Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Solutions
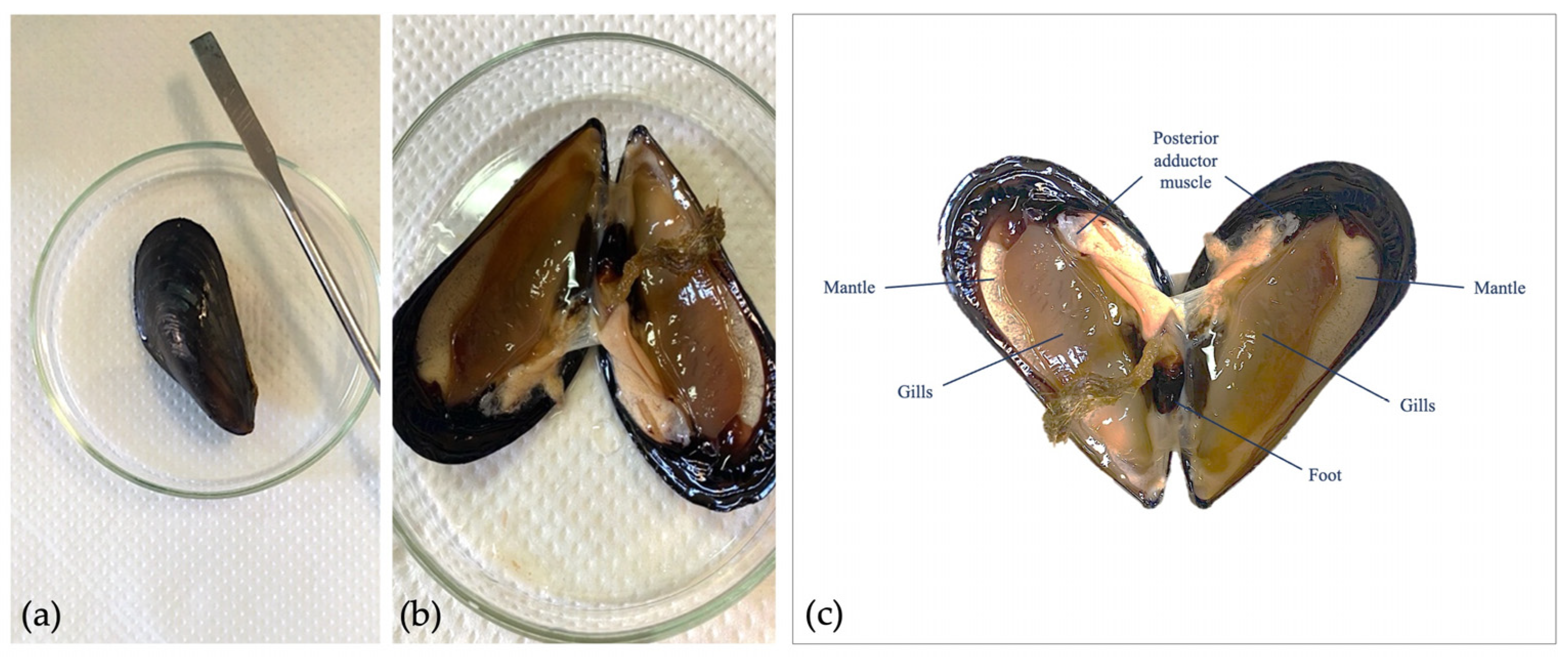
2.2. M. galloprovincialis Gametes Collection and Exposure
2.3. Sperm Motility
2.4. Integrity of Plasmatic Membrane (Eosin Y)
2.5. DNA Fragmentation (SCD Test)
2.6. Oxidative Stress
2.7. Fertilization Test
2.8. Statistical Analysis
3. Results
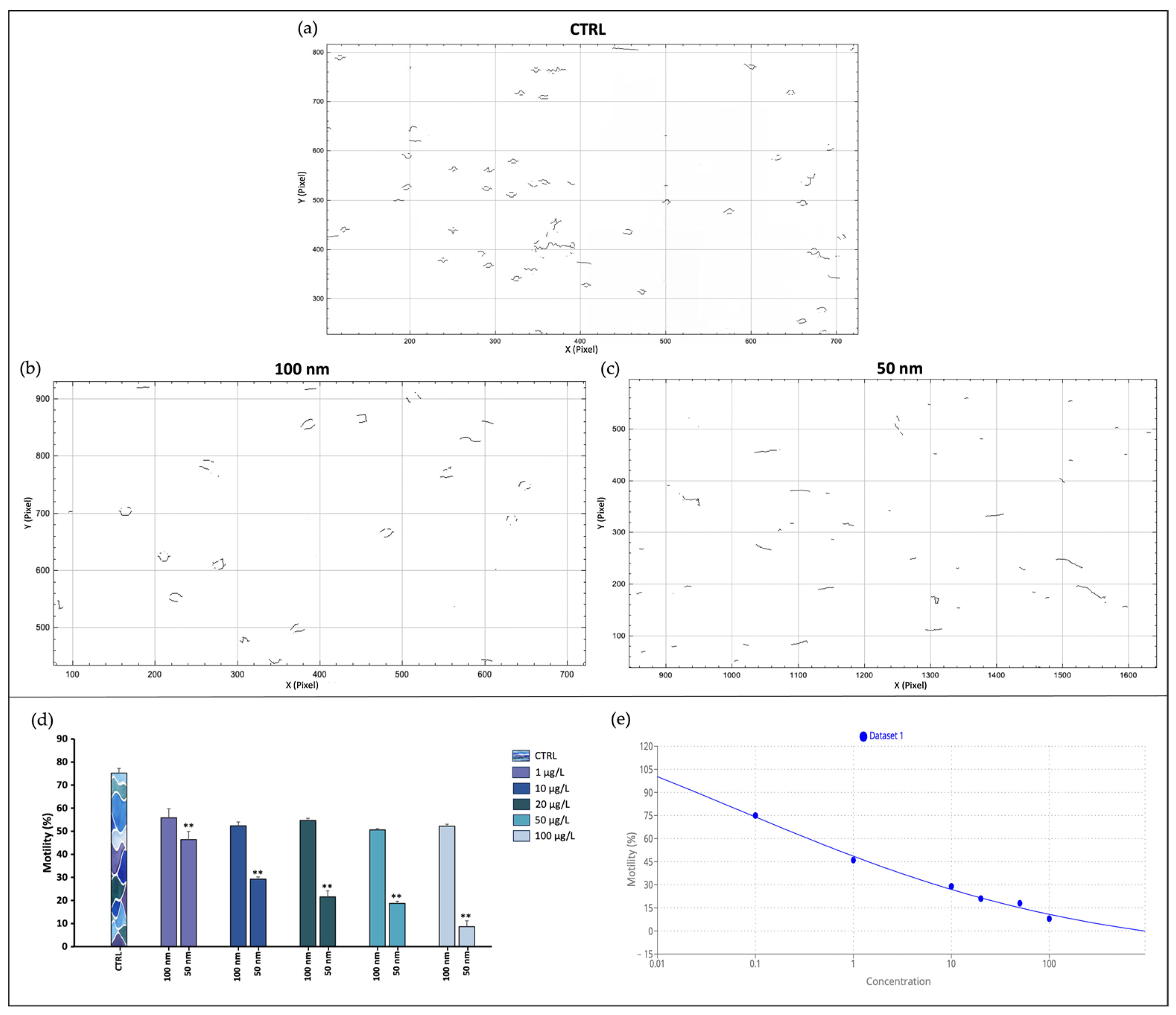
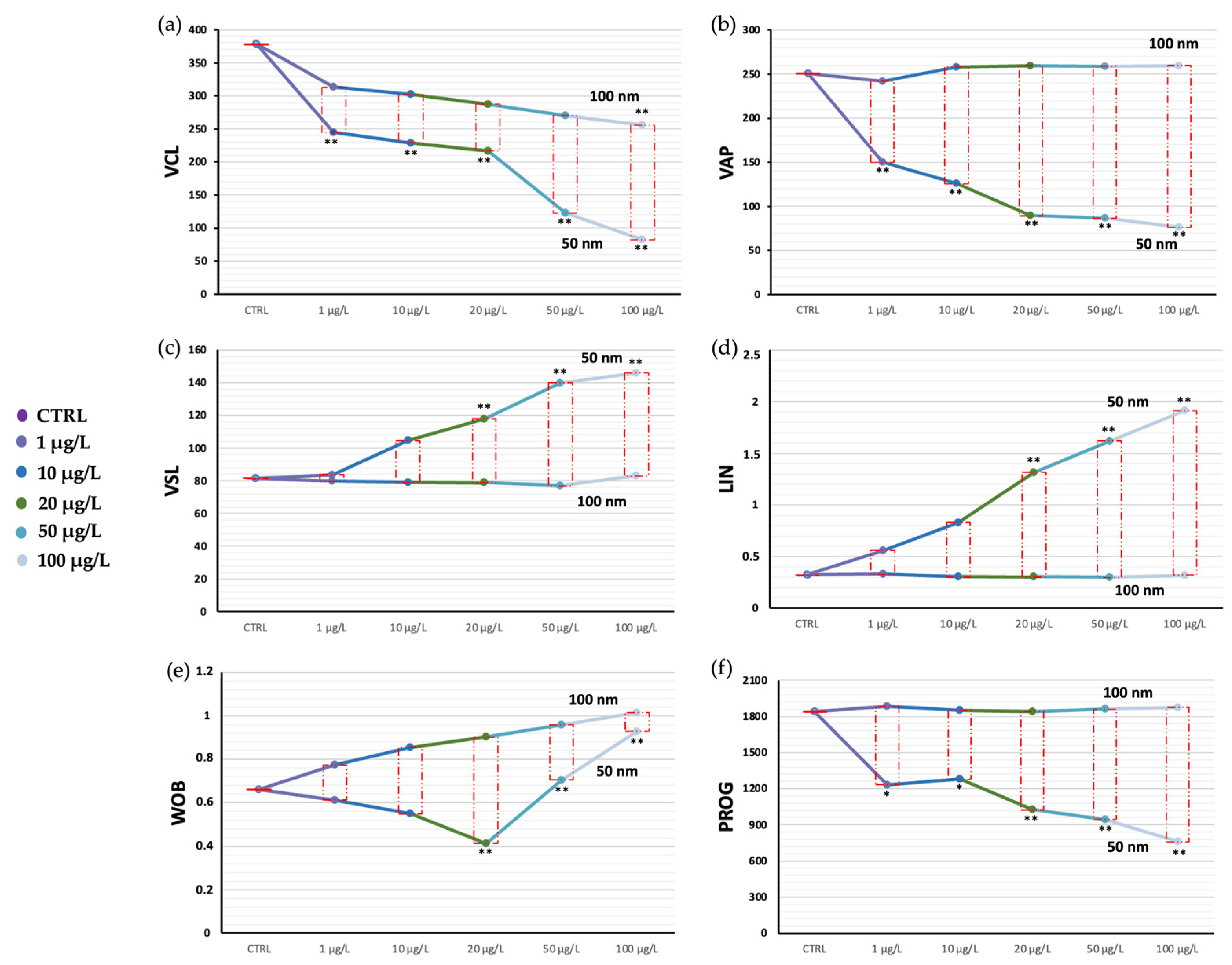
3.1. Sperm Motility
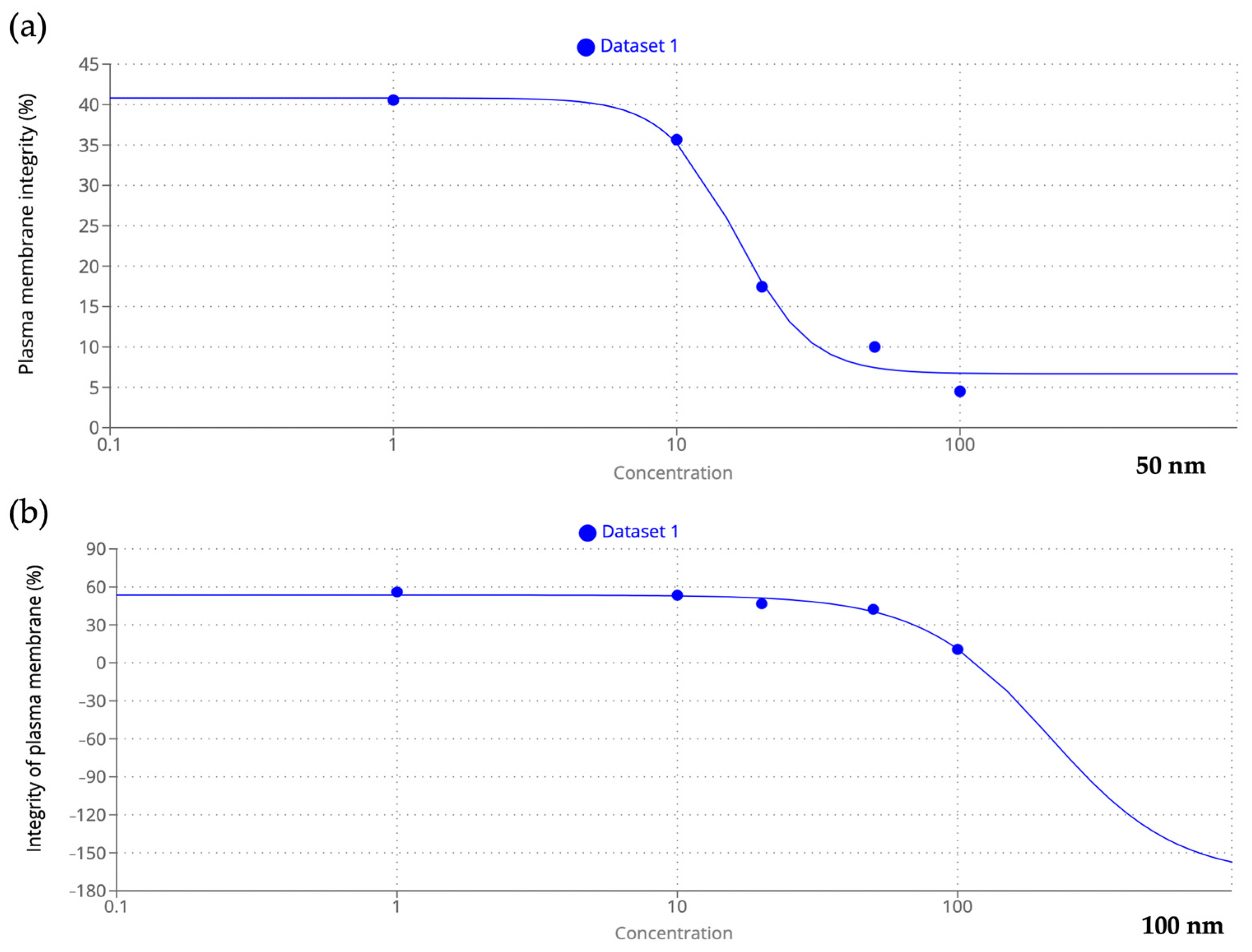
3.2. Integrity of Plasma Membrane
3.3. DNA Fragmentation
3.4. Oxidative Stress
3.5. Fertilization Toxicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eads, A.R.; Evans, J.P.; Kennington, W.J. Plasticity of fertilization rates under varying temperature in the broadcast spawning mussel, Mytilus galloprovincialis. Ecol. Evol. 2016, 6, 6578–6585. [Google Scholar] [CrossRef] [PubMed]
- Hurd Catriona, L.; Beardall, J.; Comeau, S.; Cornwall, C.E.; Havenhand, J.N.; Munday, P.L.; Parker, L.M.; Raven, J.A.; McGraw, C.M. Ocean acidification as a multiple driver: How interactions between changing seawater carbonate parameters affect marine life. Mar. Freshw. Res. 2020, 71, 263–274. [Google Scholar] [CrossRef]
- Padilla-Gamiño, J.L.; Alma, L.; Spencer, L.H.; Venkataraman, Y.R.; Wessler, L. Ocean acidification does not overlook sex: Review of understudied effects and implications of low pH on marine invertebrate sexual reproduction. Front. Mar. Sci. 2022, 9, 977754. [Google Scholar] [CrossRef]
- Eads, A.R.; Kennington, W.J.; Evans, J.P. Interactive effects of ocean warming and acidification on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2016, 562, 101–111. [Google Scholar] [CrossRef]
- James, C.A.; Lanksbury, J.; Khangaonkar, T.; West, J. Evaluating exposures of bay mussels (Mytilus trossulus) to contaminants of emerging concern through environmental sampling and hydrodynamic modeling. Sci. Total Environ. 2020, 709, 136098. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Env. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Ford, H.V.; Jones, N.H.; Davies, A.J.; Godley, B.J.; Jambeck, J.R.; Napper, I.E.; Koldewey, H.J. The fundamental links between climate change and marine plastic pollution. Sci. Total Environ. 2022, 806, 150392. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, 04709. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mojumdar, S.; Rahman, S.A.; Marimuthu, K. Plastic pollutions in the ocean: Their sources, causes, effects and control measures. J. Biol. Stud. 2023, 6, 37–52. [Google Scholar]
- Chandra, P.; Singh, D.P. Microplastic degradation by bacteria in aquatic ecosystem. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–467. [Google Scholar]
- Gangadoo, S.; Owen, S.; Rajapaksha, P.; Plaisted, K.; Cheeseman, S.; Haddara, H.; Chapman, J. Nano-plastics and their analytical characterisation and fate in the marine environment: From source to sea. Sci. Total Environ. 2020, 732, 138792. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini—Review. Clean. Eng. Technol. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Debroy, A.; George, N.; Mukherjee, G. Role of biofilms in the degradation of microplastics in aquatic environments. J. Chem. Technol. Biotechnol. 2022, 97, 3271–3282. [Google Scholar] [CrossRef]
- Syberg, K.; Knudsen, C.M.; Tairova, Z.; Khan, F.R.; Shashoua, Y.; Geertz, T.; Palmqvist, A. Sorption of PCBs to environmental plastic pollution in the North Atlantic Ocean: Importance of size and polymer type. Case Stud. Chem. Environ. Eng. 2020, 2, 100062. [Google Scholar] [CrossRef]
- Mai, L.; He, H.; Bao, L.J.; Liu, L.Y.; Zeng, E.Y. Plastics are an insignificant carrier of riverine organic pollutants to the coastal oceans. Environ. Sci. Technol. 2020, 54, 15852–15860. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Montesdeoca-Esponda, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Organic pollutants adsorbed on microplastics: Analytical methodologies and occurrence in oceans. Trends Environ. Anal. Chem. 2021, 29, e00114. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Hu, L.; Zhao, Y.; Xu, H. Trojan horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J. Hazard. Mater. 2022, 439, 129652. [Google Scholar] [CrossRef]
- Li, W.; Zu, B.; Yang, Q.; Guo, J.; Li, J. Sources, distribution, and environmental effects of microplastics: A systematic review. RSC Adv. 2023, 13, 15566–15574. [Google Scholar] [CrossRef]
- Pittura, L.; Gorbi, S.; León, V.M.; Bellas, J.; González, J.A.C.; Albentosa, M.; Regoli, F. Microplastics and nanoplastics in the marine environment. Contam. Emerg. Concern Mar. Environ. 2023, 311–348. [Google Scholar]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Tallec, K.; Blard, O.; González-Fernández, C.; Brotons, G.; Berchel, M.; Soudant, P.; Paul-Pont, I. Surface functionalization determines behavior of nanoplastic solutions in model aquatic environments. Chemosphere 2019, 225, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.A.; Rane, A.V.; Kanny, K. Applications of compatibilized polymer blends in automobile industry. In Compatibilization of Polymer Blends: Micro and Nano Scale Phase Morphologies, Interphase Characterization, and Properties; Elsevier: Amsterdam, The Netherlands, 2019; pp. 563–593. [Google Scholar]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Wathsala, R.H.G.R.; Franzellitti, S.; Scaglione, M.; Fabbri, E. Styrene impairs normal embryo development in the Mediterranean mussel (Mytilus galloprovincialis). Aquat. Toxicol. 2018, 201, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Carrasco-Braganza, M.I.; Yeste, P.M.; Vila, M.; Blasco, J. Immunotoxicity of polystyrene nanoplastics in different hemocyte subpopulations of Mytilus galloprovincialis. Sci. Rep. 2020, 10, 8637. [Google Scholar] [CrossRef]
- Jones-Williams, K. The Distribution and Fate of Microplastic Pollution in Polar Environments: From the Canadian Arctic to the South Pole. Ph.D. Thesis, University of Exeter, Exeter, United Kingdom, 2022. [Google Scholar]
- Ryan, P.G.; Lamprecht, A.; Swanepoel, D.; Moloney, C.L. The effect of fine-scale sampling frequency on estimates of beach litter accumulation. Mar. Pollut. Bull 2014, 88, 249–325. [Google Scholar] [CrossRef]
- Kwon, B.G.; Koizumi, K.; Chung, S.Y.; Kodera, Y.; Kim, J.O.; Saido, K. Global styrene oligomers monitoring as new chemical contamination from polystyrene plastic marine pollution. J. Hazard. Mat. 2015, 300, 359–367. [Google Scholar] [CrossRef]
- Kwon, B.G.; Amamiya, K.; Sato, H.; Chung, S.Y.; Kodera, Y.; Kim, S.K.; Saido, K. Monitoring of styrene oligomers as indicators of polystyrene plastic pollution in the North-West Pacific Ocean. Chemosphere 2017, 180, 500–505. [Google Scholar] [CrossRef]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of nanoplastics on hemolymph immune parameters and microbiota composition in Mytilus galloprovincialis. Mar. Environ. Res. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Gosálvez, J.; López-Fernández, C.; Hermoso, A.; Fernández, J.L.; Kjelland, M.E. Sperm DNA fragmentation in zebrafish (Danio rerio) and its impact on fertility and embryo viability—Implications for fisheries and aquaculture. Aquaculture 2014, 433, 173–182. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Zhang, Q.; Xue, R.; Qu, Y.; Wang, Q.; Dong, Z.; Zhao, J. Different patterns of hypoxia aggravate the toxicity of polystyrene nanoplastics in the mussels Mytilus galloprovincialis: Environmental risk assessment of plastics under global climate change. Sci. Total Environ. 2022, 818, 151818. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.; Pereira, K.C.; Sparaventi, E.; González-Ortegón, E.; Blasco, J. Contamination may induce behavioural plasticity in the habitat selection by shrimps: A cost-benefits balance involving contamination, shelter and predation. Environ. Pollut. 2020, 263, 114545. [Google Scholar] [CrossRef]
- Fabbri, R.; Montagna, M.; Balbi, T.; Raffo, E.; Palumbo, F.; Canesi, L. Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in Mytilus galloprovincialis. Mar. Environ. Res. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Magara, G.; Elia, A.C.; Syberg, K.; Khan, F.R. Single contaminant and combined exposures of polyethylene microplastics and fluoranthene: Accumulation and oxidative stress response in the blue mussel, Mytilus edulis. J. Toxicol. Environ. Health Part A 2018, 81, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef]
- Tallec, K.; Paul-Pont, I.; Boulais, M.; Le Goïc, N.; González-Fernández, C.; Le Grand, F.; Huvet, A. Nanopolystyrene beads affect motility and reproductive success of oyster spermatozoa (Crassostrea gigas). Nanotoxicology 2020, 14, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, C.; Tallec, K.; Le Goïc, N.; Lambert, C.; Soudant, P.; Huvet, A.; Suquet, M.; Berchel, M.; Paul-Pont, I. Cellular responses of Pacific oyster (Crassostrea gigas) gametes exposed in vitro to polystyrene nanoparticles. Chemosphere 2018, 208, 764–772. [Google Scholar] [CrossRef]
- Tallec, K.; Huvet, A.; Di Poi, C.; González-Fernández, C.; Lambert, C.; Petton, B.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018, 242, 1226–1235. [Google Scholar] [CrossRef]
- Romdhani, I.; Gallo, A.; Venditti, M.; Abelouah, M.R.; Varchetta, R.; Najahi, H.; Banni, M. Unveiling the impact of environmental microplastics on mussel spermatozoa: First evidence of prothymosin-α detection in invertebrate’s male gametes. J. Hazard. Mater. 2023, 461, 132521. [Google Scholar] [CrossRef]
- Shi, W.; Sun, S.; Han, Y.; Tang, Y.; Zhou, W.; Zhang, W.; Liu, G. Microplastics hamper the fertilization success of a broadcast spawning bivalve through reducing gamete collision and gamete fusion efficiency. Aquatic. Toxicol. 2022, 242, 106049. [Google Scholar] [CrossRef]
- UNESCO. Tenth Report of the Joint Panel on Oceanographic Tables and Standards—UNESCO Technical Papers in Marine Science; UNESCO: Paris, France, 1981; p. 25. [Google Scholar]
- Bergami, E.; Bocci, E.; Vannuccini, M.L.; Monopoli, M.; Salvati, A.; Dawson, K.A.; Corsi, I. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol. Environ. Saf. 2016, 123, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Manfra, L.; Rotini, A.; Bergami, E.; Grassi, G.; Faleri, C.; Corsi, I. Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis. Ecotoxicol. Environ. Saf. 2017, 145, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Leedy, J.G.; Ingermann, R.L. Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology 2007, 67, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Galloway, T. Reproductive consequences of paternal genotoxin exposure in marine invertebrates. Environ. Sci. Technol. 2009, 43, 928–933. [Google Scholar] [CrossRef]
- Vignier, J.; Volety, A.K.; Rolton, A.; Le Goïc, N.; Chu, F.L.; Robert, R.; Soudant, P. Sensitivity of eastern oyster (Crassostrea virginica) spermatozoa and oocytes to dispersed oil: Cellular responses and impacts on fertilization and embryogenesis. Environ. Pollut. 2017, 225, 270–282. [Google Scholar] [CrossRef][Green Version]
- Al-Thawadi, S. Microplastics and nanoplastics in aquatic environments: Challenges and threats to aquatic organisms. Arab. J. Sci. Eng. 2020, 45, 4419–4440. [Google Scholar] [CrossRef]
- Lewis, C.; Ford, A.T. Infertility in male aquatic invertebrates: A review. Aquat. Toxicol. 2012, 120, 79–89. [Google Scholar] [CrossRef]
- Vihtakari, M.; Hendriks, I.E.; Holding, J.; Renaud, P.E.; Duarte, C.M.; Havenhand, J.N. Effects of ocean acidification and warming on sperm activity and early life stages of the Mediterranean mussel (Mytilus galloprovincialis). Water 2013, 5, 1890–1915. [Google Scholar] [CrossRef]
- Boulais, M.; Demoy-Schneider, M.; Alavi, S.M.H.; Cosson, J. Spermatozoa motility in bivalves: Signaling, flagellar beating behavior, and energetics. Theriogenology 2019, 136, 15–27. [Google Scholar] [CrossRef]
- Liu, G.; Innes, D.; Thompson, R.J. Quantitative analysis of sperm plane circular movement in the blue mussels Mytilus edulis, M. trossulus and their hybrids. J. Exp. Zool. A Ecol Genet. Physiol. 2011, 315, 280–290. [Google Scholar] [CrossRef]
- Nakamura, M.; Morita, M. Sperm motility of the scleractinian coral Acropora digitifera under preindustrial, current, and predicted ocean acidification regimes. Aquat. Biol. 2012, 15, 299–302. [Google Scholar] [CrossRef]
- van der Horst, G.; Bennett, M.; Bishop, J.D. CASA in invertebrates. Reprod. Fertil. Develop. 2018, 30, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Jon, N.; Havenhand, N.O.; Williamson, J.E. Sperm swimming in the polychaete Galeolaria caespitosa shows substantial inter-individual variability in response to future ocean acidification. Mar. Pollut. Bull. 2014, 78, 213–217. [Google Scholar] [CrossRef]
- Oliver, M.; Evans, J.P. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140148. [Google Scholar] [CrossRef] [PubMed]
- Kekäläinen, J.; Larma, I.; Linden, M. Lectin staining and flow cytometry reveals female-induced sperm acrosome reaction and surface carbohydrate reorganization. Sci. Rep. 2015, 5, 15321. [Google Scholar] [CrossRef]
- Lymbery, R.A.; Kennington, W.J.; Evans, J.P. Egg chemoattractants moderate intraspecific sperm competition. Evol. Let. 2017, 1, 317–327. [Google Scholar] [CrossRef]
- Evans, J.P.; Sherman, C.D. Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 2013, 224, 166–183. [Google Scholar] [CrossRef]
- Ikenaga, J.; Yoshida, M. Chapter 3 Sperm Activation and Chemotaxis in Invertebrates. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J., Eds.; Springer: Singapore, 2020; pp. 31–46. [Google Scholar]
- Cuccaro, A.; De Marchi, L.; Oliva, M.; Battaglia, F.; Meucci, V.; Fumagalli, G.; Pretti, C. Ecotoxicological effects of the UV-filter 4-MBC on sperms and adults of the mussel Mytilus galloprovincialis. Environ. Res. 2022, 213, 113739. [Google Scholar] [CrossRef]
- Bondarenko, V.; Cosson, J. Structure and beating behavior of the sperm motility apparatus in aquatic animals. Theriogenology 2019, 135, 152–163. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef]
- Della Torre, C.; Bergami, E.; Salvati, A.; Faleri, C.; Cirino, P.; Dawson, K.A.; Corsi, I. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 2014, 48, 12302–12311. [Google Scholar] [CrossRef] [PubMed]
- Eliso, M.C.; Bergami, E.; Manfra, L.; Spagnuolo, A.; Corsi, I. Toxicity of nanoplastics during the embryogenesis of the ascidian Ciona robusta (Phylum Chordata). Nanotoxicology 2020, 14, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.M.H.; Matsumura, N.; Shiba, K.; Itoh, N.; Takahashi, K.G.; Inaba, K.; Osada, M. Roles of extracellular ions and pH in 5-HT-induced sperm motility in marine bivalve. Reproduction 2014, 147, 331–345. [Google Scholar] [CrossRef]
- Lettieri, G.; Maione, M.; Ranauda, M.A.; Mele, E.; Piscopo, M. Molecular effects on spermatozoa of Mytilus galloprovincialis exposed to hyposaline conditions. Mol. Reprod. Develop. 2019, 86, 650–660. [Google Scholar] [CrossRef]
- Nichols, Z.G.; Rikard, S.; Alavi, S.M.H.; Walton, W.C.; Butts, I.A. Regulation of sperm motility in Eastern oyster (Crassostrea virginica) spawning naturally in seawater with low salinity. PLoS ONE 2021, 16, e0243569. [Google Scholar] [CrossRef]
- Nel, A.; Mädler, L.; Velegol, D. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Mazur, A.A.; Chelomin, V.P.; Zhuravel, E.V.; Kukla, S.P.; Slobodskova, V.V.; Dovzhenko, N.V. Genotoxicity of polystyrene (PS) microspheres in short-term exposure to gametes of the sand dollar Scaphechinus mirabilis (Agassiz, 1864) (Echinodermata, Echinoidea). J. Mar. Sci. Engin. 2021, 9, 1088. [Google Scholar] [CrossRef]
- Merino, O.; Risopatrón, J.; Valdebenito, I.; Figueroa, E.; Farías, J.G. Effect of the temperature of activation medium on fish sperm quality: Impact on fertilization in vitro in aquaculture practice. Rev. Aquac. 2023, 15, 434–451. [Google Scholar] [CrossRef]
- Contino, M.; Ferruggia, G.; Indelicato, S.; Pecoraro, R.; Scalisi, E.M.; Bracchitta, G.; Brundo, M.V. In Vitro Nano-Polystyrene Toxicity: Metabolic Dysfunctions and Cytoprotective Responses of Human Spermatozoa. Biology 2023, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Barranger, A.; Akcha, F.; Rouxel, J.; Brizard, R.; Maurouard, E.; Pallud, M.; Benabdelmouna, A. Study of genetic damage in the Japanese oyster induced by an environmentally relevant exposure to diuron: Evidence of vertical transmission of DNA damage. Aquat. Toxicol. 2014, 146, 93–104. [Google Scholar] [CrossRef] [PubMed]





| Model Organism | Diameter of Nanoplastics | Concentration | Tested Parameters | Results | Author |
|---|---|---|---|---|---|
| Cassostrea gigas | 50 nm nPS-NH2 50 nm nPS-COOH | From 0.1 µg/mL to 0.25 µg/mL | Motility Velocity Fertilization | Higher toxicity of nPS-NH2 with reduction of analyzed parameters | Tallec et al., 2020 [39] |
| Cassostrea gigas | 100 nm nPS-NH2 100 nm nPS-COOH | 0.1, 1, 10 and 100 mg/ L | Motility Vitality Oxidative stress | Increased oxidative stress at the higher concentrations of nPS-COOH | González-Fernández et al., 2018 [40] |
| Cassostrea gigas | 50 nm nPS-NH2 50 nm nPS-COOH | 0.1, 1, 10 and 25 μg/mL | Fertilization Embryogenesis Metamorphosis | Higher toxicity of nPS-NH2 with reduction of fertilization rate | Tallec et al., 2018 [41] |
| Mytilus galloprovincialis | Environmental micro- and nanoplastics | 1, 10, 50, and 100 μg/L | Motility Vitality Oxidative stress Mitochondria DNA integrity Apoptosis | Decrease in all parameters tested | Romdhani et al., 2023 [42] |
| Tegillarca granosa | 500 nm and 5 μm nPS-NH2 | 0.26 and 0.69 mg/L | Motility Viability DNA integrity Apoptosis Fertilization | Increase in DNA fragmentation and decline in fertilization rate | Shi et al., 2022 [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contino, M.; Ferruggia, G.; Indelicato, S.; Pecoraro, R.; Scalisi, E.M.; Salvaggio, A.; Brundo, M.V. Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819). Toxics 2023, 11, 924. https://doi.org/10.3390/toxics11110924
Contino M, Ferruggia G, Indelicato S, Pecoraro R, Scalisi EM, Salvaggio A, Brundo MV. Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819). Toxics. 2023; 11(11):924. https://doi.org/10.3390/toxics11110924
Chicago/Turabian StyleContino, Martina, Greta Ferruggia, Stefania Indelicato, Roberta Pecoraro, Elena Maria Scalisi, Antonio Salvaggio, and Maria Violetta Brundo. 2023. "Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819)" Toxics 11, no. 11: 924. https://doi.org/10.3390/toxics11110924
APA StyleContino, M., Ferruggia, G., Indelicato, S., Pecoraro, R., Scalisi, E. M., Salvaggio, A., & Brundo, M. V. (2023). Polystyrene Nanoplastics in Aquatic Microenvironments Affect Sperm Metabolism and Fertilization of Mytilus galloprovincialis (Lamark, 1819). Toxics, 11(11), 924. https://doi.org/10.3390/toxics11110924









