Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of PFASs in Follicular Fluid and Sex Hormones in Serum
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population in Terms of Demographics
3.2. The Concentrations of PFASs in Follicular Fluid
3.3. Association between PFASs in Follicular Fluid and Hormone Levels in Serum
3.4. The Relationship between PFAS Levels in Follicular Fluid and PCOS
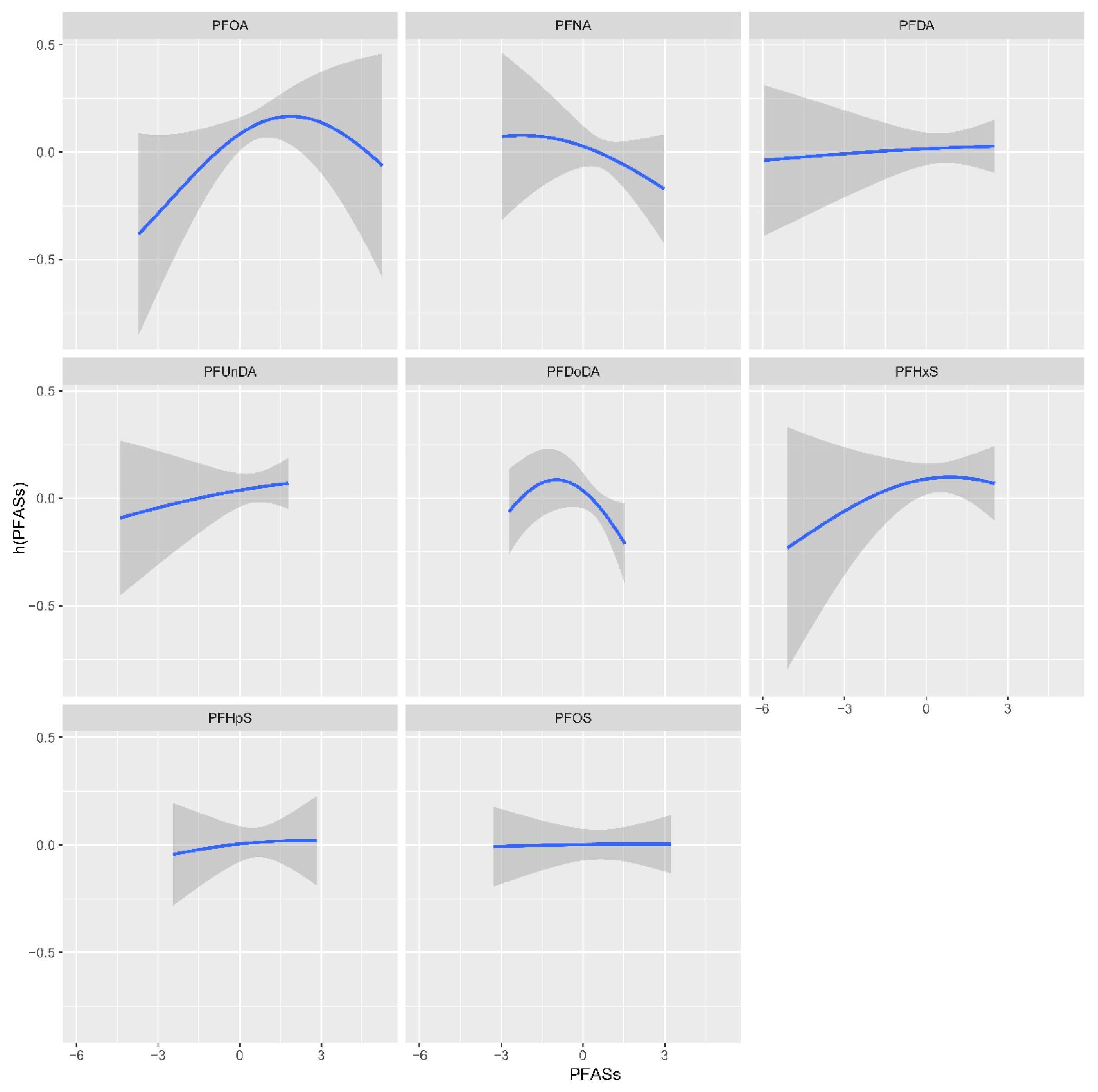
3.5. BKMR-Based Models of the Association of Individual and Joint PFAS Exposure with PCOS
3.6. Mixture Analyses Using the Quantile-Based g-Computation Method
3.7. The Mediation Effect of Testosterone in the Relationship between PFOA and PCOS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evich, M.G.; Davis, M.J.B.; Mccord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of per- and polyfluoroalkyl substances (PFAS): A review. Bioresour. Technol. 2022, 344 Pt B, 126223. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Liao, C.; Cai, Y.; Jiang, G. Perspectives on the inclusion of perfluorooctane sulfonate into the Stockholm Convention on Persistent Organic Pollutants. Environ. Sci. Technol. 2009, 43, 5171–5175. [Google Scholar] [CrossRef]
- Song, X.; Vestergren, R.; Shi, Y.; Huang, J.; Cai, Y. Emissions, Transport, and Fate of Emerging Per- and Polyfluoroalkyl Substances from One of the Major Fluoropolymer Manufacturing Facilities in China. Environ. Sci. Technol. 2018, 52, 9694–9703. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Zhang, X.; Geng, J.; Wang, T.; Shi, Y.; Hu, W.; Li, J. A review of spatial and temporal assessment of PFOS and PFOA contamination in China. Chem. Ecol. 2009, 25, 163–177. [Google Scholar] [CrossRef]
- Wang, Q.; Ruan, Y.; Lin, H.; Lam, P.K.S. Review on perfluoroalkyl and polyfluoroalkyl substances (PFASs) in the Chinese atmospheric environment. Sci. Total Environ. 2020, 737, 139804. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, J.; Wang, Z.; Zhang, L.; Qi, X.; Zhang, Y.; Chang, X.; Wu, C.; Zhou, Z. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: Findings from Sheyang Mini Birth Cohort Study. Chemosphere 2021, 273, 129664. [Google Scholar] [CrossRef] [PubMed]
- Molnar, C. Interpretable Machine Learning: A Guide for Making Black Box Models Explainable, 2nd ed.; Independently Published: Chicago, IL, USA, 2022. [Google Scholar]
- Harada, K.; Inoue, K.; Morikawa, A.; Yoshinaga, T.; Saito, N.; Koizumi, A. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ. Res. 2005, 99, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, L.; Tong, C.; Fang, F.; Zhao, S.; Tian, Y.; Tao, Y.; Zhang, J. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ. Health Perspect. 2017, 125, 067012. [Google Scholar] [CrossRef]
- Valeri, L.; Mazumdar, M.M.; Bobb, J.F.; Claus, H.B.; Rodrigues, E.; Sharif, O.I.A.; Kile, M.L.; Quamruzzaman, Q.; Afroz, S.; Golam, M.; et al. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 months of age: Evidence from rural bangladesh. Environ. Health Perspect. 2017, 125, 067015. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus, H.B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: San Francisco, CA, USA, 2016; pp. 785–794. [Google Scholar]
- Kang, Q.; Gao, F.; Zhang, X.; Wang, L.; Liu, J.; Fu, M.; Zhang, S.; Wan, Y.; Shen, H.; Hu, J. Nontargeted identification of per- and polyfluoroalkyl substances in human follicular fluid and their blood-follicle transfer. Environ. Int. 2020, 139, 105686. [Google Scholar] [CrossRef]
- European Union. European Union Amending Annex XVII of Reach as Regards PFOA, Its Salts and PFOA-Related Substances; European Union: Brussels, Belgium, 2017. [Google Scholar]
- OECD. Risk Reduction Approaches for PFASS—A Cross-Country Analysis; OECD: Paris, France, 2015. [Google Scholar]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol a in polycystic ovary syndrome: A case–control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.L.; Cunningham, T.K.; Drage, D.S.; Aylward, L.L.; Thompson, K.; Vijayasarathy, S.; Mueller, J.F.; Atkin, S.; Sathyapalan, T. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int. J. Hyg. Environ. Health 2018, 221, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Beesoon, S.; Zhu, L.; Martin, J.W. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ. Sci. Technol. 2013, 47, 10619–10627. [Google Scholar] [CrossRef] [PubMed]
- Beesoon, S.; Martin, J.W. Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ. Sci. Technol. 2015, 49, 5722–5731. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Mclaughlin, J.K.; Tarone, R.E.; Olsen, J. Perfluorinated chemicals and fetal growth: A study within the Danish national birth cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Mosch, C.; Morovitz, M.; Alba-Alejandre, I.; Boehmer, S.; Kiranoglu, M.; Faber, F.; Hannibal, I.; Genzel-Boroviczény, O.; Koletzko, B.; et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol. 2010, 44, 7123–7129. [Google Scholar] [CrossRef]
- Hanssen, L.; Röllin, H.; Odland, J.Ø.; Moe, M.K.; Sandanger, T.M. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: Results of a pilot study. J. Environ. Monit. 2010, 12, 1355–1361. [Google Scholar] [CrossRef]
- Inoue, K.; Okada, F.; Ito, R.; Kato, S.; Sasaki, S.; Nakajima, S.; Uno, A.; Saijo, Y.; Sata, F.; Yoshimura, Y.; et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: Assessment of pfos exposure in a susceptible population during pregnancy. Environ. Health Perspect. 2004, 112, 1204–1207. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.; Ji, K.; Seo, J.; Kho, Y.; Park, J.; Kim, S.; Park, S.; Hwang, I.; Jeon, J.; et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011, 45, 7465–7472. [Google Scholar] [CrossRef]
- Midasch, O.; Drexler, H.; Hart, N.; Beckmann, M.W.; Angerer, J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: A pilot study. Int. Arch. Occup. Environ. Health 2007, 80, 643–648. [Google Scholar] [CrossRef]
- Monroy, R.; Morrison, K.; Teo, K.; Atkinson, S.; Kubwabo, C.; Stewart, B.; Foster, W.G. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res. 2008, 108, 56–62. [Google Scholar] [CrossRef]
- Needham, L.L.; Grandjean, P.; Heinzow, B.; Jørgensen, P.J.; Nielsen, F.; Patterson, D.G., Jr.; Sjödin, A.; Turner, W.E.; Weihe, P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011, 45, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Butenhoff, J.L.; Zobel, L.R. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009, 27, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Whitworth, K.W.; Ydersbond, T.A.; Haug, L.S.; Haugen, M.; Knutsen, H.K.; Thomsen, C.; Meltzer, H.M.; Becher, G.; Sabaredzovic, A.; et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ. Int. 2013, 54, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Pan, Y.; Bin, L.; Liu, Y.; Huang, W.; Li, R.; Lai, K.P. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 2022, 291 Pt 2, 132892. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Wu, J.; We, X.; Xu, A.; Wang, S.; Wang, Z. Male reproductive toxicity of perfluorooctanoate (PFOA): Rodent studies. Chemosphere 2021, 270, 128608. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Y.; Zhang, L.; Wang, X. Effects of maternal exposure to PFOA on testes of male offspring mice. Chemosphere 2021, 272, 129585. [Google Scholar] [CrossRef]
- Eggert, A.; Cisneros-Montalvo, S.; Anandan, S.; Musilli, S.; Stukenborg, J.; Adamsson, A.; Nurmio, M.; Toppari, J. The effects of perfluorooctanoic acid (PFOA) on fetal and adult rat testis. Reprod. Toxicol. 2019, 90, 68–76. [Google Scholar] [CrossRef]
- Shen, H.; Gao, M.; Li, Q.; Sun, H.; Jiang, Y.; Liu, L.; Wu, J.; Yu, X.; Jia, T.; Xin, Y.; et al. Effect of PFOA exposure on diminished ovarian reserve and its metabolism. Reprod. Biol. Endocrinol. 2023, 21, 16. [Google Scholar] [CrossRef]
- Steenland, K.; Kugathasan, S.; Barr, D.B. PFOA and ulcerative colitis. Environ. Res. 2018, 165, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Zhao, L.; Winquist, A.; Parks, C. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley. Environ. Health Perspect. 2013, 121, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Vieira, V.M. Critical review on PFOA, kidney cancer, and testicular cancer. J. Air Waste Manage. Assoc. 2021, 71, 663–679. [Google Scholar] [CrossRef]
- Zahm, S.; Bonde, J.P.; Chiu, W.A.; Hoppin, J.; Kanno, J.; Abdallah, M.; Blystone, C.R.; Calkins, M.M.; Dong, G.; Dorman, D.C.; et al. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. Lancet Oncol. 2024, 25, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Awwad, O.; Rotondi, M.; Santini, F.; Imbriani, M.; Chiovato, L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J. Endocrinol. Investig. 2017, 40, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Nian, M.; Xie, M.; Zeng, L.; Qiu, W.; Zhang, J.; Yang, H. Associations of per- and polyfluoroalkyl substances and alternatives with reproductive hormones in women of childbearing age. Int. J. Hyg. Environ. Health 2023, 250, 114158. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, C.; Zhang, Y.; Fan, X.; Gao, W.; Fang, J.; Wang, Y.; Xu, Y.; Jin, L. Association of perfluoroalkyl and polyfluoroalkyl substances with sex hormones in children and adolescents 6–19 Years of age. Environ. Pollut. 2023, 329, 121707. [Google Scholar] [CrossRef]
- Henry, N.D.; Fair, P.A. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J. Appl. Toxicol. 2013, 33, 265–272. [Google Scholar] [CrossRef]
- Barrett, E.S.; Chen, C.; Thurston, S.W.; Haug, L.S.; Sabaredzovic, A.; Fjeldheim, F.N.; Frydenberg, H.; Lipson, S.F.; Ellison, P.T.; Thune, I. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril. 2015, 103, 1261–1270.e3. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef]
- Feng, X.; Cao, X.; Zhao, S.; Wang, X.; Hua, X.; Chen, L.; Chen, L. Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring. Toxicol. Sci. 2016, 155, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Johns, L.E.; Meeker, J.D. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011–2012. Int. J. Environ. Res. Public Health 2015, 12, 6098–6114. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Vested, A.; Jørgensen, K.T.; Bonde, J.P.E.; Henriksen, T.B.; Toft, G. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: A systematic review. Crit. Rev. Toxicol. 2016, 46, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shi, Y.; Wang, J.; Cai, Y.; Wu, Y. Concentrations of perfluorinated compounds in human blood from twelve cities in China. Environ. Toxicol. Chem. 2010, 29, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, Y.; Vestergren, R.; Wang, T.; Liang, Y.; Cai, Y. Highly elevated serum concentrations of perfluoroalkyl substances in fishery employees from Tangxun lake, China. Environ. Sci. Technol. 2014, 48, 3864–3874. [Google Scholar] [CrossRef]
- Harada, K.H.; Hitomi, T.; Niisoe, T.; Takanaka, K.; Kamiyama, S.; Watanabe, T.; Moon, C.; Yang, H.; Hung, N.N.; Koizumi, A. Odd-numbered perfluorocarboxylates predominate over perfluorooctanoic acid in serum samples from Japan, Korea and Vietnam. Environ. Int. 2011, 37, 1183–1189. [Google Scholar] [CrossRef]

| Controls (n = 218) | Cases (n = 73) | p-Value | |
|---|---|---|---|
| n (%) or Mean (SD) | n (%) or Mean (SD) | ||
| Age (years) | 35.4 (5.0) | 32.4 (4.0) | 0.000 |
| BMI (kg/m2) | 0.002 | ||
| <18.5 | 18 (8.3) | 5 (6.8) | |
| 18.5–23.9 | 146 (67.0) | 35 (47.9) | |
| 24–27.9 | 44 (20.2) | 21 (28.8) | |
| ≥28 | 10 (4.6) | 12 (16.4) | |
| Employment status | 0.236 | ||
| Unemployed | 18 (8.3) | 3 (4.1) | |
| Employed | 200 (91.7) | 70 (95.9) | |
| Educational level | 0.839 | ||
| ≤High school | 80 (36.7) | 24 (32.9) | |
| College graduate | 116 (53.2) | 41 (56.2) | |
| >College graduate | 22 (10.1) | 8 (11.0) | |
| Active/passive smoking | 0.003 | ||
| No | 142 (70.0) | 61 (83.6) | |
| Yes | 76 (34.9) | 12 (16.4) | |
| Pregnancy | 0.000 | ||
| 0 | 97 (44.5) | 57 (78.1) | |
| ≥1 | 121 (55.5) | 16 (21.9) | |
| Delivery | 0.002 | ||
| 0 | 175 (80.3) | 70 (95.9) | |
| ≥1 | 43 (19.7) | 3 (4.1) | |
| FSH | 8.3 (3.4) | 7.2 (1.7) | 0.030 |
| LH | 4.1 (1.9) | 9.5 (5.5) | 0.000 |
| E2 | 134.2 (64.4) | 140.5 (79.4) | 0.840 |
| P | 1.9 (2.4) | 1.7 (0.9) | 0.372 |
| T | 1.3 (0.7) | 2.3 (1.1) | 0.000 |
| PRL | 16.0 (12.0) | 17.3 (19.3) | 0.657 |
| PFASs | Measure ≥ LOD * | Median (IQR) | |||
|---|---|---|---|---|---|
| ng/mL | (%) | Controls (n = 218) | Cases (n = 73) | Total (n = 291) | p-Value |
| PFOA | 100 | 6.90 (4.64, 9.96) | 7.22 (5.84, 9.74) | 7.04 (5.05, 9.84) | 0.316 |
| PFNA | 100 | 1.04 (0.68, 1.55) | 1.00 (0.74, 1.34) | 1.04 (0.70, 1.49) | 0.475 |
| PFDA | 99.6 | 1.18 (0.76, 2.02) | 1.20 (0.65, 1.80) | 1.19 (0.70, 1.92) | 0.586 |
| PFUnDA | 99.0 | 0.70 (0.39, 1.16) | 0.65 (0.38, 0.97) | 0.67 (0.39, 1.09) | 0.509 |
| PFDoDA | 89.7 | 0.17 (0.12, 0.23) | 0.15 (0.08, 0.19) | 0.16 (0.11, 0.22) | 0.019 |
| PFHxS | 99.6 | 1.41 (0.74, 2.71) | 1.44 (0.97, 2.86) | 1.41 (0.87, 2.84) | 0.661 |
| PFHpS | 92.8 | 0.09 (0.05, 0.14) | 0.09 (0.05, 0.12) | 0.09 (0.05, 0.14) | 0.323 |
| PFOS | 100 | 6.04 (4.06, 9.27) | 5.48 (3.69, 7.80) | 5.99 (3.94, 9.11) | 0.265 |
| ∑PFAS | (12.44, 27.68) | 17.74 (13.61, 24.00) | 18.67 (13.20, 26.45) | ||
| PFOA | PFNA | PFDA | PFUnDA | PFDoDA | PFHxS | PFHpS | |
|---|---|---|---|---|---|---|---|
| PFNA | 0.677 ** | ||||||
| PFDA | 0.523 ** | 0.886 ** | |||||
| PFUnDA | 0.471 ** | 0.847 ** | 0.913 ** | ||||
| PFDoDA | 0.323 ** | 0.575 ** | 0.591 ** | 0.594 ** | |||
| PFHxS | 0.563 ** | 0.460 ** | 0.400 ** | 0.275 ** | 0.146 * | ||
| PFHpS | 0.621 ** | 0.744 ** | 0.684 ** | 0.596 ** | 0.426 ** | 0.553 ** | |
| PFOS | 0.564 ** | 0.829 ** | 0.882 ** | 0.790 ** | 0.540 ** | 0.431 ** | 0.823 ** |
| FSH | LH | E2 | P | T | PRL | |
|---|---|---|---|---|---|---|
| PFOA ** | −0.08 (−0.22, 0.05) | −0.06 (−0.20, 0.07) | 0.10 (−0.03, 0.24) | −0.02 (−0.16, 0.12) | 0.18 (0.05, 0.31) * | 0.03 (−0.11, 0.17) |
| PFNA ** | −0.10 (−0.23, 0.03) | −0.001 (−0.14, 0.13) | −0.02 (−0.15, 0.12) | 0.001 (−0.14, 0.14) | 0.16 (0.03, 0.28) * | 0.14 (0.00, 0.27) |
| PFDA ** | −0.12 (−0.25, 0.01) | −0.05 (−0.18, 0.08) | −0.007 (−0.14, 0.12) | −0.01 (−0.15, 0.13) | 0.11 (−0.02, 0.23) | 0.08 (−0.05, 0.22) |
| PFUnDA ** | −0.09 (−0.22, 0.04) | 0.009 (−0.12, 0.14) | −0.05 (−0.19, 0.08) | −0.03 (−0.16, 0.11) | 0.10 (−0.03, 0.23) | 0.11 (−0.02, 0.24) |
| PFDoDA ** | −0.08 (−0.21, 0.05) | −0.09 (−0.23, 0.04) | −0.007 (−0.14, 0.13) | −0.04 (−0.18, 0.10) | 0.03 (−0.10, 0.16) | −0.02 (−0.15, 0.12) |
| PFHxS ** | −0.01 (−0.14, 0.12) | −0.02 (−0.16, 0.11) | −0.04 (−0.18, 0.09) | 0.13 (−0.008, 0.26) | 0.05 (−0.07, 0.18) | 0.02 (−0.11, 0.16) |
| PFHpS ** | −0.07 (−0.20, 0.06) | 0.03 (−0.10, 0.16) | 0.02 (−0.11, 0.15) | 0.08 (−0.05, 0.22) | 0.07 (−0.06, 0.19) | 0.06 (−0.07, 0.20) |
| PFOS ** | −0.08 (−0.21, 0.04) | 0.000 (−0.13, 0.13) | −0.03 (−0.16, 0.10) | 0.05 (−0.08, 0.19) | 0.08 (−0.05, 0.21) | 0.03 (−0.10, 0.16) |
| PFASs | Quartiles | Crude OR | p-Value | Adjusted OR * | p-Value |
|---|---|---|---|---|---|
| (ng/mL) | (95% CI) | (95% CI) | |||
| PFOA | 1st (≤5.05) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>5.05, 7.04) | 3.09 (1.35, 7.05) | 0.008 | 3.65 (1.47, 9.05) | 0.005 | |
| 3rd (>7.04, 9.84) | 2.59 (1.12, 6.00) | 0.026 | 2.91 (1.17, 7.26) | 0.022 | |
| 4th (>9.84) | 2.06 (0.88, 4.84) | 0.097 | 3.13 (1.21, 8.09) | 0.019 | |
| P for trend ** | 0.053 | 0.032 | |||
| PFNA | 1st (≤0.70) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.70, 1.04) | 1.64 (0.78, 3.44) | 0.192 | 2.55 (1.10, 5.90) | 0.029 | |
| 3rd (>1.04, 1.49) | 1.28 (0.60, 2.74) | 0.530 | 1.50 (0.63, 3.59) | 0.364 | |
| 4th (>1.49) | 0.92 (0.42, 2.04) | 0.840 | 1.18 (0.49, 2.83) | 0.718 | |
| P for trend ** | 0.419 | 0.134 | |||
| PFDA | 1st (≤0.70) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.70, 1.19) | 0.49 (0.23, 1.08) | 0.076 | 0.56 (0.23, 1.38) | 0.210 | |
| 3rd (>1.19, 1.92) | 1.11 (0.55, 2.25) | 0.770 | 1.38 (0.63, 3.01) | 0.423 | |
| 4th (>1.92) | 0.60 (0.28, 1.28) | 0.185 | 0.68 (0.30, 1.56) | 0.364 | |
| P for trend ** | 0.115 | 0.194 | |||
| PFUnDA | 1st (≤0.39) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.39, 0.67) | 1.27 (0.62, 2.63) | 0.512 | 1.51 (0.66, 3.44) | 0.329 | |
| 3rd (>0.67, 1.09) | 1.11 (0.54, 2.32) | 0.774 | 1.18 (0.52, 2.71) | 0.694 | |
| 4th (>1.09) | 0.57 (0.25, 1.28) | 0.173 | 0.70 (0.28, 1.72) | 0.435 | |
| P for trend ** | 0.237 | 0.367 | |||
| PFDoDA | 1st (≤0.11) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.11, 0.16) | 0.86 (0.42, 1.73) | 0.669 | 0.88 (0.39, 1.95) | 0.747 | |
| 3rd (>0.16, 0.22) | 0.63 (0.30, 1.32) | 0.220 | 0.62 (0.27, 1.43) | 0.265 | |
| 4th (>0.22) | 0.42 (0.19, 0.92) | 0.030 | 0.46 (0.19, 1.11) | 0.084 | |
| P for trend ** | 0.145 | 0.309 | |||
| PFHxS | 1st (≤0.87) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.87, 1.41) | 1.82 (0.84, 3.92) | 0.127 | 2.38 (0.97, 5.79) | 0.057 | |
| 3rd (>1.41, 2.84) | 1.40 (0.64, 3.10) | 0.399 | 2.17 (0.87, 5.41) | 0.095 | |
| 4th (>2.84) | 1.48 (0.68, 3.24) | 0.324 | 2.04 (0.85, 4.88) | 0.11 | |
| P for trend ** | 0.502 | 0.226 | |||
| PFHpS | 1st (≤0.05) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>0.05, 0.09) | 1.06 (0.51, 2.19) | 0.880 | 1.38 (0.61, 3.10) | 0.436 | |
| 3rd (>0.09, 0.14) | 1.00 (0.48, 2.09) | 0.990 | 1.13 (0.49, 2.61) | 0.782 | |
| 4th (>0.14) | 0.65 (0.30, 1.42) | 0.281 | 0.95 (0.40, 2.26) | 0.905 | |
| P for trend ** | 0.611 | 0.823 | |||
| PFOS | 1st (≤3.94) | 1.00 (reference) | 1.00 (reference) | ||
| 2nd (>3.94, 5.99) | 0.73 (0.35, 1.52) | 0.401 | 1.20 (0.52, 2.78) | 0.671 | |
| 3rd (>5.99, 9.11) | 0.86 (0.42, 1.78) | 0.692 | 0.98 (0.43, 2.22) | 0.953 | |
| 4th (>9.11) | 0.55 (0.26, 1.18) | 0.127 | 0.73 (0.31, 1.71) | 0.469 | |
| P for trend ** | 0.471 | 0.732 |
| PCOS | |||
|---|---|---|---|
| Items | Weight | [OR (95% CI)] | p-Value |
| All subjects * (n = 291) | |||
| PFAS mixture | 0.96 (0.73, 1.26) | 0.771 | |
| PFOA | 0.56 | 1.74 (1.17, 2.64) | 0.007 |
| PFNA | −0.08 | 0.92 (0.48, 1.75) | 0.802 |
| PFDA | 0.42 | 1.52 (0.74, 3.22) | 0.260 |
| PFUnDA | −0.38 | 0.68 (0.37, 1.24) | 0.209 |
| PFDoDA | −0.32 | 0.73 (0.51, 1.03) | 0.075 |
| PFHxS | 0.05 | 1.05 (0.74, 1.49) | 0.795 |
| PFHpS | −0.10 | 0.90 (0.55, 1.46) | 0.674 |
| PFOS | −0.20 | 0.82 (0.46, 1.45) | 0.489 |
| BMI < 24 kg/m2 ** (n = 204) | |||
| PFAS mixture | 1.20 (0.80, 1.80) | 0.369 | |
| PFOA | 0.47 | 1.59 (0.97, 2.65) | 0.067 |
| PFNA | −0.49 | 0.61 (0.26, 1.37) | 0.245 |
| PFDA | 0.63 | 1.87 (0.75, 4.90) | 0.187 |
| PFUnDA | −0.34 | 0.71 (0.33, 1.48) | 0.367 |
| PFDoDA | 0.003 | 1.00 (0.66, 1.54) | 0.988 |
| PFHxS | 0.09 | 1.10 (0.70, 1.70) | 0.682 |
| PFHpS | 0.08 | 1.08 (0.55, 2.14) | 0.821 |
| PFOS | −0.18 | 0.84 (0.39, 1.77) | 0.642 |
| BMI ≥ 24 kg/m2 ** (n = 87) | |||
| PFAS mixture | 0.79 (0.49, 1.28) | 0.339 | |
| PFOA | 1.24 | 3.46 (1.20, 13.56) | 0.044 |
| PFNA | −0.44 | 0.64 (0.09, 4.36) | 0.650 |
| PFDA | −0.50 | 0.61 (0.08, 4.10) | 0.605 |
| PFUnDA | 0.76 | 2.14 (0.49, 10.23) | 0.315 |
| PFDoDA | −1.40 | 0.25 (0.08, 0.58) | 0.004 |
| PFHxS | −0.08 | 0.93 (0.45, 1.93) | 0.836 |
| PFHpS | −0.58 | 0.56 (0.19, 1.50) | 0.253 |
| PFOS | 0.42 | 1.53 (0.40, 5.94) | 0.531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, G.; Lin, Y.; Sun, F.; Zheng, L.; Yu, Y.; Xu, H. Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women. Toxics 2024, 12, 104. https://doi.org/10.3390/toxics12020104
Li S, Li G, Lin Y, Sun F, Zheng L, Yu Y, Xu H. Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women. Toxics. 2024; 12(2):104. https://doi.org/10.3390/toxics12020104
Chicago/Turabian StyleLi, Sen, Guojing Li, Yu Lin, Feng Sun, Liqiang Zheng, Yingying Yu, and Hong Xu. 2024. "Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women" Toxics 12, no. 2: 104. https://doi.org/10.3390/toxics12020104
APA StyleLi, S., Li, G., Lin, Y., Sun, F., Zheng, L., Yu, Y., & Xu, H. (2024). Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women. Toxics, 12(2), 104. https://doi.org/10.3390/toxics12020104






