Airborne Pesticides—Deep Diving into Sampling and Analysis
Abstract
:1. Introduction
2. Pesticide Concentrations in Air
2.1. Indoor Air
2.2. Outdoor Air
3. Advancements in Pesticide Sampling Techniques: Current Technology and Limitations
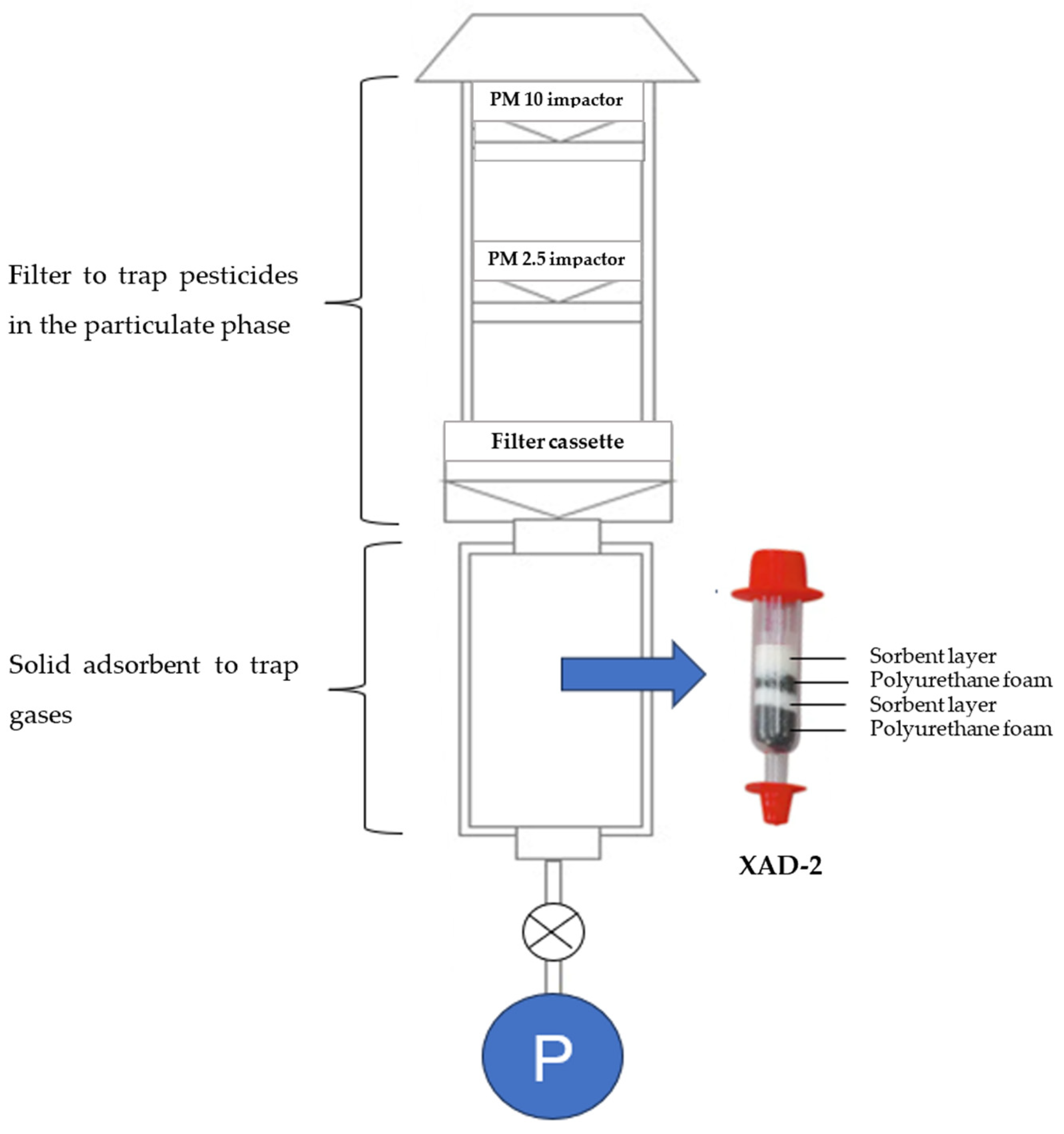
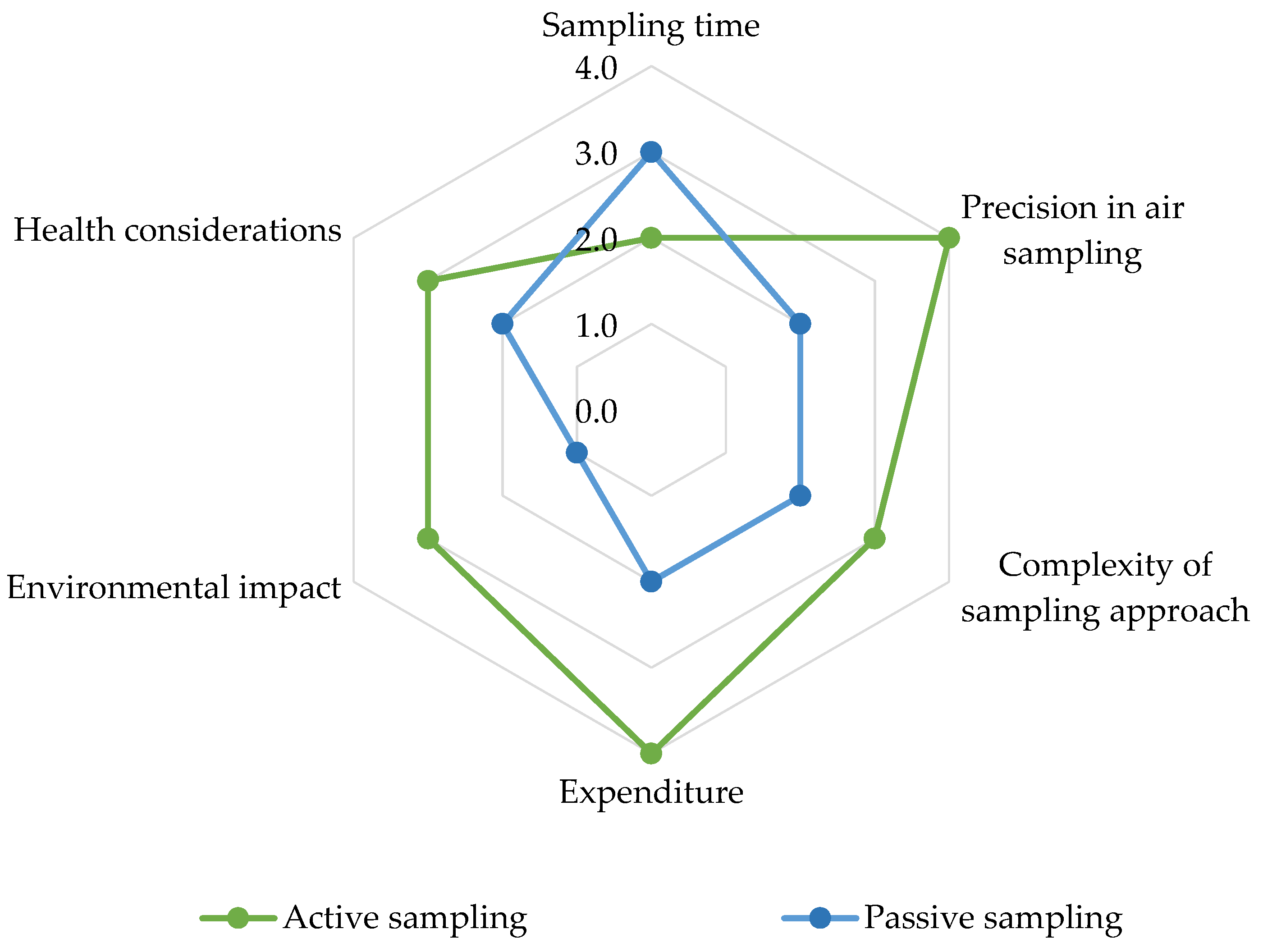
3.1. Active Sampling Applications and Limitations
3.2. Passive Sampling Applications and Limitations
3.3. Evaluation of Sampling Techniques for Measuring Airborne Pesticides
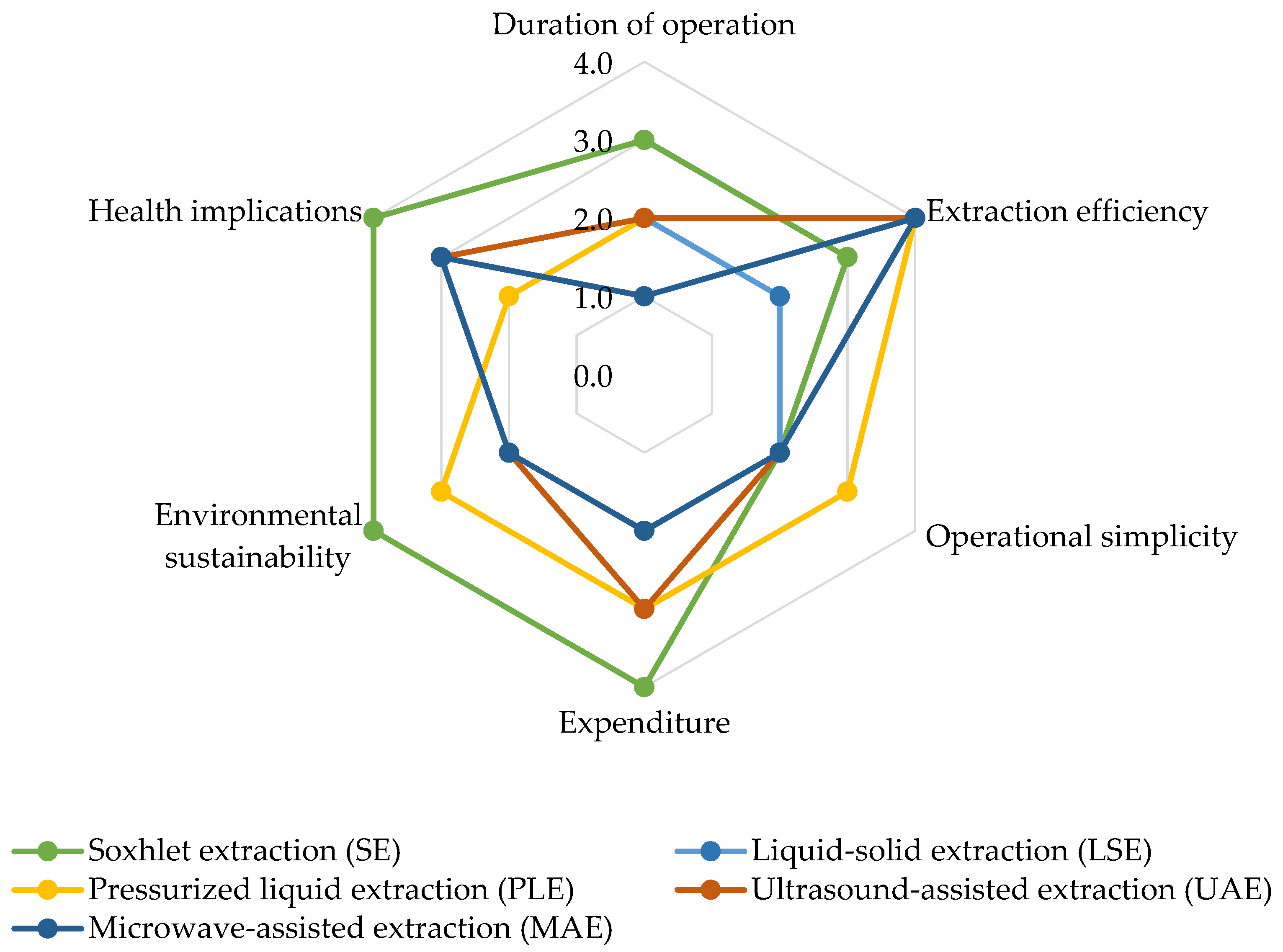
4. Emerging Trends in Pesticide Extraction Techniques: Enhancing Efficiency and Analytical Performance
4.1. Pesticide Extraction Techniques
4.2. Analytical Performance of Pesticide Extraction Methods
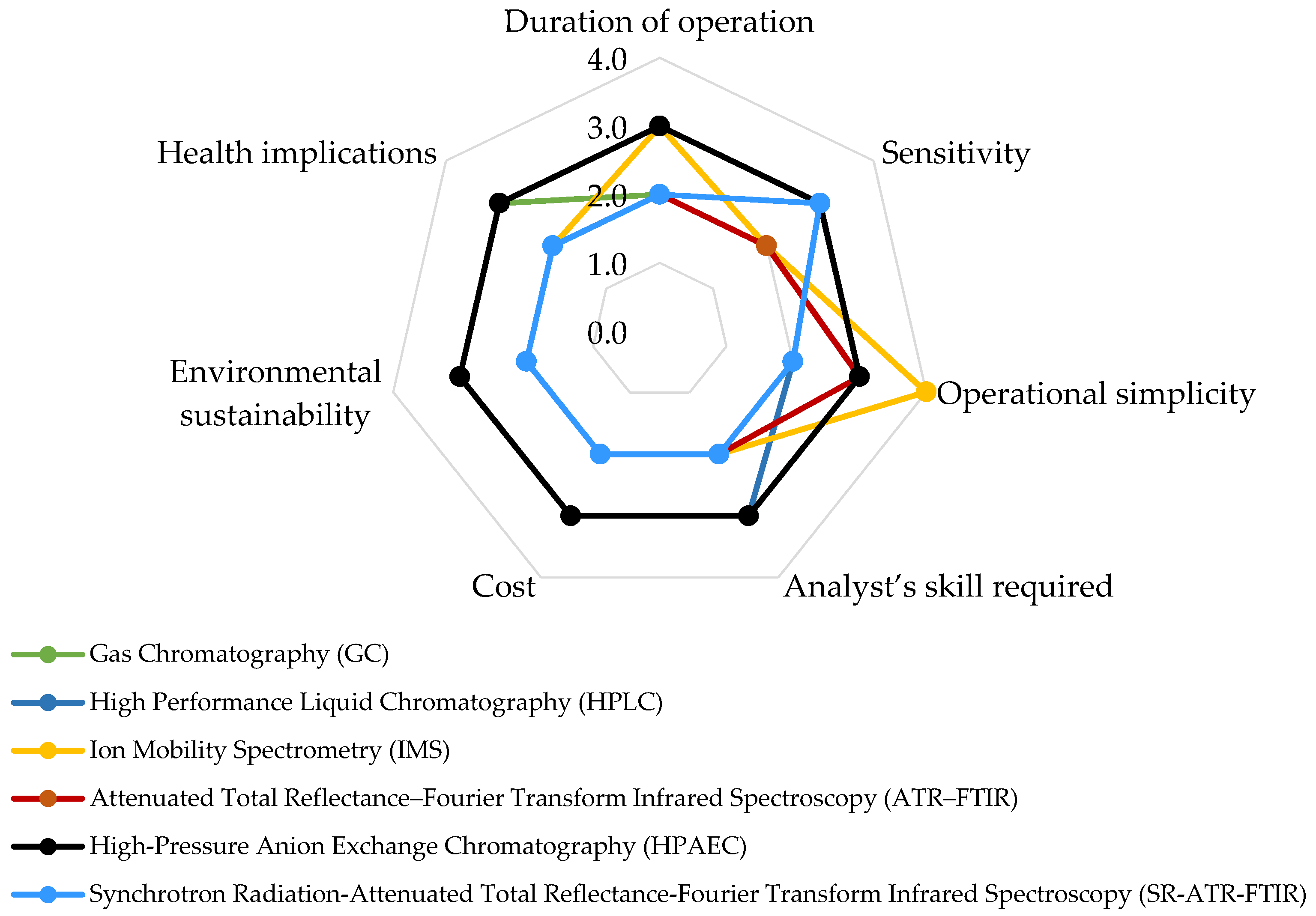
5. Advancements in Pesticide Analytical Methods: Exploring Classical and Recent Technologies
5.1. Advances in GC for Pesticide Detection in Air
5.2. Advances in LC for Pesticide Detection in Air
5.3. Other Advances in Pesticide Detection Methods
5.4. Analytical Performance of Pesticide Detection Techniques
6. Future Directions and Opportunities in Pesticide Detection and Monitoring
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamel, F. Paths from pesticides to Parkinson’s. Science 2013, 341, 722–723. [Google Scholar] [CrossRef] [PubMed]
- Mascarelli, A. Growing up with pesticides. Science 2013, 341, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol. Lett. 2013, 230, 157–165. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Yan, H. Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32222. [Google Scholar] [CrossRef]
- Brühl, C.A.; Bakanov, N.; Köthe, S.; Eichler, L.; Sorg, M.; Hörren, T. Direct pesticide exposure of insects in nature conservation areas in Germany. Sci. Rep. 2021, 11, 24144. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Amstrong, J.L.; Yost, M.G.; Fenske, R.A. Development of passive air sampler to measure airborne organophosphorus pesticides and oxygen analogs in an agricultural community. Chemosphere 2014, 111, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Dévier, M.-H.; Cruz, J.; Duporté, G.; Barron, E.; Gaillard, J.; Le Menach, K.; Pardon, P.; Augagneur, S.; Flaud, P.-M. Passive sampling as a tool to assess atmospheric pesticide contamination related to vineyard land use. Atmosphere 2022, 13, 504. [Google Scholar] [CrossRef]
- Raeppel, C.; Fabritius, M.; Nief, M.; Appenzeller, B.M.R.; Millet, M. Coupling ASE, sylilation and SPME–GC/MS for the analysis of current-used pesticides in atmosphere. Talanta 2014, 121, 24–29. [Google Scholar] [CrossRef]
- Masiá, A.; Vásquez, K.; Campo, J.; Picó, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges: Application to the Túria River Basin. J. Chromatogr. 2015, 1378, 19–31. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC-Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639. [Google Scholar] [CrossRef] [PubMed]
- El-Zahhar, A.A.; Idris, A.M.; Fawy, K.F.; Arshad, M. SEM, SEM-EDX, μ-ATR-FTIR and XRD for urban street dust characterization. Int. J. Environ. Anal. Chem. 2021, 101, 988–1006. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V.; Armenta, S.; Guardia, M.; Esteve-Turrillas, F.A. Comprehensive analysis of airborne pesticides using hard cap espresso extraction-liquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2017, 1506, 27–36. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Bedos, C.; Cellier, P.; Calvet, R.; Barriuso, E. Occurrence of pesticides in the atmosphere in France. Agronomie 2002, 22, 35–49. [Google Scholar] [CrossRef]
- Van den Berg, F.; Kubiak, R.; Benjey, W.G.; Majewsjki, M.S.; Yates, S.R.; Reeves, G.L.; Smelt, J.H.; Van der Linden, A.M.A. Emission of pesticides in the air. In Fate of Pesticides in the Atmosphere, Implications for Environmental Risk Assessment; Water, Air, Soil Pollution; van Dijk, H.F.G., van Pul, W.A.J., de Voogt, P., Eds.; Kluwer Academic Publishers: Dordrecht/Boston/London, UK, 1999; Volume 115, pp. 195–218. [Google Scholar]
- Rudel, R.A.; Dodson, R.E.; Perovich, L.J.; Morello-Frosch, R.; Camann, D.E.; Zuniga, M.M.; Yau, A.Y.; Just, A.C.; Brody, J.G. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two Northern California communities. Environ. Sci. Technol. 2010, 44, 6583–6590. [Google Scholar] [CrossRef]
- Veludo, A.F.; Figueiredo, D.M.; Degrendele, C.; Masinyana, L.; Curchod, L.; Kohoutek, J.; Kukučka, P.; Martiník, J.; Přibylová, P.; Klánová, J.; et al. Seasonal variations in air concentrations of 27 organochlorine pesticides (OCPs) and 25 current-use pesticides (CUPs) across three agricultural areas of South Africa. Chemosphere 2022, 289, 133162. [Google Scholar] [CrossRef]
- Li, J.; Zhu, T.; Wang, F.; Qiu, X.H.; Lin, W.L. Observation of organochlorine pesticides in the air of the Mt. Everest region. Ecotoxicol. Environ. Saf. 2006, 63, 33–41. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, Z.; Die, Q.; Tian, Y.; Liu, F.; He, J.; Huang, Q. Organochlorine pesticides in soil, air and vegetation at and around a contaminated site in southwestern of China: Concentration, transmission and risk evaluation. Chemosphere 2017, 178, 340–349. [Google Scholar] [CrossRef]
- Holt, E.; Audy, O.; Booij, P.; Melymuk, L.; Prokes, R.; Klánová, J. Organochlorine pesticides in the indoor air of a theatre and museum in the Cezch Republic: Inhalation exposure and cancer risk. Sci. Total Environ. 2017, 609, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.; Bush, B.; David, O.; Carpenter, D.O. PCBs in indoor air and human blood in Pittsfield Massachusetts. Chemosphere 2022, 293, 133551. [Google Scholar] [CrossRef] [PubMed]
- Audy, O.; Melymuk, L.; Venier, M.; Vojta, S.; Becanova, J.; Romanak, K.; Vykoukalova, M.; Prokes, R.; Kukucka, P.; Diamond, M.L.; et al. PCBs and organochlorine pesticides in indoor environments—A comparison of indoor contamination in Canada and Czech Republic. Chemosphere 2018, 206, 622–631. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, R.; Yu, K.; Han, M.; Wang, Y.; Huang, X.; Wang, R.; Liu, F. First report on organochlorine pesticides (OCPs) in coral tissues and the surrounding air-seawater system from the South China Sea: Distribution, source, and environmental fate. Chemosphere 2022, 286, 131711. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, G.; Blanchard, O.; Momas, I.; Seta, N. Pesticide exposure of non-occupationally exposed subjects compared to some occupational exposure: A French pilot study. Sci. Total Environ. 2006, 366, 74–91. [Google Scholar] [CrossRef]
- Coscollà, C.; Colin, P.; Yahyaoui, A.; Petrique, O.; Yusà, V.; Mellouki, A.; Pastor, A. Occurrence of currently used pesticides in ambient air of Centre Region (France). Atmos. Environ. 2010, 44, 3915–3925. [Google Scholar] [CrossRef]
- Syed, J.H.; Malik, R.N.; Liu, D.; Xu, Y.; Wang, Y.; Li, J.; Zhang, G.; Jones, K.C. Organochlorine pesticides in air and soil and estimated air-soil exchange in Punjab, Pakistan. Sci. Total Environ. 2013, 444, 491–497. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Garfinkel, R.; Hoepner, L.A.; Holmes, D.; Borjas, M.; Williams, M.K.; Reyes, A.; Rauh, V.; Perera, F.P.; Camann, D.E. Within- and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York city. Environ. Health Perspect. 2007, 115, 383–389. [Google Scholar] [CrossRef]
- Coscollà, C.; Muñoz, A.; Borrás, E.; Vera, T.; Ródenas, M.; Yusà, V. Particle size distributions of currently used pesticides in ambient air of an agricultural Mediterranean area. Atmos. Environ. 2014, 95, 29–35. [Google Scholar] [CrossRef]
- Gamboa, C.L.; Diaz, S.K.; Ruepert, C.; van Wendel de Joode, B. Passive monitoring techniques to evaluate environmental pesticide exposure: Results from the Infant’s Environmental Health study (ISA). Environmetal Res. 2020, 184, 109243. [Google Scholar] [CrossRef] [PubMed]
- Degrendele, C.; Kanduč, T.; Kocman, D.; Lammel, G.; Cambelová, A.; Dos Santos, S.G.; Horvat, M.; Kukučka, P.; Šmejkalvá, A.H.; Mikeš, O.; et al. NPAHs and OPAHs in the atmosphere of two central European cities: Seasonality, urban-to-background gradients, cancer risks and gas-to-particle partitioning. Sci. Total Environ. 2021, 793, 148528. [Google Scholar] [CrossRef] [PubMed]
- Degrendele, C.; Klánová, J.; Prokeš, R.; Příbylová, P.; Šenk, P.; Šudoma, M.; Röösli, M.; Dalvie, M.A.; Fuhrimann, S. Current use pesticides in soil and air from two agricultural sites in South Africa: Implications for environmental fate and human exposure. Sci. Total Environ. 2022, 807, 150455. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, H.H.; Lee, J.I.; Lee, J.H.; Kang, H.; Lee, J.Y. Indoor contamination from pesticides used for outdoor insect control. Sci. Total Environ. 2018, 625, 994–1002. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Kontoroupis, P.; Solomou, E.S.; Nikolaki, S.; Karabelas, A.J. Inventory of pesticide emissions into the air in Europe. Atmos. Environ. 2013, 75, 6–14. [Google Scholar] [CrossRef]
- Chang, F.-C.; Simcik, M.F.; Capel, P.D. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ. Toxicol. Chem. 2010, 30, 548–555. [Google Scholar] [CrossRef]
- Morshed, M.; Omar, R.D.; Mohamad, S.; Abd, S.W. Determination of glyphosate through passive and active sampling methods in a treated field atmosphere. Afr. J. Agric. Res. 2011, 6, 4010–4018. [Google Scholar]
- Ravier, S.; Désert, M.; Gille, G.; Armengaud, A.; Wortham, H. Monitoring of Glyphosate, Glufosinate-ammonium, and (Aminomethyl) phosphonic acid in ambient air of Provence-Alpes-Côte-d’ Azur Region, France. Atmos. Environ. 2019, 204, 102–109. [Google Scholar] [CrossRef]
- Obendorf, S.K.; Lemley, A.T.; Hedge, A.; Kline, A.A.; Tan, K.; Dokuchayeva, T. Distribution of pesticide residues within homes in central New York State. Arch. Environ. Contam. Toxicol. 2006, 50, 31–44. [Google Scholar] [CrossRef]
- Berger-Preiss, E.; Elflein, L. Determination of household insecticides in indoor air by Gas Chromatography-Mass Spectrometry. In Pesticide Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 179–190. [Google Scholar] [CrossRef]
- Ramesh, A.; Vijayalakshmi, A. Monitoring of allethrin, deltamethrin, esbiothrin, prallethrin and transfluthrin in air during the use of household mosquito repellents. J. Environ. Monit. 2001, 3, 191–193. [Google Scholar] [CrossRef]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Banks, A.P.W.; He, C.; Drage, D.S.; Gallen, C.L.; Li, Y.; Li, Q.; Thai, P.K.; Mueller, J.F. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and legacy and current pesticides in indoor environment in Australia–occurrence, sources and exposure risks. Sci. Total Environ. 2019, 693, 133588. [Google Scholar] [CrossRef]
- Reed, L.; Buchner, V.; Tchounwou, P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health 2007, 22, 213–243. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.; Ribeiro, C.; Delgado, I.; Peleteiro, B.; Aggerbeck, M.; Distel, E.; Annesi-Maesano, I.; Sarigiannis, D.; Ramos, E. The association between environmental exposures to chlordanes, adiposity and diabetes-related features: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 14546. [Google Scholar] [CrossRef]
- Yoshida, T.; Mimura, M.; Sakon, N. Estimating household exposure to pyrethroids and the relative contribution of inhalation pathway in a sample of Japanese children. Environ. Sci. Pollut. Res. Int. 2021, 28, 19310–19324. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; Yusà, V.; Beser, I.M.; Pastor, A. Multi-residue analysis of 30 currently used pesticides in fine airborne particulate matter (PM 2.5) by microwave-assisted extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8817–8827. [Google Scholar] [CrossRef] [PubMed]
- Dhuldhaj, U.P.; Singh, R.; Singh, V.K. Pesticide contamination in agro-ecosystems: Toxicity, impacts, and bio-based management strategies. Environ. Sci. Pollut. Res. 2023, 30, 9243–9279. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—A review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Schummer, C.; Mothiron, E.; Appenzeller, B.M.R.; Rizet, A.L.; Wennig, R.; Millet, M. Temporal variations of concentrations of currently used pesticides in the atmosphere of Strasbourg, France. Environ. Pollut. 2010, 158, 576–584. [Google Scholar] [CrossRef]
- Lévy, M.; Ba, H.; Pallares, C.; Pham-Huu, C.; Millet, M. Comparison and calibration of diverse passive samplers used for the air sampling of pesticides during a regional sampling monitoring campaign. Atmos. Pollut. Res. 2020, 11, 1217–1225. [Google Scholar] [CrossRef]
- Zhang, X.; Saini, A.; Hao, C.; Harner, T. Passive air sampling and nontargeted analysis for screening POP-like chemicals in the atmosphere: Opportunities and challenges. TrAC Trends Anal. Chem. 2020, 132, 116052. [Google Scholar] [CrossRef]
- Hung, H.; Katsoyiannis, A.A.; Brorstrom-Lunden, E.; Olafsdottir, K.; Aas, W.; Breivik, K.; Bohlin-Nizzetto, P.; Sigurdsson, A.; Hakola, H.; Bossi, R.; et al. Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP). Environ. Pollut. 2016, 217, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Yusà, V.; Coscollà, C.; Mellouki, W.; Pastor, A.; de la Guardia, M. Sampling and analysis of pesticides in ambient air. J. Chromatogr. A 2009, 1216, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Sauret, N.; Wortham, H.; Putaud, J.P.; Mirabel, P. Study of the effects of environmental parameters on the gas/particle partitioning of current-use pesticides in urban air. Atmos. Environ. 2008, 42, 544–553. [Google Scholar] [CrossRef]
- Peters, A.J.; Lane, D.A.; Gundel, L.A.; Northcott, G.L.; Jones, K.C. A comparison of high volume and diffusion denuder samplers for measuring semivolatile organic compounds in the atmosphere. Environ. Sci. Technol. 2000, 34, 5001–5006. [Google Scholar] [CrossRef]
- Feng, Y.L.; Mu, C.C.; Fu, Z.R.; Chen, Y.J. Determination of airborne dicarbonyls by HPLC analysis using annular denuder/filter system coated with 2,4-dinitrophenylhydrazine. Chin. J. Anal. Chem. 2011, 39, 1653–1658. [Google Scholar] [CrossRef]
- Goriaux, M.; Jourdain, B.; Temime, B.; Besombes, J.L.; Marchand, N.; Albinet, A.; Leoz-Garziandia, E.; Wortham, H. Field comparison of particulate PAH measurements using a low-flow denuder device and conventional sampling systems. Environ. Sci. Technol. 2006, 40, 6398–6404. [Google Scholar] [CrossRef]
- Melymuk, L.; Bohlin, P.; Sáňka, O.; Pozo, K.; Klánová, J. Current challenges in air sampling of semivolatile organic contaminants: Sampling artifacts and their influence on data comparability. Environ. Sci. Technol. 2014, 48, 14077–14091. [Google Scholar] [CrossRef]
- Bai, J.; Yang, W.; Zhang, C.; Zhao, Y.; Gong, C.; Sun, X.; Zhang, Q.; Wang, W. Theoretical study on the OH-initiated atmospheric reaction of 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT). Atmos. Environ. 2013, 67, 177–183. [Google Scholar] [CrossRef]
- John, E.; Coburn, S.; Liu, C.; McAughey, J.; Mariner, D.; McAdam, K.G.; Sebestyén, Z.; Bakos, I.; Dóbé, S. Effect of temperature and humidity on the gas-particle partitioning on nicotine in mainstream cigarette smoke: A diffusion denuder study. J. Aerosol Sci. 2018, 117, 100–117. [Google Scholar] [CrossRef]
- Yao, Y.; Tuduri, L.; Blanchard, P.; Harner, P.; Waite, D.; Poissantd, L.; Murphy, C.; Belzerf, W.; Aulagnierd, F.; Li, F.; et al. Spatial and temporal distribution of pesticide in air concentrations in Canadian agricultural regions. Atmos. Environ. 2006, 40, 4339–4351. [Google Scholar] [CrossRef]
- Alegria, H.; Bidleman, T.F.; Figueroa, M.S. Organochlorine pesticides in the ambient air of Chiapas, Mexico. Environ. Pollut. 2006, 140, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.; Arzel, S.; Le Bot, B.; Seux, R.; Millet, M. Adsorption/thermal desorption-GC/MS for the analysis of pesticides in the atmosphere. Chemosphere 2000, 40, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Al-Alam, J.; Lévy, M.; Ba, H.; Pham-Huu, C.; Millet, M. Measuring current-use pesticides in air: A comparison of silicon carbide foam to XAD as passive air samplers. Environ. Technol. Innov. 2021, 24, 101876. [Google Scholar] [CrossRef]
- Pellicer-Castell, E.; Belenguer-Sapiña, C.; Amorós, P.; El Haskouri, J.; Herrero-Martínez, J.M.; Mauri-Aucejo, A.R. Comparison of silica-based materials for organophosphorus pesticides sampling and occupational risk assessment. Anal. Chim. Acta 2020, 1110, 26–34. [Google Scholar] [CrossRef]
- Mari, M.; Harrison, R.M.; Schuhmacher, M.; Domingo, J.L.; Pongpiachan, S. Inferences over the sources and processes affecting polycyclic aromatic hydrocarbons in the atmosphere derived from measured data. Sci. Total Environ. 2010, 408, 2387–2393. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Ho, K.F.; Lee, S.C. A study of gas-particle partitioning of PAH according to adsorptive models and season. In Air Pollution Xviii; Wit Press: Southampton, UK, 2010; pp. 37–48. [Google Scholar]
- Pongpiachan, S.; Wiriwutikorn, T.; Rungruang, C.; Yodden, K.; Duangdee, N.; Sbrilli, A.; Gobbi, M.; Centeno, C. Impacts of micro-emulsion system on polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) reduction from industrial boilers. Fuel 2016, 172, 58–64. [Google Scholar] [CrossRef]
- López, A.; Coscollà, C.; Yusà, V. Evaluation of sampling adsorbents and validation of a LC-HRMS method for determination of 28 airborne pesticides. Talanta 2018, 189, 211–219. [Google Scholar] [CrossRef]
- Dobson, R.; Scheyer, A.; Rizet, A.L.; Mirabel, P.; Millet, M. Comparison of the efficiencies of different types of adsorbents at trapping currently used pesticides in the gaseous phase using the technique of high-volume sampling. Anal. Anal. Chem. 2006, 386, 1781–1789. [Google Scholar] [CrossRef]
- Lévy, M.; Al-Alam, J.; Ridacker, C.; Massemin, S.; Millet, M. Use of XAD®-2 passive air samplers for monitoring environmental trends of PAHs, PCBs and pesticides in three different sites in Strasbourg and its vicinity (east of France). Atmos. Environ. 2018, 195, 12–23. [Google Scholar] [CrossRef]
- Decuq, C.; Bourdat-Deschamps, M.; Benoit, P.; Bertrand, C.; Benabdallah, R.; Esnault, B.; Durand, B.; Loubet, B.; Fritsch, C.; Pelosi, C.; et al. A multiresidue analytical method on air and rainwater for assessing pesticide atmospheric contamination in untreated areas. Sci. Total Environ. 2022, 823, 153582. [Google Scholar] [CrossRef] [PubMed]
- Namiesnik, J.; Zabiegala, B.; Kot-Wasik, A.; Partyka, M.; Wasik, A. Passive sampling and/or/extraction techniques in environmental analysis, a review. Anal. Bioanal. Chem. 2005, 381, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, P.; Jones, K.C.; Strandberg, B. Occupational and indoor air exposure to persistent organic pollutants: A review of passive sampling techniques and needs. J. Environ. Monit. 2007, 9, 501–509. [Google Scholar] [CrossRef]
- Raeppel, C.; Appenzeller, B.M.; Millet, M. Determination of seven pyrethroids biocides and their synergist in indoor air by thermal-desorption gas chromatography/mass spectrometry after sampling on Tenax TA® passive tubes. Talanta 2015, 131, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Alam, J.; Lévy, M.; Ba, H.; Pham-Huu, C.; Millet, M. Passive air samplers based on ceramic adsorbent for monitoring of organochlorine pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in outdoor air. Environ. Technol. Innov. 2020, 20, 11094. [Google Scholar] [CrossRef]
- Chaemfa, C.; Barber, J.L.; Gocht, T.; Harner, T.; Holoubek, I.; Klanova, J.; Jones, K.C. Field calibration of polyurethane foam (PUF) disk passive air samplers for PCBs and OC pesticides. Environ. Pollut. 2008, 156, 1290–1297. [Google Scholar] [CrossRef]
- Koblizkova, M.; Lee, S.C.; Harner, T. Sorbent impregnated polyurethane foam disk passive air samplers for investigating current-use pesticides at the global scale. Atmos. Pollut. Res. 2012, 3, 456–462. [Google Scholar] [CrossRef]
- Galon, L.; Bragagnolo, L.; Korf, E.P. Mobility and environmental monitoring of pesticides in the atmosphere—A review. Environ. Sci. Pollut. Res. 2021, 28, 32236–32255. [Google Scholar] [CrossRef]
- Kruse-Plaß, M.; Hofmann, F.; Wosniok, W.; Schlechtriemen, U.; Kohlschütter, N. Pesticides and pesticide-related products in ambient air in Germany. Environ. Sci. Eur. 2021, 33, 1–21. [Google Scholar] [CrossRef]
- Lai, F.Y.; Rauert, C.; Gobelius, L.; Ahrens, L. A critical review on passive sampling in air and water for per- and polyfluoroalkyl substances (PFASs). TrAC Trends Anal. Chem. 2019, 121, 115311. [Google Scholar] [CrossRef]
- Lee, S.C.; Harner, T.; Pozo, K.; Shoeib, M.; Wania, F.; Muir, D.C.G.; Barrie, L.A.; Jones, K.C. Polychlorinated naphthalenes in the global atmospheric passive sampling (GAPS) study. Environ. Sci. Technol. 2007, 41, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Shoeib, M.; Harner, T. Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 2002, 36, 4142–4151. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, B.; Österman, C.; Koca Akdeva, H.; Moldanová, J.; Langer, S. The use of Polyurethane Foam (PUF) passive air samplers in exposure studies to PAHs in Swedish seafarers polycyclic aromatic compounds. TrAC-Trends Anal. Chem. 2022, 42, 448–459. [Google Scholar] [CrossRef]
- Alani, R.; Zhao, S.; Liu, X.; Akinrinade, O.; Agunbiade, F.; Ayejuyo, O.; Zhang, G. Concentrations, profiles and exposure risks of polycyclic aromatic hydrocarbons (PAHs) in passive air samples from Lagos, Nigeria. Atmos. Pollut. Res. 2021, 12, 101162. [Google Scholar] [CrossRef]
- Vuong, Q.T.; Kim, S.J.; Nguyen, T.N.T.; Thang, P.Q.; Lee, S.J.; Ohura, T.; Choi, S.D. Passive air sampling of halogenated polycyclic aromatic hydrocarbons in the largest industrial city in Korea: Spatial distributions and source identification. J. Hazard. Mater. 2020, 382, 121238. [Google Scholar] [CrossRef]
- Pozo, K.; Harner, T.; Lee, S.C.; Sinha, R.K.; Sengupta, B.; Loewen, M.; Geethalakshmi, V.; Kannan, K.; Volpi, V. Assessing seasonal and spatial trends of persistent organic pollutants (POPs) in Indian agricultural regions using PUF disk passive air samplers. Environ. Pollut. 2011, 159, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Zhang, G.; Cheng, H.; Balasubramanian, P.; Li, J.; Jones, K.C. Passive air sampling of polybrominated diphenyl ethers in New Delhi, Kolkata, Mumbai and Chennai: Levels, homologous profiling and source apportionment. Environ. Pollut. 2017, 231, 1181–1187. [Google Scholar] [CrossRef]
- Odabasi, M.; Bayram, A.; Elbir, T.; Dumanoglu, Y.; Kara, M.; Altiok, H.; Cetin, B. Investigation of seasonal variations and sources of atmospheric polychlorinated naphthalenes (PCNs) in an urban area. Atmos. Pollut. Res. 2012, 3, 477–484. [Google Scholar] [CrossRef]
- Hayward, S.J.; Gouin, T.; Wania, F. Comparison of four active and passive sampling techniques for pesticides in air. Environ. Sci. Technol. 2010, 44, 3410–3416. [Google Scholar] [CrossRef]
- Hazrati, S.; Harrad, S. Causes of variability in concentrations of polychlorinated biphenyls and polybrominated diphenyl ethers in indoor air. Environ. Sci. Technol. 2006, 40, 7584–7589. [Google Scholar] [CrossRef]
- Chaemfa, C.; Wild, E.; Davison, B.; Barber, J.L.; Jones, K.C. A study of aerosol entrapment and the influence of wind speed, chamber design and foam density on polyurethane foam passive air samplers used for persistent organic pollutants. J. Environ. Monit. 2009, 11, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Persoon, C.; Hornbuckle, K.C. Calculation of passive sampling rates from both native PCBs and depuration compounds in indoor and outdoor environments. Chemosphere 2009, 74, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Melymuk, L.; Robson, M.; Helm, P.A.; Diamond, M.L. Evaluation of passive air sampler calibrations: Selection of sampling rates and implications for the measurement of persistent organic pollutants in air. Atmos. Environ. 2011, 45, 1867–1875. [Google Scholar] [CrossRef]
- Pellicer-Castell, E.; Llompart, M.; Cáceres, J. Passive air sampling of organochlorine pesticides using a mesoporous sorbent. J. Chromatogr. A 2000, 870, 411–418. [Google Scholar]
- Lacroix, M.; Dreibine, L.; de Tymowski, B.; Vigneron, F.; Edouard, D.; Bégin, D.; Nguyen, P.; Pham, C.; Savin-Poncet, S.; Luck, F.; et al. Silicon carbide foam composite containing cobalt as a highly selective and re-usable Fischer Tropsch synthesis catalyst. Appl. Catalysis. A Gen. 2011, 397, 62–72. [Google Scholar] [CrossRef]
- Tuduri, L.; Harner, T.; Hung, H. Polyurethane foam (PUF) disks passive air samplers: Wind effect on sampling rates. Environ. Pollut. 2006, 144, 377–383. [Google Scholar] [CrossRef]
- Zhang, X.; Brown, T.N.; Ansari, A.; Yeun, B.; Kitaoka, K.; Kondo, A.; Lei, Y.D.; Wania, F. Effect of wind on the chemical uptake kinetics of a passive air sampler. Environ. Sci. Technol. 2013, 47, 7868–7875. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Cinelli, G.; Notardonato, I. Sampling of organophosphorus pesticides at trace levels in the atmosphere using XAD-2 adsorbent and analysis by gas chromatography coupled with nitrogen-phosphorus and ion trap mass spectrometry detectors. Anal. Bioanal. Chem. 2012, 404, 1517–1527. [Google Scholar] [CrossRef]
- Adou, K.; Bontoyan, W.R.; Sweeney, P.J. Multiresidue method for the analysis of pesticide residues in fruits and vegetables by accelerated solvent xxtraction and capillary gas chromatography. J. Agric. Food Chem. 2001, 49, 4153–4160. [Google Scholar] [CrossRef]
- Rodrigues, A.; Delhomme, O.; Millet, M. Use of PLE-ATD-GC/MSMS for the quantification of airborne pesticides in active and passive samples and in dust. J. Chromatogr. Sci. 2023, bmad020. [Google Scholar] [CrossRef]
- Beristain-Montiel, E.; Villalobos-Pietrini, R.; Arias-Loaiza, G.E.; Gómez-Arroyo, S.L.; Amador-Muñoz, O. An innovative ultrasound assisted extraction micro-scale cell combined with gas chromatography/mass spectrometry in negative chemical ionization to determine persistent organic pollutants in air particulate matter. J. Chromatogr. A 2016, 1477, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Yusà, V.; Coscollà, C.; Millet, M. New screening approach for risk assessment of pesticides in ambient air. Atmos. Environ. 2014, 96, 322–330. [Google Scholar] [CrossRef]
- Lesueur, C.; Gartner, M.; Mentler, A.; Fuerhacker, M. Comparison of four extraction methods for the analysis of 24 pesticides in soil samples with gas chromatography–mass spectrometry and liquid chromatography–ion trap–mass spectrometry. Talanta 2008, 75, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.; Kim, S.D. Determination of toxic organic pollutants in fine particulate matter using selective pressurized liquid extraction and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2019, 1590, 39–46. [Google Scholar] [CrossRef]
- Galmiche, M.; Delhomme, O.; François, Y.N.; Millet, M. Environmental analysis of polar and non-polar Polycyclic Aromatic Compounds in airborne particulate matter, settled dust and soot: Part I: Sampling and sample preparation. TrAC Trends Anal. Chem. 2021, 134, 116099. [Google Scholar] [CrossRef]
- Coscollà, C.; Yusà, V.; Martí, P.; Pastor, A. Analysis of currently used pesticides in fine airborne particulate matter (PM 2.5) by pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1200, 100–107. [Google Scholar] [CrossRef]
- Mercier, F.; Gilles, E.; Saramito, G.; Glorennec, P.; Le Bot, B. A multi-residue method for the simultaneous analysis in indoor dust of several classes of semi-volatile organic compounds by pressurized liquid extraction and gas chromatography/tandem mass spectrometry. J. Chromatogr. A 2014, 1336, 101–111. [Google Scholar] [CrossRef]
- Lintelmann, J.; Fischer, K.; Karg, E. Determination of selected polycyclic aromatic hydrocarbons and oxygenated polycyclic aromatic hydrocarbons in aerosol samples by high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2005, 381, 508–519. [Google Scholar] [CrossRef]
- Aydin, M.E.; Ozcan, S.; Tor, A. Ultrasonic solvent extraction of persistent organic pollutants from airborne particles. Clean 2007, 35, 660–668. [Google Scholar] [CrossRef]
- Bendicho, C.; De La Calle, I.; Pena, F.; Costas, M.; Cabaleiro, N.; Lavilla, I. Ultrasound-assisted pretreatment of solid samples in the context of green analytical chemistry. TrAC Trends Anal. Chem. 2012, 31, 50–60. [Google Scholar] [CrossRef]
- Nascimento, M.M.; da Rocha, G.O.; de Andrade, J.B. Pesticides in fine airborne particles: From a green analysis method to atmospheric characterization and risk assessment. Sci. Rep. 2017, 7, 2267. [Google Scholar] [CrossRef] [PubMed]
- Dvoršćak, M.; Bešlić, I.; Fingler, S.; Godec, R.; Šega, K.; Vasilić, Ž.; Drevenkar, V. Organochlorine Pesticides and Polychlorinated Biphenyls in Atmospheric Particles Collected in Zagreb, Croatia. Croat. Chem. Acta 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Jonker, M.T.O.; Koelmans, A.A. Extraction of polycyclic aromatic hydrocarbons from soot and sediment: Solvent evaluation and implications for sorption mechanism. Environ. Sci. Technol. 2002, 36, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Llompart, M.; Celeiro, M.; Garcia-Jares, C.; Dagnac, T. Chapter Six-Microwave-Assisted Extraction of Pesticides and Emerging Pollutants in the Environment. In Comprehensive Analytical Chemistry; Ibáñez, E., Cifuentes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 76, pp. 131–201. [Google Scholar] [CrossRef]
- Wurl, O.; Obbard, J.P. Organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in Singapore’s coastal marine sediments. Chemosphere 2005, 58, 925–933. [Google Scholar] [CrossRef]
- Yusà, V.; Pastor, A.; de la Guardia, M. Microwave-assisted extraction of polybrominated diphenyl ethers and polychlorinated naphthalenes concentrated on semipermeable membrane devices. Anal. Chim. Acta 2006, 565, 103–111. [Google Scholar] [CrossRef]
- Dean, J.R. Microwave extraction. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Naccarato, A.; Tassone, A.; Martino, M.; Elliani, R.; Sprovieri, F.; Pirrone, N.; Tagarelli, A. An innovative green protocol for the quantification of benzothiazoles, benzotriazoles and benzosulfonamides in PM10 using microwave-assisted extraction coupled with solid-phase microextraction gas chromatography tandem-mass spectrometry. Environ. Pollut. 2021, 285, 117487. [Google Scholar] [CrossRef]
- Garrido Frenich, A.; Martínez Vidal, J.L.; Moreno Frías, M. Determination of organochlorine pesticides by GC-ECD and GC-MS-MS techniques including an evaluation of the uncertainty associated with the results. Chromatographia 2003, 57, 213–220. [Google Scholar] [CrossRef]
- Wang, G.L.; Ma, L.M.; Sun, J.H.; Zhang, G. Occurrence and distribution of organochlorine pesticides (DDT and HCH) in sediments from the middle and lower reaches of the Yellow River, China. Environ. Monit. Assess. 2010, 168, 511–521. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Tao, S.; Liu, L.; Li, W.; Meng, B. Comparison and analysis of organochlorine pesticides and hexabromobiphenyls in environmental samples by gas chromatography-electron capture detector and gas chromatography-mass spectrometry. J. Chromatogr. Sci. 2015, 53, 197–203. [Google Scholar] [CrossRef]
- García-Bellido, J.; Freije-Carrelo, L.; Moldovan, M.; Encinar, J.R. Recent advances in GC-ICP-MS: Focus on the current and future impact of MS/MS technology. TrAC Trends Anal. Chem. 2020, 130, 115963. [Google Scholar] [CrossRef]
- Maceira, A.; Marcé, R.M.; Borrull, F. Analytical methods for determining organic compounds present in the particulate matter from outdoor air. TrAC Trends Anal. Chem. 2019, 122, 115707. [Google Scholar] [CrossRef]
- Coscollà, C.; Castillo, M.; Pastor, A.; Yusà, V. Determination of 40 currently used pesticides in airborne particulate matter (PM 10) by microwave-assisted extraction and gas chromatography coupled to triple quadrupole mass spectrometry. Anal. Chim. Acta 2011, 693, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Katzman, D.; Bohbot-Raviv, Y.; Dubowski, Y. Does polyacrylamide-based adjuvant actually reduce primary drift of airborne pesticides? Sci. Total Environ. 2021, 775, 145816. [Google Scholar] [CrossRef] [PubMed]
- Kazos, E.A.; Stalikas, C.D.; Nanos, C.G.; Konidari, C.N. Determination of dithiocarbamate fungicide propineb and its main metabolite propylenethiourea in airborne samples. Chemosphere 2007, 68, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, C.; Wang, W.; Fan, R.; Zhang, Z.; Guo, Y. Rapid simultaneous screening and identification of multiple pesticide residues in vegetables. Anal. Chim. Acta 2012, 757, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Jo, E.K. Multiresidue pesticide analysis in Korean ginseng by gas chromatography–triple quadrupole tandem mass spectrometry. Food Chem. 2012, 134, 2497–2503. [Google Scholar] [CrossRef]
- Wu, C.C. Multiresidue method for the determination of pesticides in Oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 580–587. [Google Scholar] [CrossRef]
- Scheyer, A.; Morville, S.; Mirabel, P.; Millet, M. A multiresidue method using ion-trap gas chromatography tandem mass spectrometry with or without derivatization with pentafluorobenzylbromide for the analysis of pesticides in the atmosphere. Anal. Bioanal. Chem. 2005, 381, 1226–1233. [Google Scholar] [CrossRef]
- Somoano-Blanco, L.; Rodriguez-Gonzalez, P.; Profrock, D.; Prange, A.; Alonso, J.I.G. Comparison of different mass spectrometric techniques for the determination of polychlorinated biphenyls by isotope dilution using 37Cl-labelled analogues. Anal. Methods 2015, 7, 9068–9075. [Google Scholar] [CrossRef]
- Li, H.P.; Li, J.H.; Li, G.C.; Jen, J.F. Simultaneous determination of airborne carbamates in workplace by high performance liquid chromatography with fluorescence detection. Talanta 2004, 63, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Hall, P. Comparison of Gas Chromatography-Mass Spectrometry and Gas Chromatography-Tandem Mass Spectrometry with Electron Ionization and Negative-Ion Chemical Ionization for Analyses of Pesticides at Trace Levels in Atmospheric Samples. Anal. Chem. Insights 2008, 3, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Kaur, P.; Bansal, P. Chapter 8—High-performance liquid chromatographic techniques for determination of organophosphate pesticides in complex matrices. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, R.B., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–196. [Google Scholar] [CrossRef]
- Rajski, L.; Gómez-Ramos, M.M.; Fernández-Alba, A.R. Large pesticide multiresidue screening method by liquid chromatography-Orbitrap mass spectrometry in full scan mode applied to fruit and vegetables. J. Chromatogr. A 2014, 1360, 119–127. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.M.; Ferrer, C.; Malato, O.; Agüera, A.; Fernández-Alba, A.R. Liquid chromatography-high-resolution mass spectrometry for pesticide residue analysis in fruit and vegetables: Screening and quantitative studies. J. Chromatogr. A 2013, 1287, 24–37. [Google Scholar] [CrossRef]
- López, A.; Yusà, V.; Millet, M.; Coscollà, C. Retrospective screening of pesticide metabolites in ambient air using liquid chromatography coupled to high-resolution mass spectrometry. Talanta 2016, 150, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Coscollà, C.; León, N.; Pastor, A.; Yusà, V. Combined target and post-run target strategy for a comprehensive analysis of pesticides in ambient air using liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2014, 1368, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef]
- Mathew, M.L.; Gopalakrishnan, A.; Aravindakumar, C.T.; Aravind, U.K. Low—Cost multilayered green fiber for the treatment of textile industry waste water. J. Hazard. Mater 2019, 365, 297–305. [Google Scholar] [CrossRef]
- Obinaju, B.E.; Martin, F.L. ATR-FTIR spectroscopy reveals polycyclic aromatic hydrocarbon contamination despite relatively pristine site characteristics: Results of a field study in the Niger Delta. Environ. Int. 2016, 89–90, 93–101. [Google Scholar] [CrossRef]
- Sreejith, M.V.; Aradhana, K.S.; Varsha, M.; Cyrus, M.K.; Aravindakumar, C.T.; Aravind, U.K. ATR-FTIR and LC-Q-ToF-MS analysis of indoor dust from different micro-environments located in a tropical metropolitan area. Sci. Total Environ. 2021, 783, 147066. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Thumanu, K.; Chantharakhon, C.; Phoomalee, C.; Tharasawatpipat, C.; Apiratikul, R.; Poshyachinda, S. Applying synchrotron radiation-based attenuated total reflection-Fourier transform infrared to evaluate the effects of shipping emissions on fluctuations of PM10-bound organic functional groups and ionic species. Atmos. Pollut. Res. 2022, 13, 101517. [Google Scholar] [CrossRef]
- Yu, P. Application of advanced synchrotron radiation-based Fourier transform infrared (SR-FTIR) microspectroscopy to animal nutrition and feed science: A novel approach. Br. J. Nutr. 2004, 92, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Feltracco, M.; Barbaro, E.; Maule, F.; Bortolini, M.; Gabrieli, J.; De Blasi, F.; Cairns, W.R.L.; Dallo, F.; Zangrando, R.; Barbante, C.; et al. Airborne polar pesticides in rural and mountain sites of North-Eastern Italy: An emerging air quality issue. Environ. Pollut. 2022, 308, 119657. [Google Scholar] [CrossRef] [PubMed]

| Pesticide | Name | Indoor Air Concentration Range (ng m−3) | Outdoor Air Concentration Range (ng m−3) | Investigated Country | References |
|---|---|---|---|---|---|
| Organochlorine | Chlordane | 3.50–4.80 | 0.01–0.02 | USA, South Africa | [19,20] |
| 4,4′-DDE | 1.20–190 | 0.002–25.60 | Nepal, USA, China, Czech Republic | [19,21,22,23] | |
| 4,4′-DDD | ND 1–5.00 | 0.011–154 | China, Czech Republic, USA, South Africa | [20,22,23,24] | |
| HCB | 0.16–1.80 | ND–0.43 | Czech Republic, Canada, China | [23,24,25,26] | |
| HCH | 0.05–7.82 | 0.00012–0.0184 | Czech Republic, Canada, China, South Africa | [20,24,25,26] | |
| Heptachlor | 5.00 | 0.00065–0.0015 | USA, South Africa | [19,20] | |
| Endosulfan | 0.3–0.8 | 0.00009–81.31 | France, Nepal, Pakistan, China | [21,22,26,27,28,29] | |
| Mirex | ND–0.00477 | ND–9.94 | USA, South Africa | [20,24] | |
| Lindane | 1.4–11.8 | NI 2 | France | [27] | |
| Aldrin | NI | 0.36–1.18 | China | [22] | |
| 4,4′-methoxychlor | NI | 0.00040–0.00883 | China | [26] | |
| Oxychlordane | NI | 0.00048 | South Africa | [20] | |
| Organophosphate | Chlorpyriphos | 0.4–83.4 | 0.1–36.1 | USA, Spain, Costa Rica, Czech Republic, South Africa | [20,30,31,32,33,34] |
| Diazinon | 1.0–427.5 | 0.28–1.49 | USA, France, South Africa | [19,20,28,30] | |
| Malathion | 0.2–16.1 | 0.010–1.0 | France, USA, Spain, Czech Republic, South Africa | [20,27,30,31,33] | |
| Parathion-methyl | 14.3 | NI | France | [27] | |
| Dichlorvos | 22.9–53,000 | NI | France, South Korea | [27,35] | |
| Dimethoate | NI | 0.1–1.0 | South Africa | [20,34] | |
| Ethoprophos | NI | 0.21–0.48 | France | [28] | |
| Carbamates | Carbaryl | NI | 1.30 | South Africa | [20] |
| Acetamides | Metazachlor | NI | 0.0092–3.13 | 25 member states of the European Union (EU-25) 3, South Africa | [20,36] |
| Triazinones/ Triazines/ Triazoles | Metribuzin | NI | 0.03 | South Africa | [20] |
| Atrazine | NI | 0.04 | South Africa | [20] | |
| Simazine | NI | 0.88 | South Africa | [20] | |
| Terbuthylazine | NI | 0.79 | South Africa | [20] | |
| Propiconazole | NI | 0.08 | South Africa | [20] | |
| Tebuconazole | NI | 0.43–22.2 | South Africa | [20,34] | |
| Herbicide | Alachlor | NI | 0.12–6.03 | France | [28] |
| Alconifen | NI | 0.23–4.15 | 25 member states of the European Union (EU-25) | [36] | |
| Diuron | NI | 0.12 | South Africa | [20] | |
| Glyphosate | NI | 0.18–510.0 | USA, Malaysia, France | [37,38,39] | |
| Trifluralin | NI | 0.12–40.74 | 25 member states of the European Union (EU-25) | [36] | |
| Fungicide | Captan | NI | 1.19–67.62 | 25 member states of the European Union (EU-25) | [28,36] |
| Chlorothalonil | NI | 0.11–107.93 | France | [28] | |
| Carbendazim | NI | 0.028 | Spain | [31] | |
| Epoxiconazole | NI | 0.12–3.99 | France | [28] | |
| Folpet | NI | 7.91–82.22 | 25 member states of the European Union (EU-25) | [36] | |
| Tebuconazole | NI | 22.2 | Czech Republic | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udomkun, P.; Boonupara, T.; Sumitsawan, S.; Khan, E.; Pongpichan, S.; Kajitvichyanukul, P. Airborne Pesticides—Deep Diving into Sampling and Analysis. Toxics 2023, 11, 883. https://doi.org/10.3390/toxics11110883
Udomkun P, Boonupara T, Sumitsawan S, Khan E, Pongpichan S, Kajitvichyanukul P. Airborne Pesticides—Deep Diving into Sampling and Analysis. Toxics. 2023; 11(11):883. https://doi.org/10.3390/toxics11110883
Chicago/Turabian StyleUdomkun, Patchimaporn, Thirasant Boonupara, Sulak Sumitsawan, Eakalak Khan, Siwatt Pongpichan, and Puangrat Kajitvichyanukul. 2023. "Airborne Pesticides—Deep Diving into Sampling and Analysis" Toxics 11, no. 11: 883. https://doi.org/10.3390/toxics11110883
APA StyleUdomkun, P., Boonupara, T., Sumitsawan, S., Khan, E., Pongpichan, S., & Kajitvichyanukul, P. (2023). Airborne Pesticides—Deep Diving into Sampling and Analysis. Toxics, 11(11), 883. https://doi.org/10.3390/toxics11110883







