Case-Control Study of the Association between Single Nucleotide Polymorphisms of Genes Involved in Xenobiotic Detoxification and Antioxidant Protection with the Long-Term Influence of Organochlorine Pesticides on the Population of the Almaty Region
Abstract
1. Introduction
2. Materials and Methods
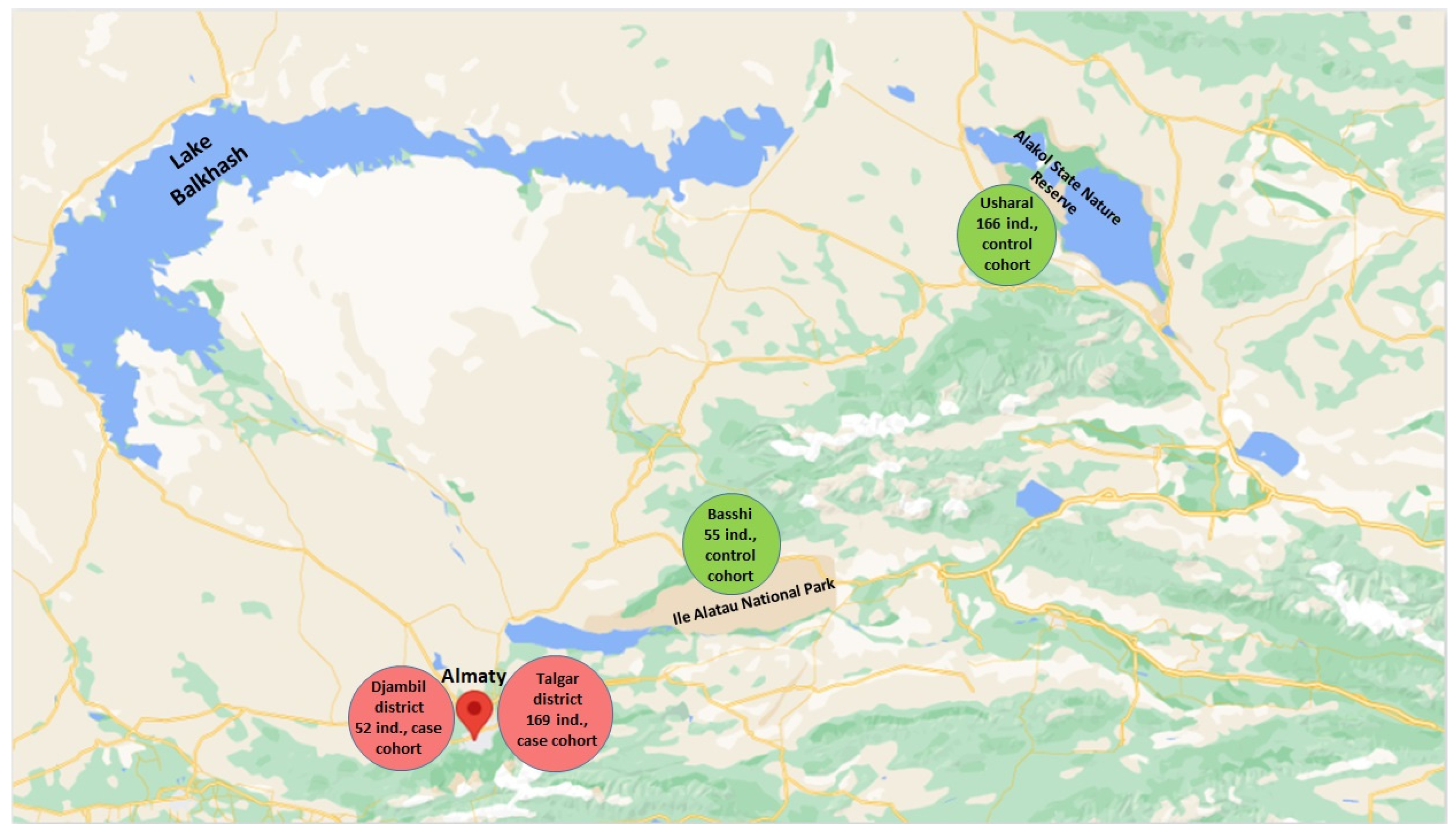
2.1. Study Objects
2.2. DNA Extraction
2.3. Genotyping by PCR-RFLP Method
2.4. Genome-Wide Genotyping of SNPs and Bioinformatic Processing
2.5. Statistical Methods
3. Results
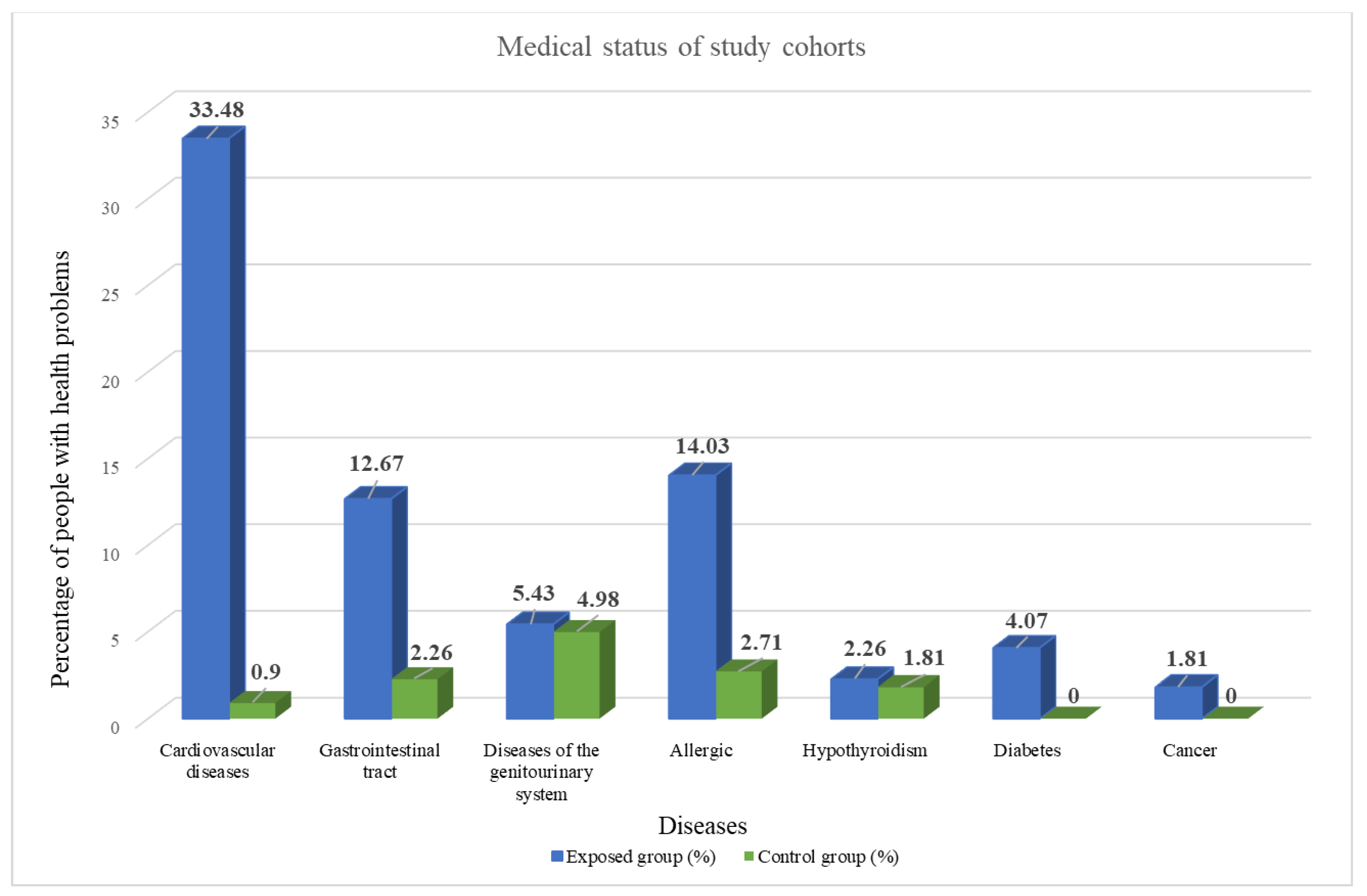
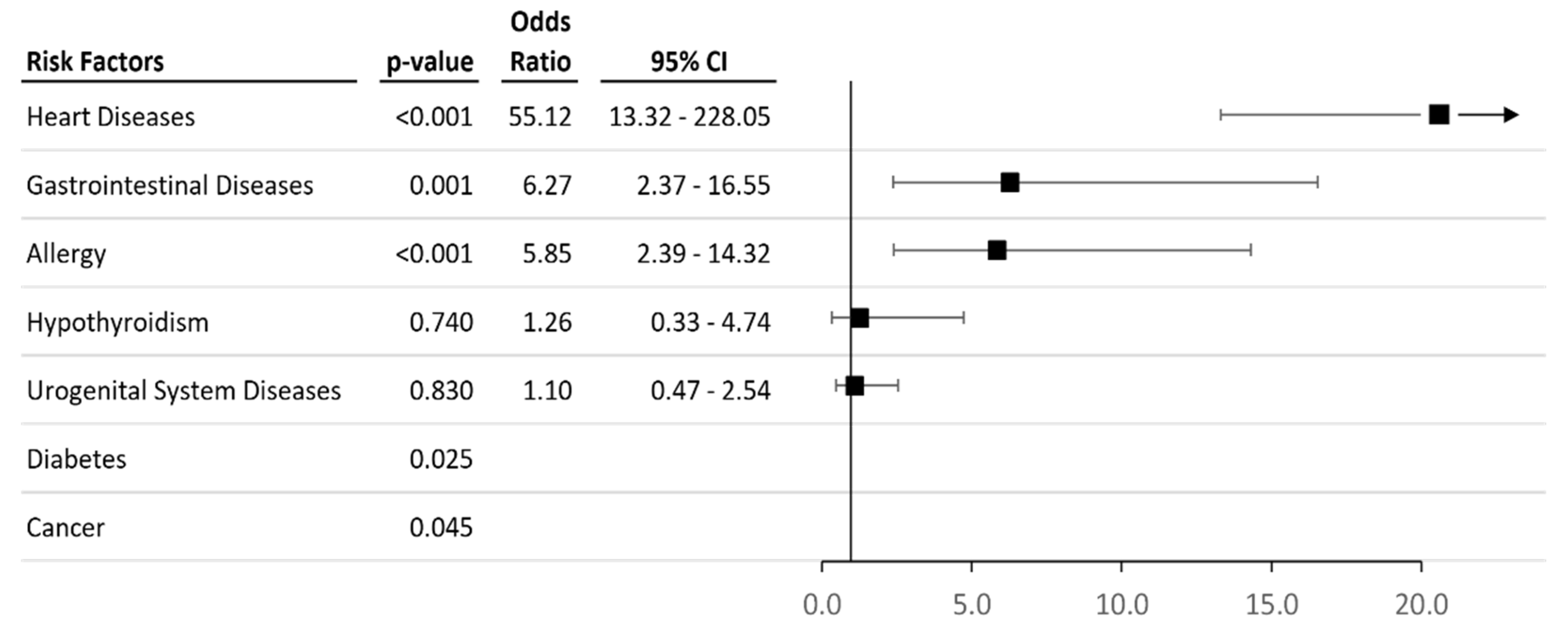
3.1. Characteristics of Case-Control Cohorts
3.2. Genotyping Results
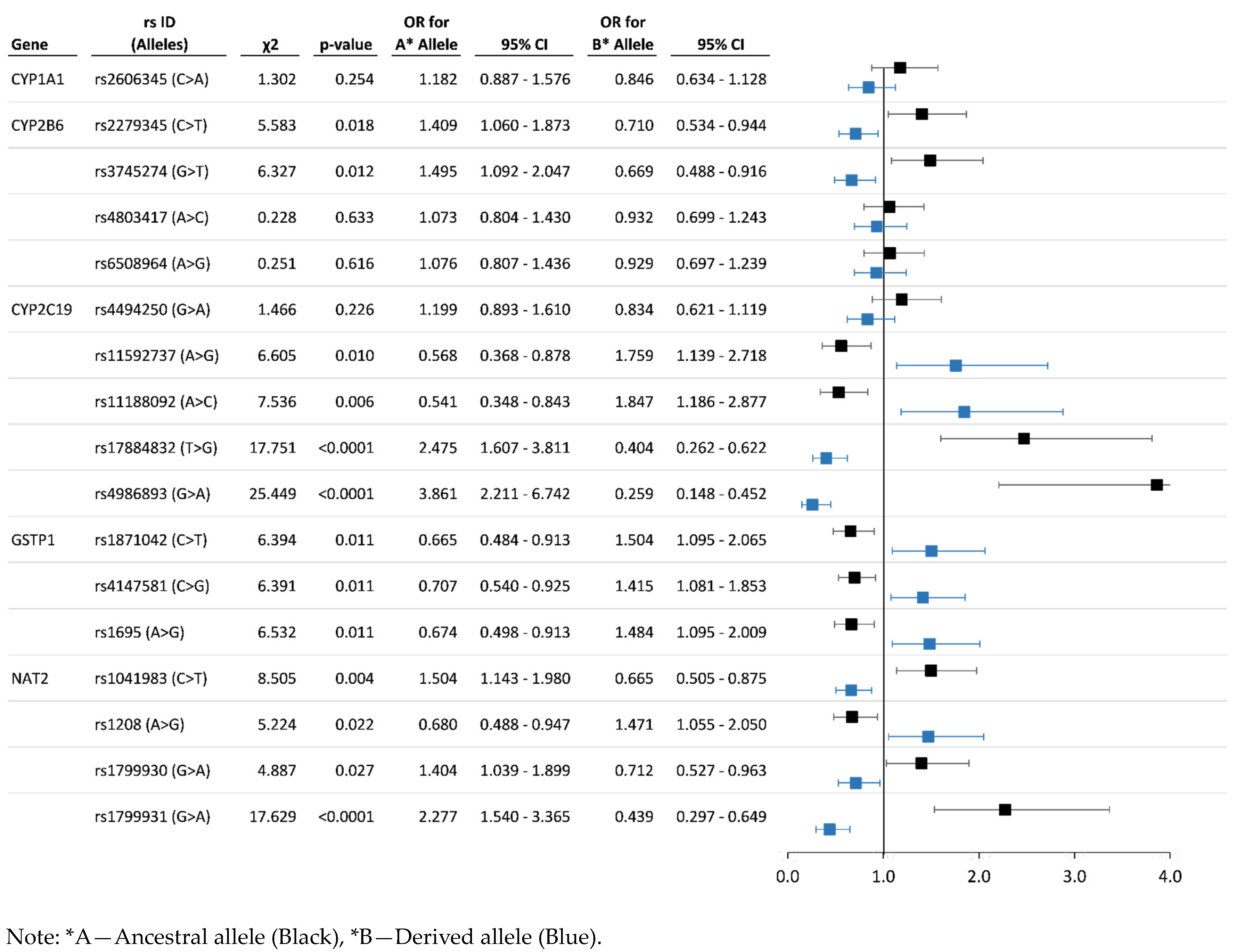
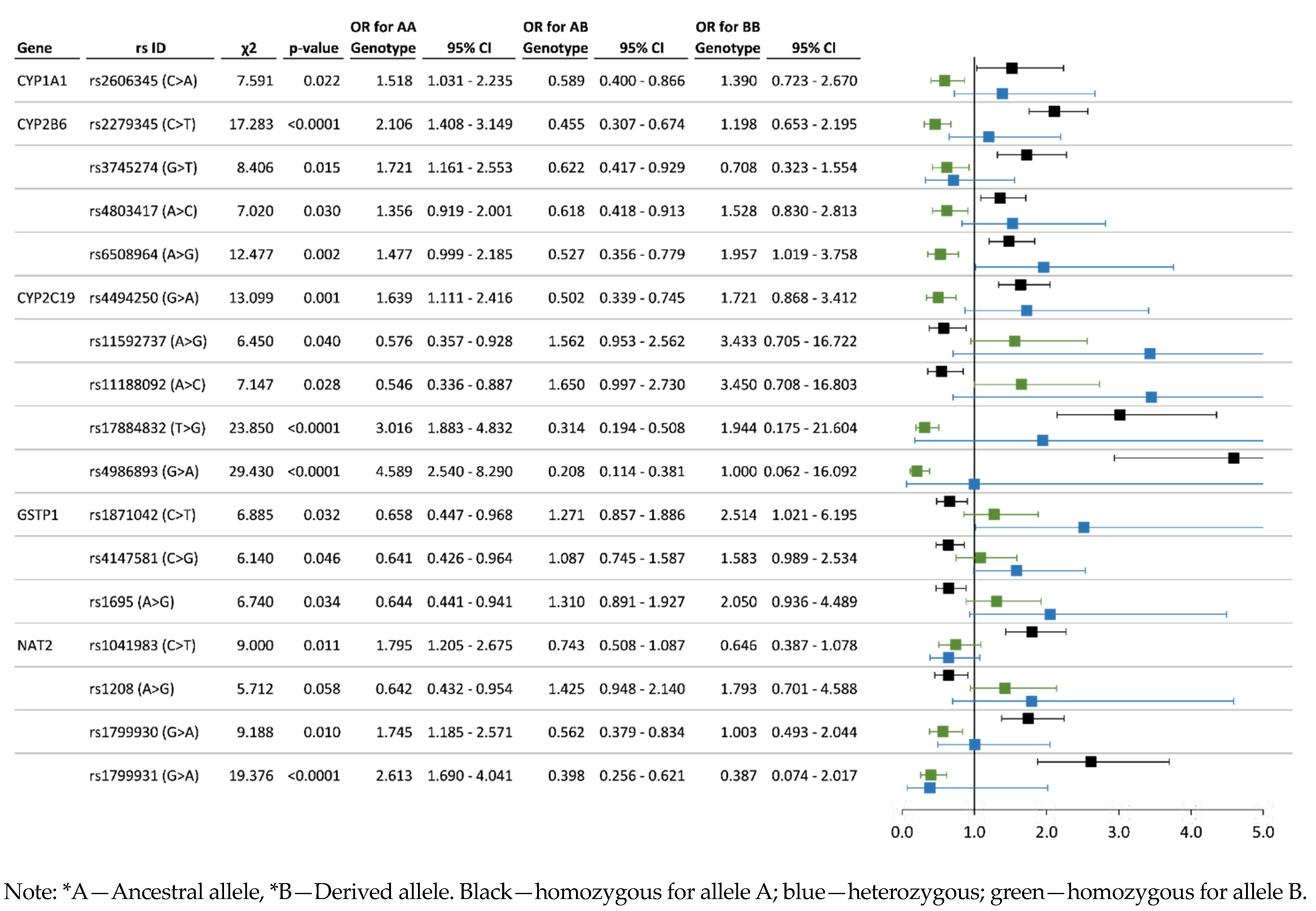
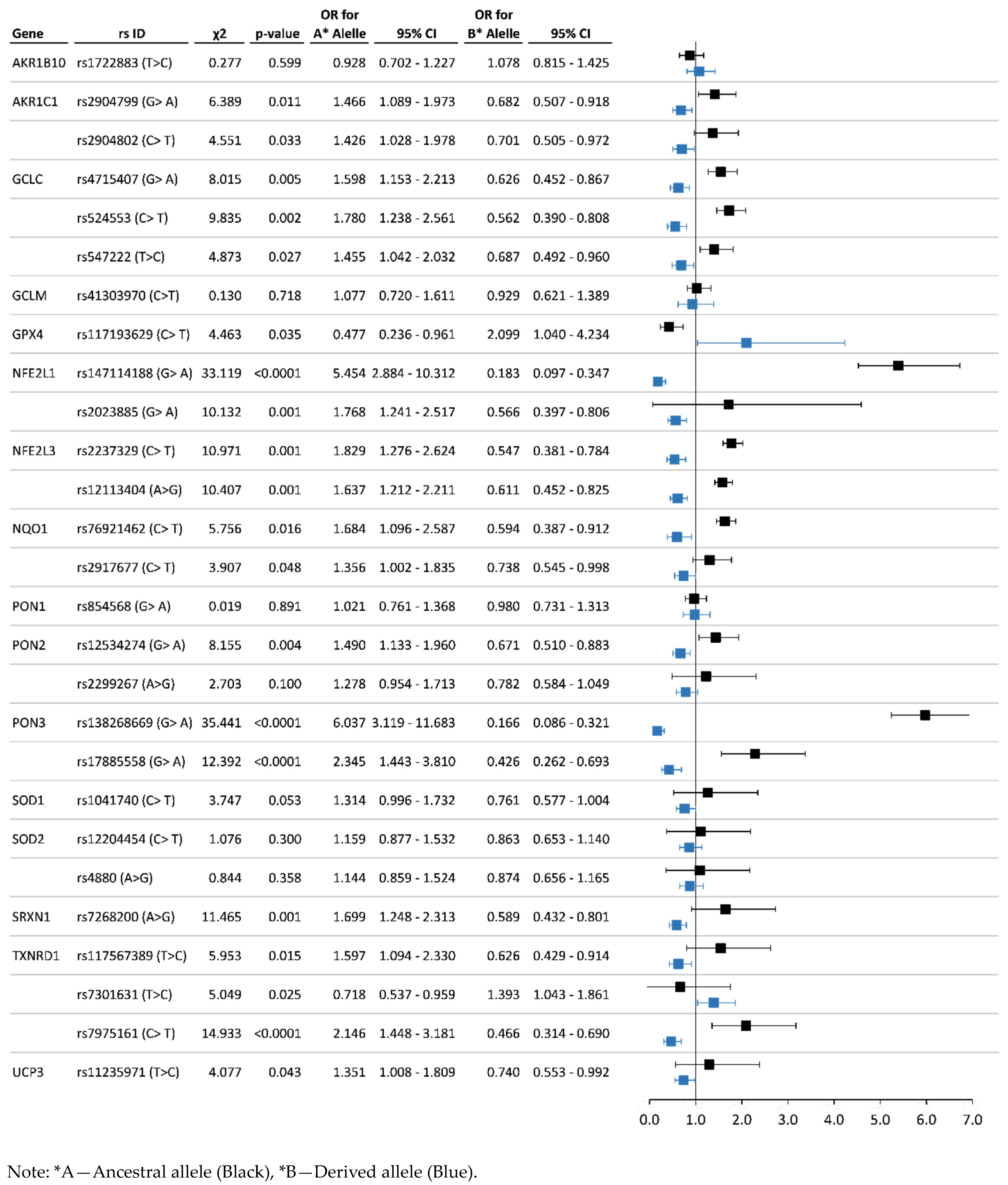
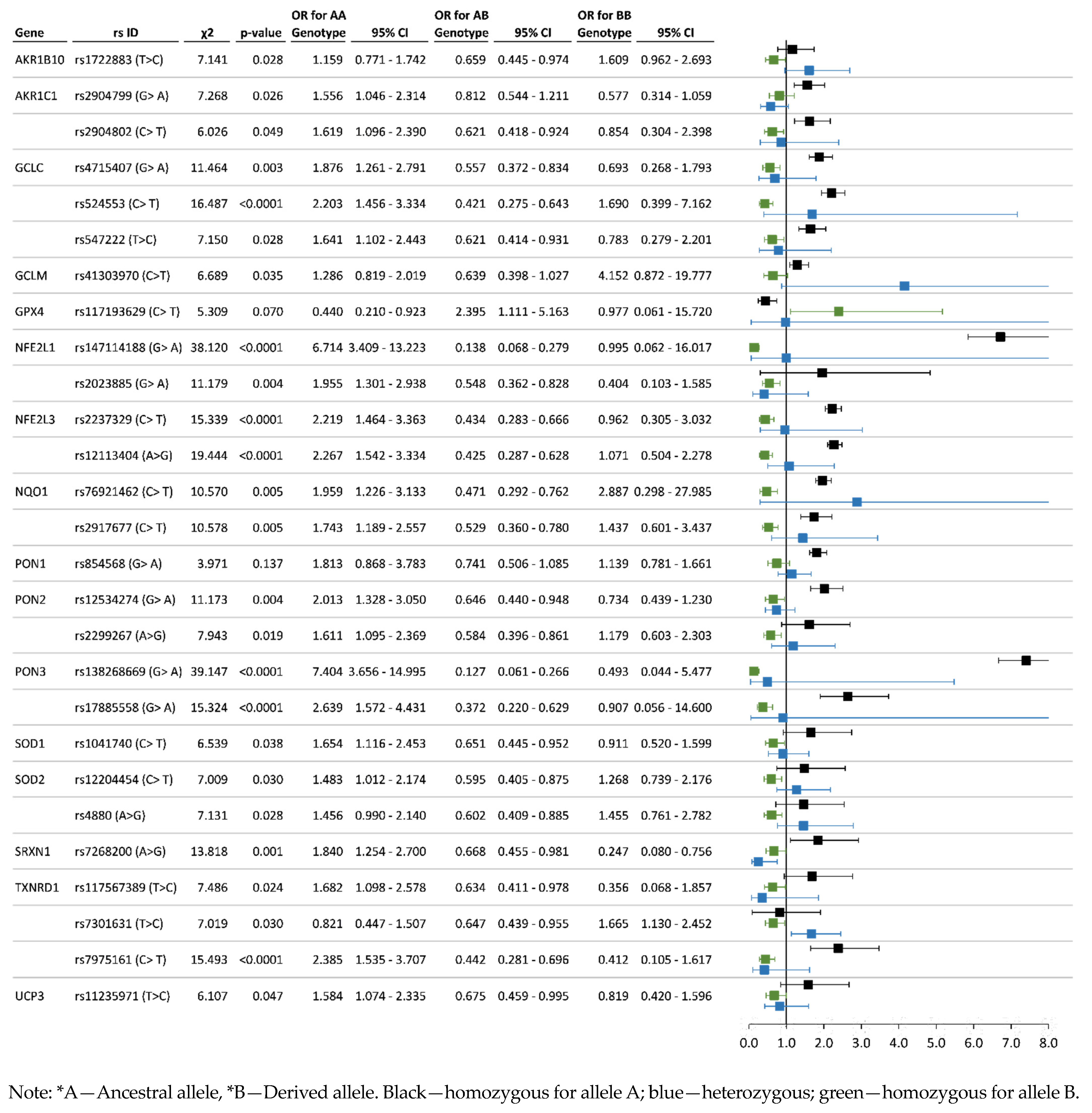
3.3. Analysis of Associations between the Allelic and Genotype Variants and Chronic Pesticide Exposure
4. Discussion
4.1. Background for Control Cohort Selection
4.2. Detoxification Genes
4.3. Antioxidant System Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mieldazys, A.; Mieldazys, R.; Vilkevicius, G.; Stulginskis, A. Agriculture—Use of Pesticides/Plant Protection Products; EU-OSHA: Bilbao, Spain, 2015; available in September. [Google Scholar]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, T.M.A.; Benvindo-Souza, M.; de Araújo Nascimento, F.; Woch, J.; dos Reis, F.G.; de Melo e Silva, D. Cancer and occupational exposure to pesticides: A bibliometric study of the past 10 years. Environ. Sci. Pollut. Res. 2022, 29, 17464–17475. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, A.F.; Wachtel, C.C.; Ghisi, N.C. Are our farm workers in danger? Genetic damage in farmers exposed to pesticides. Int. J. Environ. Res. Public Health 2019, 16, 358. [Google Scholar] [CrossRef]
- Nurzhanova, A.A.; Inelova, Z.A.; Dzhansugurova, L.B.; Nesterova, S.G. Problema ustarevshyh pesticidov v Kazakhstane [The problem of obsolete pesticides in Kazakhstan (review)]. Izvestiya NAN RK 2018, 328, 86–96. (In Russian) [Google Scholar]
- Stanley, L.A. Chapter 27—Drug Metabolism. In Pharmacognosy Fundamentals, Applications and Strategies; Academic Press: Cambridge, MA, USA, 2017; pp. 527–545. [Google Scholar] [CrossRef]
- Teodoro, M.; Briguglio, G.; Fenga, C.; Costab, C. Genetic polymorphisms as determinants of pesticide toxicity: Recent advances. Toxicol. Rep. 2019, 6, 564–570. [Google Scholar] [CrossRef] [PubMed]
- El Rifai, N.; Moustafa, N.; Degheidy, N.; Wilson, M. Glutathione S transferase theta1 and mu1 gene polymorphisms and phenotypic expression of asthma in Egyptian children: A case-control study. Ital. J. Pediatr. 2014, 40, 22. [Google Scholar] [CrossRef]
- Shah, H.K.; Sharma, T.; Banerjee, B.D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wołejko, E.; Wydro, U.; Butarewicz, A. The impact of pesticides on oxidative stress level in human organism and their activity as an endocrine disruptor. J. Environ. Sci. Health B 2017, 52, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Miozzi, E.; Teodoro, M.; Fenga, C. Influence of genetic polymorphism on pesticide-induced oxidative stress. Curr. Opin. Toxicol. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Nakanishi, G.; Pita-Oliveira, M.; Bertagnolli, L.S.; Torres-Loureiro, S.; Scudeler, M.M.; Cirino, H.S.; Chaves, M.L.; Miwa, B.; Rodrigues-Soares, F. Worldwide Systematic Review of GSTM1 and GSTT1 Null Genotypes by Continent, Ethnicity, and Therapeutic Area. OMICS A J. Integr. Biol. 2022, 26, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Mamirova, A.; Baubekova, A.; Pidlisnyuk, V.; Shadenova, E.; Djansugurova, L.; Jurjanz, S. Phytoremediation of Soil Contaminated by Organochlorine Pesticides and Toxic Trace Elements: Prospects and Limitations of Paulownia tomentosa. Toxics 2022, 10, 465. [Google Scholar] [CrossRef]
- Djangalina, E.; Altynova, N.; Mit, N.; Djansugurova, L. Chapter 7—Complex approaches to assessing the pesticides risk on human health and environment. In Pesticides in the Natural Environment; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 163–198. ISBN 9780323904896. [Google Scholar] [CrossRef]
- Djangalina, E.; Altynova, N.; Bakhtiyarova, S.; Kapysheva, U.; Zhaksymov, B.; Shadenova, E.; Baizhanov, M.; Sapargali, O.; Garshin, A.; Seisenbayeva, A.; et al. Comprehensive assessment of unutilized and obsolete pesticides impact on genetic status and health of population of Almaty region. Ecotoxicol. Environ. Saf. 2020, 202, 110905. [Google Scholar] [CrossRef]
- Mit, N.; Cherednichenko, O.; Mussayeva, A.; Khamdiyeva, O.; Amirgalieva, A.; Begmanova, M.; Tolebaeva, A.; Koishekenova, G.; Zaypanova, S.; Pilyugina, A.; et al. Ecological risk assessment and long-term environmental pollution caused by obsolete undisposed organochlorine pesticides. J. Environ. Sci. Health B. 2021, 56, 490–502. [Google Scholar] [CrossRef]
- Garshin, A.; Altynova, N.; Djangalina, E.; Khamdiyeva, O.; Baratzhanova, G.; Tolebaeva, A.; Zhaniyazov, Z.; Khussainova, E.; Cakir-Kiefer, C.; Jurjanz, S.; et al. Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides. Toxics 2023, 11, 482. [Google Scholar] [CrossRef]
- Cherednichenko, O.; Pilyugina, A.; Nuraliev, S. Chapter 10—Cytogenetical bioindication of pesticidal contamination. In Pesticides in the Natural Environment; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 227–260. ISBN 9780323904896. [Google Scholar] [CrossRef]
- Usman, M.B.; Priya, K.; Pandit, S.; Gupta, P.K.; Agrawal, S.; Sarma, H.; Prasad, R. Genetic Polymorphisms and Pesticide-Induced DNA Damage: A Review. Open Biotechnol. J. 2021, 15, 119–130. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Bottà, G.; Boffetta, P.; Antonelli, G.; Venanzetti, F. Glutathione S-transferase M1 null genotype, household pesticides exposure and cutaneous melanoma. Melanoma Res. 2016, 26, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Manco, G.; Porzio, E.; Carusone, T.M. Human Paraoxonase-2 (PON2): Protein Functions and Modulation. Antioxidants. 2021, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Adiga, U.; Banawalikar, N.; Menambath, D.T. Association of paraoxonase 1 activity and insulin resistance models in type 2 diabetes mellitus: Cross-sectional study. J. Chin. Med. Assoc. 2022, 85, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Hernandez, A.F.; Liesivuori, J.; Tsatsakis, A.M. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology 2013, 307, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Galvez, H.R.C.; Flores, J.S.; Sanchez, E.D.T.; Bravo, D.R.; Villela, M.Z.R.; Uribe, E.R. Genetic profile for the detection of susceptibility to poisoning by exposure to pesticides. Ann. Agric. Environ. Med. 2021, 28, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Weeramange, C.E.; Shu, D.; Tang, K.D.; Batra, J.; Ladwa, R.; Kenny, L.; Vasani, S.; Frazer, I.H.; Dolcetti, R.; Ellis, J.J.; et al. Analysis of human leukocyte antigen associations in human papillomavirus–positive and –negative head and neck cancer: Comparison with cervical cancer. Cancer 2022, 128, 1937–1947. [Google Scholar] [CrossRef]
- Andersen, P.L.; Jemec, G.B.; Erikstrup, C.; Didriksen, M.; Dinh, K.M.; Mikkelsen, S.; Sørensen, E.; DBDS Genetic Consortium; Nielsen, K.R.; Bruun, M.T.; et al. Human leukocyte antigen system associations in Malassezia-related skin diseases. Arch. Dermatol. Res. 2023, 315, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, W.; Maugeri, U. Epidemiologic Methods in Studying Chronic Diseases: Teaching Manual; Center for Biomedicine: Krakow, Poland, 2000; pp. 245–247. ISBN 8390489635. [Google Scholar]
- Skvortsova, L.; Perfelyeva, A.; Khussainova, E.; Mansharipova, A.; Forman, H.J.; Djansugurova, L. Association of GCLM -588C/T and GCLC -129T/C Promoter Polymorphisms of Genes Coding the Subunits of Glutamate Cysteine Ligase with Ischemic Heart Disease Development in Kazakhstan Population. Dis. Markers 2017, 2017, 4209257. [Google Scholar] [CrossRef]
- Berg, Z.K.; Rodriguez, B.; Davis, J.; Katz, A.R.; Cooney, R.V.; Masaki, K. Association between Occupational Exposure to Pesticides and Cardiovascular Disease Incidence: The Kuakini Honolulu Heart Program. J. Am. Heart Assoc. 2019, 8, e012569. [Google Scholar] [CrossRef]
- Valera, B.; Jørgensen, M.E.; Jeppesen, C.; Bjerregaard, P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013, 122, 65–73. [Google Scholar] [CrossRef]
- Tvermosegaard, M.; Dahl-Petersen, I.K.; Nielsen, N.O.; Bjerregaard, P.; Jørgensen, M.E. Cardiovascular Disease Susceptibility and Resistance in Circumpolar Inuit Populations. Can. J. Cardiol. 2015, 31, 1116–1123. [Google Scholar] [CrossRef]
- Chittrakul, J.; Sapbamrer, R.; Sirikul, W. Insecticide Exposure and Risk of Asthmatic Symptoms: A Systematic Review and Meta-Analysis. Toxics 2021, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.Y.; Hoppin, J.; Mora, A.M.; Soto-Martinez, M.E.; Gamboa, L.C.; Castañeda, J.E.P.; Reich, B.; Lindh, C.; de Joode, B.V.W. Respiratory and allergic outcomes among 5-year-old children exposed to pesticides. Thorax 2023, 78, 41–49. [Google Scholar] [CrossRef]
- Andreotti, G.; Silverman, D.T. Occupational risk factors and pancreatic cancer: A review of recent findings. Mol. Carcinog. 2012, 51, 98–108. [Google Scholar] [CrossRef]
- Goud, M.; Nayal, B.L.; Deepa, R.; Devi, O.S.; Devaki, R.N.; Anitha, M. A Case of Acute Pancreatitis with Occupational Exposure to Organophosphorus Compound. Toxicol. Int. 2012, 19, 223–224. [Google Scholar] [CrossRef][Green Version]
- Rizos, E.; Liberopoulos, E.; Kosta, P.; Efremidis, S.; Elisaf, M. Carbofuran-Induced acute pancreatitis. JOP 2004, 5, 44–47. [Google Scholar]
- Perera, F.; Tang, D.; Whyatt, R.; Lederman, S.A.; Jedrychowski, W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo [a] pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomark. Prev. 2005, 14, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Qiu, X.; Jin, L.; Ma, J.; Li, Z.; Zhang, L.; Zhu, H.; Finnell, R.H.; Zhu, T. Association of selected persistent organic pollutants in the placenta with the risk of neural tube defects. Proc. Natl. Acad. Sci. USA 2011, 108, 12770–12775. [Google Scholar] [CrossRef] [PubMed]
- Kumarakulasingham, M.; Rooney, P.H.; Dundas, S.R.; Telfer, C.; Melvin, W.T.; Curran, S.; Murray, G.I. Cytochrome p450 profile of colorectal cancer: Identification of markers of prognosis. Clin. Cancer Res. 2005, 11, 3758–3765. [Google Scholar] [CrossRef]
- Hidaka, A.; Sasazuki, S.; Matsuo, K.; Ito, H.; Charvat, H.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Inoue, M.; et al. JPHC Study Group CYP1A1, GSTM1 and GSTT1 genetic polymorphisms and gastric cancer risk among Japanese: A nested case-control study within a large-scale population-based prospective study. Int. J. Cancer 2016, 139, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dogra, N.; Singh, S. Health Risk Assessment of Occupationally Pesticide-Exposed Population of Cancer Prone Area of Punjab. Toxicol. Sci. 2018, 165, 157–169. [Google Scholar] [CrossRef] [PubMed]
- McGraw, J.; Waller, D. Cytochrome P450 variations in different ethnic populations. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 371–382. [Google Scholar] [CrossRef]
- Perovani, S.; Barbetta, M.F.S.; da Silva, R.M.; Lopes, N.P.; de Oliveira, A.R.M. In vitro-in vivo correlation of the chiral pesticide prothioconazole after interaction with human CYP450 enzymes. Food Chem. Toxicol. 2022, 163, 112947. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.A.; Abou El-Ella, S.S.; Tawfik, M.; Lein, P.J.; Olson, J.R. Allele and genotype frequencies of CYP2B6 and CYP2C19 polymorphisms in Egyptian agricultural workers. J. Toxicol Environ Health A 2012, 75, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Human cytochrome P450 enzyme 5–51 as targets of drugs, natural, and environmental compounds: Mechanisms, induction, and inhibition, toxic effects and benefits. Drug Metab. Rev. 2018, 50, 256–342. [Google Scholar] [CrossRef]
- Pinhel, M.A.D.S.; Sado, C.L.; Longo, G.D.S.; Gregorio, M.L.; Amorim, G.S.; Florim, G.M.D.S.; Mazeti, C.M.; Martins, D.P.; Oliveira, F.D.N.; Nakazone, M.A.; et al. Nullity of GSTT1/GSTM1 related to pesticides is associated with Parkinson’s disease. Arq. Neuro-Psiquiatr. 2013, 71, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Suke, S.G.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.; Guleria, K.; Sharma, C.; Makhijani, S.; Banerjee, B. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum. Exp. Toxicol. 2010, 29, 351–358. [Google Scholar] [CrossRef]
- Sharma, T.; Jain, S.; Verma, A.; Sharma, N.; Gupta, S.; Arora, V.K.; Banerjee, B.D. Gene environment interaction in urinary bladder cancer with special reference to organochlorine pesticide: A case control study. Cancer Biomark. 2013, 13, 243–251. [Google Scholar] [CrossRef]
- Zepeda-Arce, R.; Rojas-García, A.E.; Benitez-Trinidad, A.; Herrera-Moreno, J.F.; Medina-Díaz, I.M.; Barrón-Vivanco, B.S.; Villegas, G.P.; Hernández-Ochoa, I.; Heredia, M.d.J.S.; Bernal-Hernández, Y.Y. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environ. Toxicol. 2017, 32, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fang, J.; Liu, W. Superoxide dismutase coding of gene polymorphisms associated with susceptibility to Parkinson’s disease. J. Integr. Neurosci. 2019, 18, 299–303. [Google Scholar] [CrossRef]
- Naganuma, T.; Nakayama, T.; Sato, N.; Fu, Z.; Soma, M.; Aoi, N.; Hinohara, S.; Doba, N.; Usami, R. Association of extracellular superoxide dismutase gene with cerebral infarction in women: A haplotype-based case-control study. Hereditas 2008, 145, 283–292. [Google Scholar] [CrossRef]
- Marginean, C.; Streata, I.; Ioana, M.; Marginean, O.M.; Padureanu, V.; Saftoiu, A.; Petrescu, I.; Tudorache, S.; Tica, O.S.; Petrescu, F. Assessment of Oxidative Stress Genes SOD2 and SOD3 Polymorphisms Role in Human Colorectal Cancer. Curr. Health Sci. J. 2016, 42, 356–358. [Google Scholar] [CrossRef]
- Yari, A.; Saleh-Gohari, N.; Mirzaee, M.; Hashemi, F.; Saeidi, K. A Study of Associations between rs9349379 (PHACTR1), rs2891168 (CDKN2B-AS), rs11838776 (COL4A2) and rs4880 (SOD2) Polymorphic Variants and Coronary Artery Disease in Iranian Population. Biochem. Genet. 2021, 60, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.M.; Monteiro, M.B.; Marques, T.; Luna, A.M.; A Fortes, M.; Nery, M.; Queiroz, M.; A Dib, S.; Vendramini, M.F.; Azevedo, M.J.; et al. Association of genetic variants in the promoter region of genes encoding p22phox (CYBA) and glutamate cysteine ligase catalytic subunit (GCLC) and renal disease in patients with type 1 diabetes mellitus. BMC Med. Genet. 2011, 12, 129. [Google Scholar] [CrossRef]
- Gysin, R.; Kraftsik, R.; Sandell, J.; Bovet, P.; Chappuis, C.; Conus, P.; Deppen, P.; Preisig, M.; Ruiz, V.; Steullet, P.; et al. Impaired glutathione synthesis in schizophrenia: Convergent genetic and functional evidence. Proc. Natl. Acad. Sci. USA 2007, 104, 16621–16626. [Google Scholar] [CrossRef]
- Tang, W.; Bentley, A.R.; Kritchevsky, S.B.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Meibohm, B.; Cassano, P.A. Genetic variation in antioxidant enzymes, cigarette smoking, and longitudinal change in lung function. Free. Radic. Biol. Med. 2013, 63, 304–312. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Ivanov, V.P.; Bogomazov, A.D.; Freidin, M.B.; Illig, T.; Solodilova, M.A. Antioxidant Defense Enzyme Genes and Asthma Susceptibility: Gender-Specific Effects and Heterogeneity in Gene-Gene Interactions between Pathogenetic Variants of the Disease. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Katakami, N.; Kaneto, H.; Matsuoka, T.-A.; Takahara, M.; Imamura, K.; Ishibashi, F.; Kanda, T.; Kawai, K.; Osonoi, T.; Kashiwagi, A.; et al. Accumulation of gene polymorphisms related to oxidative stress is associated with myocardial infarction in Japanese type 2 diabetic patients. Atherosclerosis 2010, 212, 534–538. [Google Scholar] [CrossRef]
- Ma, N.; Liu, W.; Zhang, X.; Gao, X.; Yu, F.; Guo, W.; Meng, Y.; Gao, P.; Zhou, J.; Yuan, M.; et al. Oxidative Stress-Related Gene Polymorphisms Are Associated with Hepatitis B Virus-Induced Liver Disease in the Northern Chinese Han Population. Front. Genet. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Javeres, M.N.L.; Habib, R.; Judith, N.; Iqbal, M.; Nepovimova, E.; Kuca, K.; Batool, S.; Nurulain, S.M. Analysis of PON1 gene polymorphisms (rs662 and rs854560) and inflammatory markers in organophosphate pesticides exposed cohorts from two distinct populations. Environ. Res. 2020, 191, 110210. [Google Scholar] [CrossRef]
- Furlong, C.E.; Cole, T.B.; Jarvik, G.P.; Pettan-Brewer, C.; Geiss, G.K.; Richter, R.J.; Shih, D.M.; Tward, A.D.; Lusis, A.J.; Costa, L.G. Role of Paraoxonase (PON1) Status in Pesticide Sensitivity: Genetic and Temporal Determinants. Neurotoxicology 2005, 26, 651–659. [Google Scholar] [CrossRef]
- Eroglu, M.; Yilmaz, N.; Yalcinkaya, S.; Ay, N.; Aydin, O.; Sezer, C. Enhanced HDL-cholesterol-associated anti-oxidant PON-1 activity in prostate cancer patients. Kaohsiung J. Med. Sci. 2013, 29, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Huang, L.; Li, M.; Mo, D.; Liang, Y.; Liu, Z.; Huang, Z.; Huang, L.; Liu, J.; Zhu, B. The Association between PON1 (Q192R and L55M) Gene Polymorphisms and Risk of Cancer: A Meta-Analysis Based on 43 Studies. BioMed Res. Int. 2019, 2019, 5897505. [Google Scholar] [CrossRef]
- Kumar, R.; Saini, V.; Kaur, C.; Isser, H.S.; Tyagi, N.; Sahoo, S. Association between PON1 rs662 gene polymorphism and serum paraoxonase1 level in coronary artery disease patients in Northern India. Egypt. J. Med. Hum. Genet. 2021, 22, 74. [Google Scholar] [CrossRef]
- Paragh, G.; Balla, P.; Katona, E.; Seres, I.; Égerházi, A.; Degrell, I. Serum paraoxonase activity changes in patients with Alzheimer’s disease and vascular dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 63–67. [Google Scholar] [CrossRef]
- Saeed, M.; Lee, R.J.; Weber, O.; Do, L.; Martin, A.; Ursell, P.; Saloner, D.; Higgins, C.B. Scarred myocardium imposes additional burden on remote viable myocardium despite a reduction in the extent of area with late contrast MR enhancement. Eur. Radiol. 2005, 16, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Huen, K.; Marks, A.; Harley, K.G.; Bradman, A.; Barr, D.B.; Holland, N. PON1 and Neurodevelopment in Children from the CHAMACOS Study Exposed to Organophosphate Pesticides in Utero. Environ. Health Perspect. 2010, 118, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wan, H.; Zhu, L.; Mi, Y. Research on the effects of rs1800566 C/T polymorphism of NAD(P)H quinone oxidoreductase 1 gene on cancer risk involves analysis of 43,736 cancer cases and 56,173 controls. Front. Oncol. 2022, 12, 980897. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-Activated Cap’n’collar Transcription Factors in Aging and Human Disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef]
- Chabert, C.; Vitte, A.-L.; Iuso, D.; Chuffart, F.; Trocme, C.; Buisson, M.; Poignard, P.; Lardinois, B.; Debois, R.; Rousseaux, S.; et al. AKR1B10, One of the Triggers of Cytokine Storm in SARS-CoV2 Severe Acute Respiratory Syndrome. Int. J. Mol. Sci. 2022, 23, 1911. [Google Scholar] [CrossRef]
- Ma, J.; Luo, D.X.; Huang, C.; Shen, Y.; Bu, Y.; Markwell, S.; Gao, J.; Liu, J.; Zu, X.; Cao, Z.; et al. AKR1B10 overexpression in breast cancer: Association with tumor size, lymph node metastasis and patient survival and its potential as a novel serum marker. Int. J. Cancer 2012, 131, E862–E871. [Google Scholar] [CrossRef]
- Ko, H.H.; Peng, H.H.; Cheng, S.J.; Kuo, M.Y.P. Increased salivary AKR1B10 level: Association with progression and poor prognosis of oral squamous cell carcinoma. Head Neck 2018, 40, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Hall, N.; Narendran, N.; Yang, Y.C.; Paraoan, L. Identification of candidate protective variants for common diseases and evaluation of their protective potential. BMC Genom. 2017, 18, 575. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Paniagua, D.; Parrón, T.; Alarcón, R.; Requena, M.; Gil, F.; López-Guarnido, O.; Lacasaña, M.; Hernández, A.F. Biomarkers of oxidative stress in blood of workers exposed to non-cholinesterase inhibiting pesticides. Ecotoxicol. Environ. Saf. 2018, 162, 121–128. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNPs | Primer, 5′→3′ | PCR Conditions | PCR Products Length |
|---|---|---|---|---|
| GPX4 | Leu220Leu | f–GAGAAGGACCTGCCCCACTA r–GTCATGAGTGCCGGTGGAAG | 95 °C—4 min. 95 °C—30 s. 61 °C—30 s. 35 cycles 72 °C—45 s. 72 °C—5 min. | 96 bp |
| GCLC | −129T/C | f–TCGTCCCAAGTCTCACAGTC r–CGCCCTCCCCGCTGCTCCTC | 95 °C—4 min. 95 °C—30 s. 61 °C—30 s. 35 cycles 72 °C—45 s. 72 °C—5 min. | 613 bp |
| GCLM | −588C/T | f–CTCAAGGGCAAAGACTCA r–CCGCCTGGTGAGGTAGACAC | 95 °C—4 min. 95 °C—30 s. 58 °C—30 s. 35 cycles 72 °C—45 s. 72 °C—5 min. | 329 bp |
| GSTP1 | Ile105Val | f–ACCCCAGGGCTCTATGGGAA r–TGAGGGCACAAGAAGCCCCT | 94 °C—4 min. 94 °C—30 s. 55 °C—30 s. 30 cycles 72 °C—30 s. 72 °C—8 min. | 176 bp |
| GSTT1 | Deletion | f–CCTTACTGGTCCTCACATCTC r–TCACCGGATCATGGCCACA | 94 °C—4 min. 94 °C—4 min. 59 °C—1 min. 35 cycles 72 °C—1 min. 72 °C—8 min. | +/+;+/−: 480 bp |
| GSTM1 | Deletion | f–GAACTCCCTGAAAAGCTAAAGC r–GTTGGGCTCAAATATACGGTGG | +/+;+/−: 215 bp | |
| β-globin | f–CAACTTCATCCACGTTCACC r–GAAGAGCCTAGGACAGGTAC | +/+: 268 bp |
| Gene/rs | Substitutions | Restriction Endonucleases | Genotypes and DNA Fragments Length (bp) |
|---|---|---|---|
| GPX4 (rs713041) | Leu220Leu | StyI | TT: 68 + 28; TC: 96 + 68 + 28; CC: 96 |
| GCLC (rs524553) | −129T/C | Tsp45I | CC: 500 + 113; CT: 500 + 302 + 198 + 113; TT: 302 + 198 + 113 |
| GCLM (rs41303970) | −588C/T | MspI | CC: 200 + 84 + 45; CT: 200 + 129 + 84 + 45; TT: 200 + 129 |
| GSTP1 (rs1695) | Ile105Val | Alw26I | AA: 91 + 85; AG: 176 + 91 + 85; GG: 176 |
| Characteristics | Case, % | Control, % | tst | p-Value | |
|---|---|---|---|---|---|
| N | 221 | 221 | |||
| Age (years) | 1939–2004 (51.60 ± 12.26) | 1940–1987 (54.16 ± 7.68) | 0.178 | 0.899 | |
| Sex, n (%) | Males | 70 (31.67) | 70 (31.67) | 0 | 1 *** |
| Females | 151(68.33) | 151(68.33) | 0 | 1 *** | |
| Ethnicity, n (%) | Kazakhs | 184 (83.26) | 185 (83.71) | 0.052 | 0.967 * |
| Russians | 23 (10.41) | 19 (8.60) | 0.610 | 0.651 | |
| Other nationalities | 14 (6.33) | 17 (7.69) | 0.533 | 0.688 | |
| Smoking, n (%) | Smokers | 40(18.09) | 37 (16.74) | 0.270 | 0.832 |
| Non-smokers | 181 (81.91) | 184 (83.26) | 0.262 | 0.837 | |
| Gene | rs ID | CASE | CONTROL | European/Asian Frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele Frequencies | Genotype Frequencies | Allele Frequencies | Genotype Frequencies | |||||||||
| A* | B* | AA | AB | BB | A* | B* | AA | AB | BB | |||
| CYP1A1 | rs2606345 (C > A) | 0.690 | 0.310 | 0.491 | 0.398 | 0.111 | 0.653 | 0.347 | 0.388 | 0.529 | 0.083 | C = 0.37 A = 0.63 C = 0.95 A = 0.05 |
| CYP2B6 | rs6508964 (A > G) | 0.671 | 0.329 | 0.479 | 0.382 | 0.138 | 0.654 | 0.346 | 0.384 | 0.540 | 0.076 | C = 0.62 T = 0.33 C = 0.67 T = 0.33 |
| rs3745274 (G > T) | 0.775 | 0.225 | 0.606 | 0.338 | 0.056 | 0.697 | 0.303 | 0.472 | 0.451 | 0.078 | G = 0.716 T = 0.284 G = 0.815 T = 0.185 | |
| rs4803417 (A > C) | 0.669 | 0.331 | 0.477 | 0.384 | 0.139 | 0.653 | 0.347 | 0.402 | 0.503 | 0.095 | C = 0.41 A = 0.58 C = 0.34 A = 0.66 | |
| rs2279345 (C > T) | 0.680 | 0.320 | 0.484 | 0.392 | 0.124 | 0.601 | 0.399 | 0.308 | 0.586 | 0.106 | C = 0.62 T = 0.33 C = 0.67 T = 0.33 | |
| CYP2C19 | rs4494250 (G > A) | 0.710 | 0.290 | 0.535 | 0.350 | 0.115 | 0.671 | 0.329 | 0.412 | 0.518 | 0.070 | G = 0.65 A = 0.34 G = 0.83 A = 0.17 |
| rs11592737 (A > G) | 0.857 | 0.143 | 0.747 | 0.221 | 0.032 | 0.913 | 0.087 | 0.837 | 0.154 | 0.010 | A = 0.78 G = 0.22 A = 0.77 G = 0.23 | |
| rs4986893 (G > A) | 0.961 | 0.039 | 0.926 | 0.069 | 0.005 | 0.863 | 0.137 | 0.731 | 0.264 | 0.005 | G = 0.9998 A = 0.0002 G = 0.974 A = 0.026 | |
| rs11188092 (A > C) | 0.859 | 0.141 | 0.751 | 0.217 | 0.032 | 0.919 | 0.081 | 0.847 | 0.144 | 0.010 | A = 0.81 C = 0.19 A = 0.99 C = 0.02 | |
| rs17884832 (T > G) | 0.922 | 0.078 | 0.853 | 0.138 | 0.009 | 0.826 | 0.174 | 0.657 | 0.338 | 0.005 | T = 0.93 G = 0.06 T = 0.81 G = 0.19 | |
| GSTP1 | rs1871042 (C > T) | 0.723 | 0.277 | 0.526 | 0.395 | 0.079 | 0.797 | 0.203 | 0.627 | 0.340 | 0.033 | C = 0.66 T = 0.33 C = 0.82 T = 0.18 |
| rs4147581 (C > G) | 0.512 | 0.488 | 0.270 | 0.484 | 0.247 | 0.597 | 0.403 | 0.366 | 0.463 | 0.171 | C = 0.49 G = 0.51 C = 0.32 G = 0.68 | |
| rs1695 (A > G) | 0.695 | 0.305 | 0.482 | 0.427 | 0.091 | 0.772 | 0.228 | 0.591 | 0.363 | 0.047 | A = 0.67 G = 0.33 A = 0.81 G = 0.19 | |
| NAT2 | rs1799930 (G > A) | 0.749 | 0.251 | 0.577 | 0.344 | 0.079 | 0.680 | 0.320 | 0.438 | 0.483 | 0.079 | G = 0.715 A = 0.285 G = 0.678 A = 0.322 |
| rs1799931 (G > A) | 0.898 | 0.102 | 0.806 | 0.185 | 0.009 | 0.795 | 0.205 | 0.613 | 0.363 | 0.024 | G = 0.974 A = 0.026 G = 0.890 A = 0.110 | |
| rs1208 (A > G) | 0.750 | 0.250 | 0.560 | 0.380 | 0.060 | 0.815 | 0.185 | 0.665 | 0.300 | 0.034 | G = 0.434 A = 0.566 G = 0.247 A = 0.753 | |
| rs1041983 (C > T) | 0.647 | 0.353 | 0.433 | 0.429 | 0.138 | 0.550 | 0.450 | 0.299 | 0.502 | 0.199 | C = 0.689 T = 0.311 C = 0.578 T = 0.422 | |
| Gene | rs ID | CASE | CONTROL | European>Asian Frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allele Frequencies | Genotype Frequencies | Allele Frequencies | Genotype Frequencies | |||||||||
| A* | B* | AA | AB | BB | A* | B* | AA | AB | BB | |||
| AKR1B10 | rs1722883 T > C | 0.576 | 0.424 | 0.369 | 0.415 | 0.217 | 0.594 | 0.406 | 0.335 | 0.518 | 0.147 | T = 0.54 C = 0.46 T = 0.58 C = 0.42 |
| AKR1C1 | rs2904799 A > G | 0.712 | 0.288 | 0.519 | 0.386 | 0.095 | 0.628 | 0.372 | 0.410 | 0.436 | 0.154 | G = 0.960 A = 0.040 G = 0.71 A = 0.29 |
| rs2904802 T > C | 0.813 | 0.188 | 0.657 | 0.310 | 0.032 | 0.752 | 0.248 | 0.542 | 0.420 | 0.038 | T = 0.333 C = 0.667 T = 0.192 C = 0.808 | |
| GCLC | rs4715407 A > G | 0.804 | 0.196 | 0.645 | 0.318 | 0.037 | 0.720 | 0.280 | 0.492 | 0.455 | 0.052 | G = 0.864 A = 0.136 G = 0.736 A = 0.264 |
| rs524553 T > C | 0.871 | 0.129 | 0.765 | 0.212 | 0.023 | 0.791 | 0.209 | 0.596 | 0.390 | 0.014 | C = 0.76 T = 0.24 C = 0.85 T = 0.15 | |
| rs547222 T > C | 0.816 | 0.184 | 0.664 | 0.304 | 0.032 | 0.753 | 0.247 | 0.546 | 0.413 | 0.041 | T = 0.942 C = 0.059 T = 0.74 C = 0.26 | |
| GCLM | rs41303970 C > T | 0.881 | 0.119 | 0.799 | 0.164 | 0.037 | 0.873 | 0.127 | 0.756 | 0.235 | 0.009 | G = 0.833 A = 0.167 G = 0.812 A = 0.188 |
| GPX4 | rs117193629 T > C | 0.942 | 0.058 | 0.889 | 0.106 | 0.005 | 0.972 | 0.028 | 0.948 | 0.047 | 0.005 | C = 0.95 T = 0.05 C = 0.98 T = 0.01 |
| NFE2L1 | rs2023885 A > G | 0.856 | 0.144 | 0.726 | 0.260 | 0.014 | 0.771 | 0.229 | 0.575 | 0.391 | 0.034 | G = 0.895 A = 0.105 G = 0.768 A = 0.232 |
| rs147114188 A > G | 0.972 | 0.028 | 0.949 | 0.046 | 0.005 | 0.866 | 0.134 | 0.736 | 0.259 | 0.005 | G = 0.9998 A = 0.0002 G = 0.991 A = 0.009 | |
| NFE2L3 | rs12113404 A > G | 0.769 | 0.231 | 0.606 | 0.324 | 0.069 | 0.670 | 0.330 | 0.405 | 0.530 | 0.065 | A = 0.890 G = 0.110 A = 0.674 G = 0.326 |
| rs2237329 T > C | 0.866 | 0.134 | 0.760 | 0.212 | 0.028 | 0.780 | 0.220 | 0.589 | 0.383 | 0.029 | C = 0.989 T = 0.011 C = 0.962 T = 0.037 | |
| NQO1 | rs2917677 T > C | 0.757 | 0.243 | 0.574 | 0.366 | 0.060 | 0.697 | 0.303 | 0.436 | 0.521 | 0.043 | C = 0.57 T = 0.43 C = 0.90T = 0.09 |
| rs76921462 T > C | 0.910 | 0.090 | 0.833 | 0.153 | 0.014 | 0.857 | 0.143 | 0.718 | 0.277 | 0.005 | C = 0.977 T = 0.023 C = 0.9997 T = 0.0003 | |
| PON1 | rs854568 A > G | 0.295 | 0.705 | 0.097 | 0.396 | 0.507 | 0.291 | 0.709 | 0.056 | 0.470 | 0.474 | G = 0.199 A = 0.801 G = 0.303 A = 0.697 |
| PON2 | rs12534274 A > G | 0.628 | 0.372 | 0.400 | 0.456 | 0.144 | 0.531 | 0.469 | 0.249 | 0.565 | 0.187 | G = 0.756 A = 0.244 G = 0.516 A = 0.484 |
| rs2299267 A > G | 0.717 | 0.283 | 0.530 | 0.373 | 0.097 | 0.664 | 0.336 | 0.412 | 0.505 | 0.083 | A = 0.83 G = 0.16 A = 0.68 G = 0.32 | |
| PON3 | rs17885558 A > G | 0.938 | 0.063 | 0.880 | 0.116 | 0.005 | 0.865 | 0.135 | 0.735 | 0.260 | 0.005 | G = 0.993 A = 0.007 G = 0.964 A = 0.036 |
| rs138268669 A > G | 0.973 | 0.027 | 0.952 | 0.043 | 0.005 | 0.859 | 0.141 | 0.727 | 0.263 | 0.010 | G = 0.9998 A = 0.0002 G = 0.9997 A = 0.0003 | |
| SOD1 | rs1041740 T > C | 0.655 | 0.345 | 0.435 | 0.440 | 0.125 | 0.591 | 0.409 | 0.318 | 0.547 | 0.136 | C = 0.644 T = 0.356 C = 0.602 T = 0.398 |
| SOD2 | rs12204454 T > C | 0.663 | 0.337 | 0.484 | 0.358 | 0.158 | 0.629 | 0.371 | 0.387 | 0.484 | 0.129 | C = 0.80 T = 0.20 C = 0.65 T = 0.35 |
| rs4880 A > G | 0.687 | 0.313 | 0.488 | 0.396 | 0.115 | 0.657 | 0.343 | 0.396 | 0.522 | 0.082 | A = 0.50 G = 0.50 A = 0.86 G = 0.13 | |
| SRXN1 | rs7268200 A > G | 0.788 | 0.212 | 0.594 | 0.387 | 0.018 | 0.686 | 0.314 | 0.443 | 0.486 | 0.071 | A = 0.671 G = 0.329 A = 0.747 G = 0.253 |
| TXNRD1 | rs117567389 T > C | 0.867 | 0.133 | 0.744 | 0.246 | 0.009 | 0.804 | 0.196 | 0.634 | 0.340 | 0.026 | T = 0.9996 C = 0.0004 T = 1 C = 0 |
| rs7301631 T > C | 0.291 | 0.709 | 0.102 | 0.377 | 0.521 | 0.363 | 0.637 | 0.122 | 0.483 | 0.395 | T = 0.49 C = 0.51 T = 0.22C = 0.78 | |
| rs7975161 T > C | 0.898 | 0.102 | 0.810 | 0.176 | 0.014 | 0.804 | 0.196 | 0.642 | 0.325 | 0.033 | T = 0.159 C = 0.841 T = 0.140 C = 0.860 | |
| UCP3 | rs11235971 T > C | 0.714 | 0.286 | 0.512 | 0.406 | 0.083 | 0.649 | 0.351 | 0.398 | 0.502 | 0.100 | T = 0.847 C = 0.153 T = 0.697 C = 0.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altynova, N.; Khamdiyeva, O.; Garshin, A.; Baratzhanova, G.; Amirgaliyeva, A.; Seisenbayeva, A.; Abylkassymova, G.; Yergali, K.; Tolebaeva, A.; Skvortsova, L.; et al. Case-Control Study of the Association between Single Nucleotide Polymorphisms of Genes Involved in Xenobiotic Detoxification and Antioxidant Protection with the Long-Term Influence of Organochlorine Pesticides on the Population of the Almaty Region. Toxics 2023, 11, 948. https://doi.org/10.3390/toxics11120948
Altynova N, Khamdiyeva O, Garshin A, Baratzhanova G, Amirgaliyeva A, Seisenbayeva A, Abylkassymova G, Yergali K, Tolebaeva A, Skvortsova L, et al. Case-Control Study of the Association between Single Nucleotide Polymorphisms of Genes Involved in Xenobiotic Detoxification and Antioxidant Protection with the Long-Term Influence of Organochlorine Pesticides on the Population of the Almaty Region. Toxics. 2023; 11(12):948. https://doi.org/10.3390/toxics11120948
Chicago/Turabian StyleAltynova, Nazym, Ozada Khamdiyeva, Aleksandr Garshin, Gulminyam Baratzhanova, Almira Amirgaliyeva, Akerke Seisenbayeva, Gulnar Abylkassymova, Kanagat Yergali, Anar Tolebaeva, Liliya Skvortsova, and et al. 2023. "Case-Control Study of the Association between Single Nucleotide Polymorphisms of Genes Involved in Xenobiotic Detoxification and Antioxidant Protection with the Long-Term Influence of Organochlorine Pesticides on the Population of the Almaty Region" Toxics 11, no. 12: 948. https://doi.org/10.3390/toxics11120948
APA StyleAltynova, N., Khamdiyeva, O., Garshin, A., Baratzhanova, G., Amirgaliyeva, A., Seisenbayeva, A., Abylkassymova, G., Yergali, K., Tolebaeva, A., Skvortsova, L., Zhunussova, G., Bekmanov, B., Cakir-Kiefer, C., & Djansugurova, L. (2023). Case-Control Study of the Association between Single Nucleotide Polymorphisms of Genes Involved in Xenobiotic Detoxification and Antioxidant Protection with the Long-Term Influence of Organochlorine Pesticides on the Population of the Almaty Region. Toxics, 11(12), 948. https://doi.org/10.3390/toxics11120948







