Butylparaben Exposure Induced Darker Skin Pigmentation in Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Exposure
2.2. Fish Maintenance and Sampling
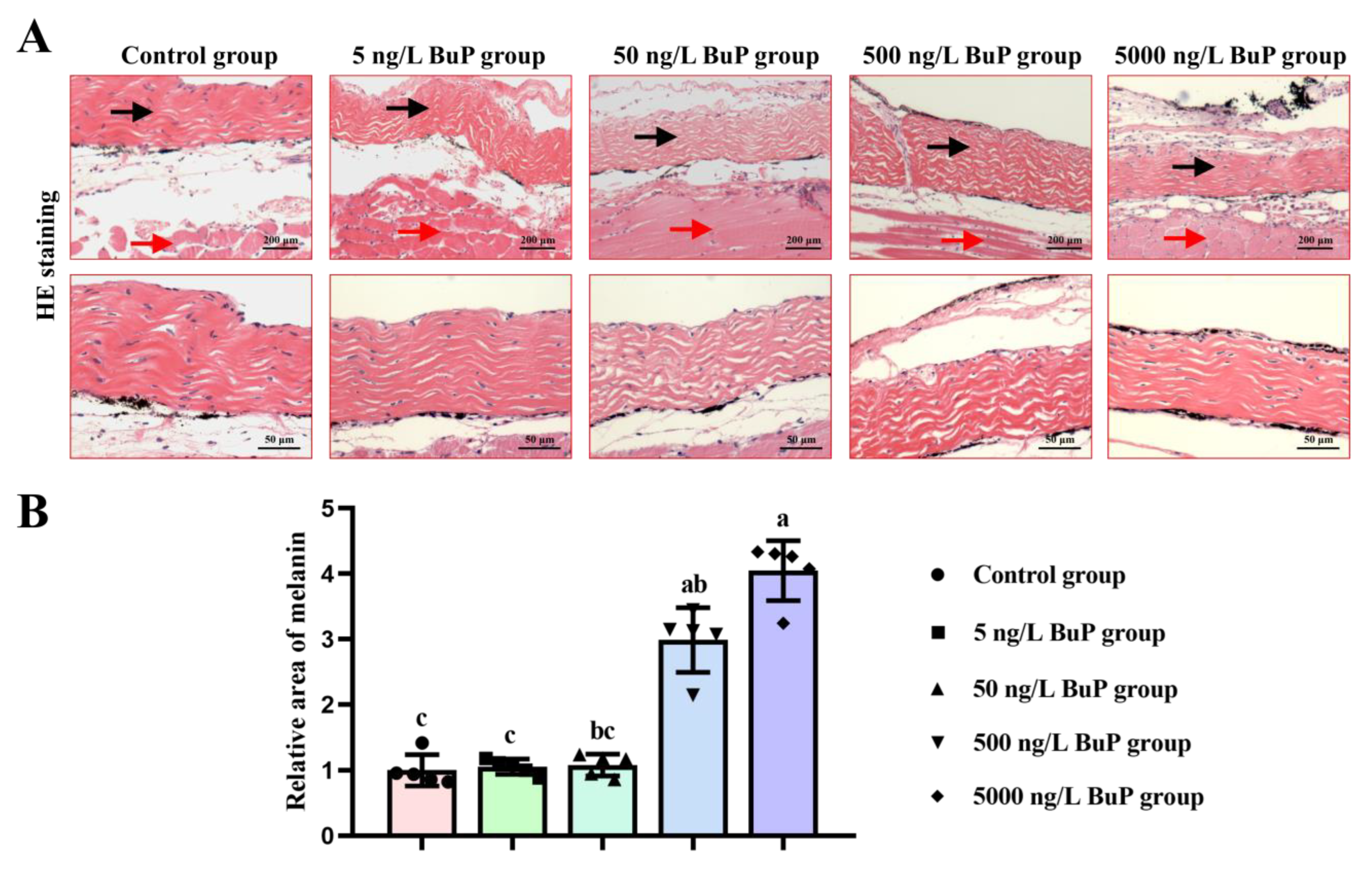
2.3. Histology Analysis
2.4. ELISA
2.5. RT-qPCR Analysis
2.6. Statistical Analysis
3. Results
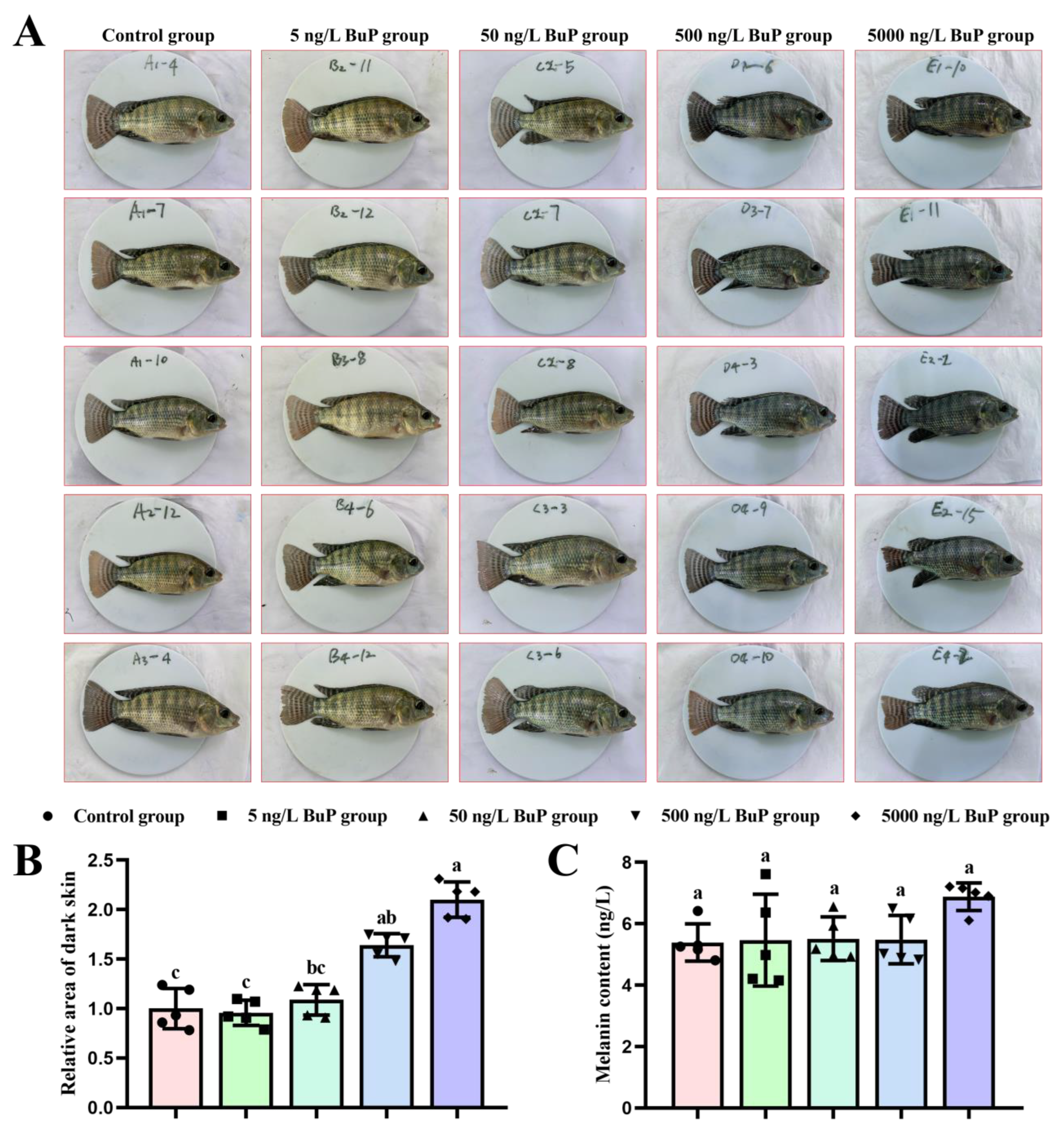
3.1. Melanin Content
3.2. Enzymes and Genes Related to Melanin Synthesis
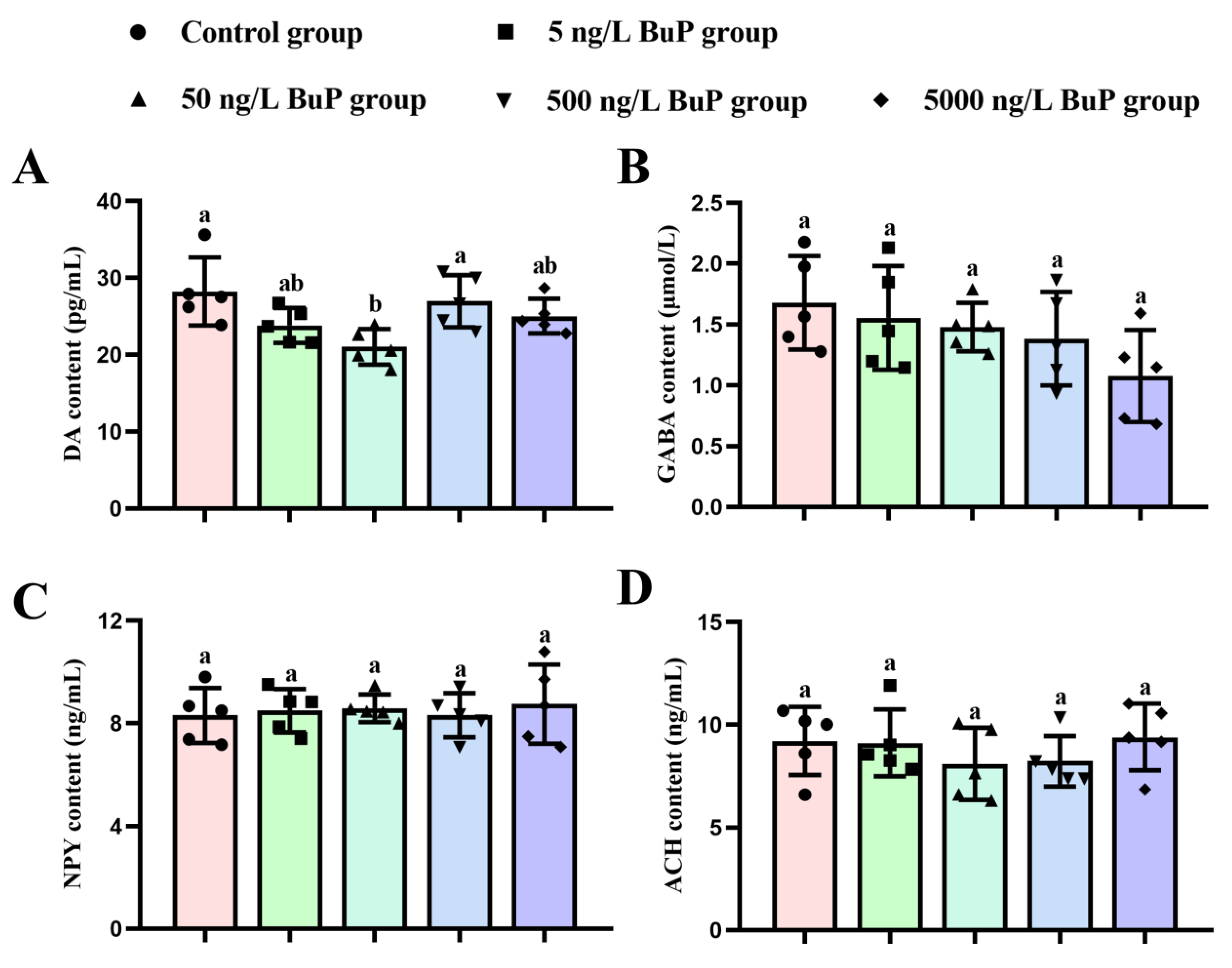
3.3. Neurotransmitter Content
3.4. Relative Gene Expression Levels in the Phototransduction Pathway
3.5. Correlation Analysis
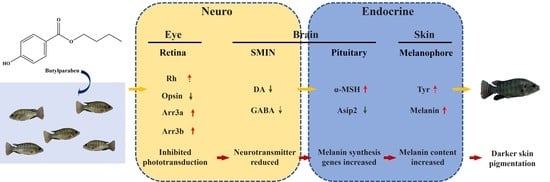
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cal, L.; Suarez-Bregua, P.; Cerdá-Reverter, J.M.; Braasch, I.; Rotllant, J. Fish pigmentation and the melanocortin system. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 211, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Vissio, P.G.; Darias, M.J.; Di Yorio, M.P.; Pérez Sirkin, D.I.; Delgadin, T.H. Fish skin pigmentation in aquaculture: The influence of rearing conditions and its neuroendocrine regulation. Gen. Comp. Endocrinol. 2021, 301, 113662. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Washio, Y.; Fujinami, Y.; Shimizu, D.; Uji, S.; Yokoi, H.; Suzuki, T. Continuous illumination through larval development suppresses dopamine synthesis in the suprachiasmatic nucleus, causing activation of α-MSH synthesis in the pituitary and abnormal metamorphic skin pigmentation in flounder. Gen. Comp. Endocrinol. 2012, 176, 215–221. [Google Scholar] [CrossRef]
- Fransway, A.F.; Fransway, P.J.; Belsito, D.V.; Yiannias, J.A. Paraben Toxicology. Dermatitis 2019, 30, 32–45. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Song, Y.; Pan, Y. Skin irritation potential of cosmetic preservatives: An exposure-relevant study. J. Cosmet. Dermatol. 2021, 20, 195–203. [Google Scholar] [CrossRef]
- Chen, C.Y.; Sun, C.Y.; Hsu, H.J.; Wu, I.W.; Chen, Y.C.; Lee, C.C. Xenoestrogen exposure and kidney function in the general population: Results of a community-based study by laboratory tests and questionnaire-based interviewing. Environ. Int. 2021, 155, 106585. [Google Scholar] [CrossRef]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; Albornoz, M.P.D.; et al. Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef]
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef]
- Ko, M.Y.; Hyun, S.A.; Jang, S.; Seo, J.W.; Rho, J.; Lee, B.S.; Ka, M. Butylparaben Induces the Neuronal Death Through the ER Stress-Mediated Apoptosis of Primary Cortical Neurons. Neurotox. Res. 2022, 40, 36–43. [Google Scholar] [CrossRef]
- Wei, F.; Cheng, H.; Sang, N. Comprehensive assessment of estrogenic activities of parabens by in silico approach and in vitro assays. Sci. Total. Environ. 2022, 845, 157194. [Google Scholar] [CrossRef] [PubMed]
- Firat, O.; Kargin, F. Individual and combined effects of heavy metals on serum biochemistry of Nile tilapia Oreochromis niloticus. Arch. Environ. Contam. Toxicol. 2010, 58, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, B.; Li, T.; Liang, G.; Xu, M.; Liu, X.; Tao, W.; Zhou, L.; Kocher, T.D.; Wang, D. Nile Tilapia: A Model for Studying Teleost Color Patterns. J. Hered. 2021, 112, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.A.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jia, Y.; Han, F.; Xia, C.; Zhao, Q.; Zhang, J.; Li, E. Toxic effects of waterborne benzylparaben on the growth, antioxidant capacity and lipid metabolism of Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2022, 248, 106197. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tamura, I.; Hirata, Y.; Kato, J.; Kagota, K.; Katsuki, S.; Yamamoto, A.; Kagami, Y.; Tatarazako, N. Aquatic toxicity and ecological risk assessment of seven parabens: Individual and additive approach. Sci. Total. Environ. 2011, 410–411, 102–111. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.J.; Jin, Y.H.; Guo, R.Y.; Yang, L.; Li, E.C.; Zhang, J.L. Triphenyltin exposure induced abnormal morphological colouration in adult male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2022, 242, 113912. [Google Scholar] [CrossRef]
- Lennquist, A.; Mårtensson Lindblad, L.G.; Hedberg, D.; Kristiansson, E.; Förlin, L. Colour and melanophore function in rainbow trout after long term exposure to the new antifoulant medetomidine. Chemosphere 2010, 80, 1050–1055. [Google Scholar] [CrossRef]
- Peng, L.; Wang, C.; Li, P.; Cheng, B.; Hu, Y.; Cheng, Y.; Zheng, Q. Evaluation of hypopigmentation in embryonic zebrafish induced by emerging disinfection byproduct, 3, 5-di-I-tyrosylalanine. Aquat. Toxicol. 2020, 225, 105525. [Google Scholar] [CrossRef] [PubMed]
- Bertolesi, G.E.; Song, Y.N.; Atkinson-Leadbeater, K.; Yang, J.J.; McFarlane, S. Interaction and developmental activation of two neuroendocrine systems that regulate light-mediated skin pigmentation. Pigment Cell Melanoma Res. 2017, 30, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Ollmann, M.M.; Lamoreux, M.L.; Wilson, B.D.; Barsh, G.S. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998, 12, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Verburg-Van Kemenade, B.M.; Jenks, B.G.; Driessen, A.G. GABA and dopamine act directly on melanotropes of Xenopus to inhibit MSH secretion. Brain Res. Bull. 1986, 17, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Roubos, E.W.; Van Wijk, D.C.; Kozicz, T.; Scheenen, W.J.; Jenks, B.G. Plasticity of melanotrope cell regulations in Xenopus laevis. Eur. J. Neurosci. 2010, 32, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Jenks, B.G.; Kidane, A.H.; Scheenen, W.J.; Roubos, E.W. Plasticity in the melanotrope neuroendocrine interface of Xenopus laevis. Neuroendocrinology 2007, 85, 177–185. [Google Scholar] [CrossRef]
- Bertolesi, G.E.; Hehr, C.L.; McFarlane, S. Melanopsin photoreception in the eye regulates light-induced skin colour changes through the production of α-MSH in the pituitary gland. Pigment Cell Melanoma Res. 2015, 28, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, T.; Kojima, D.; Fukada, Y. Light-induced body color change in developing zebrafish. Photochem. Photobiol. Sci. 2010, 9, 1498–1504. [Google Scholar] [CrossRef]
- Buczyłko, J.; Saari, J.C.; Crouch, R.K.; Palczewski, K. Mechanisms of opsin activation. J. Biol. Chem. 1996, 271, 20621–20630. [Google Scholar] [CrossRef]
- Fan, G.; Siebert, F.; Sheves, M.; Vogel, R. Rhodopsin with 11-cis-locked chromophore is capable of forming an active state photoproduct. J. Biol. Chem. 2002, 277, 40229–40234. [Google Scholar] [CrossRef]
- Burns, M.E.; Arshavsky, V.Y. Beyond counting photons: Trials and trends in vertebrate visual transduction. Neuron. 2005, 48, 387–401. [Google Scholar] [CrossRef]
- Fain, G.L. Adaptation of mammalian photoreceptors to background light: Putative role for direct modulation of phosphodiesterase. Mol. Neurobiol. 2011, 44, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E. Shedding light on dark adaptation. Biochemist 2020, 42, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Woodruff, M.L.; Chen, F.S.; Chen, Y.; Cilluffo, M.C.; Tranchina, D.; Fain, G.L. Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J. Neurosci. 2012, 32, 15998–16006. [Google Scholar] [CrossRef]
- Calvert, P.D.; Klenchin, V.A.; Bownds, M.D. Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J. Biol. Chem. 1995, 270, 24127–24129. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, N.; Liang, Z.; Li, E.-c.; Wang, Y.; Zhang, S.; Zhang, J. Butylparaben Exposure Induced Darker Skin Pigmentation in Nile Tilapia (Oreochromis niloticus). Toxics 2023, 11, 119. https://doi.org/10.3390/toxics11020119
Liu S, Zhang N, Liang Z, Li E-c, Wang Y, Zhang S, Zhang J. Butylparaben Exposure Induced Darker Skin Pigmentation in Nile Tilapia (Oreochromis niloticus). Toxics. 2023; 11(2):119. https://doi.org/10.3390/toxics11020119
Chicago/Turabian StyleLiu, Song, Nan Zhang, Zhifang Liang, Er-chao Li, Yong Wang, Shijie Zhang, and Jiliang Zhang. 2023. "Butylparaben Exposure Induced Darker Skin Pigmentation in Nile Tilapia (Oreochromis niloticus)" Toxics 11, no. 2: 119. https://doi.org/10.3390/toxics11020119
APA StyleLiu, S., Zhang, N., Liang, Z., Li, E.-c., Wang, Y., Zhang, S., & Zhang, J. (2023). Butylparaben Exposure Induced Darker Skin Pigmentation in Nile Tilapia (Oreochromis niloticus). Toxics, 11(2), 119. https://doi.org/10.3390/toxics11020119