Impact of a Polymer-Based Nanoparticle with Formoterol Drug as Nanocarrier System In Vitro and in an Experimental Asthmatic Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Synthesis and Characterization Analyses
2.2.1. Synthesis of Lys-p(HEMA) Nanoparticles
2.2.2. Synthesis of Lys-p(HEMA) NPs–Formoterol Formulation
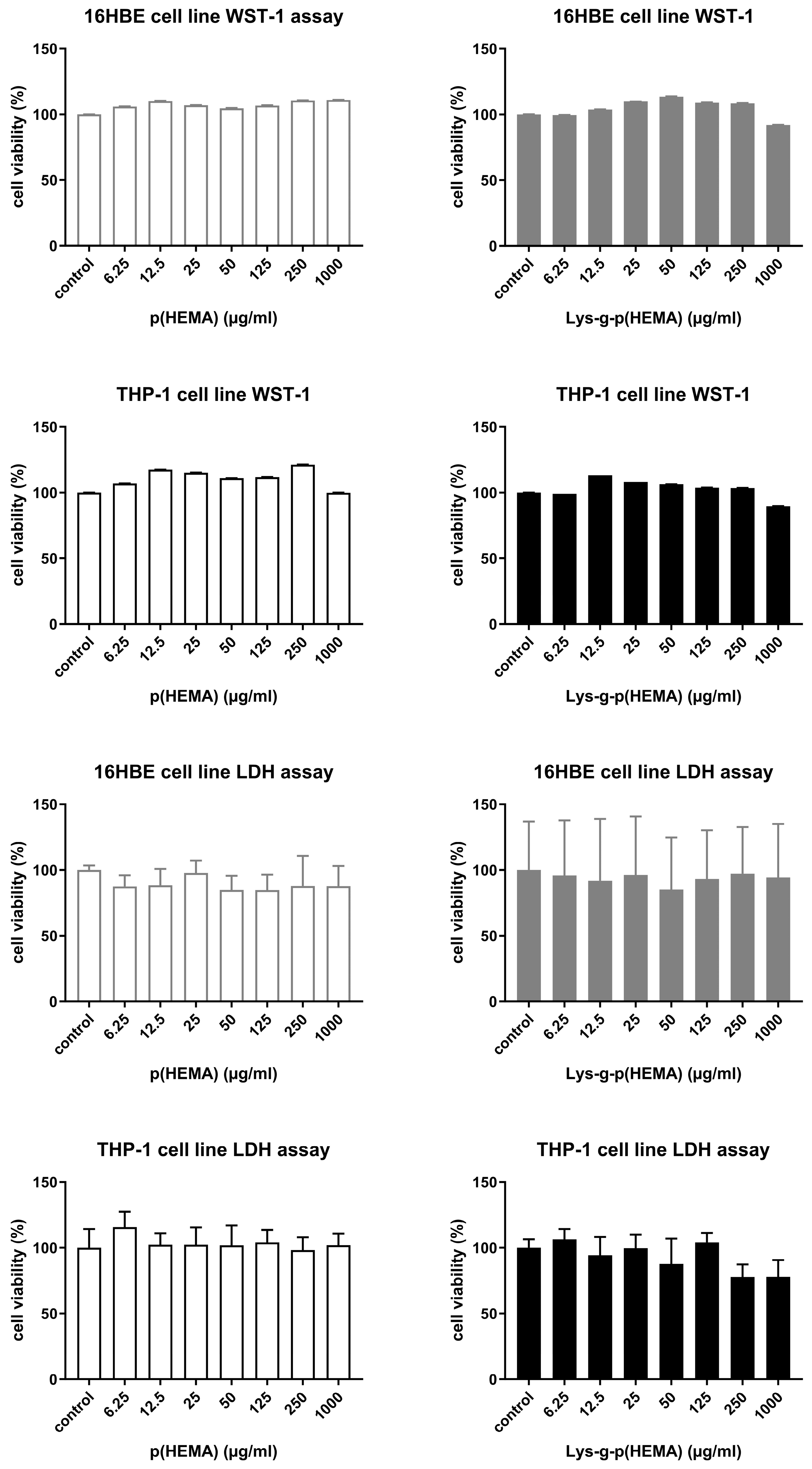
2.3. Cell Culture and Cytotoxicity Assay of Lys-p(HEMA) NPs
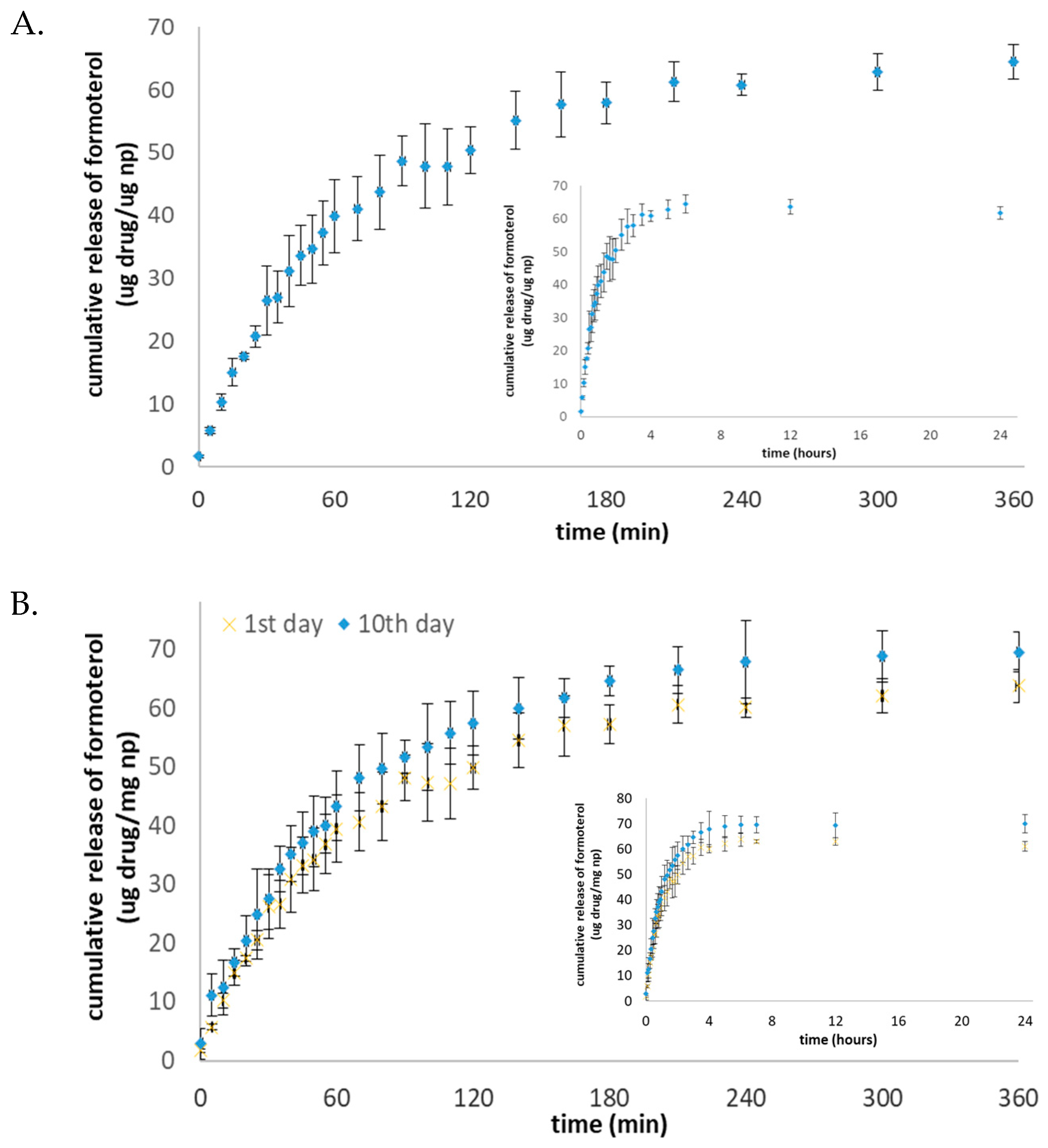
2.4. In Vitro Release of Formoterol from the Nanocarrier
2.5. Asthma Mouse Model
2.5.1. Experimental Protocol of OVA-Induced Asthma Model
2.5.2. Lung Function Measurement
2.5.3. Lung Inflammation
2.6. Data Analysis
3. Results
3.1. Synthesis of Lys-p(HEMA) NPs–Formoterol Formulation
3.2. Cytotoxicity Test Results
3.3. In Vitro Release Profile Results
3.4. OVA-Induced Asthma Model
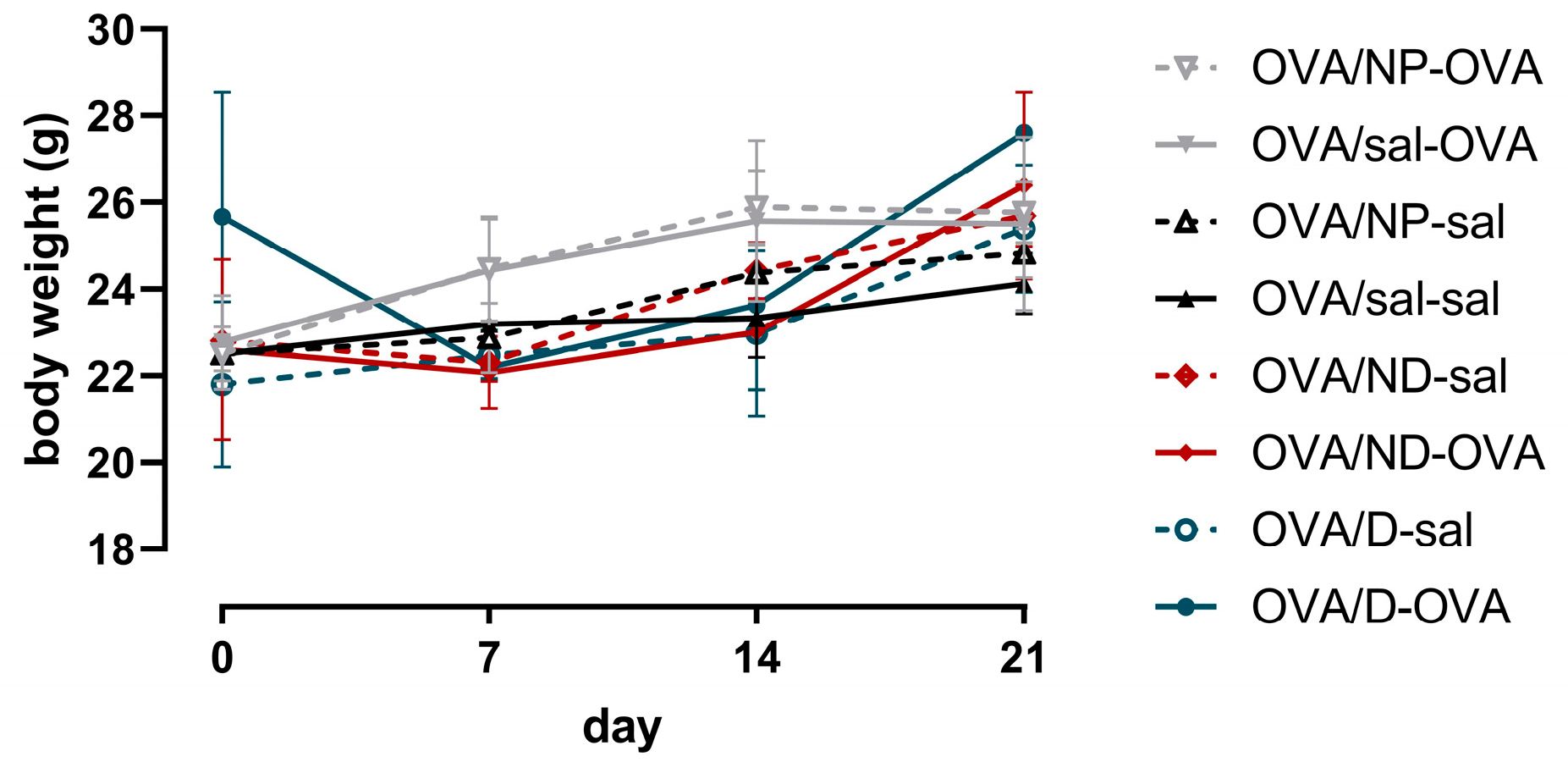
3.4.1. Organ Weights of Mice
3.4.2. Lung Function Measurement Results
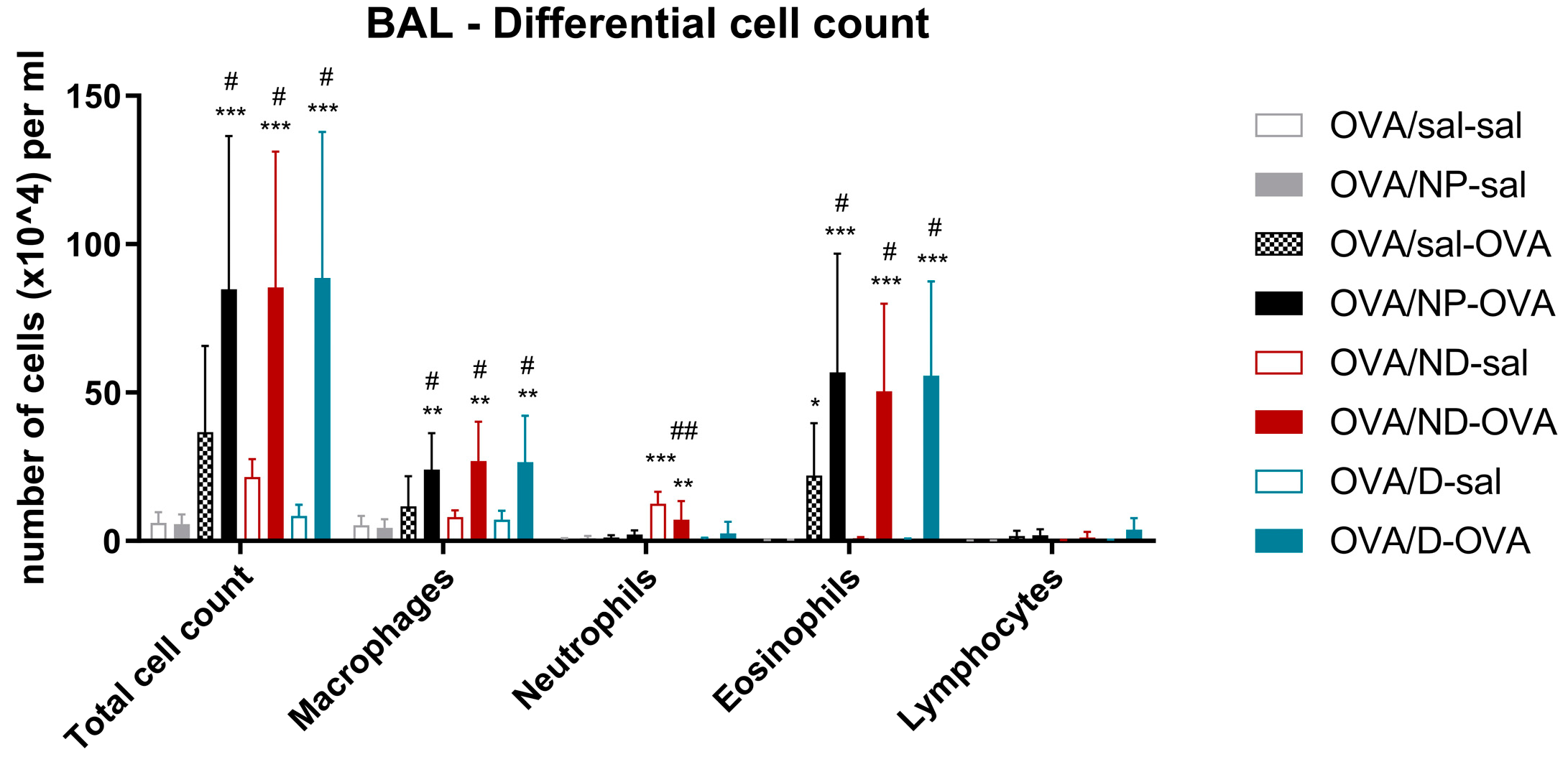
3.4.3. Bronchoalveolar Lavage Fluid Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thanawala, V.J.; Forkuo, G.S.; Al-Sawalha, N.; Azzegagh, Z.; Nguyen, L.P.; Eriksen, J.L.; Tuvim, M.J.; Lowder, T.W.; Dickey, B.F.; Knoll, B.J.; et al. Β2-Adrenoceptor Agonists Are Required for Development of the Asthma Phenotype in a Murine Model. Am. J. Respir. Cell Mol. Biol. 2013, 48, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Murad, H.A.; Habib, H.S.; Rafeeq, M.M.; Sulaiman, M.I.; Abdulrahman, A.S.; Khabaz, M.N. Co-Inhalation of Roflumilast, Rather than Formoterol, with Fluticasone More Effectively Improves Asthma in Asthmatic Mice. Exp. Biol. Med. 2017, 242, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, É.Y.; Simon, A.; da Silva, A.L.; Amaro, M.I.; de Almeida, G.S.; Agra, L.C.; Cabral, L.M.; Rocco, P.R.M.; Healy, A.M.; de Sousa, V.P. Effects of a Novel Roflumilast and Formoterol Fumarate Dry Powder Inhaler Formulation in Experimental Allergic Asthma. Int. J. Pharm. 2020, 588, 119771. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, B.-K.; Lee, Y.-C. Antiasthmatic Effects of Hesperidin, a Potential Th2 Cytokine Antagonist, in a Mouse Model of Allergic Asthma. Mediat. Inflamm. 2011, 2011, e485402. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allerg. Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Edwards, M.R.; Haas, J.; Panettieri, R.A.; Johnson, M.; Johnston, S.L. Corticosteroids and Β2 Agonists Differentially Regulate Rhinovirus-Induced Interleukin-6 via Distinct Cis-Acting Elements*. J. Biol. Chem. 2007, 282, 15366–15375. [Google Scholar] [CrossRef] [PubMed]
- Rothe, T.; Spagnolo, P.; Bridevaux, P.-O.; Clarenbach, C.; Eich-Wanger, C.; Meyer, F.; Miedinger, D.; Möller, A.; Nicod, L.P.; Nicolet-Chatelain, G.; et al. Diagnosis and Management of Asthma—The Swiss Guidelines. Respiration 2018, 95, 364–380. [Google Scholar] [CrossRef]
- Cates, C.J.; Schmidt, S.; Ferrer, M.; Sayer, B.; Waterson, S. Inhaled Steroids with and without Regular Salmeterol for Asthma: Serious Adverse Events. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Heffler, E.; Madeira, L.N.G.; Ferrando, M.; Puggioni, F.; Racca, F.; Malvezzi, L.; Passalacqua, G.; Canonica, G.W. Inhaled Corticosteroids Safety and Adverse Effects in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 776–781. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Keating, G.M. Budesonide/Formoterol. Drugs 2004, 64, 1597–1618. [Google Scholar] [CrossRef]
- Rezaei, R.; Safaei, M.; Mozaffari, H.R.; Moradpoor, H.; Karami, S.; Golshah, A.; Salimi, B.; Karami, H. The Role of Nanomaterials in the Treatment of Diseases and Their Effects on the Immune System. Open Access Maced. J. Med. Sci. 2019, 7, 1884–1890. [Google Scholar] [CrossRef]
- Wang, L.; Feng, M.; Li, Q.; Qiu, C.; Chen, R. Advances in Nanotechnology and Asthma. Ann. Transl. Med. 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. An Overview of Clinical and Commercial Impact of Drug Delivery Systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ehsan, I.; Mukherjee, B.; Mondal, L.; Roy, S.; Saha, K.D.; Paul, B.; Debnath, M.C.; Bera, T. Therapeutic Potential of Andrographolide-Loaded Nanoparticles on a Murine Asthma Model. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102006. [Google Scholar] [CrossRef] [PubMed]
- Gandhimathi, C.; Venugopal, J.R.; Sundarrajan, S.; Sridhar, R.; Tay, S.S.W.; Ramakrishna, S.; Kumar, S.D. Breathable Medicine: Pulmonary Mode of Drug Delivery. J. Nanosci. Nanotechnol. 2015, 15, 2591–2604. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Biswas, A.; Shukla, A.; Maiti, P. Biomaterials for Interfacing Cell Imaging and Drug Delivery: An Overview. Langmuir 2019, 35, 12285–12305. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bajpai, J.; Bajpai, A.K. Optimizing the Release Process and Modelling of in Vitro Release Data of Cis-Dichlorodiamminoplatinum (II) Encapsulated into Poly(2-Hydroxyethyl Methacrylate) Nanocarriers. Mater. Sci. Eng. C 2016, 58, 852–862. [Google Scholar] [CrossRef]
- Bakan, B.; Kayhan, C.T.; Koksal Karayildirim, C.; Dagdeviren, M.; Gulcemal, S.; Yildirim, Y.; Akgol, S.; Karabay Yavasoglu, N.U. Synthesis, Characterization, Toxicity and in Vivo Imaging of Lysine Graft Polymeric Nanoparticles. J. Polym. Res. 2019, 26, 239. [Google Scholar] [CrossRef]
- Öner, D.; Moisse, M.; Ghosh, M.; Duca, R.C.; Poels, K.; Luyts, K.; Putzeys, E.; Cokic, S.M.; Van Landuyt, K.; Vanoirbeek, J.; et al. Epigenetic Effects of Carbon Nanotubes in Human Monocytic Cells. Mutagenesis 2017, 32, 181–191. [Google Scholar] [CrossRef]
- Bakan, B.; Gülcemal, S.; Akgöl, S.; Hoet, P.H.M.; Karabay Yavaşoğlu, N.Ü. Synthesis, Characterization and Toxicity Assessment of a New Polymeric Nanoparticle, l-Glutamic Acid-g-p(HEMA). Chem. Biol. Interact. 2020, 315, 108870. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative Toxicity of 24 Manufactured Nanoparticles in Human Alveolar Epithelial and Macrophage Cell Lines. Part. Fibre Toxicol. 2009, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Devos, F.C.; Maaske, A.; Robichaud, A.; Pollaris, L.; Seys, S.; Lopez, C.A.; Verbeken, E.; Tenbusch, M.; Lories, R.; Nemery, B.; et al. Forced Expiration Measurements in Mouse Models of Obstructive and Restrictive Lung Diseases. Respir. Res. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Najlah, M.; D’Emanuele, A.; Elhissi, A. PAMAM Dendrimers as Aerosol Drug Nanocarriers for Pulmonary Delivery via Nebulization. Int. J. Pharm. 2014, 461, 242–250. [Google Scholar] [CrossRef]
- Kuzmov, A.; Minko, T. Nanotechnology Approaches for Inhalation Treatment of Lung Diseases. J. Control. Release 2015, 219, 500–518. [Google Scholar] [CrossRef]
- Kan, S.; Hariyadi, D.M.; Grainge, C.; Knight, D.A.; Bartlett, N.W.; Liang, M. Airway Epithelial-Targeted Nanoparticles for Asthma Therapy. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L500–L509. [Google Scholar] [CrossRef]
- Van Rijt, S.H.; Bein, T.; Meiners, S. Medical Nanoparticles for next Generation Drug Delivery to the Lungs. Eur. Respir. J. 2014, 44, 765–774. [Google Scholar] [CrossRef]
- Malcolmson, R.J.; Embleton, J.K. Dry Powder Formulations for Pulmonary Delivery. Pharm. Sci. Technol. Today 1998, 1, 394–398. [Google Scholar] [CrossRef]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, P.; Shekunov, B.Y. Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and Unknown Health Risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Pembrey, L.; Barreto, M.L.; Douwes, J.; Cooper, P.; Henderson, J.; Mpairwe, H.; Ardura-Garcia, C.; Chico, M.; Brooks, C.; Cruz, A.A.; et al. Understanding Asthma Phenotypes: The World Asthma Phenotypes (WASP) International Collaboration. ERJ Open Res. 2018, 4, 00013–02018. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Vanoirbeek, J.A.J.; Luyts, K.; Vooght, V.D.; Verbeken, E.; Thomassen, L.C.J.; Martens, J.A.; Dinsdale, D.; Boland, S.; Marano, F.; et al. Lung Exposure to Nanoparticles Modulates an Asthmatic Response in a Mouse Model. Eur. Respir. J. 2011, 37, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Frigas, E.; Loegering, D.A.; Wassom, D.L.; Steinmuller, D. Cytotoxic Properties of the Eosinophil Major Basic Protein. J. Immunol. 1979, 123, 2925–2927. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.G.; Irvin, C.G. Mechanisms of Airway Hyper-Responsiveness in Asthma: The Past, Present and yet to Come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Chiappara, G.; Gagliardo, R.; Siena, A.; Bonsignore, M.R.; Bousquet, J.; Bonsignore, G.; Vignola, A.M. Airway Remodelling in the Pathogenesis of Asthma. Curr. Opin. Allergy Clin. Immunol. 2001, 1, 85–93. [Google Scholar] [CrossRef]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of Asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [PubMed]
- Kips, J.C. Cytokines in Asthma. Eur. Respir. J. 2001, 18, 24s–33s. [Google Scholar] [CrossRef]
- Dorscheid, D.R.; Low, E.; Conforti, A.; Shifrin, S.; Sperling, A.I.; White, S.R. Corticosteroid-Induced Apoptosis in Mouse Airway Epithelium: Effect in Normal Airways and after Allergen-Induced Airway Inflammation. J. Allergy Clin. Immunol. 2003, 111, 360–366. [Google Scholar] [CrossRef]
- Hirota, J.A.; Hackett, T.L.; Inman, M.D.; Knight, D.A. Modeling Asthma in Mice: What Have We Learned about the Airway Epithelium? Am. J. Respir. Cell Mol. Biol. 2011, 44, 431–438. [Google Scholar] [CrossRef]
- Kim, D.I.; Song, M.-K.; Lee, K. Comparison of Asthma Phenotypes in OVA-Induced Mice Challenged via Inhaled and Intranasal Routes. BMC Pulm. Med. 2019, 19, 241. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Zhao, G.; Liu, Y.; Wang, F.; Song, H.; Sun, Y.; Zhou, Z.; Lu, X.; Hu, H.; et al. Mechanistic Study of Salidroside on Ovalbumin-Induced Asthmatic Model Mice Based on Untargeted Metabolomics Analysis. Food Funct. 2023, 14, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Bartlett, N.W.; Clarke, D.; Birrell, M.; Belvisi, M.; Johnston, S.L. Targeting the NF-ΚB Pathway in Asthma and Chronic Obstructive Pulmonary Disease. Pharmacol. Ther. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Ryan, D. The Role of Budesonide/Formoterol for Maintenance and Relief in the Management of Asthma. Pulm. Pharmacol. Ther. 2010, 23, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, W.; Wong, B.C.K.; Yin, L.; Wong, Y.F.; Xu, M.; Yang, Z. Liposomes Prolong the Therapeutic Effect of Anti-Asthmatic Medication via Pulmonary Delivery. Int. J. Nanomed. 2012, 7, 1139–1148. [Google Scholar] [CrossRef]
- Abraha, D.; Cho, S.H.; Agrawal, D.K.; Park, J.M.; Oh, C.K. (S,S)-Formoterol Increases the Production of IL-4 in Mast Cells and the Airways of a Murine Asthma Model. Int. Arch. Allergy Immunol. 2004, 133, 380–388. [Google Scholar] [CrossRef]



| Cytokines (pg/mL) | OVA/sal-sal | OVA/NP-sal | OVA/sal-OVA | OVA/NP-OVA | OVA/ND-sal | OVA/ND-OVA | OVA/D-sal | OVA/D-OVA |
|---|---|---|---|---|---|---|---|---|
| IL-4 | <LOD | <LOD | 2.52 ± 2.38 | 3.10 ± 2.38 | <LOD | 4.60 ± 1.86 | <LOD | 5.06 ± 1.21 |
| IL-5 | <LOD | <LOD | 1.85 ± 1.03 | 2.62 ± 1.89 | 0.18 ± 0.16 | 6.74 ± 5.38 | 0.91 ± 1.35 | 14.57 ± 13.33 # |
| IL-13 | <LOD | <LOD | 7.08 ± 5.67 | 10.04 ± 4.85 | <LOD | 10.26 ± 6.74 | 3.78 ± 1.60 | 23.49 ± 20.22 |
| IL-17A | <LOD | <LOD | 0.13 ± 0.09 | 0.31 ± 0.12 | 0.20 ± 0.19 | 0.51 ± 0.38 | <LOD | 0.31 ± 0.18 |
| IL-17F | <LOD | <LOD | <LOD | <LOD | 23.27 ± 15.58 | <LOD | 26.48 ± 24.94 | <LOD |
| IL-33 | 10.07 ± 6.42 | 9.34 ± 10.78 | 34.62 ± 25.50 | 4.44 ± 2.23 ## | 11.18 ± 8.36 | 11.68 ± 8.28 | 14.44 ± 6.19 | 11.76± 10.30 |
| IFN-γ | <LOD | <LOD | <LOD | <LOD | <LOD | 0.16 ± 0.15 | <LOD | <LOD |
| TNF-α | 2.16 ± 0.12 | 3.08 ± 1.23 | 3.99 ± 2.84 | 6.36 ± 5.44 | 13.21 ± 5.93 ** | 5.64 ± 3.36 | 1.75 ± 1.12 | 5.50 ± 1.60 |
| Il-6 | <LOD | <LOD | <LOD | <LOD | <LOD | 5.22 ± 8.46 | <LOD | <LOD |
| KC/GRO | 14.52 ± 1.61 | 30.44 ± 12.59 * | 51.20 ± 22.16 * | 46.07 ± 18.84 * | 52.80 ± 15.43 ** | 51.58 ± 19.48 * | 12.39 ± 3.58 | 50.32 ± 21.92 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakan, B.; Jonckheere, A.-C.; Decaesteker, T.; Marain, N.F.; Murugadoss, S.; Karabay Yavasoglu, N.U.; Şahar, U.; Şenay, R.H.; Akgöl, S.; Göksel, Ö.; et al. Impact of a Polymer-Based Nanoparticle with Formoterol Drug as Nanocarrier System In Vitro and in an Experimental Asthmatic Model. Toxics 2023, 11, 974. https://doi.org/10.3390/toxics11120974
Bakan B, Jonckheere A-C, Decaesteker T, Marain NF, Murugadoss S, Karabay Yavasoglu NU, Şahar U, Şenay RH, Akgöl S, Göksel Ö, et al. Impact of a Polymer-Based Nanoparticle with Formoterol Drug as Nanocarrier System In Vitro and in an Experimental Asthmatic Model. Toxics. 2023; 11(12):974. https://doi.org/10.3390/toxics11120974
Chicago/Turabian StyleBakan, Buket, Anne-Charlotte Jonckheere, Tatjana Decaesteker, Nora F. Marain, Sivakumar Murugadoss, Nefise Ulku Karabay Yavasoglu, Umut Şahar, Raziye Hilal Şenay, Sinan Akgöl, Özlem Göksel, and et al. 2023. "Impact of a Polymer-Based Nanoparticle with Formoterol Drug as Nanocarrier System In Vitro and in an Experimental Asthmatic Model" Toxics 11, no. 12: 974. https://doi.org/10.3390/toxics11120974
APA StyleBakan, B., Jonckheere, A.-C., Decaesteker, T., Marain, N. F., Murugadoss, S., Karabay Yavasoglu, N. U., Şahar, U., Şenay, R. H., Akgöl, S., Göksel, Ö., Hoet, P. H. M., & Vanoirbeek, J. A. J. (2023). Impact of a Polymer-Based Nanoparticle with Formoterol Drug as Nanocarrier System In Vitro and in an Experimental Asthmatic Model. Toxics, 11(12), 974. https://doi.org/10.3390/toxics11120974







