Effect of Butyl Paraben on Oxidative Stress in the Liver of Mauremys sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, BuP Exposure, and Sampling
2.2. Biochemical Analysis
2.3. Histological Structure
2.4. Total RNA Extraction and qRT-PCR
2.5. Data Analysis
3. Results
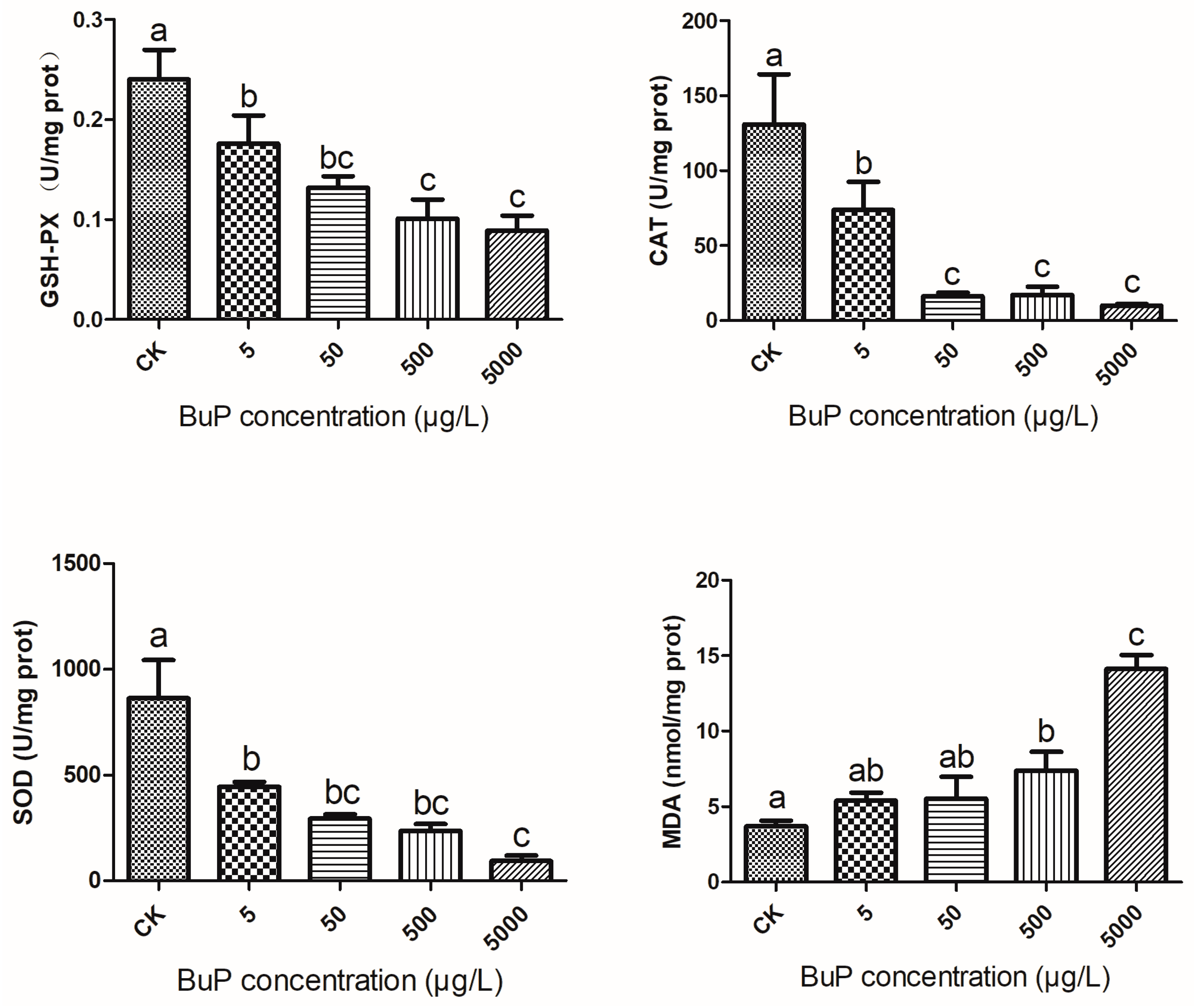
3.1. Effects of BuP on Antioxidant Enzyme Activity and MDA Content in the Liver
3.2. Effects of BuP on the mRNA Expression of Oxidant Stress Genes in the Liver
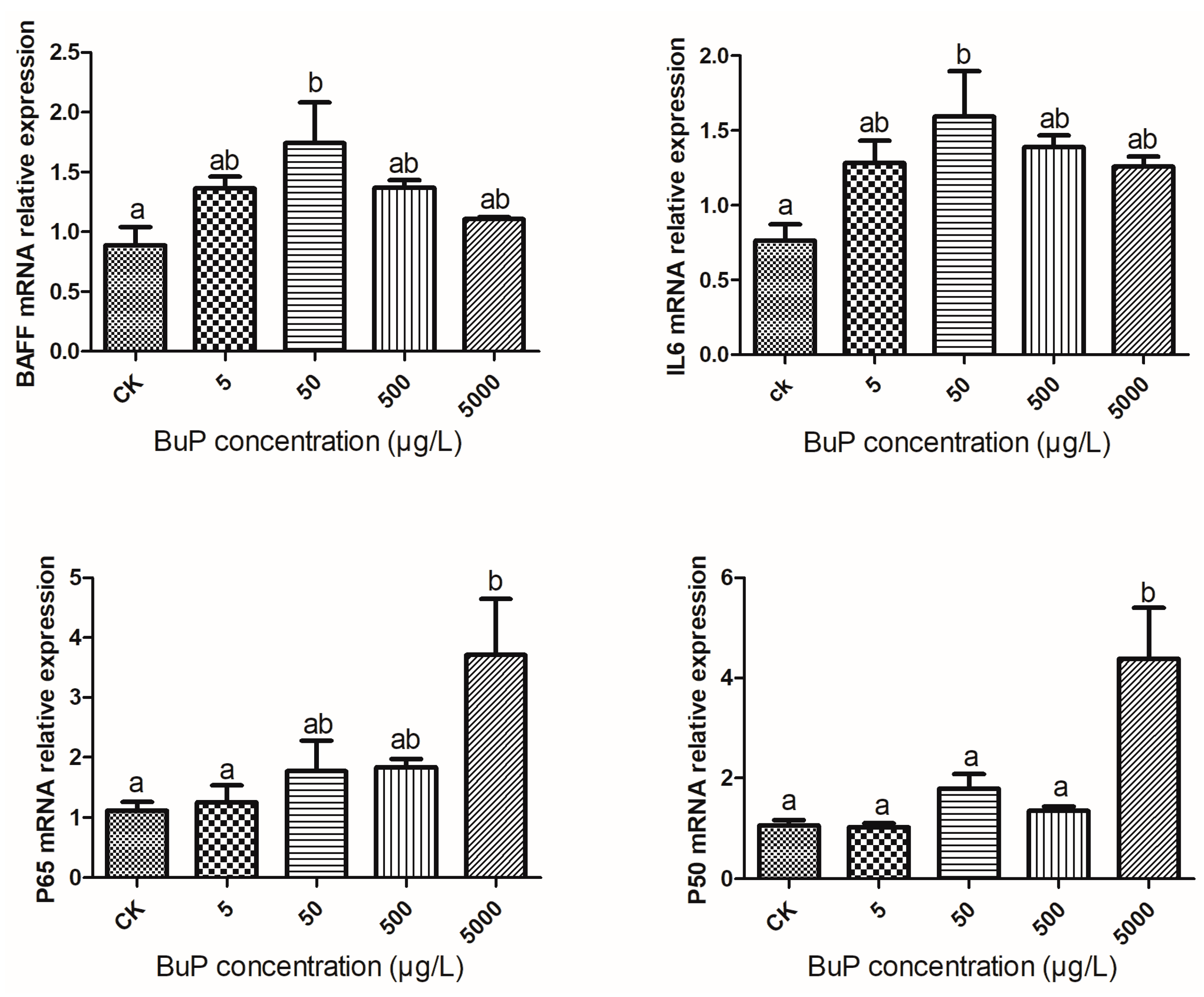
3.3. Effect of BuP on the mRNA Expression of Inflammatory Genes in the Liver
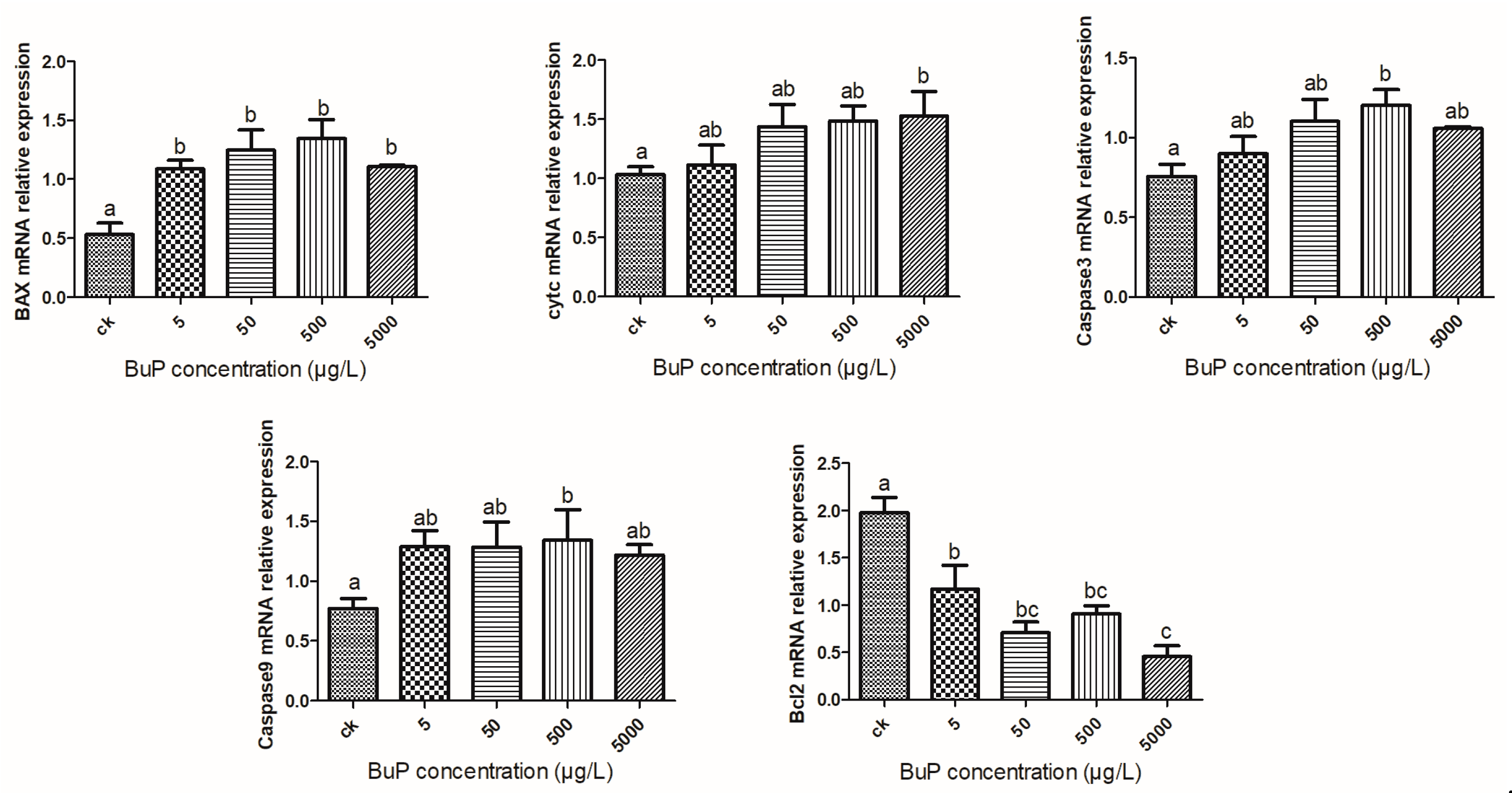
3.4. Effect of Bup on the mRNA Expression of Apoptosis Genes in the Liver
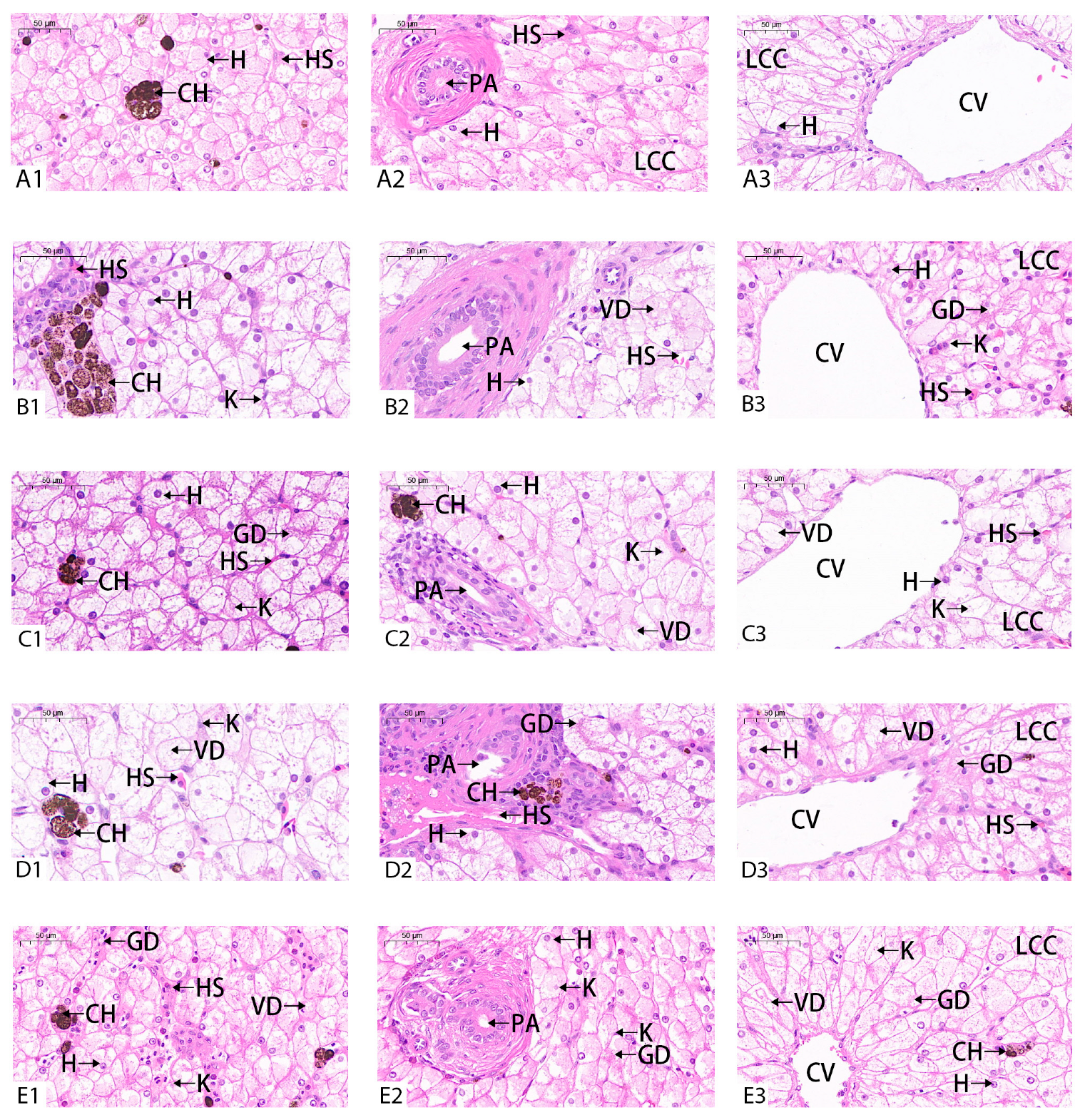
3.5. Effect of BuP on the Histological Structure of the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elder, R.L. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. J. Am. Coll. Toxicol. 1984, 3, 147–209. [Google Scholar]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2010, 24, 5–13. [Google Scholar]
- Karwacka, A.; Zamkowska, D.; Radwan, M.; Jurewicz, J. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: An overview of current epidemiological evidence. Hum. Fertil. 2019, 22, 2–25. [Google Scholar]
- Jiang, Y.Q.; Zhao, H.Z.; Xia, W.; Li, Y.Y.; Liu, H.X.; Hao, K.; Chen, J.; Sun, X.J.; Liu, W.Y.; Li, J.F.; et al. Prenatal exposure to benzophenones, parabens and triclosan and neurocognitive development at 2 years. Environ. Int. 2019, 126, 413–421. [Google Scholar] [PubMed]
- Meeker, J.D.; Yang, T.; Ye, X.; Calafat, A.M.; Hauser, R. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ. Health Perspect. 2011, 119, 252–257. [Google Scholar] [PubMed]
- Vo, T.T.B.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod. Toxicol. 2010, 29, 306–316. [Google Scholar]
- Commission, E.; Health, D.G.F. Consumers. Opinion on Polidocanol—Addendum to the SCCP Opinion on Polidocanol (SCCP/1130/07); European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Yna, D.; Rstb, D.; Tpa, D. Toxicity assessment of parabens in Caenorhabditis elegans. Chemosphere 2020, 246, 125730. [Google Scholar]
- Feng, J.L.; Zhao, J.H.; Xi, N.N.; Guo, W.; Sun, J.H. Parabens and their metabolite in surface water and sediment from the Yellow River and the Huai River in Henan Province: Spatial distribution, seasonal variation and risk assessment. Ecotox Environ. Safe 2019, 172, 480–487. [Google Scholar]
- Wang, N.; Hu, X.; Lu, S.Y.; Ma, S.T.; Kang, L.; Liao, S.C.; Yu, Y.X. Interrelationship of anthropogenic activity and parabens in fish from Taihu Lake during 2009–2017. Environ. Pollut 2019, 252, 1002–1009. [Google Scholar]
- Yamamoto, H.; Watanabe, M.; Hirata, Y.; Nakamura, Y.; Nakamura, Y.; Kitani, C.; Sekizawa, J.; Uchida, M.; Nakamura, H.; Kagami, Y. Preliminary ecological risk assessment of butyl paraben and benzyl paraben -1. Removal efficiency in wastewater treatment, acute/chronic toxicity for aquatic organisms, and effects on medaka gene expression. Environ. Sci. 2007, 14, 73–87. [Google Scholar]
- Adoamnei, E.; Mendiola, J.; Monino-Garcia, M.; Vela-Soria, F.; Iribarne-Duran, L.M.; Fernandez, M.F.; Olea, N.; Jorgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary concentrations of parabens and reproductive parameters in young men. Sci. Total Environ. 2018, 621, 201–209. [Google Scholar] [PubMed]
- Wang, L.; Wu, Y.; Zhang, W.; Kannan, K. Characteristic profiles of urinary p-Hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ. Sci. Technol. 2013, 47, 2069–2076. [Google Scholar] [PubMed]
- Dobbins, L.L.; Usenko, S.; Brain, R.A.; Brooks, B.W. Probabilistic ecological hazard assessment of parabens using Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2010, 28, 2744–2753. [Google Scholar]
- Sakuragui, M.M.; Paulino, M.G.; Pereira, C.D.S.; Carvalho, C.S.; Sadauskas-Henrique, H.; Fernandes, M.N. Integrated use of antioxidant enzymes and oxidative damage in two fish species to assess pollution in man-made hydroelectric reservoirs. Environ. Pollut. 2013, 178, 41–51. [Google Scholar]
- Kang, S.; Kim, S.; Park, J.; Kim, H.J.; Lee, J.; Choi, G.; Choi, S.; Kim, S.; Kim, S.Y.; Moon, H.B. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci. Total Environ. 2013, 461–462, 214–221. [Google Scholar]
- Kroll, C.; Mastroeni, S.; Veugelers, P.J.; Mastroeni, M.F. Associations of ADIPOQ and LEP gene variants with energy intake: A systematic review. Nutrients 2019, 11, 750. [Google Scholar]
- Shah, K.H.; Verma, R.J. Butyl p-hydroxybenzoic acid induces oxidative stress in mice liver—An in vivo study. Acta Pol. Pharm. 2011, 68, 875–879. [Google Scholar]
- Wen, A.X.; Zhou, D.G. Effects of stress on turtles and turtles. J. Econ. Anim. 2009, 13, 108–114. [Google Scholar]
- Jiang, M.; Zeng, G.M.; Chang, Z.; Ma, X.Y.; Ming, C.; Zhang, J.C.; Lu, L.H.; Yu, Q.; Hu, L.P.; Liu, L.F. Assessment of heavy metal contamination in the surrounding soils and surface sediments in Xiawangang River, Qingshuitang District. PLoS ONE 2013, 8, e71176. [Google Scholar]
- Ma, K.; Li, C.; Shi, H.T.; Wang, J.; Liu, D.; Wang, J.C. Comparative study on the home territory of red-eared turtle and Chinese striped-neck turtle in Qionghai section of Wanquan River in Hainan. Chin. J. Zool. 2013, 48, 331–337. (In Chinese) [Google Scholar]
- Read, S.M.; Northcote, D.H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 1981, 116, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, W.H.; Liang, L.Y.; Huang, Z.B.; Hong, M.L. Modulation of the intestinal barrier adaptive functions in red-eared slider (Trachemys scripta elegans) invading brackish waters. Sci. Total Environ. 2020, 751, 141744. [Google Scholar] [CrossRef] [PubMed]
- Beazley, W.D.; Gaze, D.; Panske, A.; Panzig, E.; Schallreuter, K.U. Serum selenium levels and blood glutathione peroxidase activities in vitiligo. Br. J. Dermatol. 1999, 141, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M. Oxygen in biology and biochemistry: Role of free radicals. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Almeida, E.A.; Silva, H.; Grunig, D.; Bainy, A.C.D.; Freitas, F.P.; Motta, F.D.; Gomes, O.F.; Medeiros, M.H.G.; Di Mascio, P. Evaluation of glutathione status in aquatic organisms. In Oxidative Stress in Aquatic Ecosystems; Abele, D., Vázquez-Medina, J.P., Zenteno-Savín, T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Silva, D.C.; Serrano, L.; Oliveira, T.M.A.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus ). Ecotox Environ. Safe 2018, 162, 85–91. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes. Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Hayashi, A.; Suzuki, H.; Itoh, K.; Yamamoto, M.; Sugiyama, Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Bioph Res. Co. 2003, 310, 824–829. [Google Scholar] [CrossRef]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Primiano, T.; Li, Y.; Kensler, T.W.; Trush, M.A.; Sutter, T.R. Identification of dithiolethione-inducible gene-1 as a leukotriene B4 12-hydroxydehydrogenase: Implications for chemoprevention. Carcinogenesis 1998, 19, 999–1005. [Google Scholar] [CrossRef][Green Version]
- Ke, B.B.; Shen, X.D.; Zhang, Y.; Ji, H.F.; Gao, F.; Yue, S.; Kamo, N.; Zhai, Y.; Yamamoto, M.; Busuttil, R.W. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J. Hepatol. 2013, 59, 1200–1207. [Google Scholar] [CrossRef]
- Gunal, C.; Kksal, G.; Zkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar]
- Ye, C.X.; Yang, F.F.; Miao, Y.T.; Ling, R.Z.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar]
- Wang, H.H.; Lei, Z.R. Research progress of insect heat shock proteins. Sci. Agric. Sin. 2005, 38, 12. [Google Scholar]
- Paul, I.; Priscilla, M.; Babatunji, O.; Abidemi, K. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar]
- Morimoto, R.; Santoro, M. Stress-inducible responses and heat shock proteins: New pharmacologic targets for cytoprotection. Nat. Biotechnol. 1998, 16, 833–838. [Google Scholar] [CrossRef]
- Liang, L.; Huang, Z.; Li, N.; Wang, D.; Hong, M. Effects of ammonia exposure on antioxidant function, immune response and NF-κB pathway in Chinese Strip-necked Turtle (Mauremys sinensis). Aquat. Toxicol. 2020, 229, 105621. [Google Scholar] [CrossRef]
- Pratt William, B. The role of the HSP90-based chapeone system in signal transduction by nuclear recepors and receptors signaling via MAP kinase. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 297. [Google Scholar] [CrossRef]
- Kregel, K.C. Invited Review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Ai, H.X.; Shen, Y.F.; Min, C.; Pang, S.Y.; Zhang, J.X.; Zhang, S.Q.; Zhao, Z.Z. Molecular structure, expression and bioactivity characterization of TNF13B (BAFF) gene in mefugu (Takifugu obscures). Fish. Shellfish. Immunol. 2011, 30, 1265–1274. [Google Scholar] [CrossRef]
- Lam, W.S.; Wu, S.Y.; Lin, S.J.; Lin, C.C.; Chen, Y.M.; Wang, H.C.; Chen, T.Y.; Lin, H.T.; Lin, H.Y. The expression of two novel orange-spotted grouper (Epinephelus coioides) TNF genes in peripheral blood leukocytes, various organs, and fish larvae. Fish. Shellfish. Immunol. 2011, 30, 618–629. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Baltimore, D. NF-kappaB: Ten years after. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef]
- Lin, G.A.; Xu, N.; Xi, R.W. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J. Mol. Cell Biol. 2010, 2, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Estaquier, J.; Vallette, F.; Vayssiere, J.L.; Mignotte, B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012, 942, 157–183. [Google Scholar] [PubMed]
- Yuan, F. Effects of hawthorn leaves favonoids on oxidative stress and cardiomyocytes apoptosis of neonatal rat cadiocytes induced by H2O2. Mod. J. Integr. Tradit. Chin. West. Med. 2016, 25, 5. [Google Scholar]
- Burke, P.J. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liang, L.; Li, N.; Li, W.; Yu, Z.; Zhang, J.; Shi, H.; Ding, L.; Hong, M. Ammonia exposure induces endoplasmic reticulum stress and apoptosis in Chinese striped-necked turtle (Mauremys sinensis). Aquat. Toxicol. 2021, 237, 105903. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.R.; He, B.; Wang, Y.L.; Shi, H.T. Toxic effects of nitrate stress on embryos of red-eared turtle and Chinese striped turtle. Fish. Sci. 2012, 31, 4. (In Chinese) [Google Scholar]
- Huang, Z.B.; Liang, L.; Li, W.H.; Li, N.; Ding, L.; Hong, M.L. Changes of tissue structure of main organs of Chinese striped turtle after acute ammonia nitrogen stress and recovery. Fish. Sci. 2022, 41, 143–149. (In Chinese) [Google Scholar]

| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Nrf2 | GAGGCCCAGCTTGCACTTA | GGCAGTTTTGAGCAGCCAC |
| Keap1 | GCTCATCGAGTTCGCCTACA | CCTTGACCACGCTGTCGAT |
| HSP70 | CCACCTCTTCGCAGTGTTCT | AAGCCGGGGACAAAAATAGCA |
| HSP90 | TGGAAGGGTTTACCGACGAG | GCCGCTCCTCCTCTTCATAC |
| P50 | GTGACTGCTGGACTGGGAAA | TTTCAGCAAAAGTGCTTGCCA |
| P65 | CTCCAGAAGCCACAGGTTG | GGGGAAGAAGGGGTCAAAGTT |
| BAFF | CAGAACTAGCAACTCTCCGCAT | ACCCCAGTCCCAGGATAAGAG |
| IL-6 | TAGGTTCTACCGGCTGCACT | CCAGGCATCATGGATCACCA |
| Bcl-2 | GAGGGGATACGATTGGGCTG | AGCAACAGTAGGGGGAGAGA |
| Bax | GTCGTGGCGCTTTTCTACTT | CCAGCTCGGGGACCTTG |
| CytC | CTGCGGGCTTCTCTTACACA | TCCATCAGTGTTTCCTCACCC |
| Caspase-3 | TGTGTTAAGTCATGGTGAAGATGGA | AGTTTTGGCTTTCCCACAAGAC |
| Caspase-9 | GACCGAGGATTTGAGGTGGAT | AAAGGGAGTTGCATCGGACT |
| β-actin | GCACCCTGTGCTGCTTACA | CACAGTGTGGGTGACACCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Xie, Z.; Sun, X.; Wu, X.; Zhang, J.; Shi, H.; Ding, L.; Hong, M. Effect of Butyl Paraben on Oxidative Stress in the Liver of Mauremys sinensis. Toxics 2023, 11, 915. https://doi.org/10.3390/toxics11110915
Yin Y, Xie Z, Sun X, Wu X, Zhang J, Shi H, Ding L, Hong M. Effect of Butyl Paraben on Oxidative Stress in the Liver of Mauremys sinensis. Toxics. 2023; 11(11):915. https://doi.org/10.3390/toxics11110915
Chicago/Turabian StyleYin, Yaru, Zhenzi Xie, Xiao Sun, Xia Wu, Jiliang Zhang, Haitao Shi, Li Ding, and Meiling Hong. 2023. "Effect of Butyl Paraben on Oxidative Stress in the Liver of Mauremys sinensis" Toxics 11, no. 11: 915. https://doi.org/10.3390/toxics11110915
APA StyleYin, Y., Xie, Z., Sun, X., Wu, X., Zhang, J., Shi, H., Ding, L., & Hong, M. (2023). Effect of Butyl Paraben on Oxidative Stress in the Liver of Mauremys sinensis. Toxics, 11(11), 915. https://doi.org/10.3390/toxics11110915






