Serpentine Overburden Products—Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil
Abstract
:1. Introduction
2. Materials and Methods
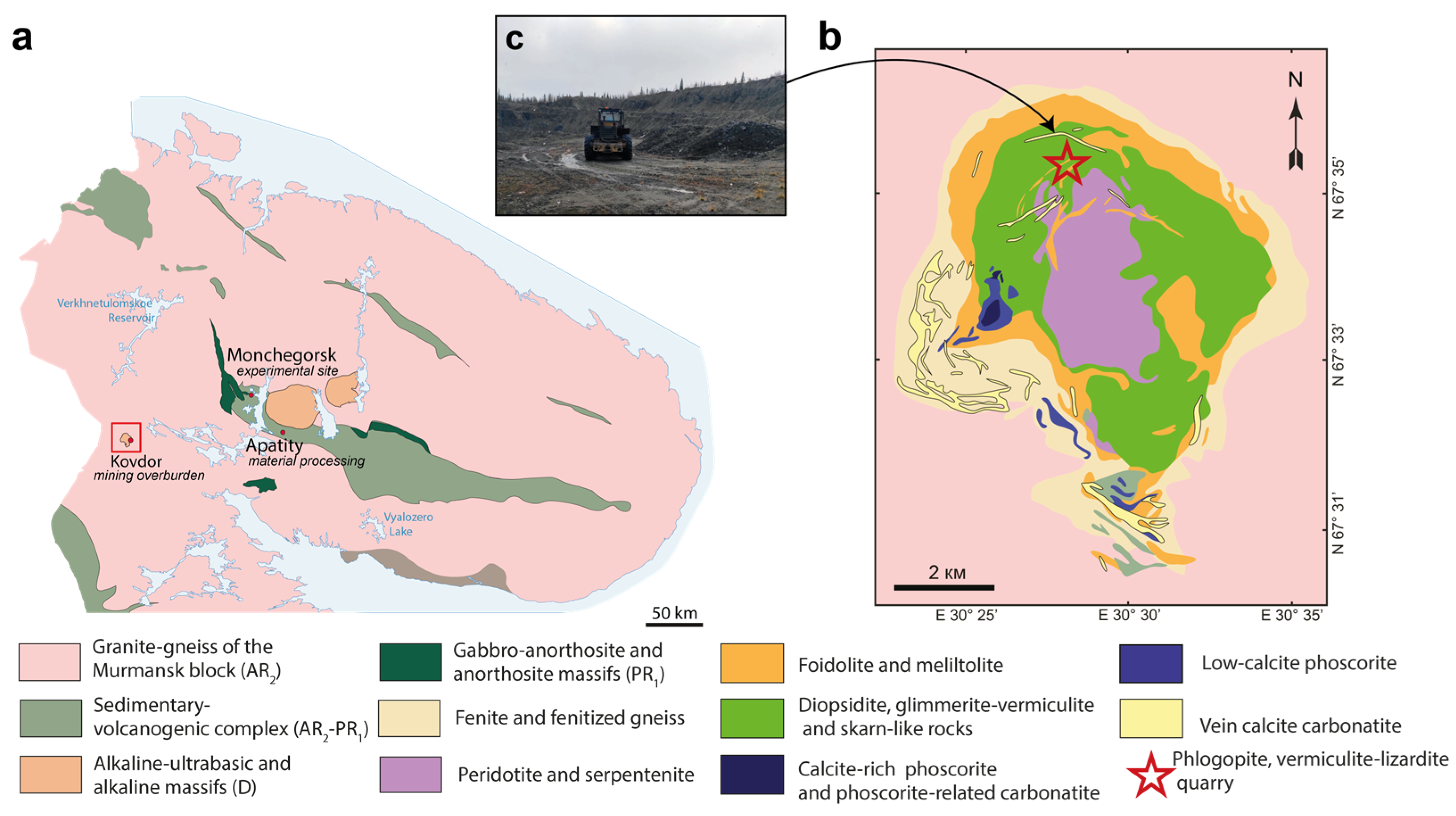
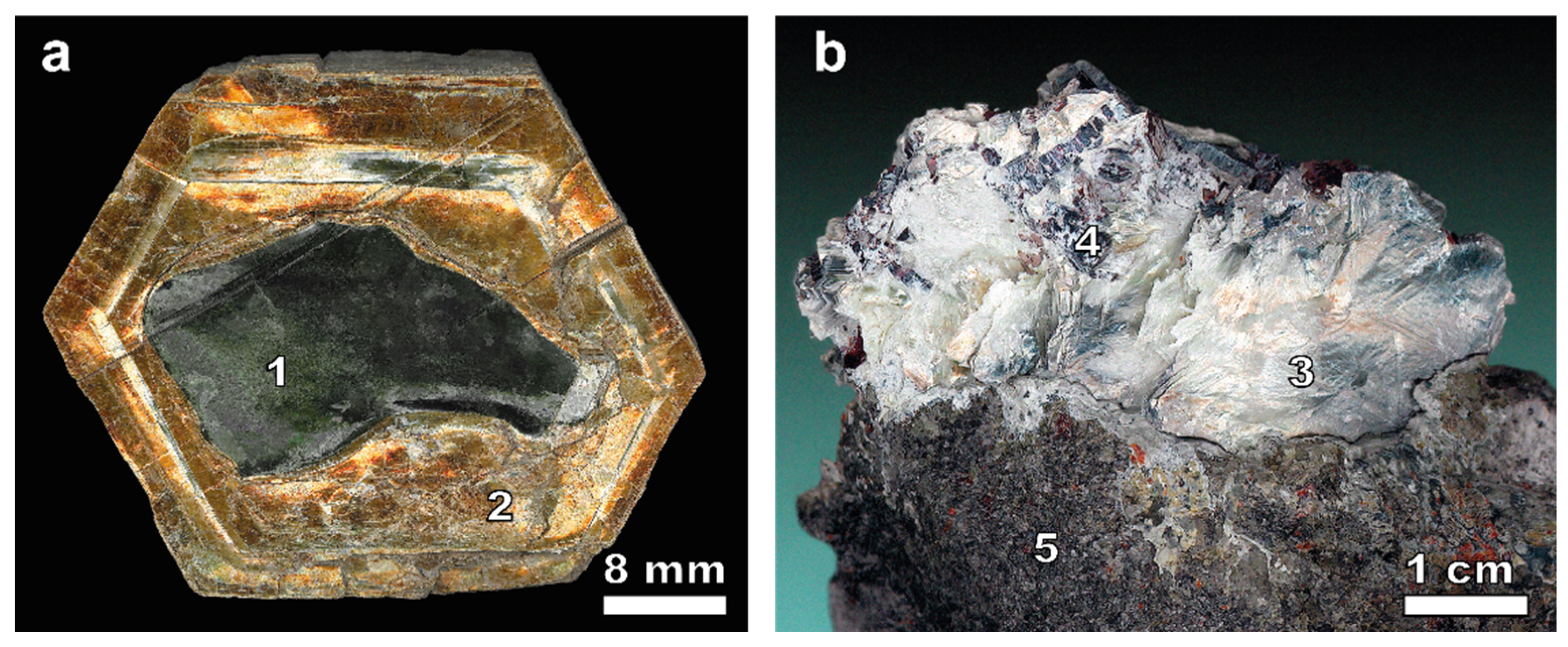
2.1. Materials
2.2. Design of Field Experiment
2.3. Methods
2.3.1. Chemical Analyses
2.3.2. Interpretation of the Sequential Fractioning Results
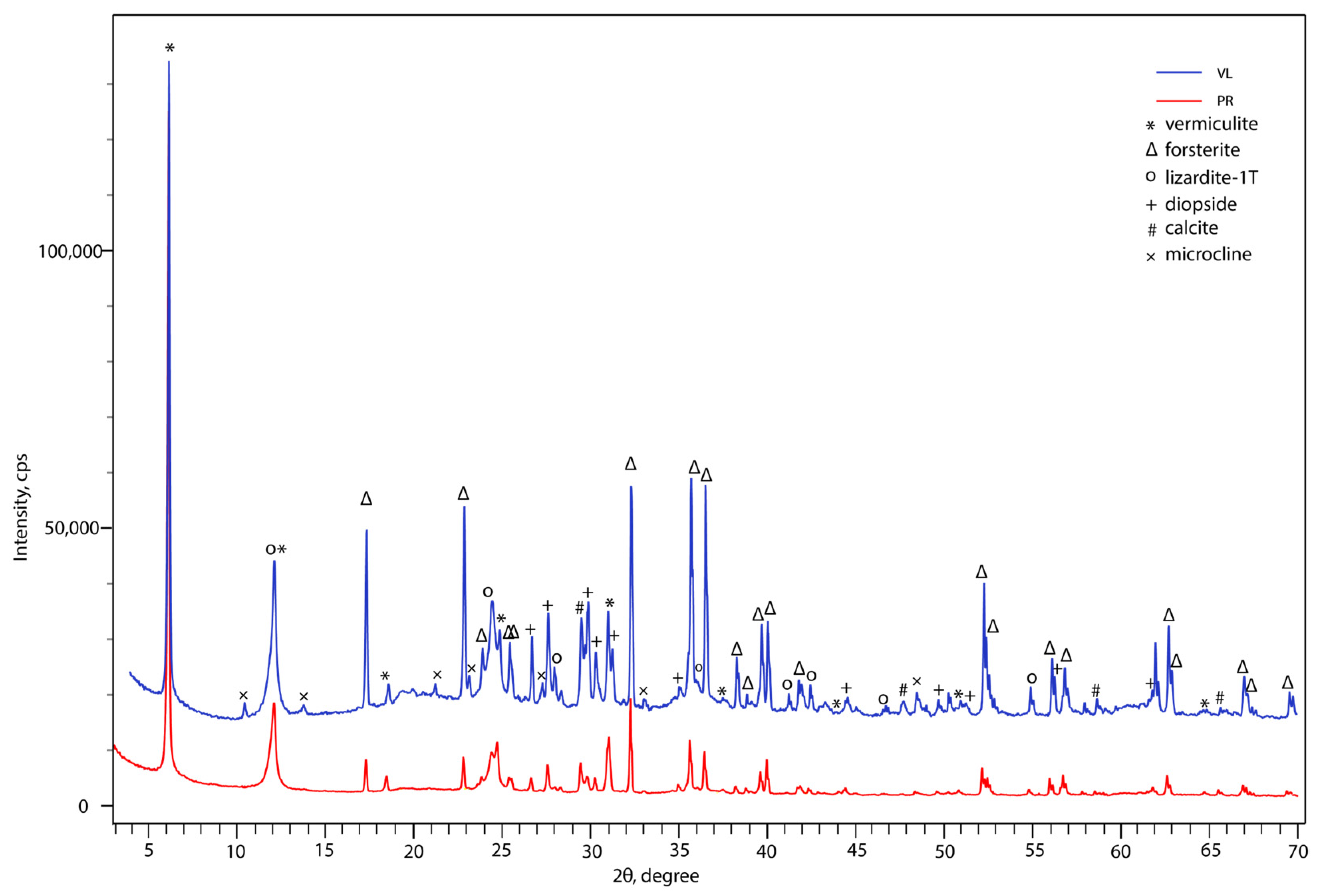
2.4. Powder X-ray Diffraction
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Serpentine-Containing Soil Mixtures
3.2. Composition of Geochemical Fractions of Serpentine-Containing Soil Mixtures
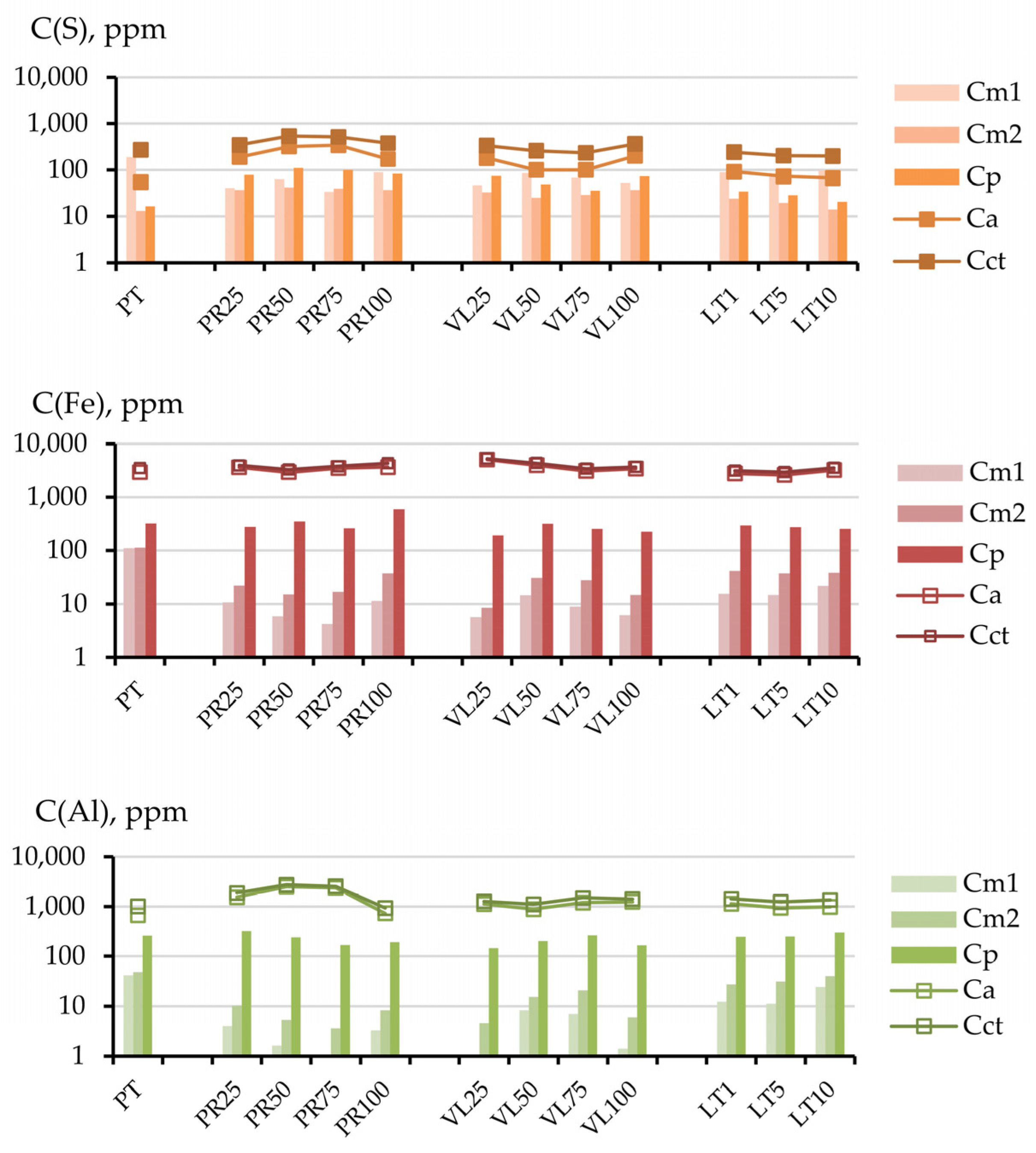
3.2.1. Influence of Mineral Composition and Share of Serpentine-Containing Materials in Soil Mixture on the Distribution of Chemical Elements by Fraction
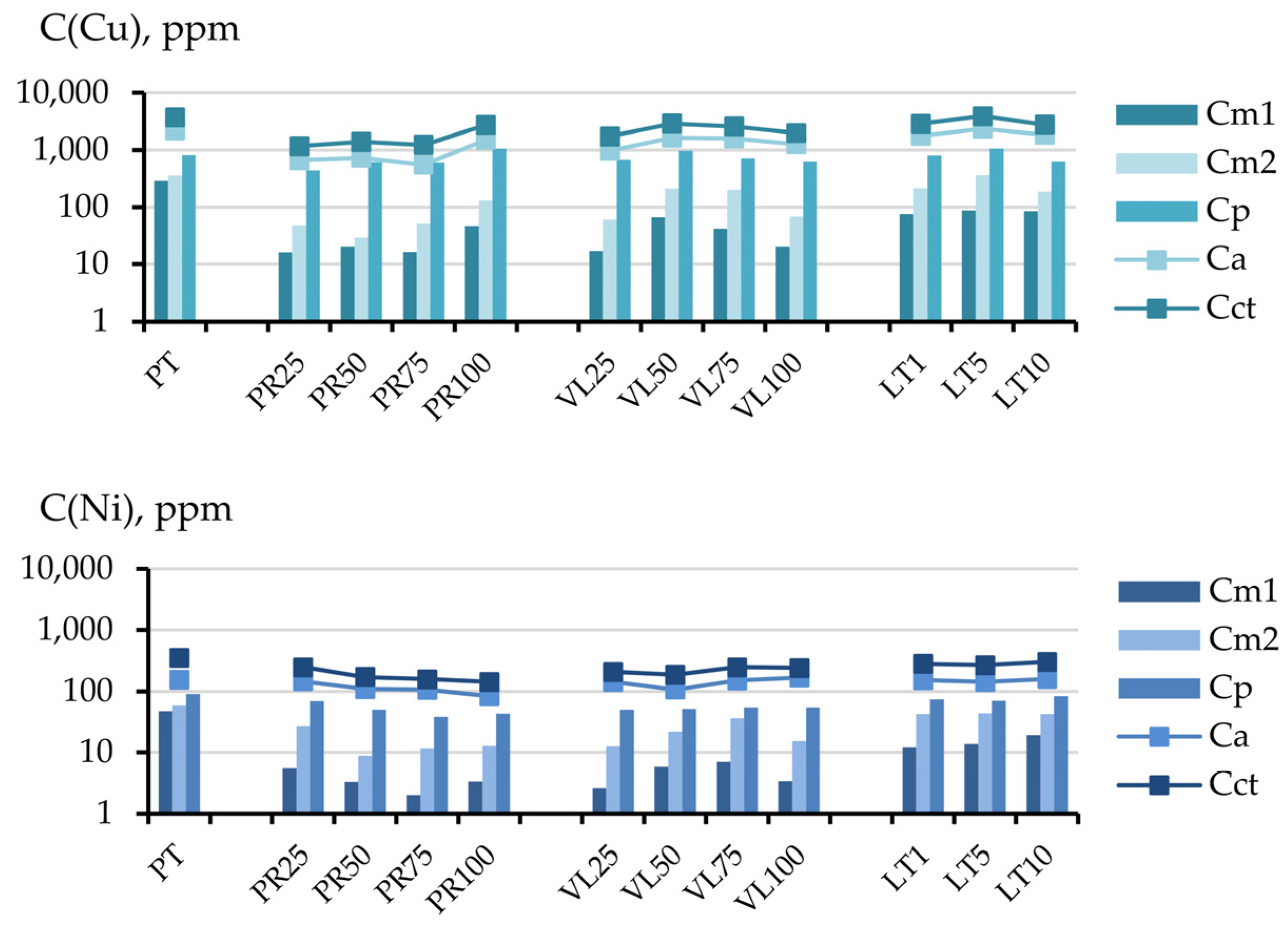
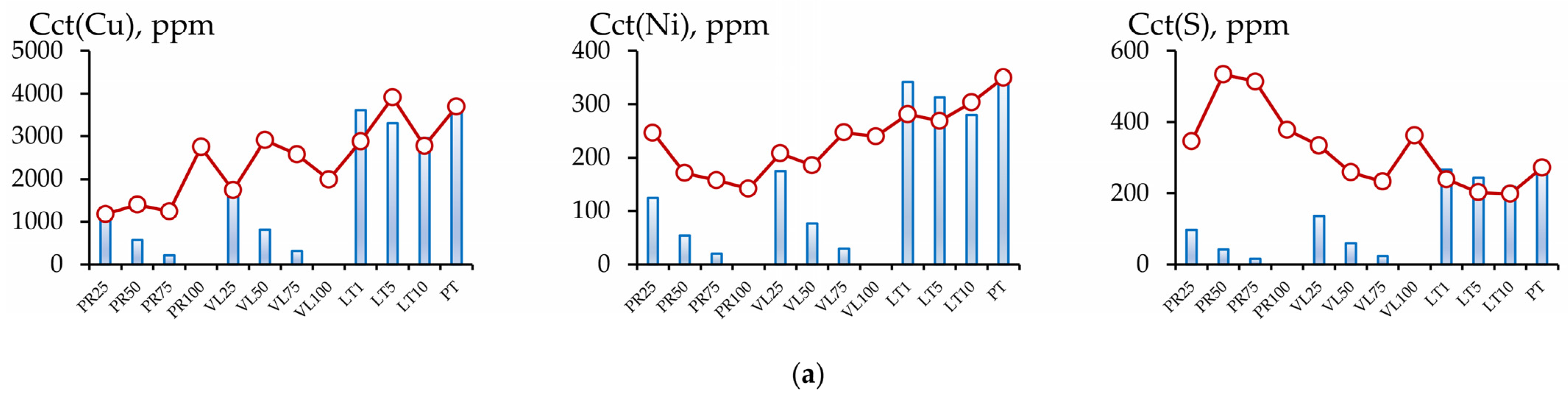
3.2.2. Influence of the Dilution Factor of Technogenic Peat with Serpentine-Containing Materials on the Contents of the Cu, Ni, and S Fractions
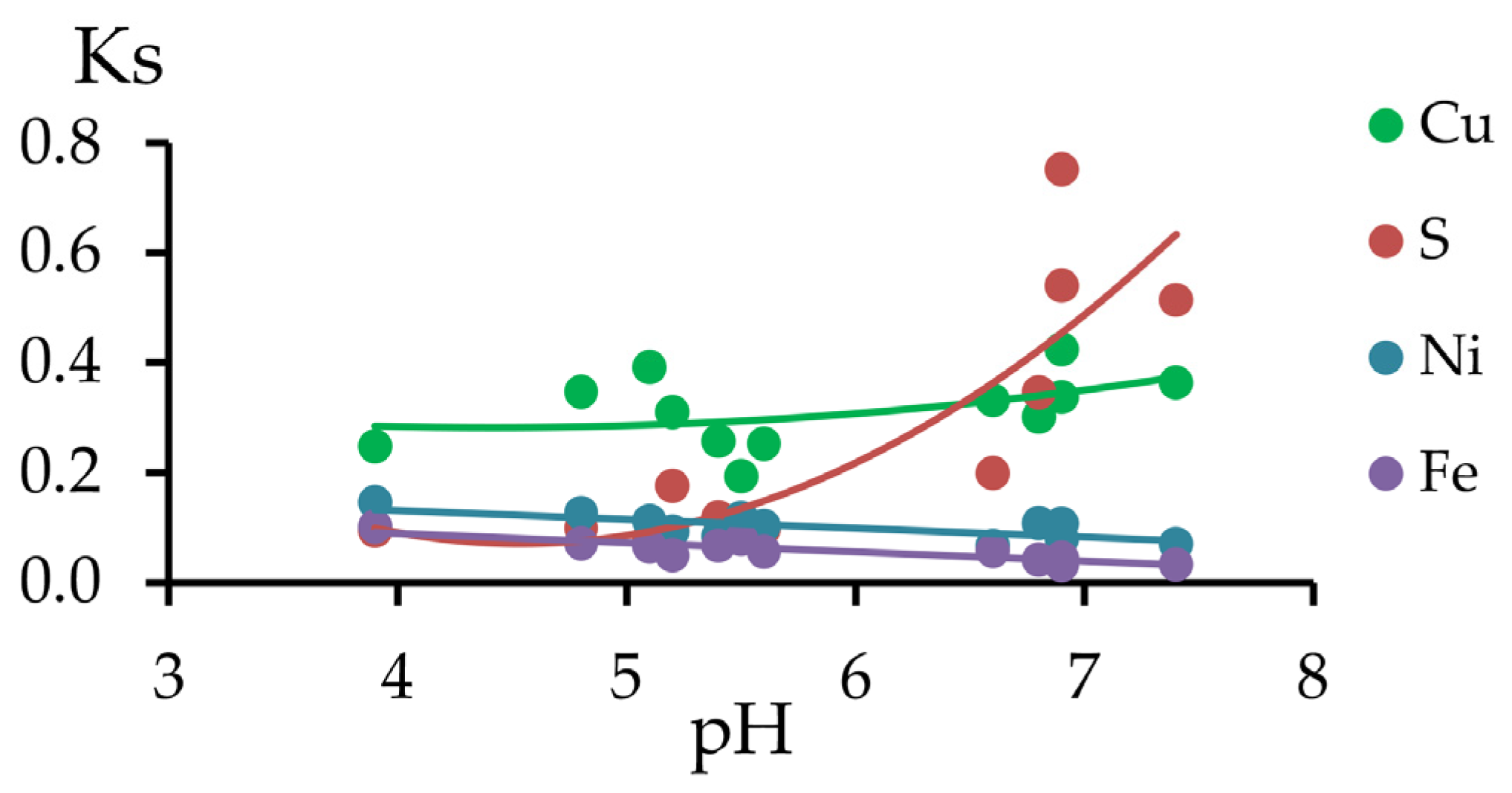
3.2.3. The Migration Coefficients of Pollutants
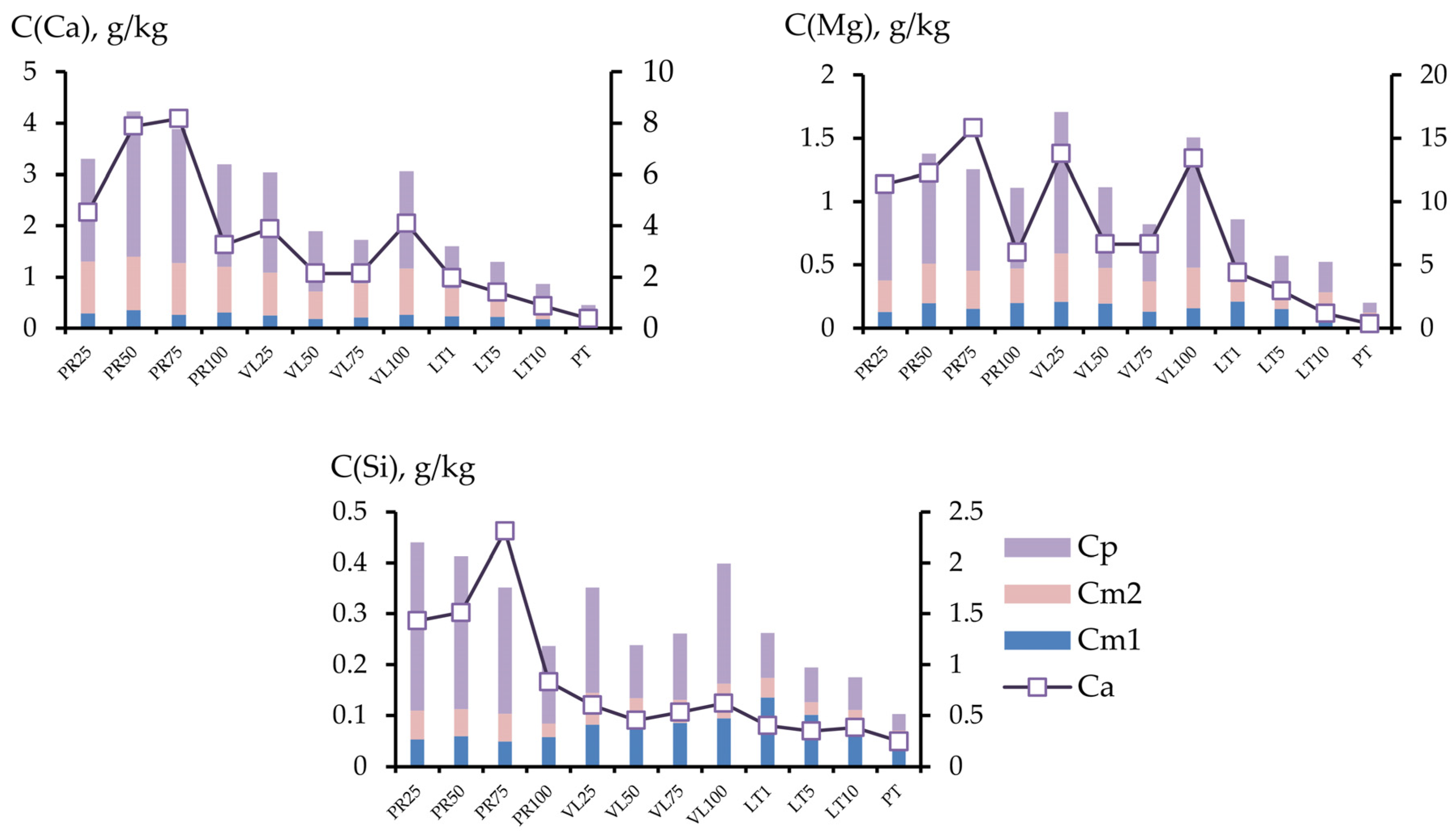
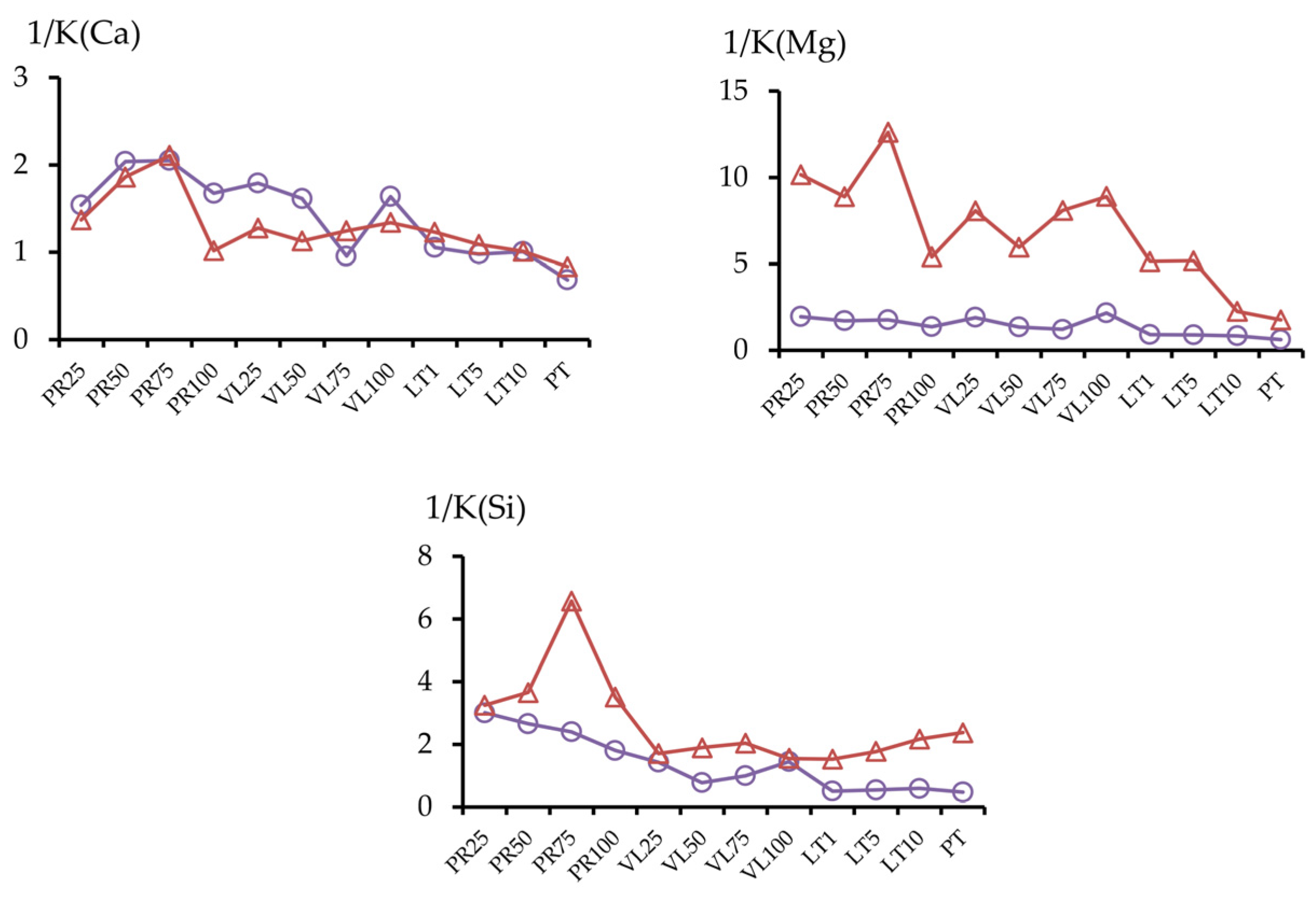
3.2.4. Provision of Soil Mixtures with Alkaline Components and Silicon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visioli, G.; Menta, C.; Gardi, C.; Conti, F.D. Metal toxicity and biodiversity in serpentine soils: Application of bioassay tests and microarthropod index. Chemosphere 2013, 90, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Carmignano, O.R.; Vieira, S.S.; Brandao, P.R.; Bertoli, A.C.; Lago, R.M. Serpentinites: Mineral structure, properties and technological applications. J. Braz. Chem. Soc. 2020, 31, 2–14. [Google Scholar] [CrossRef]
- Aziz, R.A.; Rahim, S.A.; Sahid, I.; Idris, W.M.R. Speciation and Availability of Heavy Metals On Serpentinized Paddy Soil and Paddy Tissue. Procedia-Soc. Behav. Sci. 2015, 195, 1658–1665. [Google Scholar] [CrossRef]
- Miranda, M.; Benedito, J.L.; Blanco-Penedo, I.; Lopez-Lamas, C.; Merino, A.; Lopez-Alonso, M. Metal accumulation in cattle raised in a serpentine-soil area: Relationship between metal concentrations in soil, forage and animal tissues. J. Trace Elem. Med. Biol. 2009, 23, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Vithanage, M.; Oze, C.; Bandara, W.M.A.T.; Weerasooriya, R. Nickel and manganese release in serpentine soil from the Ussangoda Ultramafic Complex, Sri Lanka. Geoderma 2012, 189–190, 1–9. [Google Scholar] [CrossRef]
- Siebecker, M.G.; Chaney, R.L.; Sparks, D.L. Nickel speciation in several serpentine (ultramafic) topsoils via bulk synchrotron-based techniques. Geoderma 2017, 298, 35–45. [Google Scholar] [CrossRef]
- Infante, E.F.; Dulfo, C.P.; Dicen, G.P.; Hseu, Z.Y.; Navarrete, I.A. Bioaccumulation and human health risk assessment of chromium and nickel in paddy rice grown in serpentine soils. Environ. Sci. Pollut. Res. 2021, 28, 17146–17157. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Morrison, J.M.; Goldhaber, M.B.; Mills, C.T.; Breit, G.N.; Hooper, R.L.; Holloway, J.M.; Diehl, S.F.; Ranville, J.F. Weathering and transport of chromium and nickel from serpentinite in the Coast Range ophiolite to the Sacramento Valley, California, USA. Appl. Geochem. 2015, 61, 72–86. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive removal of nickel(II) ions from aqueous environment: A review. J. Environ. Manag. 2016, 179, 1–20. [Google Scholar] [CrossRef]
- Matsui, Y.; Syono, Y.; Akimoto, S.; Kitayama, K. Unit cell dimensions of some synthetic orthopyroxene group solid solutions. Geochem. J. 1968, 2, 61–70. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Dickfos, M.J. Characterisation of Ni silicate-bearing minerals by UV–vis–NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 71, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.S.; King, H.E.; John, T.; Kusebauch, C.; Putnis, A. Pseudomorphic replacement of diopside during interaction with (Ni,Mg)Cl2 aqueous solutions: Implications for the Ni-enrichment mechanism in talc- and serpentine-type phases. Chem. Geol. 2014, 380, 27–40. [Google Scholar] [CrossRef]
- Roque-Rosell, J.; Villanova-de-Benavent, C.; Proenza, J.A. The accumulation of Ni in serpentines and garnierites from the Falcondo Ni-laterite deposit (Dominican Republic) elucidated by means of μXAS. Geochim. Cosmochim. Acta 2017, 198, 48–69. [Google Scholar] [CrossRef]
- Momcilovic, M.Z.; Randelovic, M.S.; Purenovic, M.M.; Dordevic, J.S.; Onjia, A.; Matovic, B. Morpho-structural, adsorption and electrochemical characteristics of serpentinite. Sep. Purif. Technol. 2016, 163, 72–78. [Google Scholar] [CrossRef]
- Kremenetskaya, I.; Korytnaya, O.; Vasil’eva, T.; Belyaevskii, A.; Bubnova, T. Peculiar features of preparation and application of fractionated magnesia-silicate reagent. Russ. J. Appl. Chem. 2012, 85, 1493–1500. [Google Scholar] [CrossRef]
- Kremenetskaya, I.; Belyavskiy, A.; Vasilieva, T.; Korytnaya, O.; Makarova, T. Amorfizatsiya serpentinovyih mineralov v tehnologii polucheniya magnezialno-silikatnogo reagenta dlya immobilizatsii tyazhelyih metallov [Amorphization of serpentine minerals in the technology of obtaining a magnesian-silicate reagent for immobilization of heavy metals]. Chem. Sustain. Dev. 2010, 18, 41–49. (In Russian) [Google Scholar]
- Zulumyan, N.; Mirgorodski, A.; Isahakyan, A.; Beglaryan, H. The mechanism of decomposition of serpentines from peridotites on heating. J. Therm. Anal. Calorim. 2014, 115, 1003–1012. [Google Scholar] [CrossRef]
- Slukovskaya, M.V.; Kremenetskaya, I.P.; Mosendz, I.A.; Ivanova, T.K.; Drogobuzhskaya, S.V.; Ivanova, L.A.; Novikov, A.I.; Shirokaya, A.A. Thermally activated serpentine materials as soil additives for copper and nickel immobilization in highly polluted peat. Environ. Geochem. Health 2023, 45, 67–83. [Google Scholar] [CrossRef]
- Reddy, R.A.; Balkwill, K.; McLellan, T. Are plant taxa found on the Witwatersrand serpentine ecotypes or substrate-generalists? S. Afr. J. Bot. 2012, 80, 81–95. [Google Scholar] [CrossRef]
- Ufimtseva, M.D. The patterns in accumulation of chemical elements by higher plants and their responses in biogeochemical provinces. Geochem. Int. 2015, 53, 441–455. [Google Scholar] [CrossRef]
- Kazakou, E.; Adamidis, G.C.; Baker, A.J.M.; Reeves, R.D.; Godino, M. Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant Soil 2010, 332, 369–385. [Google Scholar] [CrossRef]
- Koleli, N.; Demir, A.; Kantar, C.; Atag, G.A.; Kusvuran, K.; Binzet, R. Heavy Metal Accumulation in Serpentine Flora of Mersin-Findikpinari (Turkey)—Role of Ethylenediamine Tetraacetic Acid in Facilitating Extraction of Nickel. In Soil Remediation and Plants; Academic Press: Cambridge, MA, USA, 2015; pp. 629–659. ISBN 9780127999371. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, S.; Shah, M.T.; Khan, K.; Huang, Q.; Ali, R. Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. Int. J. Phytoremediation 2015, 17, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, I.; Alekseeva-Popova, N.; Kataeva, M.; Bech, J.; Roca, N. A study of trace elements in plants of the Polar Urals and Chukotka in the search for metallophyte hyperaccumulators. Geochem. Explor. Environ. Anal. 2019, 19, 138–145. [Google Scholar] [CrossRef]
- Harter, R.D. Effect of soil pH on adsorption of lead, copper, zinc, and nickel. Soil Sci. Soc. Am. J. 1983, 47, 47–51. [Google Scholar] [CrossRef]
- Siromlya, T.I. K voprosu o podvizhnyih formah soedineniy himicheskih elementov v pochvah [About the mobile forms of the compounds of chemical elements in soils]. Sib. Ekol. Zhurnal 2009, 2, 307–318. (In Russian) [Google Scholar]
- Malakhov, V.V.; Vlasov, A.A.; Dovlitova, L.S. Determination of the phase composition of atmospheric aerosol isung the reference-free method of differentiating dissolution. Chem. Sustain. Dev. 2002, 10, 615–620. [Google Scholar]
- Kierczak, J.; Neel, C.; Aleksander-Kwaterczak, U.; Helios-Rybicka, E.; Bril, H.; Puziewicz, J. Solid speciation and mobility of potentially toxic elements from natural and contaminated soils: A combined approach. Chemosphere 2008, 73, 776–784. [Google Scholar] [CrossRef]
- Slukovskaya, M.V.; Vasenev, V.I.; Ivashchenko, K.V.; Dolgikh, A.V.; Novikov, A.I.; Kremenetskaya, I.P.; Ivanova, L.A.; Gubin, S.V. Organic matter accumulation by alkaline-constructed soils in heavily metal-polluted area of Subarctic zone. J. Soils Sediments 2021, 21, 2071–2088. [Google Scholar] [CrossRef]
- Kremenetskaya, I.; Alekseeva, S.; Slukovskaya, M.; Mosendz, I.; Drogobuzhskaya, S.; Ivanova, L. Expanded vermiculite-reached product obtained from mining waste: The effect of roasting temperature on the agronomic properties. Physicochem. Probl. Miner. Process. 2020, 56, 103–113. [Google Scholar] [CrossRef]
- Ivanyuk, G.; Konopleva, N.; Yakovenchuk, V.; Pakhomovsky, Y.; Panikorovskii, T.; Kalashnikov, A.; Bocharov, V.; Bazai, A.; Mikhailova, J.; Goryainov, P. Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: III. Pyrochlore Supergroup Minerals. Minerals 2018, 8, 277. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Kovdor; Laplandia Minerals: Apatity, Russia, 2002; ISBN 5900395413. [Google Scholar]
- Atamanov, A.V.; Afanas’ev, A.P. Structure and Ore Typesof the Kovdor Vermiculite Deposit. In Geology, Properties and Adaptation of Vermiculite; Nauka: Leningrad, Russia, 1967; pp. 15–17. (In Russian) [Google Scholar]
- Ivanova, L.A.; Slukovskaya, M.V.; Kremenetskaya, I.P.; Gorbacheva, T.T. Pora ozelenyat Arktiku. Innovatsionnyie gazonnyie tehnologii dlya sozdaniya travyanogo pokrova razlichnogo naznacheniya v usloviyah Zapolyarya: Metodicheskie rekomendatsii [It’s Time to Green the Arctic. Innovative Lawn Technologies for Creating Grass Cover for Different Purposes in the Polar Regions: Methodological Recommendations]; FRC CSC RAS: Apatity, Russia, 2020; 37p, (In Russian). [Google Scholar] [CrossRef]
- Nizhegorodov, A.I. Theory and practical use of modular-pouring electric furnaces for firing vermiculite. Refract. Ind. Ceram. 2015, 56, 361–365. [Google Scholar] [CrossRef]
- SmartLab Studio Ver. 1.4.3.0 2023. Available online: https://www.rigaku.com/support/software/slstudio (accessed on 21 February 2023).
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E. Vegan: Community Ecology Package; R Package Version 2.6-4. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 29 May 2022).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. 2022. Available online: https://github.com/tidyverse/dplyr (accessed on 9 May 2022).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Wickham, H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antic-Mladenovic, S.; Frohne, T.; Stark, H.-J.; Tomic, Z.; Licina, V. Nickel in a serpentine-enriched Fluvisol: Redox affected dynamics and binding forms. Geoderma 2016, 263, 203–214. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Burachevskaya, M.V.; Bauer, T.V.; Sushkova, S.N. Method of determining loosely bound compounds of heavy metals in the soil. MethodsX 2018, 5, 217–226. [Google Scholar] [CrossRef]
- Mondaca, P.; Neaman, A.; Sauvé, S.; Salgado, E.; Bravo, M. Solubility, partitioning and activity of copper in contaminated soils in a semiarid zone. J. Plant Nutr. Soil Sci. 2015, 178, 452–459. [Google Scholar] [CrossRef]
- Ladonin, D.; Karpukhin, M. Fractional composition of nickel, copper, zinc, and lead compounds in soils contaminated with oxides and soluble metal salts. Eurasian Soil Sci. 2011, 44, 953–965. [Google Scholar] [CrossRef]
- Oze, C.; Skinner, C.; Schroth, A.W.; Coleman, R.G. Growing up green on serpentine soils: Biogeochemistry of serpentine vegetation in the Central Coast Range of California. Appl. Geochem. 2008, 23, 3391–3403. [Google Scholar] [CrossRef]
- Whittaker, R.J. An Application of Detrended Correspondence Analysis and Non-Metric Multidimensional Scaling to the Identification and Analysis of Environmental Factor Complexes and Vegetation Structures. J. Ecol. 1987, 75, 363–376. [Google Scholar] [CrossRef]
- Zelano, I.; Sivry, Y.; Quantin, C.; Gelabert, A.; Tharaud, M.; Nowak, S.; Garnier, J.; Malandrino, M.; Benedetti, M.F. Study of Ni exchangeable pool speciation in ultramafic and mining environments with isotopic exchange kinetic data and models. Appl. Geochem. 2016, 64, 146–156. [Google Scholar] [CrossRef]








| C(HCl), mol/L | Differential Fractions | Integral Fractions | Coefficients of Migration | ||||
|---|---|---|---|---|---|---|---|
| 0.001 | Cm1 | Readily mobile | Csm | Short-term mobile | Cm1 + Cm2 | ||
| 0.01 | Cm2 | Mobile | |||||
| 0.1 | Cp | Potentially mobile | Cps | Sum of mobile fractions | Csm + Cp | Km | Csm/Cps |
| 1 | Ca | Acid-soluble | Cct | Conditionally total | Cps + Ca | ||
| Cin | Inert | Cto-Cct | Kp | Cps/Cin | |||
| Cto | Total | Ks | Cct/Cto | ||||
| Label | Material | Bulk Density, g/cm3 | Moisture Capacity, wt.% | pH (H2O) | pH (KCl) |
|---|---|---|---|---|---|
| PR | Pyroxenite material | 1.57 | 48 | 8.85 | 6.67 |
| VL | Vermiculite–lizardite material | 1.03 | 49 | 8.77 | 7.54 |
| LT | Thermally activated vermiculite–lizardite material | 0.65 | 90 | 9.72 | 9.54 |
| PT | Industrially polluted peat soil | 0.29 | 280 | 4.1 | 3.7 |
| Components | PT | PR | VL |
|---|---|---|---|
| SiO2 | 2.14 | 36.79 | 38.04 |
| MgO | 0.27 | 34.49 | 36.04 |
| CaO | 0.05 | 2.80 | 2.81 |
| Al2O3 | 2.01 | 1.68 | 2.00 |
| Fe2O3 | 3.77 | 10.5 | 5.71 |
| MnO | 0.03 | 0.34 | 0.18 |
| TiO2 | 0.10 | 0.13 | 0.12 |
| NiO | 0.30 | 0.05 | 0.05 |
| Cr2O3 | 0.02 | 0.04 | 0.03 |
| P2O5 | 0.27 | 0.10 | 0.05 |
| K2O | 0.27 | 0.16 | 0.01 |
| Cl | 0.10 | 0.14 | 0.14 |
| CuO | 0.75 | – | – |
| LOI | 89.00 | 12.75 | 14.82 |
| Label | Proportion of Serpentinite | Bulk Density, kg∙dm−3 | pH (H2O) | Eh | |
|---|---|---|---|---|---|
| vol.% | wt.% | ||||
| PR25 | 25 | 65 | 0.87 | 6.8 | 275 |
| PR50 | 50 | 85 | 1.07 | 6.9 | 240 |
| PR75 | 75 | 94 | 1.42 | 7.4 | 248 |
| PR100 | 100 | 100 | 1.69 | 6.6 | 268 |
| VL25 | 25 | 50 | 0.57 | 5.2 | 251 |
| VL50 | 50 | 78 | 0.96 | 5.4 | 302 |
| VL75 | 75 | 92 | 1.03 | 5.6 | 282 |
| VL100 | 100 | 100 | 1.23 | 6.9 | 261 |
| LT1(1) | 1 | 2.5 | 0.30 | 5.8 | 296 |
| LT1(2) | 1 | 2.5 | 0.30 | 5.1 | 268 |
| LT5(1) | 5 | 11 | 0.38 | 4.9 | 268 |
| LT5(2) | 5 | 11 | 0.38 | 5.2 | 271 |
| LT10(1) | 10 | 20 | 0.38 | 4.8 | 304 |
| LT10(2) | 10 | 20 | 0.38 | 4.6 | 278 |
| PT(1) | 0 | 0 | 0.29 | 3.9 | 239 |
| PT(2) | 0 | 0 | 0.29 | 4.3 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slukovskaya, M.V.; Petrova, A.G.; Ivanova, L.A.; Ivanova, T.K.; Mosendz, I.A.; Novikov, A.I.; Shirokaya, A.A.; Kovorotniaia, M.V.; Panikorovskii, T.L.; Kremenetskaya, I.P. Serpentine Overburden Products—Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil. Toxics 2023, 11, 957. https://doi.org/10.3390/toxics11120957
Slukovskaya MV, Petrova AG, Ivanova LA, Ivanova TK, Mosendz IA, Novikov AI, Shirokaya AA, Kovorotniaia MV, Panikorovskii TL, Kremenetskaya IP. Serpentine Overburden Products—Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil. Toxics. 2023; 11(12):957. https://doi.org/10.3390/toxics11120957
Chicago/Turabian StyleSlukovskaya, Marina V., Anna G. Petrova, Liubov A. Ivanova, Tatiana K. Ivanova, Irina A. Mosendz, Andrey I. Novikov, Anna A. Shirokaya, Mariia V. Kovorotniaia, Taras L. Panikorovskii, and Irina P. Kremenetskaya. 2023. "Serpentine Overburden Products—Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil" Toxics 11, no. 12: 957. https://doi.org/10.3390/toxics11120957
APA StyleSlukovskaya, M. V., Petrova, A. G., Ivanova, L. A., Ivanova, T. K., Mosendz, I. A., Novikov, A. I., Shirokaya, A. A., Kovorotniaia, M. V., Panikorovskii, T. L., & Kremenetskaya, I. P. (2023). Serpentine Overburden Products—Nature-Inspired Materials for Metal Detoxification in Industrially Polluted Soil. Toxics, 11(12), 957. https://doi.org/10.3390/toxics11120957







