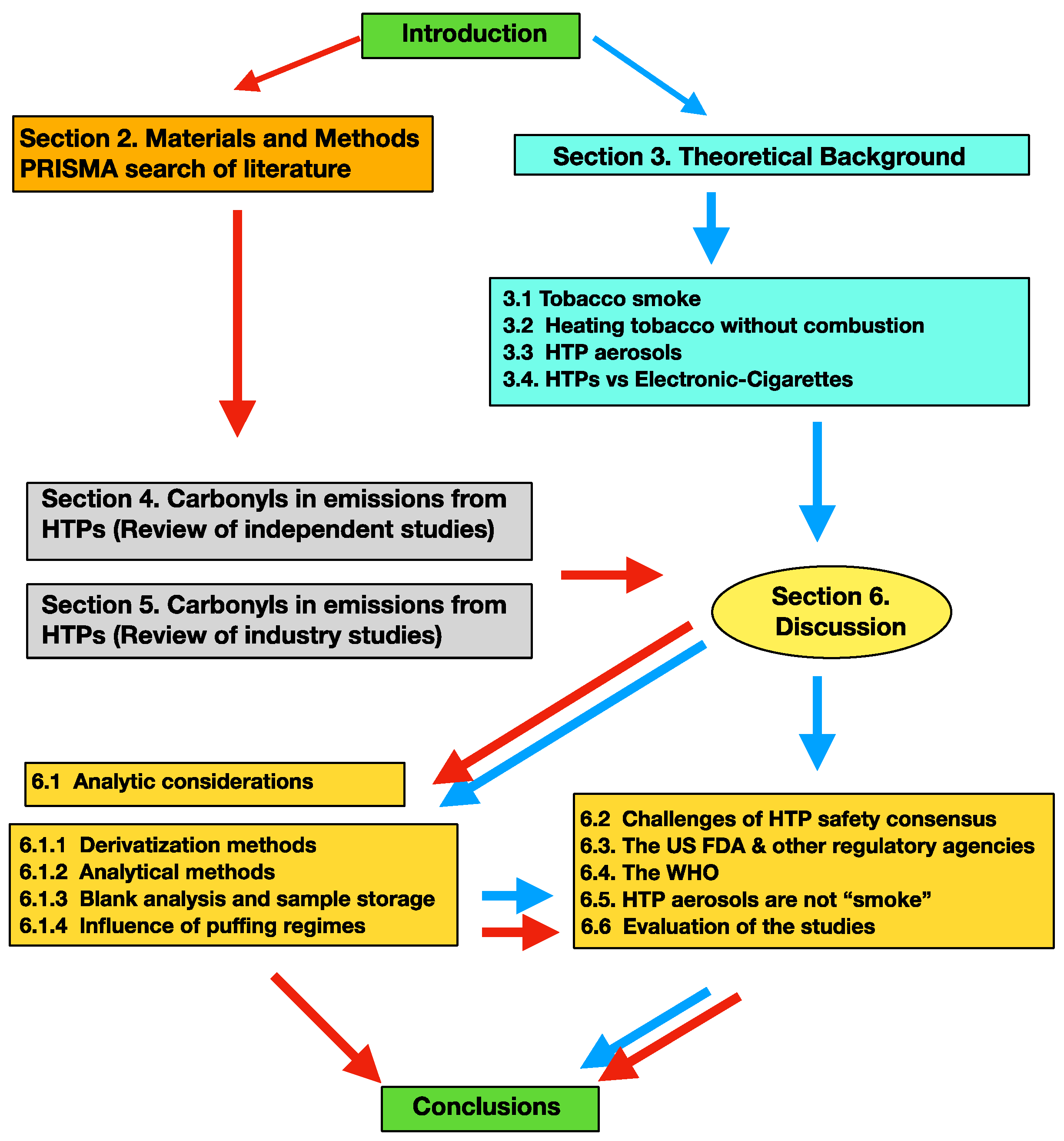
Aerosol Emissions from Heated Tobacco Products: A Review Focusing on Carbonyls, Analytical Methods, and Experimental Quality
Abstract
:1. Introduction
- eHTP: aerosols are generated by directly heating specially treated tobacco sticks (or “heets”). The gender includes two series of devices, IQOS™ Philip Morris International (PMI) (Stamford, U.S.A.) and gloTM, manufactured by and British American Tobacco (BAT) (London, UK);
- aHTP: an independently generated aerosol is filtered through tobacco sticks. There are two devices of this type, eFuse™ (BAT, London, UK) and Ploom™ (JTI, Geneva, Switzerland), respectively, manufactured by BAT and JTI;
- cHTP: a carbon rod is used to heat tobacco sticks. The now obsolete Eclipse™ (BAT, London, UK).
- 18.4% in 2017 used a single tobacco product (78.8% cigarettes and 5.2% HTPs), while 3.2% used multiple tobacco products (with 10.5% using HTPs) [16];
- In 2018, monthly HTP use was 2.7% (1.7% daily use), with 67.8% and 25.0% current and former smokers, respectively, and 1.0% never smokers. IQOS and menthol flavor were used at 64.5% and menthol flavor (of all products) at 41.5%, respectively. IQOS was preferred by younger respondents and Ploom TECH by older respondents and non-daily HTP users [17].
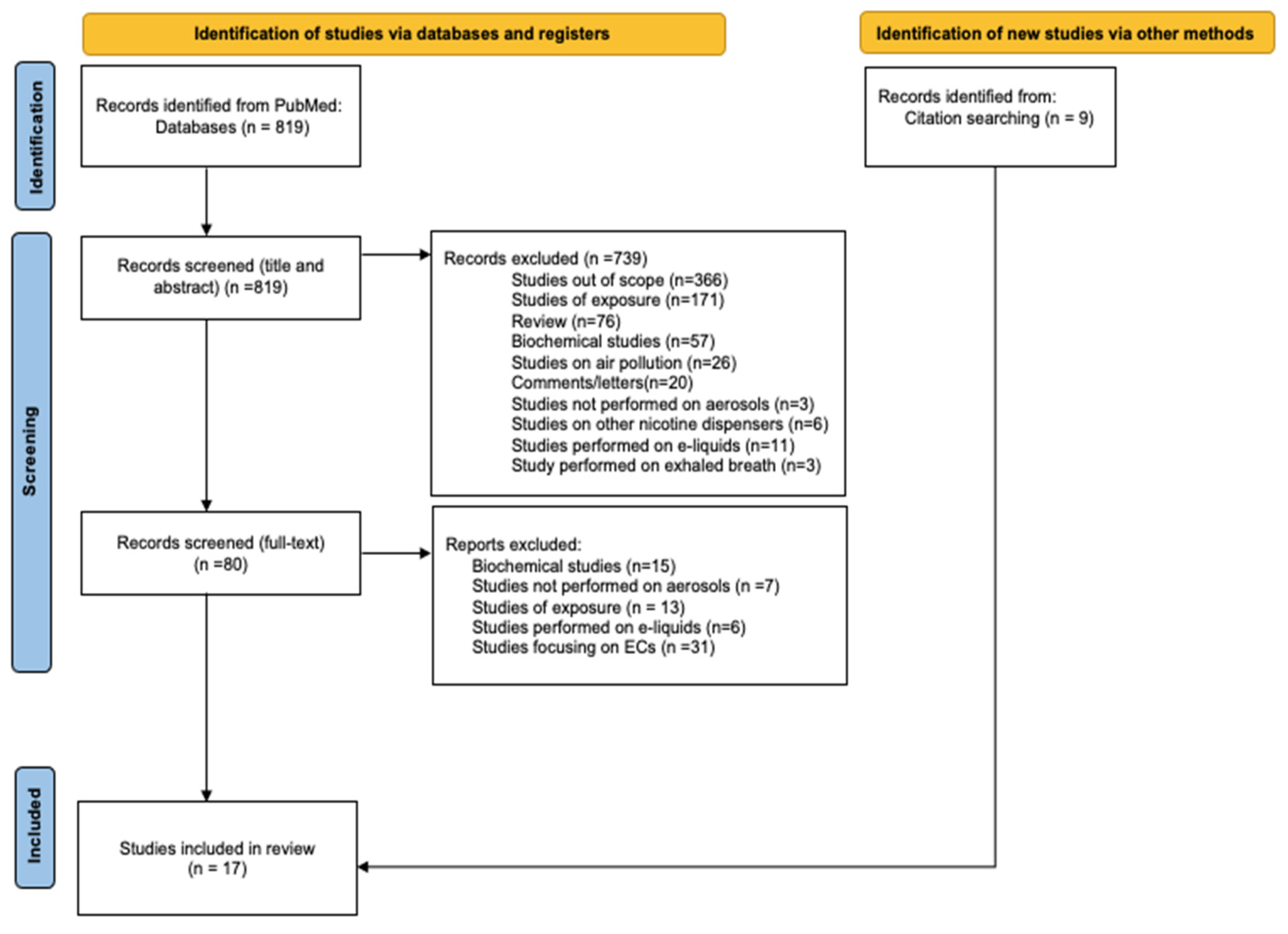
2. Materials and Methods
- Studies were conducted on aerosols collected according to the standardized recommended puffing protocol of the Cooperation Centre for Scientific Research Relative to Tobacco (CORESTA);
- Aerosols were adequately treated for carbonyl entrapment;
- Analytical methods were adequate and reproducible, with particular attention to blank analyses;
- Samples were stored adequately prior to analysis.
3. Theoretical Background
3.1. Tobacco Smoke
- “mainstream emission”: the inhaled smoke from the mouth end of the puffed cigarette, cooled and transported by the cigarette rod and exhaled by the smoker;
- “side stream emission”: the smoke released directly into the environment from the burning/smoldering tip of the cigarette;
- “environmental tobacco smoke” (ETS): the smoke formed by an aging mixture of exhaled mainstream and sidestream emissions released into the environment, which diffuse, dilute, and react with ambient air chemicals.
3.2. Heating Tobacco without Combustion
3.3. HTP Aerosols
3.4. HTPs vs. ECs
4. Carbonyls in Emissions from HTPs (Independent Studies)
4.1. Previous Reviews
4.2. Emission Studies
| Authors | Devices | Smoking Regimes | Analytical Methods | Derivatization Methods | Results Reported as |
|---|---|---|---|---|---|
| Auer et al. [53] | IQOS Lucky Strike Blue Lights | ISO 3308:2012 | Incomprehensible | Incomprehensible | μg/cig |
| Farsalinos et al. [55] | IQOS Nautilius Mini Marlboro Red | ISO 20778:2018 | CORESTA | DNPH solution | μg/stick: μg/12 puffs |
| Mallock et al. [57] | IQOS 1R4F | ISO 20778:2018 | HPLC-UV LC-MS/MS | DNPH solution | μg/stick: μg/12 puffs |
| Uchiyama et al. [49] | IQOS glo Ploom Tech 1R5F 3R4F CM6 | ISO 20778:2018 ISO 3308:2012 | HPLC-UV | DNPH solution | μg/stick |
| Salman et al. [58] | IQOS Marlboro Red | ISO 20778:2018 | HPLC-UV | DNPH-cartridges | μg/session |
| Heide et al. [59] | IQOS Eclipse PowerCig, Duvance Vype 2R4F | ISO 20778:2018 ISO 3308:2012 | SPI-TOFMS | No derivatization | μg/puff |
| Wang et al. [60] | Unidentified HTP and ultra-light cig, 3R4F | ISO 3308:2012 | CORESTA | DNPH solution | μg/stick |
| Dusautoir et al. [61] | IQOS EC Lounge NHOSS Mod Box 3R4F | ISO 20778:2018 | HPLC-UV | DNPH-cartridges | μg/puff |
| Authors | Method Validation | Blank Analysis |
|---|---|---|
| Auer et al. [53] | Not detected | x |
| Farsalinos et al. [55] | LOD: 0.254 μg/collection for formaldehyde, 0.290 μg/collection for acetaldehyde, 0.395 μg/collection for acrolein, 0.440 μg/ collection for propionaldehyde 0.403 μg/collection for crotonaldehyde. | x |
| Mallock et al. [57] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
| Uchiyama et al. [49] | LOD was calculated on the basis of the signal-to-noise ratios of 3: 0.76−17 μg/L. LOQ was calculated using a signal-to-noise ratio of 10: 2.5−58 μg/mL. Linearity: coefficients of determination greater than 0.9966. Reproducibility: expressed as the relative standard deviation (RSD), ranging from 1.9% to 5.1% (carbonyls) and from 0.23% to 4.4% (VOCs). | √ |
| Salman et al. [58] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
| Heide et al. [59] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
| Wang et al. [60] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
| Dusautoir et al. [61] | LOQ ranging from 6 to 15 ng/mL. | √ |
5. Studies on Heated Tobacco Products Funded by the Tobacco Industry
| Authors | Devices | Smoking Regimes | Analytical Methods | Derivatization Methods | Results Reported as |
|---|---|---|---|---|---|
| Schaller et al. [62] | IQOS 3R4F | ISO 20778:2018 | LC-MS/MS | DNPH solution | μg/stick |
| Schaller et al. [45] | IQOS 3R4F | ISO 20778:2018 | HPLC-UV | DNPH solution | μg/stick |
| Jaccard et al. [63] | IQOS CC 3R4F | ISO 20778:2018 | HPLC-UV | DNPH solution | μg/stick |
| Poynton et al. [64] | iFuse Vype ePen I 3R4F | ISO 20778:2018 | HPLC-UV | DNPH solution | μg/100 puff μg/stick |
| Buratto et al. [65] | IQOS 3R4F | ISO 20778:2018 | LC-MS/MS | DNPH solution | μg/cig |
| Crooks et al. [68] | glo 3R4F | ISO 20778:2018 | GC-MS SIM | PFBHA solutions | μg/stick |
| Eaton et al. [36] | THP 1.0 3R4F | ISO 20778:2018 | HPLC-UV | DNPH solution | μg/stick |
| Forster et al. [69] | glo IQOS 3R4F | ISO 20778:2018 | GC-MS SIM | PFBHA solutions | μg/stick |
| Bentley et al. [46] | IQOS 3R4F | ISO 20778:2018 | Untargeted methods | No derivatization | μg/stick |
| Authors | Method Validation | Blank Analysis |
|---|---|---|
| Schaller et al. [62] | The precision of the analytical method was calculated and reported as the mean value ± SD. LOQ was calculated. | x |
| Schaller et al. [45] | The precision of the analytical method was calculated and reported as the mean value ± SD. LOQ was calculated. | x |
| Jaccard et al. [63] | The precision of the analytical method was calculated and reported as the mean value ± SD. LOD and LOQ were calculated. | x |
| Poynton et al. [64] | The precision of the analytical method was calculated and reported as the mean value ± SD. LOD and LOQ were calculated. | x |
| Buratto et al. [65] | The analytical method was validated according to the International Conference on Harmonization (ICH) guidelines. | √ |
| Crooks et al. [68] | The precision of the analytical method was calculated and reported as the mean value ± SD. | √ |
| Eaton et al. [36] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
| Forster et al. [69] | The precision of the analytical method was calculated and reported as the mean value ± SD. | √ |
| Bentley et al. [46] | The precision of the analytical method was calculated and reported as the mean value ± SD. | x |
6. Discussion
6.1. Analytical Considerations
6.1.1. Derivatization Methods
- Saturation: During analysis, the DNPH cartridges can become saturated with carbonyl compounds. This can occur during the same analysis or depends on the volume of aerosols passing through the cartridge. When a cartridge reaches saturation, it may not retain additional carbonyl compounds, thereby compromising the accuracy of the analysis.
- Condensate deposits: Condensates, such as water droplets or other substances in aerosols, may be deposited on the surface of DNPH cartridges. These deposits can hinder the ability of cartridges to efficiently retain carbonyl compounds, thereby affecting the amount of compounds detected.
- Secondary reactions with oxidants, such as ozone, can react with DNPH in cartridges, causing unwanted secondary reactions and interfering with the analysis of carbonyl compounds. These reactions can lead to biased results or overestimation of the presence of carbonyl compounds.
- Polymerization byproduct formation: In unsaturated carbonyl compounds such as acrolein, the possibility of polymerization byproduct formation during derivatization with DNPH has been observed. This polymerization may prevent the accurate identification and quantification of unsaturated carbonyl compounds, thereby introducing uncertainties in the analysis.
6.1.2. Analytical Methods
6.1.3. Blank Analysis and Sample Storage
- The blank method, or blank analysis, is an important practice in quantitative analysis to check for external contamination and ensure that the data obtained are indeed attributable to the sample being analyzed. Blank analysis involves the analysis of a matrix devoid of the analytes of interest, which is processed and subjected to the same steps as those used for the analysis of real samples. This enables the identification and assessment of contaminants that can affect the analytical results.
- In the context of the reviewed studies, only five performed or described blank air analyses [49,61,65,68,69]. This means that only a small percentage of the studies considered possible external contamination and performed controls to exclude contamination from the analysis results. Blank analysis is particularly important when studying volatile compounds in air because the air itself can be subject to contamination from a variety of environmental sources. Verification that the data are derived only from the sample and not from external contamination is critical for ensuring the reliability of the analysis results. Blank analysis provides an additional check to identify and correct for any contamination that may compromise the accuracy and interpretation of the data obtained. In the remaining studies, it is unclear whether the blank analysis was performed at all or that its mention was omitted in the final article.
- In the context of the reviewed studies, there seems to be a lack of information regarding the storage conditions of the samples before analysis. This lack of detail may be a limitation in understanding and reproducing the results. In fact, only a few studies have specified that characteristic ISO conditioning was performed. The storage conditions for aerosol samples may vary depending on the compounds of interest and the analysis objectives. However, some general considerations include the following.
- Temperature storage: Aerosol samples should be stored at appropriate temperatures to preserve the analyte stability. Depending on the nature of the carbonyl compounds and solvents used, refrigerated or frozen temperatures may be necessary to prevent the decomposition or volatilization of the analytes.
- Protection from light: Some compounds may be sensitive to light and undergo undesirable photochemical reactions. Therefore, aerosol samples should be protected from direct light or stored in opaque containers to prevent alteration of analytes.
- Prevention of internal secondary reactions: It is important to ensure that collected aerosol samples are stored in a manner that minimizes the possibility of unwanted chemical reactions within the samples. This may require the use of inert containers or the use of appropriate chemical stabilizers.
- Storage time: It is important to consider the length of time that aerosol samples can be stored prior to analysis. Some compounds may be subject to degradation over time; therefore, it is necessary to assess the stability of the analytes of interest and define appropriate storage times to avoid alteration of the results.
6.1.4. Influence of Puffing Regimes
6.2. Challenges to HTP Safety Consensus: More HPHCs Than in Tobacco Smoke?
| Compound | iQOS µg/Stick | 3R4F µg/cig | % Excess in IQOS | Toxicological Marker mg/m3 | Inhalation Dose iQOS | Safety Threshold |
|---|---|---|---|---|---|---|
| 2-ethyl-5-methyl-1,4-Dioxane | 0.055 | 0.0004 | 13650 | MRL ATSDR-CDC 0.16 [87] | 1.1 µg/day | 3.19 mg/day |
| Furanmethanol | 39.2 | 7.0 | 460 | OSHA TWA 8 h 40 [88] | 274 µg/8 h | 266 mg/8 h |
| Glycidol | 5.71 | 1.76 | 224 | CalOSHA TWA 8 h 6.1 [87] | 39.9 µg/8 h | 40.67 mg/8 h |
| Furfural | 31 | 25.9 | 20 | ACGIH TLV © 8 h TWA 0.8 [87] | 217.7 µg/8 h | 5.33 mg/8 h |
6.3. The US FDA and Other Regulatory Agencies
With respect the exposure modification order request, the applicant has demonstrated that the products sold or distributed with the proposed modified risk information meet the standard under section 911(g)(2) of the FD&C Act, including that a measurable and substantial reduction in morbidity or mortality among individual tobacco users is reasonably likely in subsequent studies, and issuance of an order is expected to benefit the health of the population as a whole taking into account both users of tobacco products and persons who do not currently use tobacco products. [our emphasis]
In short, unlike the section 911(g)(1) standard, which requires scientific evidence showing actual risk reduction (e.g., a finding that the product, as actually used by consumers, will significantly reduce harm and risk to individual users; a finding that the product, as actually used by consumers, will benefit the health of the population a as whole), section 911(g)(2) establishes a lower standard, which allows FDA to issue an order when risk reduction has not yet been demonstrated but is reasonably likely based on demonstrated reductions in exposure (e.g., a finding that a reduction in morbidity or mortality among individual users is reasonably likely in subsequent studies; a finding that issuance of an order is expected to benefit the health of the population as a whole).
6.4. The WHO
- The US FDA statement noted that “Even with this action [MRTP modified exposure order], these products are not safe nor “FDA approved”;
- The US FDA authorization rejected claims that the use of the product is less harmful than other tobacco products or reduces health risks;
- The WHO reiterates that reducing exposure to harmful chemicals in Heated Tobacco Products (HTPs) does not render them harmless, nor does it translate to reduced risk to human health;
- Some toxins are present at higher levels in HTP aerosols than in conventional cigarette smoke, and there are some additional toxins present in HTP aerosols that are not present in conventional cigarette smoke. The health implications of exposure to these are unknown.
“Data submitted by the company shows that marketing these particular products with the authorized information could help addicted adult smokers transition away from combusted cigarettes and reduce their exposure to harmful chemicals, but only if they completely switch”. (Mitch Zeller, J.D., former director of the FDA’s Center for Tobacco Products)
6.5. HTP Aerosols Are Not “Smoke”
6.6. Evaluation of the Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| °C | Degrees centigrade |
| BAT | British American Tobacco |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| CORESTA | Cooperation Centre for Scientific Research Relative to Tobacco |
| DNPH | 2,4-Dinitrophenylhydrazine |
| EC | Electronic Cigarette |
| ENDS | Electronic Nicotine Delivery Systems |
| ETS | Environmental tobacco smoke |
| FDA | US Food and Drug Administration |
| FTIR | Fourier Transform Infra-Red Spectrometry |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GC-MS SIM | Gas Chromatography–Mass Spectrometry Selected Ion Monitoring |
| GVP | Gas vapor phase |
| HCI | Health Canada Intense |
| HPHC | Hazardous and Potentially Hazardous Compounds |
| HPLC-DAD | High-Performance Liquid Chromatography Diode Array Detector |
| HPLC-UV | High-Performance Liquid Chromatography Ultraviolet |
| HTP | Heated Tobacco Products |
| ISO 20778:2018 | CORESTA FTC puffing regime (2 s puff, 60 s inter puff, 35 mL puff volume) |
| ISO 20778:2018 | HCI puffing regime (2 s puff, 30 s inter puff, 55 mL puff volume) |
| ISO | International Organization for Standardization |
| JTI | Japan Tobacco International |
| LC-MS/MS | Liquid Chromatography–Mass Spectrometry |
| MRTP | Modified Risk Tobacco Product |
| NGDPM | Nicotine-free dry particulate matter |
| NNK | Tobacco specific nitrosamines |
| NNN | Tobacco specific nitrosamines |
| NOx | Nitrogen oxide denoted by “x” |
| PAHs | Polycyclic aromatic hydrocarbons |
| PBS | Phosphate-buffered salt solutions |
| PFBHA | o-(2,3,4,5,6-Pentafluorobenzyl)hydroxylamine |
| PMI | Phillip Morris International |
| SPI | TOFMS Single-photon ionization Mass Spectrometry |
| TGA | Thermogravimetric Analysis |
| THF | Tetrahydrofuran |
| THR | Tobacco Harm Reduction |
| TPM | Total particle matter |
| TPSAC | Tobacco Product Scientific Advisory Committee |
| TSNA | Tobacco specific nitrosamines |
| UHPLC-UV | Ultra High-Performance Liquid Chromatography Ultraviolet |
| UV | Ultraviolet |
| VOCs | Volatile organic compounds |
| WHO | World Health Organization |
| WS | Smoke-type samples |
References
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta GA, USA, 2010.
- World Health Organization. WHO Report on The Global Tobacco Epidemic, 2017. Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Caponnetto, P.; Keller, E.; Bruno, C.M.; Polosa, R. Handling relapse in smoking cessation: Strategies and recommendations. Intern. Emerg. Med. 2013, 8, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kotz, D.; Batra, A.; Kastaun, S. Smoking Cessation Attempts and Common Strategies Employed. Dtsch. Arztebl. Int. 2020, 117, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H. Burning Issues: Global State of Tobacco Harm Reduction 2020; Knowledge-Action-Change: London, UK, 2020. [Google Scholar]
- 2023 Third Quarter Results. Tokyo. 31 October 2023. Japan Tobacco (Pages 6 and 25). Available online: https://www.jt.com/investors/results/forecast/pdf/2023/Third_Quarter/20231031_07.pdf (accessed on 5 November 2023).
- World Health Organization. Electronic Nicotine and Non-Nicotine Delivery Systems: A Brief; World Health Organization Regional Office for Europe: Geneva, Switzerland, 2020. [Google Scholar]
- How Are Non-Combusted Cigarettes, Sometimes Called Heat-Not-Burn Products, Different from E-Cigarettes and Cigarettes? Available online: https://www.fda.gov/tobacco-products/products-ingredients-components/how-are-non-combusted-cigarettes-sometimes-called-heat-not-burn-products-different-e-cigarettes-and (accessed on 7 November 2023).
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat Not Burn Tobacco Product-A New Global Trend: Impact of Heat-Not-Burn Tobacco Products on Public Health, a Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 409. [Google Scholar] [CrossRef]
- Dempsey, R.; Rodrigo, G.; Vonmoos, F.; Gunduz, I.; Belushkin, M.; Esposito, M. Preliminary toxicological assessment of heated tobacco products: A review of the literature and proposed strategy. Toxicol. Rep. 2023, 10, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Sutanto, E.; Smith, D.M.; Hitchman, S.C.; Gravely, S.; Yong, H.H.; Borland, R.; O’Connor, R.J.; Cummings, K.M.; Fong, G.T.; et al. Awareness, trial and use of heated tobacco products among adult cigarette smokers and e-cigarette users: Findings from the 2018 ITC Four Country Smoking and Vaping Survey. Tobaco Control 2020, 31, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Duan, Z.; Bar-Zeev, Y.; Abroms, L.C.; Khayat, A.; Tosakoon, S.; Romm, K.F.; Wang, Y.; Berg, C.J. IQOS Use and Interest by Sociodemographic and Tobacco Behavior Characteristics among Adults in the US and Israel. Int. J. Environ. Res. Public Health 2023, 20, 3141. [Google Scholar] [CrossRef] [PubMed]
- FDA. Authorizes Marketing of IQOS Tobacco Heating System with ‘Reduced Exposure’ Information. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-iqos-tobacco-heating-system-reduced-exposure-information (accessed on 7 January 2023).
- Special Issue “Japan: Evaluating the Effectiveness of Tobacco Control Policies and the Use of Heated Tobacco Products”. Available online: https://www.mdpi.com/journal/ijerph/special_issues/Japan_evaluating_effectiveness_tobacco_control_policies_use_heated_tobacco_products (accessed on 10 January 2023).
- Cummings, K.M.; Nahhas, G.J.; Sweanor, D.T. What Is Accounting for the Rapid Decline in Cigarette Sales in Japan? Int. J. Environ. Res. Public Health 2020, 17, 3570. [Google Scholar] [CrossRef]
- Sugiyama, T.; Tabuchi, T. Use of Multiple Tobacco and Tobacco-Like Products Including Heated Tobacco and E-Cigarettes in Japan: A Cross-Sectional Assessment of the 2017 JASTIS Study. Int. J. Environ. Res. Public Health 2020, 17, 2161. [Google Scholar] [CrossRef]
- Sutanto, E.; Miller, C.; Smith, D.M.; O’Connor, R.J.; Quah, A.C.K.; Cummings, K.M.; Xu, S.; Fong, G.T.; Hyland, A.; Ouimet, J.; et al. Prevalence, Use Behaviors, and Preferences among Users of Heated Tobacco Products: Findings from the 2018 ITC Japan Survey. Int. J. Environ. Res. Public Health 2019, 16, 4630. [Google Scholar] [CrossRef]
- Statement on the Toxicological Evaluation of Novel Heat-Not-Burn Tobacco Products. Available online: https://cot.food.gov.uk/sites/default/files/heat_not_burn_tobacco_statement.pdf (accessed on 18 January 2023).
- McNeill, A.; Brose, L.S.; Calder, R.; Bauld, L.; Robson, D. Evidence Review of e-Cigarettes and Heated Tobacco Products 2018: A Report Commissioned by Public Health England; Public Health England: London, UK, 2018. [Google Scholar]
- Publications on Tobacco and Related Products. Available online: https://www.rivm.nl/en/tobacco/publications-on-tobacco-and-related-products (accessed on 15 January 2023).
- Fowles, J.; Dybing, E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tobaco Control 2003, 12, 424–430. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 88, 1–478. [Google Scholar]
- Cogliano, V.J.; Grosse, Y.; Baan, R.A.; Straif, K.; Secretan, M.B.; El Ghissassi, F. Working Group for Volume 88. Meeting report: Summary of IARC monographs on formaldehyde, 2-butoxyethanol, and 1-tert-butoxy-2-propanol. Environ. Health Perspect. 2005, 113, 1205–1208. [Google Scholar] [CrossRef]
- IARC Working Group on the Identification of Carcinogenic Hazards to Humans. Acrolein, Crotonaldehyde, and Arecoline. Lyon (FR): International Agency for Research on Cancer; (IARC Monographs on the Identification of Carcinogenic Hazards to Humans, No. 128). 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK589586/ (accessed on 18 November 2022).
- Simonavicius, E.; McNeill, A.; Shahab, L.; Brose, L.S. Heat-not-burn tobacco products: A systematic literature review. Tobaco Control 2019, 28, 582–594. [Google Scholar] [CrossRef] [PubMed]
- El-Kaassamani, M.; Yen, M.; Talih, S.; El-Hellani, A. Analysis of mainstream emissions, secondhand emissions and the environmental impact of IQOS waste: A systematic review on IQOS that accounts for data source. Tobaco Control 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; Massey, E.D.; Smith, G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 2004, 42, S53–S83. [Google Scholar] [CrossRef] [PubMed]
- Borgerding, M.; Klus, H. Analysis of complex mixtures—Cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Geiss, O.; Kotzias, D. Tobacco, Cigarettes and Cigarette Smoke, An Overview. Institute for Health and Consumer Protection; EUR 22783; European Commission, Directorate General, Joint Research Centre: Brussels, Belgium, 2007; ISBN 978-92-79-06000-7. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC37472 (accessed on 20 November 2022).
- Nordlund, M.; Smith, M.; Maeder, S.; McGrath, T.; Schaller, J.-P.; Pratte, P.; Picavet, P.; Peitsch, M.C. Scientific Substantiation of the Absence of Combustion in the Electrically Heated Tobacco Product (EHTP) and that the Aerosol Emitted is Not Smoke. Available online: https://www.pmiscience.com/en/research/publications-library/scientific-substantiation-of-the-absence-of-combustion-in-the-electrically-heated-tobacco-product-ehtp-and-that-the-aerosol-emitted-is-not-smoke/ (accessed on 18 December 2022).
- Malt, L.; Thompson, K.; Mason, E.; Walele, T.; Nahde, T.; O’Connell, G. The product science of electrically heated tobacco products: A narrative review of the scientific literature. F1000Research 2022, 11, 121. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Yang, H.P.; Yan, R.; Chen, H.P.; Zheng, C.G.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuel 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Forster, M.; Liu, C.; Duke, M.G.; McAdam, K.G.; Proctor, C.J. An experimental method to study emissions from heated tobacco between 100–200 °C. Chem. Cent. J. 2015, 9, 20. [Google Scholar] [CrossRef]
- Barontini, F.; Tugnoli, A.; Cozzani, V.; Tetteh, J.; Jarriault, M.; Zinovik, I. Volatile products formed in the thermal decomposition of a tobacco substrate. Ind. Eng. Chem. Res. 2013, 52, 14984–14997. [Google Scholar] [CrossRef]
- Barontini, F.; Rocchi, M.; Tugnoli, A.; Cozzani, V.; Tetteh, J.; Jarriault, M.; Zinovik, I. Quantitative analysis of evolved gas in the thermal decomposition of a tobacco substrate. Chem. Eng. Trans. 2013, 32, 703–708. [Google Scholar]
- Cozzani, V.; Barontini, F.; McGrath, T.; Mahler, B.; Nordlund, M.; Smith, M.; Schaller, J.P.; Zuber, G. An experimental investigation into the operation of an electrically heated tobacco system. Thermochim. Acta 2020, 684, 178475. [Google Scholar] [CrossRef]
- Eaton, D.; Jakaj, B.; Forster, M.; Nicol, J.; Mavropoulou, E.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C.J. Assessment of tobacco heating product THP1.0. Part 2: Product design, operation and thermophysical characterisation. Regul. Toxicol. Pharmacol. 2018, 93, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Pratte, P.; Cosandey, S.; Goujon Ginglinger, C. Investigation of solid particles in the mainstream aerosol of the Tobacco Heating System THS2.2 and mainstream smoke of a 3R4F reference cigarette. Hum. Exp. Toxicol. 2017, 36, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Pérez, A.; Cano-Casanova, L.; Román-Martínez, M.D.C.; Lillo-Ródenas, M.Á. Solid matter and soluble compounds collected from cigarette smoke and heated tobacco product aerosol using a laboratory designed puffing setup. Environ. Res. 2022, 206, 112619. [Google Scholar] [CrossRef] [PubMed]
- Kärkelä, T.; Tapper, U.; Kajolinna, T. Comparison of 3R4F cigarette smoke and IQOS heated tobacco product aerosol emissions. Environ. Sci. Pollut. Res. Int. 2022, 29, 27051–27069. [Google Scholar] [CrossRef]
- Pacitto, A.; Stabile, L.; Scungio, M.; Rizza, V.; Buonanno, G. Characterization of airborne particles emitted by an electrically heated tobacco smoking system. Environ. Pollut. 2018, 240, 248–254. [Google Scholar] [CrossRef]
- Nordlund, M.; Kuczaj, A. Modeling Aerosol Formation in an Electrically Heated Tobacco Product. World Academy of Science, Engineering and Technology, Open Science Index 112. Int. J. Chem. Mol. Eng. 2016, 10, 373–385. [Google Scholar]
- Schaller, J.P.; Pijnenburg, J.P.M.; Ajithkumar, A.; Tricker, A.R. Evaluation of the Tobacco Heating System 2.2. Part 3: Influence of the tobacco blend on the formation of harmful and potentially harmful constituents of the Tobacco Heating System 2.2 aerosol. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. 2), S48–S58. [Google Scholar] [CrossRef]
- Bentley, M.C.; Almstetter, M.; Arndt, D.; Knorr, A.; Martin, E.; Pospisil, P.; Maeder, S. Comprehensive chemical characterization of the aerosol generated by a heated tobacco product by untargeted screening. Anal. Bioanal. Chem. 2020, 412, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Soulet, S.; Sussman, R.A. A Critical Review of Recent Literature on Metal Contents in E-Cigarette Aerosol. Toxics 2022, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Soulet, S.; Sussman, R.A. Critical Review of the Recent Literature on Organic Byproducts in E-Cigarette Aerosol Emissions. Toxics 2022, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Noguchi, M.; Takagi, N.; Hayashida, H.; Inaba, Y.; Ogura, H.; Kunugita, N. Simple Determination of Gaseous and Particulate Compounds Generated from Heated Tobacco Products. Chem. Res. Toxicol. 2018, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- ISO 3308:2012; Routine Analytical Cigarette-Smoking Machine—Definitions and Standard Conditions. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 20778:2018; Cigarettes Routine Analytical Cigarette Smoking Machine—Definitions and Standard Conditions with an Intense Smoking Regime. International Organization for Standardization: Geneva, Switzerland, 2018.
- Helen, G.S.; Jacob, P., III; Nardone, N.; Benowitz, N.L. IQOS: Examination of Philip Morris international’s claim of reduced exposure. Tobaco Control 2018, 27, s30–s36. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern. Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Varlet, V.; Concha-Lozano, N.; Berthet, A.; Plateel, G.; Favrat, B.; De Cesare, M.; Lauer, E.; Augsburger, M.; Thomas, A.; Giroud, C. Drug vaping applied to cannabis: Is “Cannavaping” a therapeutic alternative to marijuana? Sci. Rep. 2016, 6, 25599. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Yannovits, N.; Sarri, T.; Voudris, V.; Poulas, K.; Leischow, S.J. Carbonyl emissions from a novel heated tobacco product (IQOS): Comparison with an e-cigarette and a tobacco cigarette. Addiction 2018, 113, 2099–2106. [Google Scholar] [CrossRef]
- Cooperation Centre for Scientific Research Relative to Tobacco Smoke Analytes Sub-Group CORESTA. Recommended Method No. 74 Determination of Selected Carbonyls in Mainstream Cigarette Smoke by HPLC; CORESTA: Paris, France, 2019. [Google Scholar]
- Mallock, N.; Böss, L.; Burk, R.; Danziger, M.; Welsch, T.; Hahn, H.; Trieu, H.; Hahn, J.; Pieper, E.; Henkler-Stephani, F.; et al. Levels of selected analytes in the emissions of “heat not burn” tobacco products that are relevant to assess human health risks. Arch. Toxicol. 2018, 92, 2145–2149. [Google Scholar] [CrossRef]
- Salman, R.; Talih, S.; El-Hage, R.; Haddad, C.; Karaoghlanian, N.; El-Hellani, A.; Saliba, N.A.; Shihadeh, A. Free-Base and Total Nicotine, Reactive Oxygen Species, and Carbonyl Emissions From IQOS, a Heated Tobacco Product. Nicotine Tob. Res. 2019, 21, 1285. [Google Scholar] [CrossRef]
- Heide, J.; Adam, T.W.; Jacobs, E.; Wolter, J.; Ehlert, S.; Walte, A.; Zimmermann, R. Puff-Resolved Analysis and Selected Quantification of Chemicals in the Gas Phase of E-Cigarettes, Heat-Not-Burn Devices, and Conventional Cigarettes Using Single-Photon Ionization Time-of-Flight Mass Spectrometry (SPI-TOFMS): A Comparative Study. Nicotine Tob. Res. 2021, 23, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Chen, L.; Liu, D.; Yu, T.; Bai, R.; Yan, L.; Zhou, J. Harmful chemicals of heat not burn product and its induced oxidative stress of macrophages at air-liquid interface: Comparison with ultra-light cigarette. Toxicol. Lett. 2020, 331, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Dusautoir, R.; Zarcone, G.; Verriele, M.; Garçon, G.; Fronval, I.; Beauval, N.; Allorge, D.; Riffault, V.; Locoge, N.; Lo-Guidice, J.; et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J. Hazard. Mater. 2021, 401, 123417. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. 2), S27–S47. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, G.; Tafin Djoko, D.; Moennikes, O.; Jeannet, C.; Kondylis, A.; Belushkin, M. Comparative assessment of HPHC yields in the Tobacco Heating System THS2.2 and commercial cigarettes. Regul. Toxicol. Pharmacol. 2017, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poynton, S.; Sutton, J.; Goodall, S.; Margham, J.; Forster, M.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017, 106, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Buratto, R.; Correia, D.; Parel, M.; Crenna, M.; Bilger, M.; Debrick, A. Determination of eight carbonyl compounds in aerosols trapped in phosphate buffer saline solutions to support in vitro assessment studies. Talanta 2018, 184, 42–49. [Google Scholar] [CrossRef]
- Miller, J.H., IV; Gardner, W.P.; Gonzalez, R.R. UHPLC separation with MS analysis for eight carbonyl compounds in mainstream tobacco smoke. J. Chromatogr. Sci. 2010, 48, 12–17. [Google Scholar] [CrossRef]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef]
- Crooks, I.; Neilson, L.; Scott, K.; Reynolds, L.; Oke, T.; Forster, M.; Meredith, C.; McAdam, K.; Proctor, C. Evaluation of flavourings potentially used in a heated tobacco product: Chemical analysis, in vitro mutagenicity, genotoxicity, cytotoxicity and in vitro tumour promoting activity. Food Chem. Toxicol. 2018, 118, 940–952. [Google Scholar] [CrossRef]
- Forster, M.; Fiebelkorn, S.; Yurteri, C.; Mariner, D.; Liu, C.; Wright, C.; McAdam, K.; Murphy, J.; Proctor, C. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018, 93, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Inaba, Y.; Kunugita, N. Derivatization of carbonyl compounds with 2,4-dinitrophenylhydrazine and their subsequent determination by high-performance liquid chromatography. J. Chromatogr. B 2011, 879, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, S.M.; Hendriksen, L.; Karst, U. Determination of aldehydes and ketones using derivatization with 2,4-dinitrophenylhydrazine and liquid chromatography-atmospheric pressure photoionization-mass spectrometry. J. Chromatogr. A 2004, 1058, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.H.; Ho, K.F.; Liu, W.; Lee, S.; Dai, W.; Cao, J.; Ip, S.H.S. Unsuitability of using the DNPH-coated solid sorbent cartridge for determination of airborne unsaturated carbonyls. Atmos. Environ. 2011, 45, 261–265. [Google Scholar] [CrossRef]
- Clench, M.R.; Tetler, L.W. Detectors: Mass Spectrometry. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 448–455. ISBN 9780122267703. [Google Scholar] [CrossRef]
- ISO 21160:2018; Cigarettes Determination of Selected Carbonyls in the Mainstream Smoke of Cigarettes—Method Using High Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2018.
- Cooperation Centre for Scientific Research Relative to Tobacco Smoke Analytes Sub-Group CORESTA. Recommended Method No. 86 Determination of Select Carbonyls in Tobacco and Tobacco Products by UHPLC-MS/MS; CORESTA: Paris, France, 2021. [Google Scholar]
- Pauwels, C.G.G.M.; Klerx, W.N.M.; Pennings, J.L.A.; Boots, A.W.; van Schooten, F.J.; Opperhuizen, A.; Talhout, R. Cigarette Filter Ventilation and Smoking Protocol Influence Aldehyde Smoke Yields. Chem. Res. Toxicol. 2018, 31, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, C.G.G.M.; Boots, A.W.; Visser, W.F.; Pennings, J.L.A.; Talhout, R.; Schooten, F.V.; Opperhuizen, A. Characteristic Human Individual Puffing Profiles Can Generate More TNCO than ISO and Health Canada Regimes on Smoking Machine When the Same Brand Is Smoked. Int. J. Environ. Res. Public Health 2020, 17, 3225. [Google Scholar] [CrossRef] [PubMed]
- Heated Tobacco Products: Information Sheet, 2nd ed; WHO: Geneva, Switzerland. 2020. Available online: https://www.who.int/publications/i/item/WHO-HEP-HPR-2020.2 (accessed on 7 July 2023).
- WHO. Statement on Heated Tobacco Products and the US FDA Decision Regarding IQOS. Available online: https://www.who.int/news/item/27-07-2020-who-statement-on-heated-tobacco-products-and-the-us-fda-decision-regarding-iqos (accessed on 7 July 2023).
- US Food & Drug Administration. Scientific Review of Modified Risk Tobacco Product Application (MRTPA) Under Section 911(d) of the FD&C Act-Technical Project Lead. Available online: https://www.fda.gov/media/139796/download (accessed on 7 July 2023).
- Chen, X.; Bailey, P.C.; Yang, C.; Hiraki, B.; Oldham, M.J.; Gillman, I.G. Targeted Characterization of the Chemical Composition of JUUL Systems Aerosol and Comparison with 3R4F Reference Cigarettes and IQOS Heat Sticks. Separations 2021, 8, 168. [Google Scholar] [CrossRef]
- Hammond, D.; Fong, G.T.; Cummings, K.M.; O’Connor, R.J.; Giovino, G.A.; McNeill, A. Cigarette yields and human exposure: A comparison of alternative testing regimens. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1495–1501. [Google Scholar] [CrossRef]
- Fagerström, K.O.; Bates, S. Compensation and effective smoking by different nicotine dependent smokers. Addict. Behav. 1981, 6, 331–336. [Google Scholar] [CrossRef]
- Djordjevic, M.V.; Hoffmann, D.; Hoffmann, I. Nicotine regulates smoking patterns. Prev. Med. 1997, 26, 435–440. [Google Scholar] [CrossRef]
- Djordjevic, M.V.; Stellman, S.D.; Zang, E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J. Natl. Cancer Inst. 2000, 92, 106–111. [Google Scholar] [CrossRef]
- Jarvis, M.J.; Boreham, R.; Primatesta, P.; Feyerabend, C.; Bryant, A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: Evidence from a representative population survey. J. Natl. Cancer. Inst. 2001, 93, 134–138. [Google Scholar] [CrossRef]
- NIOSH Pocket Guide to Chemical Hazards. Available online: https://www.cdc.gov/niosh/npg/default.html (accessed on 7 July 2023).
- 2-Furanmethanol: Human Health Tier II Assessment. Available online: https://www.industrialchemicals.gov.au/sites/default/files/2-Furanmethanol_Human%20health%20tier%20II%20assessment.pdf (accessed on 13 July 2023).
- Criteria for a Recommended Standard: Occupational Exposure to Diacetyl and 2,3-Pentanedione. Available online: https://www.cdc.gov/niosh/docs/2016-111/default.html (accessed on 13 July 2023).
- Occupational Safety and Health Administration (OSHA). Available online: https://www.osha.gov/chemicaldata/64 (accessed on 15 July 2023).
- The National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/idlh/110861.html (accessed on 13 July 2023).
- WHO Study Group on Tobacco Product Regulation. Report on the Scientific Basis of Tobacco Product Regulation: Eighth Report of a WHO Study Group. Available online: https://www.who.int/publications/i/item/9789240022720 (accessed on 13 July 2023).
- Uguna, C.N.; Snape, C.E. Should IQOS Emissions Be Considered as Smoke and Harmful to Health? A Review of the Chemical Evidence. ACS Omega 2022, 7, 22111–22124. [Google Scholar] [CrossRef]
| Compound | µg/Stick | µg/Cig (1R5F) | µg /Cig (3R4F) | Toxicological Marker mg/m3 | Exposure Dose | Safety Threshold |
|---|---|---|---|---|---|---|
| IQOS | ||||||
| Acetol | 65, 81, 84 | 4.6 ± 0.78 | 22 ± 1.4 | NA | 0.43–0.56 mg/day | NA |
| Butanal | 15, 15, 15 | 8.7 ± 0.82 | 26 ± 1.1 | NA | 0.1 mg/day | NA |
| Diacetyl | 42, 49, 41 | 34 ± 0.9 | 120 ± 8.9 | 0.017 TWA lifetime 8 h (NIOSH) [89] | 0.27–0.32 mg/8 h | 0.11 mg/8 h |
| Glyoxal | 3.6, 4.5, 4.8 | 2.2 ± 0.44 | 8.9±0.55 | CAL/OSHA PEL 0.1 [90] | 0.024–0.032 mg/8 h | 0.66 mg/8 h |
| 1-valeraldehyde | 8.8, 8.4., 8.3 | 4.6 ± 0.96 | 22 ± 1.6 | 175 NIOSH REL [87] | 0.055–0.058 mg/8 h | 1166.67 mg/8 h |
| glo | ||||||
| Acetol | 40, 46, 49 | 4.6 ± 0.78 | 22 ± 1.4 | NA | 0.26–0.32 mg/8 h | NA |
| Furfural | 68, 79, 150 | 1.2 ± 0.35 | 14 ± 2.0 | ACGIH TLV © 8 h TWA 0.8 [87] | 0.45–1.0 mg/8 h | 5.33 mg/8 h |
| Methylglyoxal | 1.4, 4.3, 4.3 | 3.0 ± 0.55 | 14 ± 1.1 | NA | 0.009–0.028 mg/8 h | NA |
| Pyridine | 16, 14, 1.2 | 1.1 ± 0.4 | 9.5 ± 1.1 | 15 ACGIH TWA 8 h [91] | 0.008–0.106 mg/8 h | 100 mg/8 h |
| Authors | Puffing Conditions | Analytical Methods | Derivatization Methods | Other Information | Score and Comments |
|---|---|---|---|---|---|
| Auer et al. [53] | √ | x | x | x Several flaws | 1.0 ⬤ |
| Farsalinos et al. [55] | √ | √ | √ | √ | 4.0 ⬤ |
| Mallock et al. [57] | √ | √ | √ | √ | 4.0 ⬤ |
| Uchiyama et al. [49] | √ | √ | ½ | √ | 3.5 ⬤ |
| Salman et al. [58] | ½ | √ | √ | x Flawed conclusions on IQOS pollution | 2.5 ⬤ |
| Heide et al. [59] | √ | √ | ½ No derivatization | x Poor validation | 2.5 ⬤ |
| Wang et al. [60] | √ | √ | √ | x Number of puffs per cigarette not specified | 3.0 ⬤ |
| Dusautoir et al. [61] | ½ | √ | √ | x HCI regime for sub-ohm devices | 2.5 ⬤ |
| Schaller et al. [62] | √ | √ | √ | √ | 4.0 ⬤ |
| Schaller et al. [45] | √ | √ | √ | √ | 4.0 ⬤ |
| Jaccard et al. [63] | √ | √ | √ | x No description of conventional cigarettes analyzed | 3.0 ⬤ |
| Poynton et al. [64] | √ | √ | √ | √ | 4.0 ⬤ |
| Buratto et al. [65] | √ | √ | √ | x Number of puffs per cigarette not specified | 3.0 ⬤ |
| Crooks et al. [68] | √ | √ | √ | ½ Internal methods not available | 3.5 ⬤ |
| Eaton et al. [36] | √ | √ | √ | ½ Internal methods not available | 3.5 ⬤ |
| Forster et al. [69] | √ | √ | √ | ½ Internal methods not available | 3.5 ⬤ |
| Bentley et al. [46] | √ | √ | ½ No derivatization | √ | 3.5 ⬤ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sussman, R.A.; Sipala, F.; Emma, R.; Ronsisvalle, S. Aerosol Emissions from Heated Tobacco Products: A Review Focusing on Carbonyls, Analytical Methods, and Experimental Quality. Toxics 2023, 11, 947. https://doi.org/10.3390/toxics11120947
Sussman RA, Sipala F, Emma R, Ronsisvalle S. Aerosol Emissions from Heated Tobacco Products: A Review Focusing on Carbonyls, Analytical Methods, and Experimental Quality. Toxics. 2023; 11(12):947. https://doi.org/10.3390/toxics11120947
Chicago/Turabian StyleSussman, Roberto A., Federica Sipala, Rosalia Emma, and Simone Ronsisvalle. 2023. "Aerosol Emissions from Heated Tobacco Products: A Review Focusing on Carbonyls, Analytical Methods, and Experimental Quality" Toxics 11, no. 12: 947. https://doi.org/10.3390/toxics11120947
APA StyleSussman, R. A., Sipala, F., Emma, R., & Ronsisvalle, S. (2023). Aerosol Emissions from Heated Tobacco Products: A Review Focusing on Carbonyls, Analytical Methods, and Experimental Quality. Toxics, 11(12), 947. https://doi.org/10.3390/toxics11120947







