Int. J. Toxicol. 2008, 27, 273–278. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, Y.; Jorgensen, L.v.G.; Strobel, B.W.; Hansen, H.C.B.; Cedergreen, N. Where does the toxicity come from in saponin extract? Chemosphere 2018, 204, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Modarresi Chahardehi, A.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Mostowy, S. The Case for Modeling Human Infection in Zebrafish. Trends Microbiol. 2020, 28, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.P.; Gantar, M.; Gibbs, P.D.L.; Schmale, M.C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2007, 145, 61–72. [Google Scholar] [CrossRef]
- Berry, J.P.; Gibbs, P.D.L.; Schmale, M.C.; Saker, M.L. Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon 2009, 53, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Fikirdesici, S.; Altindag, A.; Ozdemir, E. Investigation of acute toxicity of cadmium-arsenic mixtures to Daphnia magna with toxic units approach. Turk. J. Zool. 2012, 36, 543–550. [Google Scholar] [CrossRef]
- Kicha, A.A.; Kalinovsky, A.I.; Levina, E.V.; Stonik, V.A.; Elyakov, G.B. Asterosaponin-P1 from the starfish Patiria pectinifera. Tetrahedron Lett. 1983, 24, 3893–3896. [Google Scholar] [CrossRef]
- Iorizzi, M.; Bryan, P.; McClintock, J.; Minale, L.; Palagiano, E.; Maurelli, S.; Riccio, R.; Zollo, F. Chemical and biological investigation of the polar constituents of the starfish Luidia clathrata, collected in the gulf of mexico. J. Nat. Prod. 1995, 58, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Decorrea, R.S.; Riccio, R.; Minale, L.; Duque, C. Starfish saponins, Part 21. steroidal glycosides from the starfish Oreaster reticulatus. J. Nat. Prod. 1985, 48, 751–755. [Google Scholar] [CrossRef]
- De Luca, E.; Zaccaria, G.M.; Hadhoud, M.; Rizzo, G.; Ponzini, R.; Morbiducci, U.; Santoro, M.M. ZebraBeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Hayati, F.; Chabib, L.; Fauzi, I.S.; Awaluddin, R.; Sumayya; Faizah, W.S.; Mohd Nasir, M.H.; Nipun, T.S. Effects of Pegagan (Centella asiatica L.) Ethanolic Extract SNEDDS (Self-nanoemulsifying Drug Delivery Systems) on the Development of Zebrafish (Danio rerio) Embryos. J. Pharm. Bioallied Sci. 2020, 12, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Shariff, N.F.S.; Singgampalam, T.; Ng, C.H.; Kue, C.S. Antioxidant activity and zebrafish teratogenicity of hydroalcoholic Moringa oleifera L. leaf extracts. Br. Food J. 2020, 122, 3129–3137. [Google Scholar] [CrossRef]
- Du, Z.-C.; Xia, Z.-S.; Huang, Y.-F.; Peng, Y.; Cao, B.-B.; Li, C.-Q.; Liang, Y.-F.; Zhao, F.-H.; Zhang, M.-Z.; Chen, Z.-M.; et al. Cardiotoxicity induced by Cochinchina momordica seed extract in zebrafish. J. Appl. Toxicol. 2021, 41, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
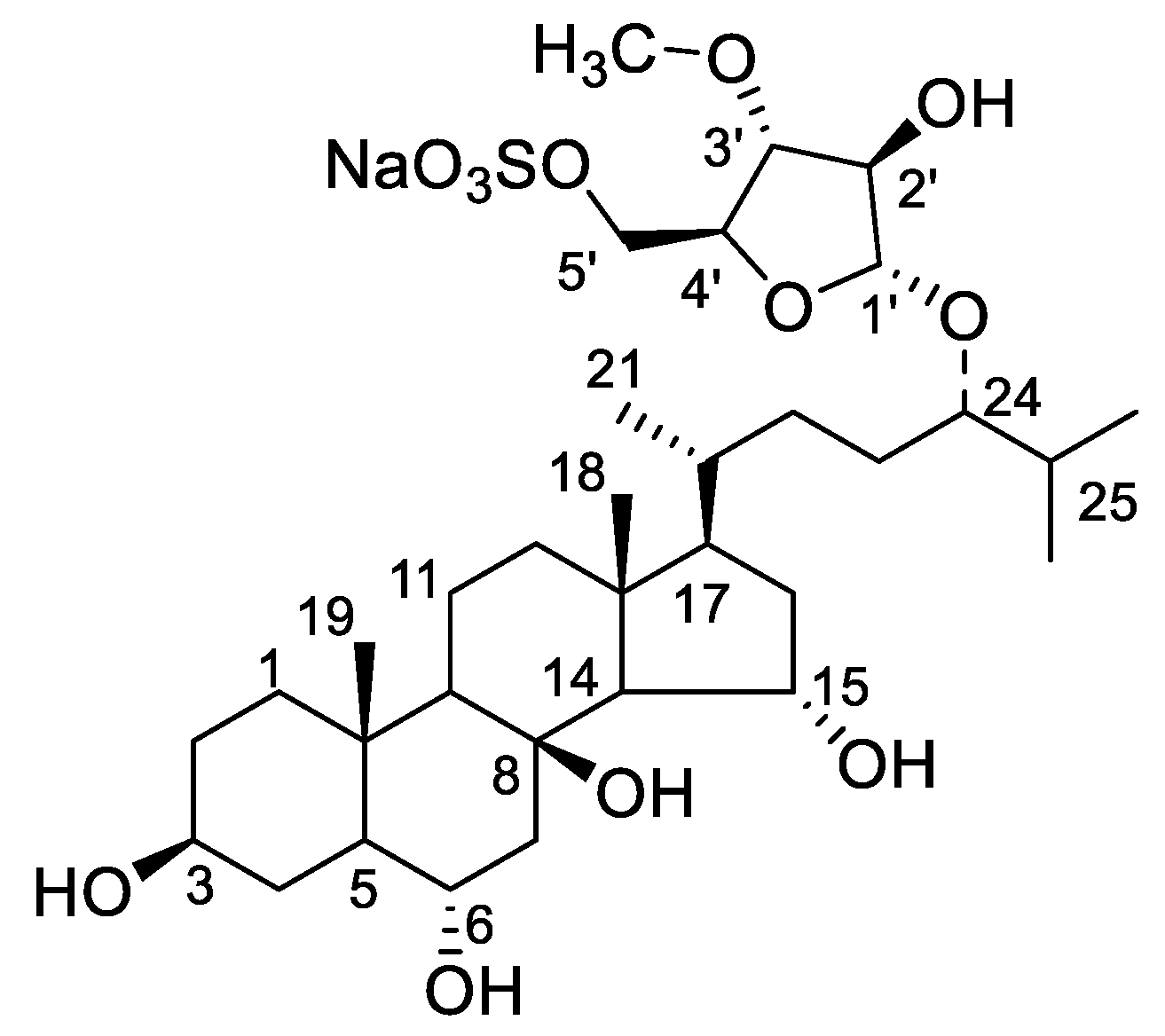
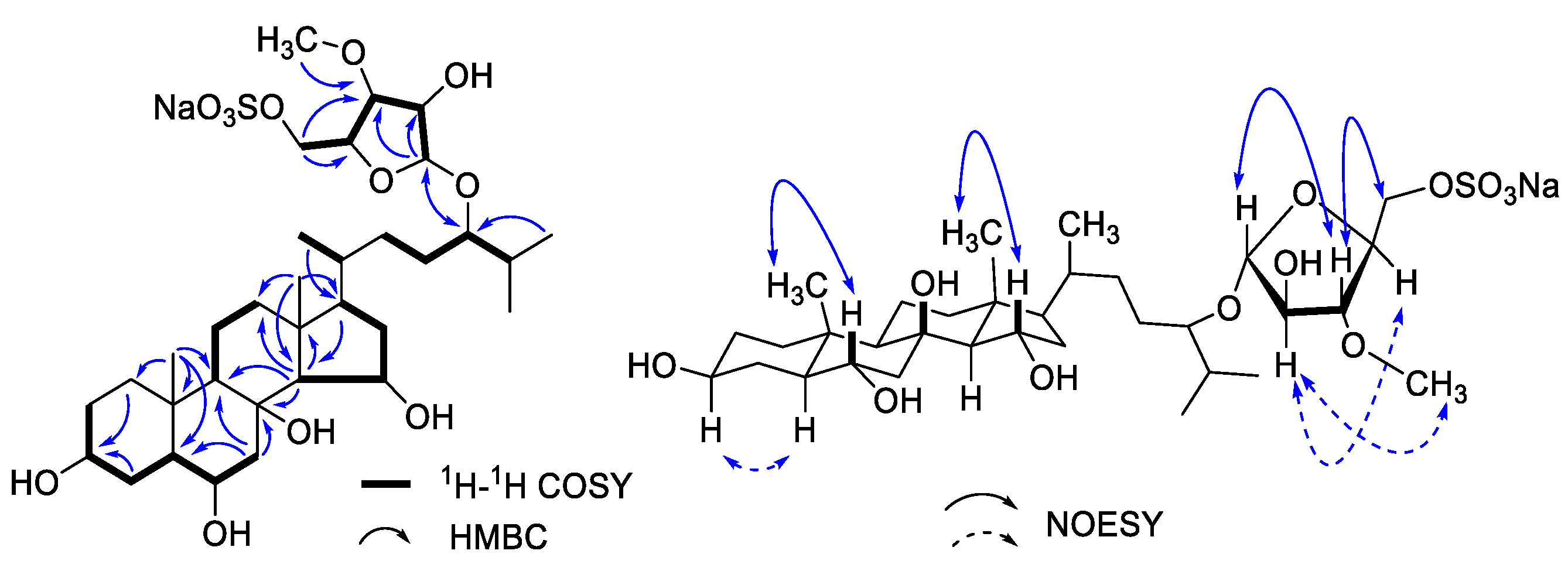

| No. | δH (J in Hz) | δC | No. | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1a | 1.73 (m) | 39.7 (CH2) | 16a | 1.90 (m) | 42.0 (CH2) |
| 1b | 0.98 (m) | 16b | 1.78 (m) | ||
| 2a | 1.73 (m) | 31.6 (CH2) | 17 | 1.35 (m) | 56.2 (CH) |
| 2b | 1.47 (m) | 18 | 0.97 (s) | 15.6 (CH3) | |
| 3 | 3.48 (m) | 72.3 (CH) | 19 | 1.02 (s) | 14.4 (CH3) |
| 4a | 2.19 (m) | 32.5 (CH2) | 20 | 1.33 (m) | 36.5 (CH) |
| 4b | 1.20 (m) | 21 | 0.90 (d, 6.5) | 19.1 (CH3) | |
| 5 | 1.03 (m) | 53.7 (CH) | 22a | 1.64 (m) | 33.3 (CH2) |
| 6 | 3.61 (m) | 67.8 (CH) | 22b | 0.92 (m) | |
| 7a | 2.39 (dd, 13.5, 4.0) | 50.4 (CH2) | 23a | 1.56 (m) | 28.9 (CH2) |
| 7b | 1.37 (m) | 23b | 1.23 (m) | ||
| 8 | -- | 76.2 (qC) | 24 | 3.29 (m) | 84.7 (CH) |
| 9 | 0.87 (m) | 57.4 (CH) | 25 | 1.84 (m) | 32.0 (CH) |
| 10 | -- | 38.0 (qC) | 26 | 0.90 (d, 6.5) | 18.3 (CH3) |
| 11a | 1.69 (m) | 19.8 (CH2) | 27 | 0.90 (d, 6.5) | 18.7 (CH3) |
| 11b | 1.50 (m) | 1′ | 4.94 (s) | 109.7 (CH) | |
| 12a | 1.95 (m) | 43.0 (CH2) | 2′ | 4.04 (m) | 81.8 (CH) |
| 12b | 1.25 (m) | 3′ | 3.59 (dd, 5.7, 2.4) | 89.5 (CH) | |
| 13 | -- | 45.6 (qC) | 4′ | 4.19 (m) | 82.0 (CH) |
| 14 | 1.29 (m) | 67.1 (CH) | 5′ | 4.12 (d, 5.1) | 69.1 (CH2) |
| 15 | 4.21 (m) | 69.9 (CH) | OMe | 3.44 (s) | 58.6 (CH3) |
| Concentrations (mg/L) | Times of Exposure (h) | ||||
|---|---|---|---|---|---|
| 12 | 24 | 48 | 72 | 96 | |
| 0 | 0 | 0 | 0 | 0 | 0 |
| 5.0 | 0 | 0 | 0 | 0 | 0 |
| 7.5 | 0 | 0 | 0 | 0 | 0 |
| 10.0 | 0 | 0 | 24.67 ± 2.23 | 58.1 ± 8.57 | 82.30 ± 8.10 |
| 15.0 | 0 | 10.76 ± 7.24 | 39.26 ± 7.15 | 75.36 ± 3.94 | NE |
| 25.0 | 0 | 24.17 ± 9.36 | 49.23 ± 9.75 | 90.25 ± 9.32 | NE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Yang, X.; Zhang, R.; Li, Y.; Yan, Z.; Li, X.; Ma, B.; Liu, Y.; Lin, A.; Han, S.; et al. Embryotoxicity and Teratogenicity of Steroidal Saponin Isolated from Ophiopholis mirabilis. Toxics 2023, 11, 137. https://doi.org/10.3390/toxics11020137
Xu Q, Yang X, Zhang R, Li Y, Yan Z, Li X, Ma B, Liu Y, Lin A, Han S, et al. Embryotoxicity and Teratogenicity of Steroidal Saponin Isolated from Ophiopholis mirabilis. Toxics. 2023; 11(2):137. https://doi.org/10.3390/toxics11020137
Chicago/Turabian StyleXu, Qian, Xiao Yang, Ranran Zhang, Yaxi Li, Zhi Yan, Xiaodong Li, Bing Ma, Yanfang Liu, Ainuo Lin, Shaoshuai Han, and et al. 2023. "Embryotoxicity and Teratogenicity of Steroidal Saponin Isolated from Ophiopholis mirabilis" Toxics 11, no. 2: 137. https://doi.org/10.3390/toxics11020137
APA StyleXu, Q., Yang, X., Zhang, R., Li, Y., Yan, Z., Li, X., Ma, B., Liu, Y., Lin, A., Han, S., Li, K., & Chen, L. (2023). Embryotoxicity and Teratogenicity of Steroidal Saponin Isolated from Ophiopholis mirabilis. Toxics, 11(2), 137. https://doi.org/10.3390/toxics11020137







