N-Acetyl-L-Cysteine Ameliorates BPAF-Induced Porcine Sertoli Cell Apoptosis and Cell Cycle Arrest via Inhibiting the ROS Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. CCK-8 Assay
2.4. Cell Apoptosis Assays
2.5. Cell Cycle Analysis
2.6. Western Blot
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Detection of Intracellular ROS
2.9. Oxidative Stress-Related Molecular Assays
2.10. Statistical Analysis
3. Results
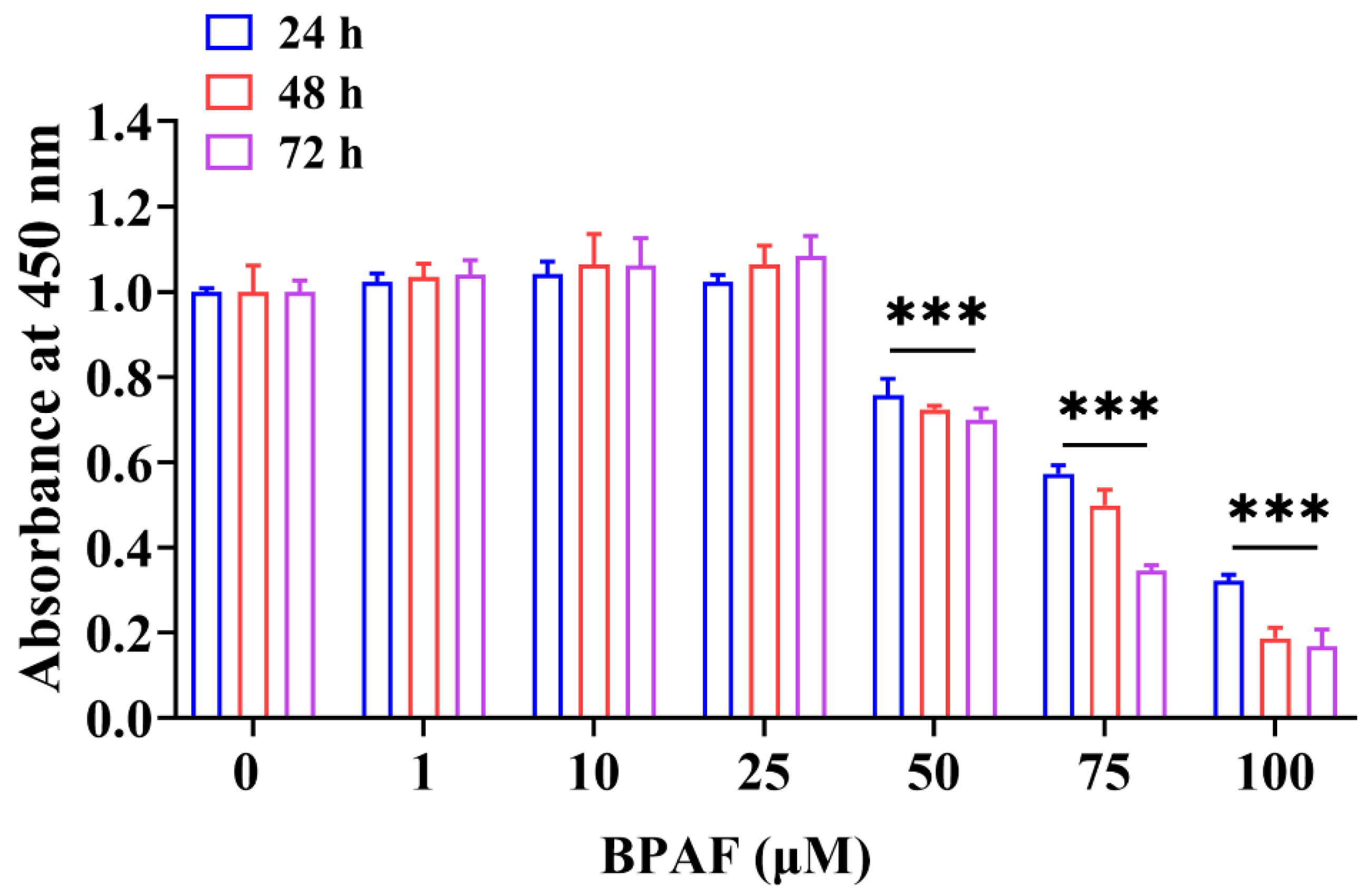
3.1. BPAF-Inhibited Porcine ST Cell Viability
3.2. BPAF Induced Cell Apoptosis and Cell Cycle Arrest at G2/M Phase of Porcine ST Cells
3.3. BPAF-Triggered Oxidative Stress in Porcine ST Cells
3.4. NAC Relieves Cell Viability and Oxidative Stress in BPAF-Induced Porcine ST Cells
3.5. NAC Alleviates BPAF-Triggered Apoptosis and Cell Cycle Arrest in Porcine ST Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Akahori, Y.; Nakai, M.; Yamasaki, K.; Takatsuki, M.; Shimohigashi, Y.; Ohtaki, M. Relationship between the results of in vitro receptor binding assay to human estrogen receptor alpha and in vivo uterotrophic assay: Comparative study with 65 selected chemicals. Toxicol. In Vitro 2008, 22, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Jiao, Z.; Shi, J.; Li, M.; Shao, B. Bisphenol AF may cause testosterone reduction by directly affecting testis function in adult male rats. Toxicol. Lett. 2012, 211, 201–209. [Google Scholar] [CrossRef]
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol. 2012, 46, 13136–13143. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary concentrations of bisphenol A and three other Bisphenols in convenience samples of U.S. adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, B.; Zhao, Y.; Zhang, J.; Shao, B. Highly sensitive and high-throughput method for the analysis of bisphenol analogues and their halogenated derivatives in breast milk. J. Agric. Food Chem. 2017, 65, 10452–10463. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, J.; Chen, Z.; Hong, Y.; Cai, Z. Occurrence and partitioning of bisphenol analogues in adults’ blood from China. Environ. Sci. Technol. 2018, 52, 812–820. [Google Scholar] [CrossRef]
- Presunto, M.; Mariana, M.; Lorigo, M.; Cairrao, E. The effects of bisphenol A on human male infertility: A review of current epidemiological studies. Int. J. Mol. Sci. 2023, 24, 12417. [Google Scholar] [CrossRef]
- Klenke, U.; Constantin, S.; Wray, S. BPA directly decreases GnRH neuronal activity via noncanonical pathway. Endocrinology 2016, 157, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, A.; Rachoń, D. Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2014, 30, 260–265. [Google Scholar] [CrossRef]
- Mahalingam, S.; Ther, L.; Gao, L.; Wang, W.; Ziv-Gal, A.; Flaws, J.A. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod. Toxicol. 2017, 74, 150–157. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lee, S.Y.; Kim, K.Y.; Hong, Y.P. Acute testis toxicity of bisphenol A diglycidyl ether in Sprague-Dawley rats. J. Prev. Med. Public Health 2010, 43, 131–137. [Google Scholar] [CrossRef]
- Huang, M.; Li, X.; Jia, S.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ. Pollut. 2021, 270, 116051. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef]
- Guo, Y.; Hai, Y.; Yao, C.; Chen, Z.; Hou, J.; Li, Z.; He, Z. Long-term culture and significant expansion of human Sertoli cells whilst maintaining stable global phenotype and AKT and SMAD1/5 activation. Cell Commun. Signal 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Liang, G.; Hawken, P.A.; Meachem, S.J.; Malecki, I.A.; Ham, S.; Stewart, T.; Guan, L.L.; Martin, G.B. Nutrition affects Sertoli cell function but not Sertoli cell numbers in sexually mature male sheep. Reprod. Fertil. Dev. 2014, 28, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell. Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg effect revisited--lesson from the Sertoli cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Li, J.L.; Palmer, C.; Maric, D.; Dean, J. Sertoli cell-only phenotype and scRNA-seq define PRAMEF12 as a factor essential for spermatogenesis in mice. Nat. Commun. 2019, 10, 5196. [Google Scholar] [CrossRef]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, X.Q.; Huang, X.; Zhou, J.; Wilson, S.M.; Yang, S.; Zhang, L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS ONE 2013, 8, e73731. [Google Scholar] [CrossRef]
- Lei, B.; Sun, S.; Xu, J.; Feng, C.; Yu, Y.; Xu, G.; Wu, M.; Peng, W. Low-concentration BPAF- and BPF-induced cell biological effects are mediated by ROS in MCF-7 breast cancer cells. Environ. Sci. Pollut. Res. Int. 2018, 25, 3200–3208. [Google Scholar] [CrossRef]
- Ma, C.; Song, H.; Guan, K.; Zhou, J.; Xia, X.; Li, F. Characterization of swine testicular cell line as immature porcine Sertoli cell line. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Sun, S.; Zhang, X.; Feng, C.; Xu, J.; Wen, Y.; Huang, Y.; Wu, M.; Yu, Y. Bisphenol AF exerts estrogenic activity in MCF-7 cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway. Chemosphere 2019, 220, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Yin, L.; Shengyang Yu, K.; Hofmann, M.C.; Yu, X. High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol A and selected analogues in mouse spermatogonial cells. Toxicol. Sci. 2017, 155, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, D.; Zhou, P.; Su, X.; Yang, R.; Shao, C.; Ma, A.; Wu, J. Individual and combined effect of bisphenol A and bisphenol AF on prostate cell proliferation through NF-κB signaling pathway. Int. J. Mol. Sci. 2022, 23, 12283. [Google Scholar] [CrossRef]
- Koti, B.C.; Nagathan, S.; Vishwanathswamy, A.; Gadad, P.C.; Thippeswamy, A. Cardioprotective effect of Vedic Guard against doxorubicin-induced cardiotoxicity in rats: A biochemical, electrocardiographic, and histopathological study. Pharmacogn. Mag. 2013, 9, 176–181. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; Heeba, G.H.; El-Moselhy, M.A. Quercetin augments the protective effect of losartan against chronic doxorubicin cardiotoxicity in rats. Environ. Toxicol. Pharmacol. 2013, 36, 443–450. [Google Scholar] [CrossRef]
- Fallarino, F.; Luca, G.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Fioretti, M.C.; Grohmann, U.; Becchetti, E.; Burgevin, A.; Kratzer, R.; et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J. Exp. Med. 2009, 206, 2511–2526. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef]
- Rossi, G.; Dufrusine, B.; Lizzi, A.R.; Luzi, C.; Piccoli, A.; Fezza, F.; Iorio, R.; D’Andrea, G.; Dainese, E.; Cecconi, S.; et al. Bisphenol A deranges the endocannabinoid system of primary Sertoli cells with an impact on inhibin B production. Int. J. Mol. Sci. 2020, 21, 8986. [Google Scholar] [CrossRef]
- Ham, J.; Yun, B.H.; Lim, W.; Song, G. Folpet induces mitochondrial dysfunction and ROS-mediated apoptosis in mouse Sertoli cells. Pestic. Biochem. Physiol. 2021, 177, 104903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, X.; Ding, F.; Wu, X.; Tang, N.; Wu, Q. Apoptosis and blood-testis barrier disruption during male reproductive dysfunction induced by PAHs of different molecular weights. Environ. Pollut. 2022, 300, 118959. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Park, H.J.; Oh, J.H.; Lee, E.H.; Park, S.M.; Yoon, S. Nonylphenol-induced apoptotic cell death in mouse TM4 Sertoli cells via the generation of reactive oxygen species and activation of the ERK signaling pathway. J. Appl. Toxicol. 2014, 34, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Mostafalou, S.; Abdollahi, M. Pesticides: An update of human exposure and toxicity. Arch. Toxicol. 2017, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Banerjee, R. Environmental toxins: Alarming impacts of pesticides on male fertility. Hum. Exp. Toxicol. 2014, 33, 1017–1039. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The impact of oxidative stress in male infertility. Front. Mol. Biosci. 2021, 8, 799294. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Guo, Y.; Moon, H.B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food. Chem. 2013, 61, 4655–4662. [Google Scholar] [CrossRef]
- Wu, D.; Huang, C.J.; Jiao, X.F.; Ding, Z.M.; Zhang, S.X.; Miao, Y.L.; Huo, L.J. Bisphenol AF compromises blood-testis barrier integrity and sperm quality in mice. Chemosphere 2019, 237, 124410. [Google Scholar] [CrossRef]
- Lei, B.; Xu, L.; Tang, Q.; Sun, S.; Yu, M.; Huang, Y. Molecular mechanism study of BPAF-induced proliferation of ERα-negative SKBR-3 human breast cancer cells in vitro/in vivo. Sci. Total Environ. 2021, 775, 145814. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Hu, C.; Yu, X.J. High-content analysis of testicular toxicity of BPA and its selected analogs in mouse spermatogonial, Sertoli cells, and Leydig cells revealed BPAF induced unique multinucleation phenotype associated with the increased DNA synthesis. Toxicol. Vitr. 2023, 89, 105589. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zeng, L.; Shen, B.; Xu, M.Y.; Zhu, A.Y.; Wu, C.W. Antioxidant defenses at transcriptional and enzymatic levels and gene expression of Nrf2-Keap1 signaling molecules in response to acute zinc exposure in the spleen of the large yellow croaker Pseudosciaena crocea. Fish Shellfish Immunol. 2016, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Mytilineou, C.; Kramer, B.C.; Yabut, J.A. Glutathione depletion and oxidative stress. Park. Relat. Disord. 2002, 8, 385–387. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhang, W.D.; Wang, J.J.; Feng, S.D. Senegenin ameliorate acute lung injury through reduction of oxidative stress and inhibition of inflammation in cecal ligation and puncture-induced sepsis rats. Inflammation 2016, 39, 900–906. [Google Scholar] [CrossRef]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. Vitr. 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert. Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Liu, X.; Nie, S.; Huang, D.; Xie, M. Nonylphenol regulates cyclooxygenase-2 expression via Ros-activated NF-κB pathway in sertoli TM4 cells. Environ. Toxicol. 2015, 30, 1144–1152. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.C.; Yang, X.; Tao, J.J.; Liang, G.; Wu, J.Z.; Wu, W.C.; Wang, Y.; Song, Z.M.; Zhang, X. The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis. Neural Regen. Res. 2018, 13, 1665–1672. [Google Scholar] [PubMed]
- Kwon, J.H.; Lee, N.G.; Kang, A.R.; Song, J.Y.; Hwang, S.G.; Um, H.D.; Kim, J.; Park, J.K. Radiosensitizer effect of β-apopicropodophyllin against colorectal cancer via induction of reactive oxygen species and apoptosis. Int. J. Mol. Sci. 2021, 22, 13514. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Zhai, Y.X.; Chen, J.; Lu, Y.; Wang, J.W.; Quan, C.S.; Zhao, R.X.; Xiao, X.; He, Q.; Werle, K.D.; et al. LKB1 reduces ROS-mediated cell damage via activation of p38. Oncogene 2015, 34, 3848–3859. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Bag, A.K.; Tripathi, R.; Samanta, S.K.; Pal, B.C.; Shaha, C.; Mandal, C. Mahanine, a novel mitochondrial complex-III inhibitor induces G0/G1 arrest through redox alteration-mediated DNA damage response and regresses glioblastoma multiforme. Am. J. Cancer Res. 2014, 4, 629–647. [Google Scholar] [PubMed]
- Wu, S.; Ai, Y.; Huang, H.; Wu, G.; Zhou, S.; Hong, W.; Akuetteh, P.D.P.; Jin, G.; Zhao, X.; Zhang, Y.; et al. A synthesized olean-28,13β-lactam targets YTHDF1-GLS1 axis to induce ROS-dependent metabolic crisis and cell death in pancreatic adenocarcinoma. Cancer Cell. Int. 2022, 22, 143. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Zhou, Z.; Wang, H.; Zhang, T.; Zang, J.; Yin, F.; Sun, W.; Chen, J.; Duan, L.; Xu, J.; et al. Alternol, a natural compound, exerts an anti-tumour effect on osteosarcoma by modulating of STAT3 and ROS/MAPK signalling pathways. J. Cell. Mol. Med. 2017, 21, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.C.; Zhang, J.P.; Huo, Y.N.; Xue, H.Y.; Wang, W.; Zhang, J.J.; Wang, X.Z. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.Q.; Shi, M.; Deng, C.C.; Wang, Y.S.; Tang, Y.; Wang, X.Z. 17β-estradiol rescues the damage of thiazolidinedione on chicken Sertoli cell proliferation via adiponectin. Ecotoxicol. Environ. Saf. 2022, 233, 113308. [Google Scholar] [CrossRef]
- Liu, D.L.; Liu, S.J.; Hu, S.Q.; Chen, Y.C.; Guo, J. Probing the potential mechanism of quercetin and kaempferol against heat stress-induced Sertoli cell injury: Through integrating network pharmacology and experimental validation. Int. J. Mol. Sci. 2022, 23, 11163. [Google Scholar] [CrossRef]





| Gene | The Sequence of the Primers |
|---|---|
| BAX | F: GCCGAAATGTTTGCTGACG R: CAGCCGATCTCGAAGGAAG |
| BAD | F: CAAAGGCCGATTCCCTTCCT R: GGCGGCGTTAGGGTTAATCT |
| BCL-2 | F: TCCAGGCAGTTTAATACATTC R: TCCCTTTATACACTGGGTGA |
| PCNA | F: ACCGCTGCGACCGCAATTTG R: ACGTGCAAATTCACCAGAAGGCATC |
| CDK4 | F: GCGGAGATTGGTGTTGGTG R: CATTGGGGACTCTTACGCTCTT |
| CDK2 | F: GTGGCTGCATCACAAGGAGG R: CCGGAAGAGCTGGTCAATCT |
| β-actin | F: CCAGGTCATCACCATCGG R: CCGTGTTGGCGTAGAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wu, J.; Lei, R.; Zhang, Y.; Qiao, M.; Zhou, J.; Xu, Z.; Li, Z.; Sun, H.; Peng, X.; et al. N-Acetyl-L-Cysteine Ameliorates BPAF-Induced Porcine Sertoli Cell Apoptosis and Cell Cycle Arrest via Inhibiting the ROS Level. Toxics 2023, 11, 923. https://doi.org/10.3390/toxics11110923
Feng Y, Wu J, Lei R, Zhang Y, Qiao M, Zhou J, Xu Z, Li Z, Sun H, Peng X, et al. N-Acetyl-L-Cysteine Ameliorates BPAF-Induced Porcine Sertoli Cell Apoptosis and Cell Cycle Arrest via Inhibiting the ROS Level. Toxics. 2023; 11(11):923. https://doi.org/10.3390/toxics11110923
Chicago/Turabian StyleFeng, Yue, Junjing Wu, Runyu Lei, Yu Zhang, Mu Qiao, Jiawei Zhou, Zhong Xu, Zipeng Li, Hua Sun, Xianwen Peng, and et al. 2023. "N-Acetyl-L-Cysteine Ameliorates BPAF-Induced Porcine Sertoli Cell Apoptosis and Cell Cycle Arrest via Inhibiting the ROS Level" Toxics 11, no. 11: 923. https://doi.org/10.3390/toxics11110923
APA StyleFeng, Y., Wu, J., Lei, R., Zhang, Y., Qiao, M., Zhou, J., Xu, Z., Li, Z., Sun, H., Peng, X., & Mei, S. (2023). N-Acetyl-L-Cysteine Ameliorates BPAF-Induced Porcine Sertoli Cell Apoptosis and Cell Cycle Arrest via Inhibiting the ROS Level. Toxics, 11(11), 923. https://doi.org/10.3390/toxics11110923





